Abstract
Integrin-mediated cell adhesion and signaling events are essential for the proper development and homeostasis of most epithelial tissues. Dysregulation of integrin expression and function can cause abnormal epithelial cell proliferation and/or differentiation, contributing to the pathogenesis of malignant epithelial cancers. Here we report on the use of a conditional knockout strategy exploiting the Cre/Lox technology to study the in vivo functions of αv integrins during epithelial cell proliferation and differentiation. We show that genetic ablation of αv integrin expression in basal epithelial cells of the eyelid skin and conjunctiva causes the formation of tumors that are strikingly similar to the malignant epithelial cancer, squamous cell carcinoma. These data suggest a mechanism whereby αv integrins normally suppress epithelial cell proliferation, likely via adhesion to ECM ligands, as well as by the modulation of intracellular signaling cascades. We propose that αv gene deletion eliminates normal integrin-mediated growth suppression, ultimately leading to cellular transformation and tumorigenesis. Hence, these studies reveal a novel tumor suppressor-like function of αv integrins and provide a genetically tractable mouse model for studying the pathogenesis of squamous cell carcinoma and related cancers of epithelial origin, as well as to test and develop novel therapeutic compounds to treat or prevent squamous cell carcinoma of the skin.
The skin is a dynamic organ composed of two multicellular layers, the dermis and epidermis, which are separated by an intervening basement membrane.1 An assortment of extracellular matrix (ECM) proteins within the basement membrane supply instructive cues that regulate basal cell proliferation, differentiation, and migration.2 Abnormal regulation of these events can lead to the pathogenesis of a variety of epithelial abnormalities, including the malignant cancer squamous cell carcinoma (SCC).3 SCC is the most common form of skin cancer and arises via defective growth regulatory pathways in stem cells or transit amplifying cells located in the epidermal basal layer.
Members of the integrin family of ECM receptors play critical roles during the development and homeostasis of most stratified epithelial tissues.4 Several integrins and their associated intracellular signaling effectors are expressed in basal epithelial cells, and gene ablation studies in mice reveal essential functional roles for these molecules in the formation and maintenance of the epithelial tissues, particularly the skin.5 For example, mice genetically null for the α6 or β4 integrin genes develop skin pathologies due to defective epidermal-dermal adhesion.6,7 Loss of α3 integrin expression leads to skin blistering phenotypes related to defective assembly of epidermal basement membranes.8 Selective ablation of the murine β1 integrin gene in the skin leads to severe defects in epidermal development and homeostasis,9 and activating mutations in the human β1 integrin gene are found in rare cases of SCC.10
The αv integrin subfamily consists of αvβ1, αvβ3, αvβ5, αvβ6, and αvβ8, and various genetic ablation studies in mice have shown that these integrins play important roles in multiple physiological and pathological contexts.11,12,13,14,15,16 However, in vivo genetic models to study αv integrin-mediated regulation of epithelial cell growth have not been reported. In this study we use Cre/lox technology to analyze the in vivo growth regulatory functions for the αv integrin subunit. We show that genetic ablation of αv expression selectively in basal cells of the eyelid skin and conjunctiva leads to development of epithelial tumors with pathological similarities to SCC. These data are direct molecular genetic evidence that αv integrins provide critical growth regulatory functions during epithelial proliferation and homeostasis. To our knowledge, these findings represent the first experimental data showing that genetic ablation of αv integrin expression and function in epithelial cells leads to dysregulation of normal cell growth and homeostasis, and suggest a physiological tumor suppressor-like function for αv integrins. Furthermore, this study provides a novel mouse genetic model to study the pathogenesis of SCC in the eyelid skin and conjunctiva.
Materials and Methods
Mouse Strains and Genotyping
This murine glial fibrillary acidic protein (mGFAP)-Cre transgene consists of a genomic fragment encompassing the minimal murine GFAP promoter, as well as regulatory intronic sequences flanking the Cre cDNA.17 Generation and characterization of mGFAP-Cre transgenic mice have been described elsewhere.18 The αv-flox mouse strain has been previously described.14 The Rosa26-LoxSTOPLox-LacZ reporter strain was purchased from The Jackson Laboratories.19 All mouse genotypes were confirmed by standard PCR-based genotyping of genomic DNA isolated from tail snips. The following primer sequences were used for PCR genotyping: Cre, 5′-ACCAGCCAGCTATCAACTC-3′, and 5′-TATACGCGTGCTAGCGAAGATCTCCATCTTCCAGCAG-3′. The Cre primers yield a single PCR product of ∼200 bp. αv-flox primers, F1: 5′-GTTGAGTATGCTCCATGCAGGTCA-3′, F2: 5′-TTCAGGACGGCACAAAGACCGTTG-3′, and R: 5′-CACAAATCAAGGATGACCAAACTGAG-3′. The F1-R primer pair generates a PCR product of approximately 350 bp. The F2-R primer pair generates an 850 bp band representing the non-recombined αv-flox allele, or a 250bp band representing the recombined αv-flox allele. The αv+/+ or αv−/− alleles yield a PCR product of 250 bp using either the F1-R primer pair, or a PCR product of 550 bp using the F2-R primer pair.
Antibodies, Immunohistochemistry, and Histology
The anti-αv antiserum was used at a 1:300 dilution.20 To minimize nonspecific immunoreactivity, the diluted antibody was first pre-absorbed using αv-null acetone-extracted protein, prepared as previously described.14 The anti-β-catenin antibody was purchased from Chemicon, Inc. For immunofluorescence and immunohistochemical analyses, samples of eye tumors from GFAP-Cre+; αvflox/− mutant animals, or eyelid tissue from control animals were fresh-frozen in Tissue Tek OCT (Miles). Sections (7 μm) were immunostained with rabbit IgG (10 μg/ml), or anti-αv antibody. A secondary antibody conjugated to horseradish peroxidase (Vector Laboratories) in combination with diaminobenzidine chromagenic substrate was used for immunohistochemistry. Alternatively, an Alexa488-conjugated goat anti-rabbit secondary antibody (Molecular Probes) was used for immunofluorescence. For histopathology studies, eyelids or eye tumors were excised and fixed overnight at 4°C with 4% paraformaldehyde in phosphate buffered saline. Tissue was subsequently dehydrated and processed for standard paraffin embedding and H&E staining. Alternatively, to visualize mucin-expressing goblet-like cells, paraffin sections from eye tumors were counterstained with periodic acid-Schiff and diastase, or Alcian Blue. Oil Red O staining was performed on sections prepared from unfixed, fresh-frozen ocular tumors.
Results
A Murine GFAP-Cre Transgene Is Expressed in Epithelial Cells of the Developing Eyelid and Conjunctiva
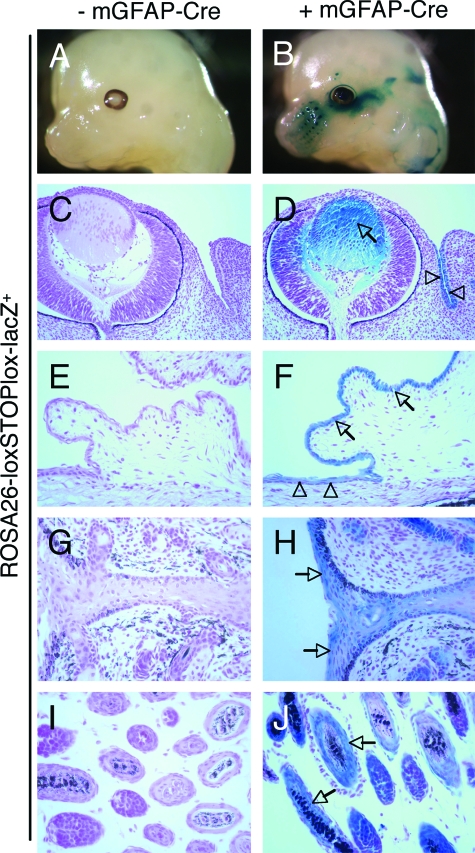
Previously, we used Cre/lox technology to selectively ablate the murine αv integrin gene in central nervous system (CNS) neural cells.14 These efforts involved analyzing the temporal and spatial expression patterns of various Cre transgenes reportedly expressed in specific cell types in the CNS. One such transgene, consisting of a fragment of the mGFAP promoter inserted 5′ to the cDNA encoding Cre recombinase (mGFAP-Cre), was reported to be expressed specifically in postnatal CNS glia and neurons.21 Indeed, using the ROSA26-loxSTOPlox-lacZ reporter mouse,19 we confirmed Cre activity in some neuronal and glial cells of the developing neural tube, as well as some cells in the subventricular zone of the brain (data not shown). However, we also detected transgene expression outside of the CNS. As shown in Figure 1, B and D, embryos (E13.5) harboring the mGFAP-Cre and ROSA26-loxSTOPlox-lacZ transgenes expressed robust levels of Cre in epithelial cells of the developing lens and conjunctiva. Additionally, analysis of neonatal (P7) transgenic mice revealed Cre expression in epithelial cells of the conjunctiva and cornea (Figure 1F), eyelid (Figure 1H), as well as occasional hair follicles in the eyelid epidermis (Figure 1J). We did not detect lacZ activity in embryos or neonates lacking the GFAP-Cre transgene (Figure 1, A, C, E, G, and I). The pattern of Cre expression was primarily localized to epithelial cells of the eyelid skin; we did not detect Cre activity in epithelia of other neonatal organs, including the intestine and lung (data not shown). It is likely that the epithelial expression pattern of the GFAP-Cre transgene is an aberrant consequence of the transgene insertion site. For example, the transgene may have inserted into a genomic region that is regulated by enhancer elements that activate gene expression in specific epithelial cells of the developing eye. A second transgene that we have previously characterized,14 consisting of the human GFAP promoter regulating Cre expression, is active primarily in CNS glia, and is not expressed in skin epithelial cells (unpublished data). However, it also remains possible that some cells in the eyelid skin and conjunctiva originate from GFAP-expressing progenitors, or that some basal epithelial cells express GFAP as others have recently shown.22
Figure 1.
A murine GFAP-Cre transgene is expressed in epithelial cells of the embryonic and postnatal eye. Embryos (E13.5) expressing the ROSA26-loxSTOPLox-LacZ reporter transgene in the absence (A) or presence (B) of the mGFAP-Cre transgene were dissected and whole-mounts were stained to determine the spatial pattern of β-galactosidase activity. β-galactosidase is minimally expressed in embryos lacking the mGFAP-Cre transgene (A); however, embryos harboring the mGFAP-Cre transgene display Cre-mediated expression of β-galactosidase (B). C, D: Embryonic heads were sectioned horizontally and the pattern of β-galactosidase activity was analyzed microscopically. Note the β-galactosidase activity in epithelial cells of the lens (arrow in D) and conjunctiva (arrowheads in D). Sagittal histological sections through the center of the neonatal eye (P7) from ROSA26-loxSTOPlox-LacZ transgenics in the absence (E, G, I) or presence (F, H, J) of the mGFAP-Cre transgene. Cre-mediated β-galactosidase activity is present in epithelial cells in the developing conjunctiva (arrows in F), cornea (arrowheads in F), eyelid (arrows in H), and hair follicles (arrows in J).
Targeted Deletion of the αv Integrin Gene Using the mGFAP-Cre Transgene
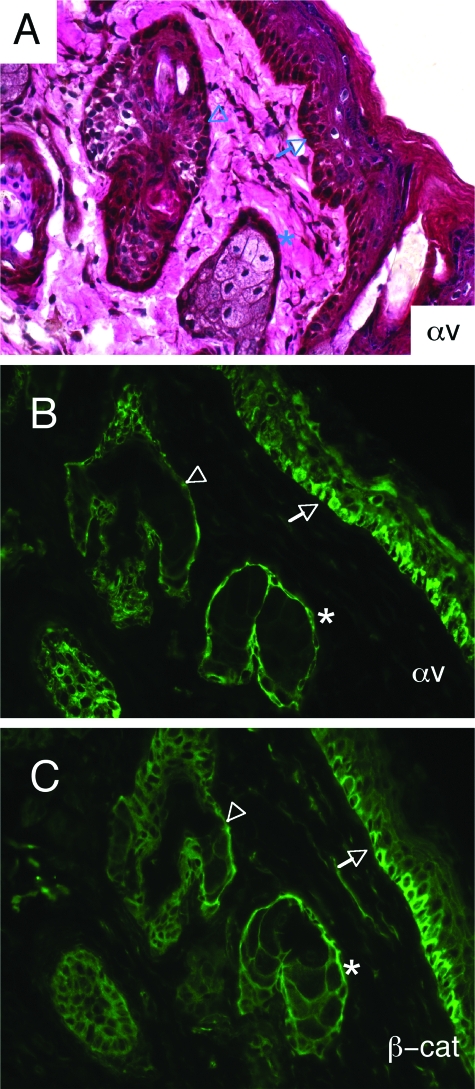
We used an anti-αv integrin antibody14,20 to analyze the spatial expression pattern of αv integrin protein in the postnatal murine eyelid. αv integrin protein expression was detected in the basal epithelium of the normal eyelid, as well as by basal epithelial cells in sebaceous glands and hair follicles (Figure 2, A and B). The expression pattern of αv integrin protein was very similar to the pattern detected for β-catenin (Figure 2C), a protein commonly expressed in basal cells of stratified epithelial tissues.23 We selectively ablated the αv integrin gene by generating mice harboring a conditional αv allele (αvflox/flox) in combination with the mGFAP-Cre transgene (Figure 3A). First, mGFAP-Cre hemizygotes were bred with αv+/− mice to generate GFAP-Cre+; αv+/− progeny, which were subsequently bred with αvflox/flox mice.13,14 The resulting mutant progeny are hemizygous for the mGFAP-Cre transgene, and carry one αv-flox allele and one αv-null allele. Littermate controls were hemizygous for the mGFAP-Cre transgene, and carry one αv-flox allele and one αv wild-type allele.
Figure 2.
αv Integrin protein is expressed in basal epithelial cells of the murine eyelid. A: Eyelids from adult mGFAP-Cre+; αvflox/+ mice were immunostained with anti-αv integrin antibody. Note the expression of αv integrin protein in basal epithelial cells of the eyelid epidermis (arrows), as well as the basal epithelial cells of hair follicles (arrowheads) and sebaceous glands (asterisks). B: Immunufluorescence staining with anti-αv integrin antibody shows αv protein expression in basal epithelial cells of the eyelid epidermis (arrows), hair follicles (arrowheads), and sebaceous glands (asterisks). C: αv integrin protein expression overlaps with β-catenin, which is commonly expressed by basal epithelial cells.
Figure 3.
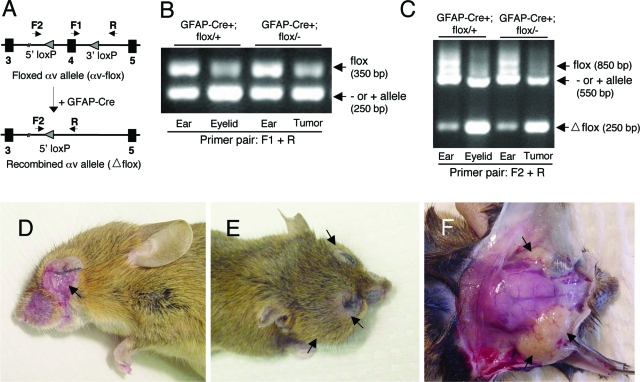
Conditional ablation of the αv integrin gene in basal epithelial cells of the eye leads to tumorigenesis. A: Experimental strategy to selectively ablate a conditional αv integrin allele. Arrows indicate primers for monitoring Cre-mediated genomic recombination. Control mice harbor one αvflox allele and one αv wild-type (+) allele and mutant mice harbor an αvflox allele and an αv null (−) allele via deletion of exon one.14 The primer pair F1 and R amplifies the 350-bp genomic region spanning exon 4 and the 3′ loxP site. The primer pair F2 and R amplifies the region (850 bp) containing both the 5′ and 3′ loxP sites. B: PCR-based amplification of genomic DNA isolated from ear or eyelid tissues from control (GFAP-Cre+;αvflox/+) mice. Ear and eyelid tumor samples from mutant (GFAP-Cre+;αvflox/−) animals were also analyzed. The primer pair, F1 and R, amplifies a 350 bp band, containing the 3′ loxP sequence. The intensity of this band is reduced in eyelid and eye tumor samples, due to cre-mediated recombination of the αv-flox allele. C: Identical genomic samples described in (B) were used with the F2 and R primer pair, which amplify an 850 bp band containing 5′ and 3′ loxP sites. In samples from control eye and mutant tumor, amplification of this 850 bp band is significantly reduced. Instead, a 250 bp band representing the recombined αv-flox allele (lower panel in A) is detected. D: A twelve month-old GFAP-Cre+; αvflox/− mutant mouse. Note the macroscopic tumor displaying obvious ulceration (arrow). E: An 18 month-old GFAP-Cre+; αvflox/− mutant mouse displaying large bilateral eye tumors (arrows). F: The mutant mouse in (E) with the skin removed to expose the skull and tumor masses encompassing both eyes (arrows).
We used genomic PCR to test for mGFAP-Cre-mediated deletion of the αv integrin gene. We isolated genomic DNA from eyelid tissue, and analyzed αv-flox deletion using PCR to monitor a 350 bp PCR band representing the αv-flox allele (Figure 3B). Analysis of ear, eyelid, or eye tumor genomic DNA samples from control and mutant mice revealed recombination of the αv-flox allele selectively in the eye or eye tumor samples. We monitored deletion of the conditional αv allele using a second primer pair designed to amplify an 850 bp band representing the non-recombined αv-flox allele (Figure 3C). The same primer pair amplified a 250 bp band representing the recombined αvflox/flox gene. Amplification of the complementary allele, which lacked loxP sites and is either wild-type or null for αv, yielded a 550 bp PCR product. Analysis of ear, eyelid, or eye tumor genomic DNA samples from control and mutant mice revealed recombination of the αv-flox allele selectively in the eye or eye tumor samples. This correlated with reduced intensity of the intact 850 bp αv-flox cassette, as well as an increase in the recombined 250 bp PCR product (Figure 3C).
Genetic Ablation of αv Integrin Causes the Formation of Eyelid Skin Tumors Displaying Pathological Characteristics of Squamous Cell Carcinoma
mGFAP-Cre+, αvflox/+ and mGFAP-Cre+, αvflox/− mutants were born in expected ratios, and displayed no grossly obvious developmental or behavioral abnormalities (data not shown). However, beginning as early as nine postnatal months, mGFAP-Cre+; αvflox/− mutant animals developed tumors surrounding one or both eyes (Figure 3, D–F). mGFAP-Cre+; αvflox/+ control littermates (17/17 analyzed thus far) did not develop eye tumors like those observed in the mutant animals. Unilateral or bilateral eye tumors have developed in 12/12 mutant animals analyzed thus far, with most tumors being grossly obvious by 12 to 18 months of age. One mutant with an apparent unilateral tumor also displayed metastatic lesions in the cervical lymph nodes (data not shown). In most cases (7/12 mice analyzed thus far), postmortem analyses of mutant animals with one grossly obvious tumor also revealed a smaller, microscopic ocular tumor. Additionally, postmortem analysis of two adult mutants lacking grossly obvious tumors in either eye revealed microscopic tumors in at least one eye (data not shown). Many mutants developed tumors that were ulcerated (Figure 3D), and tumor growth often led to complete closure of one or both eyes (Figure 3, E and F). Postmortem analyses of tumor size revealed late-stage tumors as large as 1 cm3 (Figure 3F).
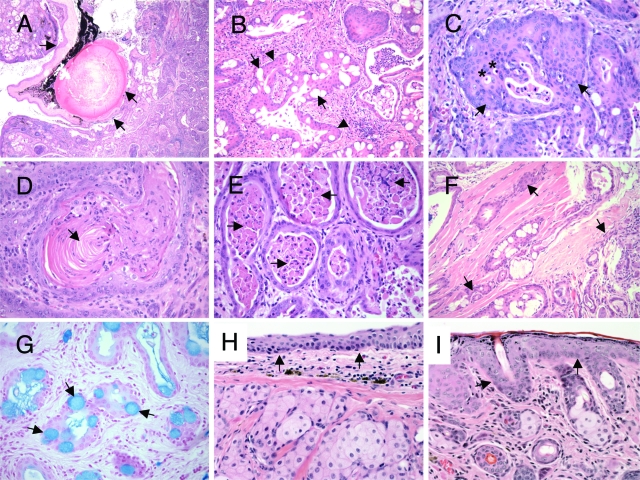
All tumors arose in the periorbital region subjacent to the palpebral and bulbar conjunctiva. Given the periorbital location of the tumors subjacent to conjunctiva and their recapitulation of the biphasic pattern of normal conjunctival epithelium, these tumors are best regarded as deriving from conjunctiva. Tumors exhibited malignant behavior, as evidenced by compression of the globe of the eye (Figure 4A), and invasion into periorbital tissues, including invasion into skeletal muscle in several cases (Figure 4F), and in one case invasion of the globe (Figure 4A). By microscopic analysis, all tumors showed similar morphological findings: an invasive squamous proliferation, many with admixed goblet-like cells displaying pale, homogeneous cytoplasms and eccentric nuclei (Figure 4B). These goblet-like cells were positive for mucin by staining with Alcian Blue (Figure 4G) and periodic acid-Schiff with diastase (data not shown), and negative for fat by Oil Red O stain performed on unfixed, frozen tumor sections. These morphological and histochemical features are most consistent with the interpretation that the goblet-like cells are mucin-secreting epithelial cells, and that they are not sebaceous in origin, nor are they macrophages recruited to the tumor for clearance of apoptotic cell debris.
Figure 4.
Genetic ablation of αv integrin in the murine eye epithelium leads to tumors with histological characteristics of squamous cell carcinoma. H&E staining of eye tumor sections from GFAP-Cre+; αvflox/− mutant animals. A: Tumor compressing and invading globe of the eye (arrows). B: Tumor showing admixed squamous cells (arrowheads) and goblet-like cells (arrows). C: Tumor with predominantly squamous differentiation (arrows) and intralumenal apoptosis (asterisks). D: Tumor showing keratinization (arrow). E: Tumor tubulo-cystic structures with marked lumenal accumulation of desquamated tumor cells (arrows). F: Tumor invading skeletal muscle of the eye (arrows). G: Tumor showing biphasic squamous and goblet-like cell differentiation. The Alcian Blue mucin stain highlights goblet-like cells (arrows). H, I: H&E stained sections from the ocular region of an αvflox/− mouse that did not harbor the GFAP-Cre transgene. Note the normal cytoarchitecture of the conjunctiva (arrows in H) and eyelid skin epidermis (arrows in I).
In all tumors the squamous component predominated and was mostly nonkeratinizing (Figure 4C), though keratinization was present in some regions in some tumors (Figure 4D). All tumors had a distinctive tubulo-cystic pattern of growth, with desquamated cells and inflammatory cells present centrally located within the tubulo-cystic structures (Figure 4E). Since the potential glandular component is not clearly malignant, it is uncertain whether these tumors meet strict criteria for adenosquamous carcinoma. Characteristic features of mucoepidermoid carcinoma are not clearly identified. These tumors represent invasive carcinomas, most in keeping with invasive squamous cell carcinoma with scattered goblet-like cells. The latter feature is of unclear morphological significance, given that similar tumors of murine or human conjunctiva have not been well characterized or described.
Discussion
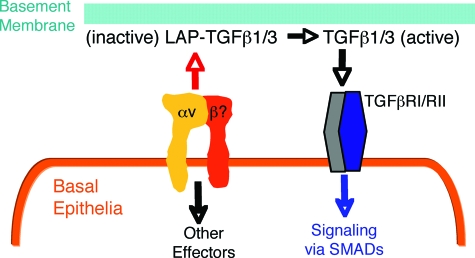
Here we have exploited Cre/Lox technology to analyze the functions of αv integrins in the eyelid skin and conjunctiva. A central result of this work is that genetic ablation of αv integrin expression in basal epithelial cells leads to SCC. Based on these data, we propose a mechanism whereby αv integrins normally suppress epithelial cell growth via adhesion to inhibitory ECM ligands within the adjacent epidermal basement membrane (Figure 5). Genetic ablation of αv integrin expression prevents normal epithelial cell growth suppression by uncoupling integrin-mediated ECM adhesion and signaling, subsequently leading to epithelial tumorigenesis.
Figure 5.
A model for αv integrin-mediated regulation of epithelial proliferation and homeostasis. αv integrins expressed in basal epithelial cells of the conjunctiva and eyelid skin bind to the latent forms of TGFβ1 and 3 (latent associated peptide-TGFβ1/3) in the epidermal basement membrane. Integrin binding leads to activation of TGFβ signaling and suppression of epithelial cell proliferation, likely via an autocrine loop. Genetic ablation of αv integrin expression in basal epithelia causes dysregulation of TGFβ growth inhibition, leading to epithelial cell hyperplasia and tumor progression.
αv Integrins and Epithelial Tumorigenesis
The αv integrin subunit heterodimerizes with five different β subunits, and at least three of these integrins, αvβ1, αvβ5, and αvβ6, are expressed at varying levels in normal and malignant epithelial cells of the skin.2,3,24 Watt and colleagues have shown that a human SCC-derived cell line lacks endogenous αv integrin expression, which most likely contributes to enhanced in vitro proliferation and survival properties.3,25 These data are consistent with our in vivo gene deletion results, and support our model that αv integrin negatively regulates normal epithelial cell proliferation, and that loss of αv integrin expression or function causes aberrant cell growth. Other reports show that increased expression of αv integrins, particularly αvβ6, in SCCs correlate with advanced tumor progression and poor patient prognosis.26 Furthermore, inhibition of αvβ6 integrin expression and function leads to reduced SCC progression and invasiveness.27 It is possible that αv integrin expression levels regulate distinct phases of tumor onset and progression. For example, reduced αv integrin expression or function, via epigenetic or post-translational modifications, may promote tumor initiation, whereas subsequent increased αv and β6 integrin gene expression may drive tumor growth and malignancy. Our mouse genetic data support a role for reduced integrin expression and function being necessary for tumor initiation. Tumor progression in the mouse model described in this paper may occur via integrin-independent pathways, or integrin-dependent pathways unrelated to αvβ6 integrin overexpression. Indeed, there is an extended latency period from the time of Cre-mediated gene deletion (embryogenesis, Figure 1) to the formation of grossly obvious eyelid tumors (12 to 18 postnatal months, Figure 3). Thus, αv integrin probably influences other critical growth regulatory cascades that are progressively altered following gene deletion; together, these events contribute to the onset and progression of eyelid SCC. We are currently investigating the molecular alterations that occur as a result of αv gene deletion, for example, whether tumor suppressor or oncogene signaling pathways are dysregulated, and how these events collectively lead to SCC. All five murine β subunit genes that pair with αv have been ablated individually or in various combinations, yet none of the published studies reveals a phenotype that relates to SCC.5 Thus, it is likely that the combined loss of two or more αv-containing integrins, eg, αvβ6, and αvβ8, may contribute to SCC. We are currently generating mice that lack multiple β integrin genes to address this possibility.
αv Integrins and Functional Links with Transforming Growth Factor β Signaling in SCC
αv integrins bind to RGD tripeptide motifs within the latent associated peptides of transforming growth factor (TGF) β1 and TGFβ3.28 Latent associated peptides non-covalently associate with TGFβ1/3 in the ECM and maintain TGFβ in an inactive form. Both αvβ6 and αvβ8 integrins mediate the physical dissociation of latent associated peptides, leading to release of bioactive TGFβ1 and TGFβ3 from the ECM.29,30,31,32 TGFβ’s and their receptors have been shown to negatively regulate epithelial cell growth.33 A recent report reveals that selective ablation of TGFβ-receptor I signaling leads to development of admixed squamous cell carcinomas and mucoepidermoid carcinomas in the periorbital and perianal regions.34 Fuchs and colleagues more recently published a study showing that genetic ablation of the TGFβ receptor II gene in basal cells of the mouse skin epidermis (via the Keratin5-Cre transgene) results in SCC development in perianal and perivaginal areas, and these results correlate with reduced expression of TGFβ receptor II in human SCC samples.35 The TGFβ receptor I/II knockout results are consistent with a previous report showing that mice lacking Smad4, an intracellular signaling protein regulated by TGFβ receptors, develop SCC of the skin.36 Interestingly, genetic deletion of αv integrins in mouse dendritic cells leads to colitis and colon tumor formation, and these events are mostly due to defective αvβ8 integrin-mediated TGFβ activation.13,16 These various data strongly support our model that αv integrins, via activation of TGFβ signaling events, normally serve to suppress epithelial cell growth (Figure 5). Genetic ablation of αv integrins or TGFβ signaling components dysregulate this balance, leading to epithelial cell hyperplasia and tumor progression.
In conclusion, our molecular genetic strategies reveal important functions for αv integrins in regulating epithelial cell proliferation and homeostasis in the eyelid skin and conjunctiva. To our knowledge, these are the first direct genetic data supporting a tumor suppressor-like function for αv integrins in epithelial cells. Since the expression of the GFAP-Cre transgene has not been detected in epithelial cells outside of the eyelid skin and conjunctiva, or other stratified epithelial organs, we cannot yet conclude that αv integrins play more general roles in suppressing basal epithelial cell growth. We are currently deleting αv integrin expression using other Cre transgenes that are expressed in a broader range of epithelial organs to test this possibility. These various integrin knockout models will likely be useful translational tools to study SCC onset and progression, as well as to test and develop novel therapeutic compounds to treat or prevent SCC of the skin.
Acknowledgments
The GFAP-Cre transgenic mice were kindly provided by Dr. Anton Berns (Netherlands Cancer Institute). The αv integrin anti-serum was provided by Dr. Louis Reichardt (UCSF). We thank Aaron Cook for technical assistance with mouse genotyping. We are also grateful to Dr. Bita Esmaeli and Dr. Alexander Lazar (both at MD Anderson Cancer Center) for helpful discussions regarding the ocular tumor pathologies.
Footnotes
Address reprint requests to Richard O. Hynes, Ph.D., Center for Cancer Research, and the Howard Hughes Medical Institute, Massachusetts Institute of Technology, Cambridge, MA 02139. E-mail: rohynes@mit.edu.
Supported by grants from the National Cancer Institute (RO1CA17007) and the National Heart Lung and Blood Institute (PO1HL066105) and by the Howard Hughes Medical Institute of which R.O.H. is an Investigator. A.L.H. was a United Kingdom Research Council Fellow.
J.H.M. and M.B. contributed equally to this work.
Current address of J.H.M.: Department of Cancer Biology, MD Anderson Cancer Center, Houston, TX. Current address of M.B.: Department of Pathology, University of New Mexico, Albuquerque, NM. Current address of A.L.-H.: Massachusetts General Hospital, Department of Pediatrics, Boston, MA.
References
- Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3:199–209. doi: 10.1038/nrg758. [DOI] [PubMed] [Google Scholar]
- Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2002;21:3919–3926. doi: 10.1093/emboj/cdf399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes SM, Watt FM. New roles for integrins in squamous-cell carcinoma. Nat Rev Cancer. 2006;6:175–183. doi: 10.1038/nrc1817. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Bouvard D, Brakebusch C, Gustafsson E, Aszodi A, Bengtsson T, Berna A, Fassler R. Functional consequences of integrin gene mutations in mice. Circ Res. 2001;89:211–223. doi: 10.1161/hh1501.094874. [DOI] [PubMed] [Google Scholar]
- Dowling J, Yu QC, Fuchs E. Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. alpha3beta1 Integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, Svensson M, Herken R, Sasaki T, Timpl R, Werner S, Fassler R. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RD, Jones J, Taylor C, Watt FM. Sequence variation in the I-like domain of the beta1 integrin subunit in human oral squamous cell carcinomas. Cancer Lett. 2004;213:189–194. doi: 10.1016/j.canlet.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT, Roes JT, Savill JS, Hynes RO. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci USA. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, Hynes RO. Selective ablation of alpha v integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration, and premature death, Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, Bluestone JA, Sheppard D. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Tamura T, Mikoshiba K. Cell-specific expression of the mouse glial fibrillary acidic protein gene: identification of the cis- and trans-acting promoter elements for astrocyte-specific expression. J Neurochem. 1990;55:1180–1188. doi: 10.1111/j.1471-4159.1990.tb03123.x. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Bossy B, Reichardt LF. Chick integrin alpha V subunit molecular analysis reveals high conservation of structural domains and association with multiple beta subunits in embryo fibroblasts. Biochemistry. 1990;29:10191–10198. doi: 10.1021/bi00496a006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14:994–1004. [PMC free article] [PubMed] [Google Scholar]
- Danielyan L, Tolstonog G, Traub P, Salvetter J, Gleiter CH, Reisig D, Gebhardt R, Buniatian GH. Colocalization of glial fibrillary acidic protein, metallothionein, and MHC II in human, rat. NOD/SCID, and nude mouse skin keratinocytes and fibroblasts. J Invest Dermatol. 2007;127:555–563. doi: 10.1038/sj.jid.5700575. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp MA. Alpha9 and beta8 integrin expression correlates with the merger of the developing mouse eyelids. Dev Dyn. 1999;214:216–228. doi: 10.1002/(SICI)1097-0177(199903)214:3<216::AID-AJA5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Janes SM, Watt FM. Switch from alphavbeta5 to alphavbeta6 integrin expression protects squamous cell carcinomas from anoikis. J Cell Biol. 2004;166:419–431. doi: 10.1083/jcb.200312074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbag S, Kenter GG, Gorter A, Dreef EJ, Koopman LA, Violette SM, Weinreb PH, Fleuren GJ. Overexpression of the alpha v beta 6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J Pathol. 2007;212:316–324. doi: 10.1002/path.2168. [DOI] [PubMed] [Google Scholar]
- Nystrom ML, McCulloch D, Weinreb PH, Violette SM, Speight PM, Marshall JF, Hart IR, Thomas GJ. Cyclooxygenase-2 inhibition suppresses alphavbeta6 integrin-dependent oral squamous carcinoma invasion. Cancer Res. 2006;66:10833–10842. doi: 10.1158/0008-5472.CAN-06-1640. [DOI] [PubMed] [Google Scholar]
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. Integrin alpha(v) beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166:1883–1894. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier S, Mu DZ, O'Connell D, Boylen K, Travis W, Liu WH, Broaddus VC, Nishimura SL. A role for the integrin alphavbeta8 in the negative regulation of epithelial cell growth. Cancer Res. 2000;60:7084–7093. [PubMed] [Google Scholar]
- Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin alpha(v) beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature. 2003;422:169–173. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Honjo Y, Bian Y, Kawakami K, Molinolo A, Longenecker G, Boppana R, Larsson J, Karlsson S, Gutkind JS, Puri RK, Kulkarni AB. TGF-beta receptor I conditional knockout mice develop spontaneous squamous cell carcinoma. Cell Cycle. 2007;6:1360–1366. doi: 10.4161/cc.6.11.4268. [DOI] [PubMed] [Google Scholar]
- Guasch G, Schober M, Pasolli HA, Conn EB, Polak L, Fuchs E. Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell. 2007;12:313–327. doi: 10.1016/j.ccr.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Mao C, Teng Y, Li W, Zhang J, Cheng X, Li X, Han X, Xia Z, Deng H, Yang X. Targeted disruption of Smad4 in mouse epidermis results in failure of hair follicle cycling and formation of skin tumors. Cancer Res. 2005;65:8671–8678. doi: 10.1158/0008-5472.CAN-05-0800. [DOI] [PubMed] [Google Scholar]