Abstract
Human cancers often display an avidity for glucose, a feature that is exploited in clinical staging and response monitoring by using 18F-fluoro-deoxyglucose (FDG) positron emission tomography. Determinants of FDG accumulation include tumor blood flow, glucose transport, and glycolytic rate, but the underlying molecular mechanisms are incompletely understood. The phosphoinositide-3 kinase/Akt/mammalian target of rapamycin complex (mTORC) 1 pathway has been implicated in this process via the hypoxia-inducible factor alpha-dependent expression of vascular endothelial growth factor and glycolytic enzymes. Thus, we predicted that tumors with elevated mTORC1 activity would be accompanied by high FDG uptake. We tested this hypothesis in eight renal angiomyolipomas in which the loss of tuberous sclerosis complex (TSC) 1/2 function gave rise to constitutive mTORC1 activation. Surprisingly, these tumors displayed low FDG uptake on positron emission tomography. Exploring the underlying mechanisms in vitro revealed that Tsc2 regulates the membrane localization of the glucose transporter proteins (Glut)1, Glut2, and Glut4, and, therefore, glucose uptake. Down-regulation of cytoplasmic linker protein 170, an mTOR effector, rescued Glut4 trafficking in Tsc2−/− cells, whereas up-regulation of Akt activity in these cells was insufficient to redistribute Glut4 to the plasma membrane. The effect of mTORC1 on glucose uptake was confirmed using a liver-specific Tsc1- deletion mouse model in which FDG uptake was reduced in the livers of mutant mice compared with wild-type controls. Together, these data show that mTORC1 activity is insufficient for increased glycolysis in tumors and that constitutive mTOR activity negatively regulates glucose transporter trafficking.
Studies have shown that the biological behavior of many cancers correlates with their metabolic activities as indicated by the degree of 18F-fluoro-deoxyglucose (FDG) accumulation on positron emission tomography (PET) imaging.1 Stemming from the initial observations by Warburg, it is widely accepted that human tumors use glucose as their preferential energy source and display a high rate of glycolysis.2,3 While a number of mechanisms may contribute to increased glycolytic metabolism, recent evidence suggests that there is a proliferative advantage for tumor cells to use glycolysis rather than mitochondrial oxidation as their primary source of ATP.4 The molecular basis of how tumors acquire this phenotype is not well understood. It has been suggested that as the tumor grows, relative hypoxia leads to the induction of the hypoxia-inducible factor (HIF)-family of transcription factors that promotes glucose metabolism through the expression of glycolytic enzymes.5 However, a number of oncogenic pathways have been identified to directly influence HIF1 levels. For example, HIF1 protein expression is positively regulated by mammalian target of rapamycin (mTOR)-dependent translation of 5′TOP mRNA, and tumor HIF1 levels have been shown to predict sensitivity to mTOR inhibition by rapamycin.6,7
The mTOR pathway plays a major role in nutrient sensing, and one of its key negative regulator is the tuberous sclerosis complex (TSC)1/2 complex.8,9 mTORC1 promotes protein synthesis via two effectors, p70/p85-S6 protein kinase (S6K1) and eIF4E-binding protein (4E-BP1), and is sensitive to rapamycin treatment. On growth factor stimulation, active Akt phosphorylates TSC2 and reduces the inhibitory function of the TSC1/2 complex on Rheb GTPase, which in turn stimulates mTORC1. Cellular energy and oxygen availability also regulate mTORC1 activity through 5′-AMP-activated protein kinase (AMPK) and regulated in development and DNA damage responses (REDD)1-mediated effects on TSC2. Consequently, the loss of TSC1/2 function leads to constitutive activation of mTORC1 and results in increased cell growth that manifests clinically as predominantly benign tumors found in tuberous sclerosis.10 As expected, these tumors display up-regulation of HIF1 and vascular endothelial growth factor (VEGF), and consequently, are highly vascular. The phosphoinositide-3 kinase (PIK3)/Akt/mTOR pathway is also involved in other hereditary tumor syndromes such as Cowden’s disease and Peutz-Jeghers syndrome, and more importantly, in many forms of sporadic human cancers, thus suggesting a central role of mTOR in tumorigenesis.11
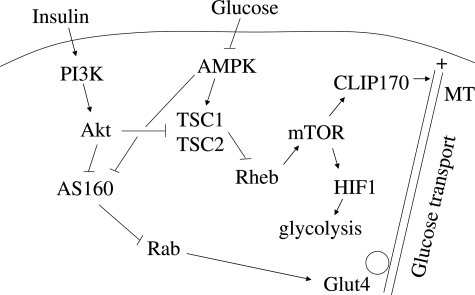
Tumor glycolysis has been linked to the Akt/mTOR pathway. In an experimental leukemia model, Elstrom et al showed that Akt activation was sufficient to promote tumor 2-fluoro-2-deoxy-D-glucose (FDG) uptake by increasing the rate of glucose metabolism.12 Furthermore, the expression of glycolytic enzymes was found to be under the influence of mTOR, and treatment with rapamycin suppressed the rate of glycolysis.13 Other determinants of FDG avidity include tumor blood flow and glucose transport. The latter is regulated by a family of facilitated glucose transporters among which glucose transporter protein (Glut)4 is best characterized for its role in insulin responsive glucose import.14 In the unstimulated state, Glut4 resides in specialized intracellular vesicles that are transported to the plasma membrane in the presence of insulin. Multiple pathways that include Akt and AMPK play a role in Glut4 trafficking (Figure 1). Recent evidence suggests that AS160 serves as a GTPase activating protein for Rab that mediates vesicular transport, and is a substrate for both Akt and AMPK.15 Interestingly, activation of Akt by growth factors and induction of AMPK by energy deprivation have been shown to promote glucose import, even though these two kinases have opposing effects on TSC2 and mTOR. Thus, the relationship between mTOR and glucose transport appears to vary according to the input from different pathways that converge on the TSC1/2 complex.
Figure 1.
Model of glucose transport regulation by the Akt/TSC pathway. Growth factors (eg, insulin) and energy substrate availability (eg, glucose) regulate TSC1/TSC2 activity through Akt and AMPK, respectively to modulate mTOR function in promoting HIF1α protein synthesis and the activity of CLIP170 on microtubule plus-end growth. Akt and AMPK further regulate trafficking of hexose transporters such as Glut4 via the AS160/Rab pathway.
We previously reported that TSC1/2 regulates post-Golgi transport of vesicular stomatitis virus glycoprotein (VSVG) and that cells lacking TSC1/2 mislocalize caveolin-1.16 Further, we found that this transport defect was due to a disruption of microtubule organization stemming from mTOR hyperactivity.17 mTOR interacts and phosphorylates cytoplasmic linker protein (CLIP)170, a microtubule-binding protein that regulates microtubule dynamics.18 Additionally, we demonstrated that down-regulation of CLIP170 was sufficient to rescue the aberrant microtubule cytoskeleton and caveolin-1 localization in the Tsc2−/− cells. To determine the influence of this pathway on other membrane proteins, we examined the effects of mTOR on the trafficking of glucose transporters. Here, we found that cells lacking Tsc2 have reduced membrane localized Glut1, Glut2, and Glut4 following growth factor stimulation. The abnormal Glut4 traffic was rescued by down-regulation of CLIP170, but not reversed by manipulating Akt or AS160 expression. We further show that glucose uptake was compromised in the presence of constitutive activation of mTORC1 in vitro and in vivo. These data provide novel insights into the role of the TSC/mTOR pathway in glucose transport and show that mTORC1 is a negative determinant of FDG uptake in PET imaging.
Materials and Methods
Cells
Tsc2+/+ and Tsc2−/− embryonic fibroblasts were derived from the Eker rat as described.16 Wild-type RK3E and 3T3L1 cells were obtained from American Type Culture Collection (Manassas, VA). Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) or DMEM/F12 supplemented with 5 to 10% fetal bovine serum (FBS). For adipocyte differentiation, 3T3L1 cells were grown in DMEM medium containing 20% FBS. Differentiation was initiated 48 hours after confluence by changing the medium to 10% FBS/DMEM with 115 μg/ml isobutylmethylxanthine, 390 ng/ml dexamethasone, and 10 μg/ml insulin. After 4 days in differentiation media, cells were placed in 10% FBS/DMEM with 10 μg/ml insulin.
Liver-Specific Tsc1 Deletion Mice
The Tsc1floxed/floxed mice were a generous gift of D. Kwiatkowski (Harvard, Boston, MA), and the AlbCre/+ mice were purchased from the Jackson Laboratory (Bar Harbor, ME). These animals were crossed to generate Tsc1floxed/floxed; AlbCre/+ mice (hereby known as Tsc1c−/−) along with littermate wild-type controls (Tsc1c+/+). Animals were weaned at 3 weeks of age and genotyped. They were maintained on regular chow ad libitum with 12-hour day/night cycles in a modified specific pathogen-free facility. In the in vivo experiments, mice were fasted overnight. On sacrifice, tissues were analyzed by Western blot analyses as previously described.19 All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Washington.
Plasmids and RNAi Constructs
Adenoviral construct encoding myr-Akt1 (gift of K. Walsh, Tufts University, Boston, MA) was prepared as described.20 Adenovirus encoding myr-Akt2 was obtained from Vector Biolabs (Philadelphia, PA). Cy3-labeled siRNA reagents for Tsc2 and CLIP170 were obtained from Ambion (Austin, TX). On-TARGET plus siRNA for Tsc2 and AS160 were from Dharmacon (Chicago, IL). Briefly, cells were plated in 6-well dishes until ∼50% confluent and then transfected with the target siRNA (100 nmol/L) using either Lipofectamine Plus (Invitrogen, Carlsbad, CA) or DharmaFECT (Dharmacon) depending on the cell type. Cells were harvested after 72 hours and analyzed by Western blot as previously described.19 Cells to be analyzed by immunofluorescence were transferred to eight-well chamber slides at 48 hours post-transfection, fixed after 16 hours with paraformaldehyde, and stained.
Immunofluorescence Confocal Analyses
Cells were seeded onto 2-well chamber slides (Nalge Nunc International, Rochester, NY) or eight-well chamber slides (Falcon; Becton Dickinson, Franklin Lakes, NJ) coated with poly L-lysine and allowed to adhere to the slide (16 hours) before they were fixed with 4% paraformaldehyde or cold (−20°C) methanol. Following PBS washing and treatment with 0.1% Triton X-100, the cells were blocked with 10% normal goat serum in PBS for 30 minutes at room temperature and then incubated with the indicated antibodies diluted in PBS for 1 hour at room temperature or 4°C overnight. Cells were washed and then incubated with Alexa 488 or 568-conjugated anti-rabbit and anti-mouse antibodies as appropriate for 1 hour at room temperature. Cells were washed in PBS, mounted, counterstained with 4′,6-diamidino-2-phenylindole, and visualized with a Leica SP/NT Spectral Confocal microscope (Leica, Wetzlar, Germany).
Glucose Uptake
In Vitro [3H]-Deoxyglucose Uptake
Cells were plated onto 60 mm dishes at ∼80% confluence. After culturing in serum free DMEM/F12 medium for 20 hours, the cells were washed three times with glucose free DMEM and then incubated in glucose-free medium. The cells were either left untreated or stimulated with 10 μmol/L insulin for various times at 37°C. 2-Deoxy-D-[1-3H]glucose was added to each dish to a final concentration of 500 nCi/ml and incubated for 10 minutes. The cells were washed twice with ice-cold dPBS and solubilized in 1.0 ml 1% SDS. The radioactive content of 500 μl of sample in a 4.0 ml of Econofluor was determined using a scintillation counter. The protein concentration was determined using the bicinchoninic acid protein assay (Pierce, Rockford, IL). Results were expressed as percentage change in cpm per mg protein. Non-specific uptake was measured in the presence of 20 μmol/L cytochalasin B and subtracted from each determination to obtain specific uptake.
Flow Cytometric Analysis
The tracer 6-N-7-nitrobenz-2-oxa-1,3-diazol-4-yl-amino- 6-deoxyglucose (NBDG; Molecular Probes, Eugene, OR) was used to monitor glucose flux. After 24 hours in serum free medium, 1 × 106 cells were trypsinized and resuspended in incubation buffer (140 mmol/L NaCl, 20 mmol/L 4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid [systematic], 5 mmol/L KCl, 2.5 mmol/L MgSO4, and 5.5 mmol/L glucose pH 7.4) and maintained at 22°C. Flow cytometric analysis was initiated immediately after the addition of tracer levels of NBDG and insulin (final concentrations 30 μmol/L and 10 μmol/L, respectively). At fixed time intervals, the uptake of the fluorescent probe was recorded as mean fluorescence intensity during a 600-second period. Flow cytometric analysis was performed using an Influx flow cytometer (Cytopeia, Seattle, Washington) equipped with three lasers at the University of Washington Flow Cytometry Facility.
In Vivo [18F]-Fluoro-Deoxyglucose Uptake
Mice were fasted overnight and placed under anesthesia using isofluorane. Approximately 2 mCi of 18F-fluoro-deoxyglucose was injected retro-orbitally and allowed to equilibrate for 40 minutes before mice were sacrificed by cervical dislocation. Radioactive contents of the organs were measured in a dose calibrator (Capintec CRC-7, Ramsey, NJ), and the results were expressed as percentage of injected dose per gram of tissue, for each organ with respect to whole body uptake, and corrected for background radioactivity and decay. For human FDG-PET, an institutional review board-approved retrospective review was performed on three subjects who had undergone PET imaging as part of their medical evaluation. Studies were performed using standard institutional clinical protocols.
Statistics
Comparisons of means were analyzed by Student’s t-test when comparing two groups, and analysis of variance when comparing more than two groups.
Results
Effects of Tsc2 on Glut4 Localization
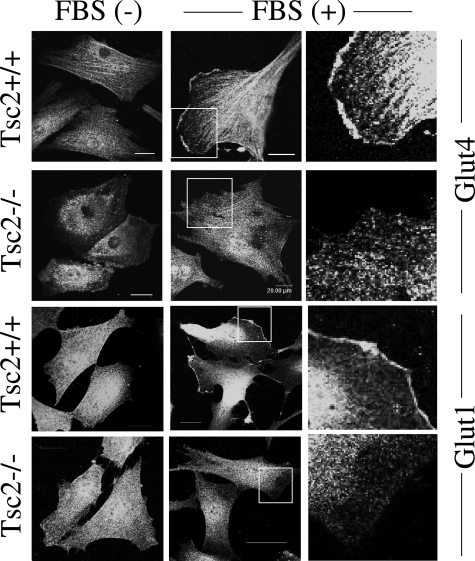
Based on our earlier observations that the TSC1/2 complex regulates intracellular localization of caveolin-1, we hypothesized that other caveolae-associated proteins may also be affected by the TSC/mTOR pathway. Among the hexose transporters, Glut4 and Glut1 have been localized to lipid raft domains such as caveolae.21,22 We examined their subcellular distribution by indirect immunofluorescence analyses in an isogenic pair of Tsc2+/+ and Tsc2−/− embryonic fibroblasts. In the wild-type cells, both transporters translocated from the cytosol to the plasma membrane on stimulation by serum (Figure 2). In contrast, Glut4 and Glut1 were largely absent at the membrane of Tsc2−/− cells in the presence of serum (Figure 2). Of note, the levels of Glut1 and Glut4 expression in the wild-type and mutant cells were essentially identical (data not shown). These observations suggest that the membrane translocation of these two transporters may be impaired with the loss of Tsc2.
Figure 2.
Glut4 and Glut1 mislocalization in Tsc2−/− fibroblasts. A pair of isogenic Tsc2−/− and Tsc2+/+ embryonic fibroblast lines were grown in the absence or presence of FBS, fixed in 4% paraformaldehyde, and processed for immunofluorescence analyses of Glut4 and Glut1. Right column shows magnified views of boxed areas. Bar is equivalent to 20 μm.
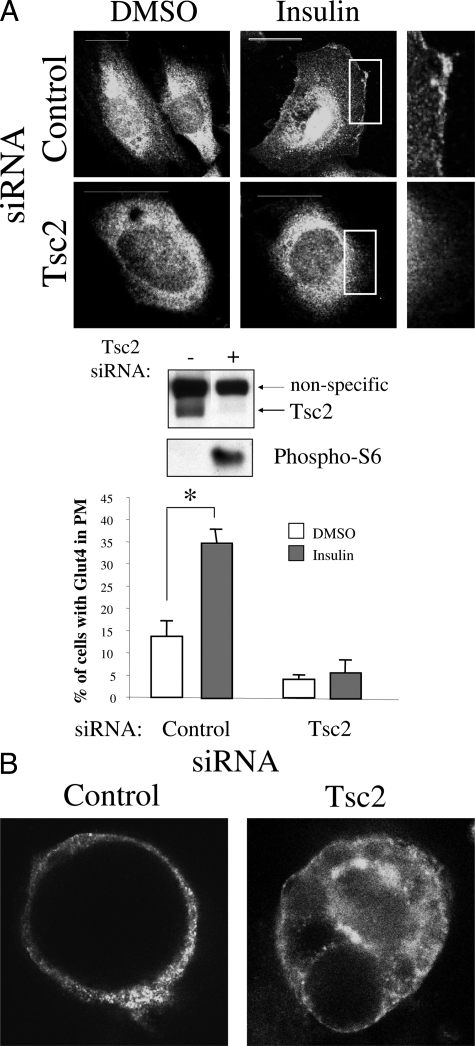
Next, to demonstrate that the observed difference in Glut4 localization was due specifically to the effects of Tsc2, we down-regulated its expression by RNA interference in three independent wild-type cell lines and analyzed Glut4 distribution by immunofluorescence analyses. Figure 3A shows the effects of Tsc2 siRNA in down-regulating the target protein and the functional consequences in mTORC1 activation based on increased phospho-S6 expression. The proportion of RK3E rat kidney epithelial cells with insulin-induced membrane Glut4 was significantly less in Tsc2 siRNA-treated cells (5%) as compared with controls (35%) (P < 0.05) (Figure 3A). Similarly, in differentiated 3T3L1 adipocytes, down-regulation of Tsc2 led to reduced insulin-stimulated Glut4 membrane translocation (Figure 3B). The same trend was observed in the Tsc2+/+ embryonic fibroblasts following Tsc2 siRNA (data not shown). Together, the data suggest that Glut4 trafficking is under the control of the TSC2 pathway.
Figure 3.
Tsc2 regulates Glut4 membrane localization. A: Effects of Tsc2 siRNA on Glut4 trafficking. Tsc2+/+ rat kidney epithelial, RK3E, cells were transfected with Cy3-labeled control or Tsc2 siRNA for 72 hours, starved overnight, and stimulated with insulin or control (dimethyl sulfoxide). Immunofluorescence analyses demonstrate Glut4 distribution. Boxed areas are magnified to highlight the presence or absence of plasma membrane Glut4. Bar is equivalent to 20 μm. Western blot analysis was performed using antibodies for TSC2 (C20, Santa Cruz, CA), and phospho-S6(235/236) (Cell Signaling, Danvers, MA). The percentages of cells with plasma membrane (PM) Glut4 are tabulated in the graph, with at least 100 cells counted in each category. *P < 0.05. B: Example of Glut4 distribution in insulin-stimulated differentiated 3T3L1 adipocytes following transfection with Cy3-labeled control or Tsc2 siRNA.
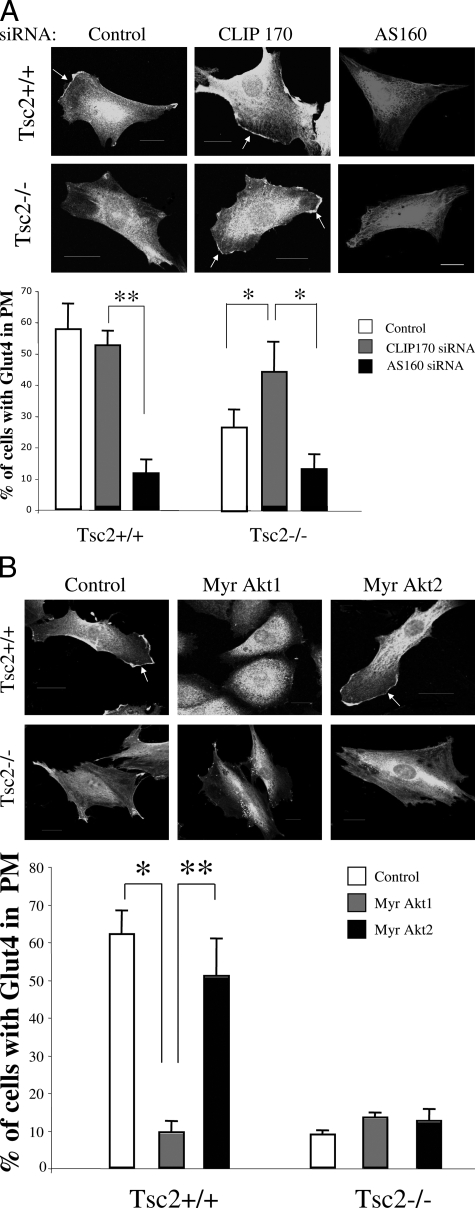
CLIP170 but Not Akt/AS160 Affects Glut4 Localization in the Tsc2−/− Cells
To determine the mechanism behind Glut4 mislocalization in the Tsc2−/− cells, we postulated that mTOR activity plays a role in Glut4 translocation through its influence on microtubule function that is mediated by CLIP170. This hypothesis was based on our previous observations that mTOR and CLIP170 regulate caveolin-1 localization through microtubules in Tsc2−/− cells.17 Others have shown that Glut4 traffic is dependent on intact microtubules.23,24 To test this hypothesis, we down-regulated CLIP170 by siRNA in the Tsc2−/− and Tsc2+/+ cells, and examined Glut4 localization following serum stimulation. Figure 4A shows that CLIP170 siRNA significantly increased the proportion of Tsc2−/− cells with membrane Glut4 but had little effect in the wild-type cells. As an alternative hypothesis, reduced Glut4 translocation may be caused by a known negative feedback of p70S6K on IRS1, thus leading to reduced Akt activity in the Tsc2 null state25,26 This, in turn, promotes AS160 GAP activity to inhibit Rab-mediated Glut4 translocation. We anticipated that down-regulation of AS160 in the Tsc2-null cells may promote Glut4 trafficking, but instead treatment of mutant and wild-type cells with AS160 siRNA led to a significant suppression of membrane Glut4 (Figure 4A). To further examine the effects of Akt, we overexpressed constitutively activated forms of Akt1 and Akt2 and assayed for Glut4 distribution. We found that neither Akt1 nor Akt2 rescued Glut4 translocation in the Tsc2−/− cells (Figure 4B). However, Myr-Akt1 but not Myr-Akt2 suppressed membrane Glut4 in wild-type cells. This is consistent with the previously reported differential effects of Akt1 and Akt2 on Glut4 trafficking.27 Together, our data suggest that the feedback inhibition of Akt in the Tsc2-null cells does not account for the mislocalization of Glut4, but rather supports a role of CLIP170-dependent microtubule function in Glut4 trafficking.
Figure 4.
CLIP170 and not Akt regulates Glut4 localization in the Tsc2−/− cells. A: Effects of CLIP170 and AS160 siRNA on Glut4 distribution. Cells were transfected with control or test siRNA 72 hours before Glut4 immunofluorescence analysis. Percentages of cells with plasma membrane Glut4 are tabulated in the graph. *P < 0.05, **P < 0.01. B: Effects of active Myr-Akt1 and Myr-Akt2 on Glut4 distribution. Glut4 immunofluorescence analysis was performed 6 days following viral transduction. For all of these studies, a minimum of 50 cells from each category were counted. Arrows indicate plasma membrane Glut4. Bar is equivalent to 20 μm. *P < 0.01, **P < 0.05.
Insulin-Stimulated Glucose Uptake in Tsc2−/− Cells
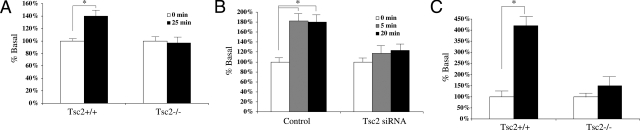
To determine the functional consequence of Glut4 mislocalization in the Tsc2−/− cells, we measured insulin-stimulated glucose uptake in these cells. Serum-starved cells were stimulated by insulin for various time intervals. 2- Deoxy-D-[1-H3]-glucose (500 nCi/ml) was added during the last 10 minutes. Figure 5A shows the relative radioactivity (as compared with unstimulated controls) at 25 minutes following 10 μmol/L insulin treatment. Wild-type fibroblasts showed approximately 40% increase in glucose uptake, whereas Tsc2−/− cells had no significant change over the same time period. To confirm the effects of Tsc2 on glucose uptake, wild-type RK3E kidney cells were transfected with control or Tsc2 siRNA, and subjected to insulin-stimulated glucose uptake experiments. The control cells imported ∼80% more glucose over baseline at 5 and 20 minutes following stimulation. In contrast, insulin stimulated only a modest change in glucose uptake after down-regulation of Tsc2 (Figure 5B). Similar results were obtained in the Tsc2+/+ fibroblasts treated with Tsc2 siRNA (data not shown). Using an independent flow cytometric assay with a fluorescent derivative of deoxyglucose, NBDG, we confirmed that the Tsc2−/− cells imported significantly less glucose compared to Tsc2+/+ cells within the first minute of insulin stimulation (Figure 5C). Consistently, our results suggest that insulin-stimulated glucose uptake in vitro is impaired in the absence of Tsc2 function.
Figure 5.
Reduced insulin-stimulated glucose uptake on loss of Tsc2. A: [3H]-deoxyglucose uptake in wild-type and mutant fibroblasts was measured following 25 minutes of insulin stimulation. Results are normalized to time 0 minutes. B: RK3E cells were transfected with control or Tsc2 siRNA for 72 hours, stimulated with insulin for 5 and 20 minutes in the presence of [3H]-deoxyglucose. Uptake levels relative to time 0 are shown. C: NBDG flow cytometric analysis of wild-type and mutant cells following one minute of insulin stimulation. Results show uptake relative to time 0. *P < 0.05.
Glucose Uptake in Tsc1c−/− Mice
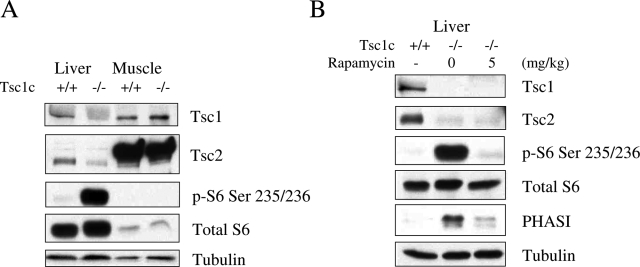
To analyze the effects of the TSC/mTOR pathway on glucose uptake in vivo, we examined the hepatic import of radiolabeled FDG in mice, given that the liver plays an important role in glucose homeostasis. We used a genetic approach to develop a state of constitutive mTORC1 activation in the liver. Since the Tsc1-null state was embryonic lethal,28 we created mice that were deleted for Tsc1 specifically in hepatocytes by breeding mice homozygous for Tsc1 floxed alleles to those carrying the Cre recombinase gene downstream of the albumin promoter. The resultant conditional Tsc1f/f;Cre+/− animals (designated as Tsc1c−/−) were viable and fertile without gross developmental abnormalities. A detailed description of the hepatic phenotype of these mice will be summarized in a separate report (manuscript in preparation). At 6 weeks of age, the Tsc1c−/− mice showed loss of Tsc1 expression and the corresponding up-regulation of phospho-S6 in the liver but not muscle (Figure 6A). Other tissues including central nervous system, heart, lung, and fat also showed comparable Tsc1 expression in the Tsc1c−/− and Tsc1c+/+ animals (data not shown). Thus, the effects of Tsc1 ablation were specific to the liver. Of note, the hepatic expression of Tsc2 in the mutant mice was also reduced secondary to increased protein degradation as previous reported.29 Further, Figure 6B shows that treatment with rapamycin (5 mg/kg) in a Tsc1c−/− mouse effectively reduced phospho-S6 and phospho-4E-BP1 (PHAS)I to that of the wild-type littermate control. These data confirm that the loss of hepatic Tsc1 results in up-regulation of mTORC1 activity.
Figure 6.
Liver-specific activation of mTORC1 in Tsc1c−/− mice. Western blot analyses showing expression of Tsc1, Tsc2, and downstream effectors in (A) liver and muscle tissues from a representative pair of wild-type and mutant mice, and (B) the effects of rapamycin on hepatic mTORC1 activity in a Tsc1c−/− mouse. Tubulin serves as a loading control.
Following an overnight fast, control and mutant mice (n = four per group) were injected intravenously with approximately 2 mCi of 18F-fluorodeoxyglucose. They remained anesthetized for 40 minutes until sacrifice, at which time, whole body and organ specific FDG uptake was quantified. Results were based on the ratio of FDG activity per weight of the organ with respect to whole body activity and corrected for decay during the experimental period. Table 1 shows the results of FDG uptake in various organs of the mutant animals compared to the wild-type controls. FDG uptake in the liver was significantly less in the Tsc1-mutant compared to the wild-type animals, whereas the relative activities in the brain, muscle, spleen and kidneys are similar between the two groups. Since hepatic glucose transport is mediated primarily by Glut2 rather than Glut1 or Glut430 (Supplemental Figure S1A at http://ajp.amjpathol.org), we further examined if Glut2 localization is under the influence of the TSC/mTOR pathway. Indeed, immunofluorescence analysis of Glut2 showed a significant reduction in plasma membrane localization in the Tsc2−/− cells compared with the Tsc2+/+ cells similar to the results of Glut1 and Glut4 (Supplemental Figure S1B at http://ajp.amjpathol.org). Together, these observations provide strong support that the TSC/mTOR pathway has an in vivo effect in regulating hepatic glucose uptake.
Table 1.
Organ-Specific FDG Uptake in the Tsc1c−/− Mice
| Organ | Tsc1c+/+*
|
Tsc1c−/−#
|
P value | ||
|---|---|---|---|---|---|
| Mean | SD | M | SD | ||
| Liver | 0.929 | 0.138 | 0.772 | 0.092 | 0.04 |
| Spleen | 1.410 | 0.259 | 1.709 | 0.277 | 0.09 |
| Kidney | 5.910 | 1.223 | 6.259 | 1.002 | 0.33 |
| Muscle | 0.289 | 0.106 | 0.261 | 0.013 | 0.34 |
| Brain | 3.596 | 1.073 | 3.740 | 0.931 | 0.42 |
Tsc1c+/+, Tsc1f/f;Cre−/−;
Tsc1c−/−, Tsc1f/f;Cre+/−.
FDG-PET Imaging of Renal Angiomyolipoma
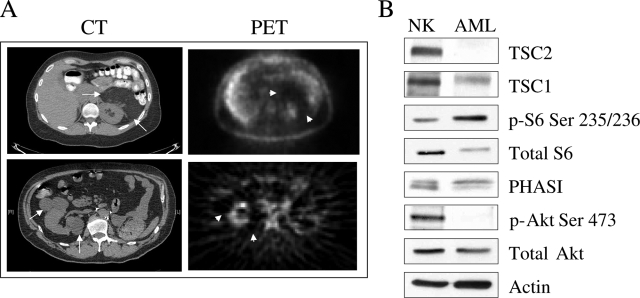
Finally, to determine the relevance of our findings in humans, we retrospectively reviewed three cases of renal angiomyolipomas (AMLs) in patients who had undergone FDG-PET during their medical evaluation. Two patients were diagnosed with tuberous sclerosis, and one with sporadic AML. Together, there were eight AMLs captured by FDG-PET. Patient and tumor characteristics along with the results of PET are shown in Table 2. In general, FDG uptake expressed as standard uptake value of >3 is interpreted as being ‘positive’ or PET-avid. None of the eight AMLs showed standard uptake value greater than 2.3. Indeed, all but one had values below 2 with a median standard uptake value of 1.25, suggesting that these tumors were not FDG-avid. Figure 7A shows examples of AMLs from cases #2 and #3. Computed tomography scans illustrate perirenal masses typical of AMLs, and the corresponding PET scans shows minimum FDG activities in the areas of the tumors. In case #3, the large lesion in the left kidney was removed surgically due to symptoms. Western blot analysis of the tumor showed high levels of phospho-S6 and PHASI along with a greatly reduced level of TSC2, confirming mTOR activation secondary to the loss of TSC2 as previously reported in sporadic AML19 (Figure 7B). The relative paucity of FDG activities in renal AMLs cannot be explained on the basis of the tumor size or blood flow since all of the lesions were larger than 2 cm (median: 2.7 cm), and AMLs are known to be hypervascular with an associated risk of spontaneous hemorrhage.31,32 Together, our in vitro and in vivo data indicate that constitutive activation of mTORC1 negatively regulates hexose transporter trafficking and glucose uptake. These findings implicate a novel role of the TSC/mTOR/CLIP170 pathway in glucose homeostasis.
Table 2.
FDG-PET of Human Renal Anigomyolipomas
| Case | Sex | Age | TSC | AML
|
FDG-PET SUV* | |
|---|---|---|---|---|---|---|
| Number | Size (cm) | |||||
| 1 | F | 16 | Yes | 4 | 2.0, 2.2, 2.3, 3.1 | 1.1, 1.3, 1.4, 2.3 |
| 2 | M | 57 | Yes | 3 | 2.6, 2.8, 3.7 | 1.1, 1.1, 1.3 |
| 3 | F | 36 | No | 1 | 6.5 | 1.2 |
SUV, standard uptake value.
Figure 7.
mTOR activation is associated with low FDG-uptake in human AML. A: Paired computerized tomography and PET images of renal AMLs from cases #3 (top) and #2 (bottom). Arrows indicate masses representing AMLs on computerized tomography, and arrowheads show corresponding areas on FDG-PET. Background FDG activities are seen within the normal kidneys as it is being secreted in the urine. B: Western blot of renal angiomyolipoma from case #3 showing expression of TSC1, TSC2, phospho-S6, total S6, PHASI, phospho-Akt, total Akt, and actin.
Discussion
This study provides the first evidence that mTORC1 activation secondary to the loss of TSC1/2 function is accompanied by a reduction in glucose uptake. The molecular basis for this phenomenon is attributed to the effects of mTOR on CLIP170-dependent microtubule-mediated protein transport. Specifically, down-regulation of Tsc2 disrupts growth-factor stimulated glucose transporter membrane translocation. This defect was rescued when cells were transfected with CLIP170 siRNA, but not with overexpression of activated Akt1 or Akt2. These findings support our earlier observations demonstrating that the TSC/mTOR pathway functions through CLIP170 to regulate microtubule organization and protein trafficking. This is consistent with previous studies showing an important role of MT in glucose transport.23,24
The negative regulation of Glut4 by mTOR is somewhat surprising given that acute activation of Akt promotes the translocation of cytosolic Glut4 vesicles toward the plasma membrane. In response to growth factors, such as insulin, PI3K activates Akt, which in turn phosphorylates AS160 to inhibit its function as a Rab GTPase activating protein.14 This results in increased membrane Glut4 via Rab-dependent vesicle docking and/or fusion. However, our data suggest that under conditions of sustained mTOR activation, glucose uptake is limited by the cytosolic retention of hexose transporters and is independent of Akt/AS160 activities. We postulate that in wild-type cells, growth factors induce a biphasic response in Glut4 trafficking. The initial activation of Akt stimulates membrane translocation, but subsequently, sustained mTOR activity inhibits Glut4 transport. This may represent an adaptive cellular response to self-regulate metabolic supply and demand. Under the pathological setting of TSC1/2 loss, persistent mTOR activity would lead to an imbalance between energy supply (ie, glucose uptake) and energy demand (ie, glycolysis). Our in vivo observations of reduced FDG uptake in the conditional liver-specific Tsc1 mutant mice and the renal AMLs in humans strongly support this model. Results of this study clearly show that constitutive mTOR activation per se is not sufficient to promote glucose uptake, and may in fact be inhibitory.
There are several predictions based on our model. Firstly, we predict that the Tsc1 or Tsc2-null cells would be ‘hyper’-sensitive to glucose deprivation due to the imbalance between supply and demand. Indeed, in vitro studies showed that these cells undergo apoptosis at a much higher rate than wild-type cells when glucose is withdrawn from the media.33 Clinically, this may be a contributing factor to the ‘benign’ nature of the TSC-related tumors. As such, glucose restriction may be exploited as a therapeutic strategy to enhance selective tumor cell killing. Secondly, we predict that under conditions of mTOR activation, inhibition of mTOR might lead to an increase in Glut4 translocation and glucose uptake. Krebs et al recently reported the effects of rapamycin on peripheral glucose uptake in healthy human volunteers.34 In the setting of mTOR stimulation (ie, hyperaminoacidemia and prandial-like peripheral hyperinsulinemia), rapamycin treatment resulted in a significant increase in glucose uptake without a change in endogenous glucose production whereas under condition of low peripheral insulinemia (ie, absence of mTORC1 activation), glucose uptake was unaltered by rapamycin. This clinical demonstration of the effects of rapamycin on glucose tolerance is highly consistent with our model.
However, in renal cell carcinoma, Thomas et al reported that treatment with rapamycin analogues is sometimes associated with reduced FDG uptake on PET imaging.7 They found that tumors with elevated HIF1A expression were most likely to respond to mTOR inhibition. This would imply that mTOR inhibition in tumors could have an opposite effect on glucose uptake compared to normal tissues. One plausible explanation for this apparent difference is that the effects of the rapamycin analogues in cancer may predominantly target tumor angiogenesis by inhibiting the HIF1/VEGF pathway rather than directly affecting the tumor cells. Indeed, multiple studies have demonstrated that rapamycin inhibits tumor formation due to its anti-angiogenic activity that correlated with VEGF production.35,36,37 The mechanism of VEGF up-regulation was found to be secondary to increased HIF1α 5′ TOP-dependent translation downstream of mTOR activation.38 As such, the observed changes in FDG-PET in response to rapamycin analogues may not be reflective of actual glucose transport of the tumor cells but rather tumor blood supply.
Other corroborating evidence in support of our observations comes from studies of FDG uptake in the brains of TSC patients. Multiple reports suggest that cortical tubers, the predominant central nervous system manifestation of TSC, are consistently hypometabolic compared to respective contralateral region.39,40 Like the AMLs, these lesions also express high levels of phospho-S6K1 or S6.41,42 Thus, it is worth emphasizing that mTOR hyperactivity per se is insufficient to predict high FDG uptake in vivo. Molecular events besides mTOR are necessary to account for the frequently observed FDG-avidity in many human cancers.
Supplementary Material
Acknowledgments
We acknowledge D. Kwiatkowski, K. Walsh, and P. Wu for reagents. We thank E. Barnes for critical comments, B. Lewellen for assistance in FDG-PET studies, and D. Martin for assistance with confocal microscopy.
Footnotes
Address reprint requests to Raymond S. Yeung, Department of Surgery, Box 356410, University of Washington, 1959 NE Pacific, Seattle, WA 98195. E-mail: ryeung@u.washington.edu.
Supported in part by grants CA077882 and CA102662 from the National Institutes of Health.
References
- Kelloff GJ, Hoffman JM, Johnson B, Scher HI, Siegel BA, Cheng EY, Cheson BD, O'Shaughnessy J, Guyton KZ, Mankoff DA, Shankar L, Larson SM, Sigman CC, Schilsky RL, Sullivan DC. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res. 2005;11:2785–2808. doi: 10.1158/1078-0432.CCR-04-2626. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Bui T, Thompson CB. Cancer’s sweet tooth. Cancer Cell. 2006;9:419–420. doi: 10.1016/j.ccr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- Li YM, Zhou BP, Deng J, Pan Y, Hay N, Hung MC. A hypoxia-independent hypoxia-inducible factor-1 activation pathway induced by phosphatidylinositol-3 kinase/Akt in HER2 overexpressing cells. Cancer Res. 2005;65:3257–3263. doi: 10.1158/0008-5472.CAN-04-1284. [DOI] [PubMed] [Google Scholar]
- Thomas GV, Tran C, Mellinghoff IK, Welsbie DS, Chan E, Fueger B, Czernin J, Sawyers CL. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006;12:122–127. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene. 2006;25:6347–6360. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- Abraham RT, Gibbons JJ. The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res. 2007;13:3109–3114. doi: 10.1158/1078-0432.CCR-06-2798. [DOI] [PubMed] [Google Scholar]
- Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, Thompson CB. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- Edinger AL, Linardic CM, Chiang GG, Thompson CB, Abraham RT. Differential effects of rapamycin on mammalian target of rapamycin signaling functions in mammalian cells. Cancer Res. 2003;63:8451–8460. [PubMed] [Google Scholar]
- Ishiki M, Klip A. Minireview: recent developments in the regulation of glucose transporter-4 traffic: new signals, locations, and partners. Endocrinology. 2005;146:5071–5078. doi: 10.1210/en.2005-0850. [DOI] [PubMed] [Google Scholar]
- Thong FS, Bilan PJ, Klip A. The Rab GTPase-activating protein AS160 integrates Akt, protein kinase C, and AMP-activated protein kinase signals regulating GLUT4 traffic. Diabetes. 2007;56:414–423. doi: 10.2337/db06-0900. [DOI] [PubMed] [Google Scholar]
- Jones KA, Jiang X, Yamamoto Y, Yeung RS. Tuberin is a component of lipid rafts and mediates caveolin-1 localization: role of TSC2 in post-Golgi transport. Exp Cell Res. 2004;295:512–524. doi: 10.1016/j.yexcr.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Jiang X, Yeung RS. Regulation of microtubule-dependent protein transport by the TSC2/mammalian target of rapamycin pathway. Cancer Res. 2006;66:5258–5269. doi: 10.1158/0008-5472.CAN-05-4510. [DOI] [PubMed] [Google Scholar]
- Choi JH, Bertram PG, Drenan R, Carvalho J, Zhou HH, Zheng XF. The FKBP12-rapamycin-associated protein (FRAP) is a CLIP-170 kinase. EMBO Rep. 2002;3:988–994. doi: 10.1093/embo-reports/kvf197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenerson H, Folpe AL, Takayama TK, Yeung RS. Activation of the mTOR pathway in sporadic angiomyolipomas and other perivascular epithelioid cell neoplasms. Hum Pathol. 2007;38:1361–1371. doi: 10.1016/j.humpath.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Fujio Y, Kureishi Y, Rudic RD, Daumerie G, Fulton D, Sessa WC, Walsh K. Acute modulation of endothelial Akt/PKB activity alters nitric oxide-dependent vasomotor activity in vivo. J Clin Invest. 2000;106:493–499. doi: 10.1172/JCI9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel AR, Pessin JE. Insulin signaling in microdomains of the plasma membrane. Traffic. 2003;4:711–716. doi: 10.1034/j.1600-0854.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- Sakyo T, Naraba H, Teraoka H, Kitagawa T. The intrinsic structure of glucose transporter isoforms Glut1 and Glut3 regulates their differential distribution to detergent-resistant membrane domains in nonpolarized mammalian cells. Febs J. 2007;274:2843–2853. doi: 10.1111/j.1742-4658.2007.05814.x. [DOI] [PubMed] [Google Scholar]
- Huang J, Imamura T, Babendure JL, Lu JC, Olefsky JM. Disruption of microtubules ablates the specificity of insulin signaling to GLUT4 translocation in 3T3–L1 adipocytes. J Biol Chem. 2005;280:42300–42306. doi: 10.1074/jbc.M510920200. [DOI] [PubMed] [Google Scholar]
- Eyster CA, Duggins QS, Gorbsky GJ, Olson AL. Microtubule network is required for insulin signaling through activation of Akt/protein kinase B: evidence that insulin stimulates vesicle docking/fusion but not intracellular mobility. J Biol Chem. 2006;281:39719–39727. doi: 10.1074/jbc.M607101200. [DOI] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF. The TSC1–2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Logsdon MN, Lipovsky AI, Abbott D, Kwiatkowski DJ, Cantley LC. Feedback inhibition of Akt signaling limits the growth of tumors lacking Tsc2. Genes Dev. 2005;19:1773–1778. doi: 10.1101/gad.1314605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZY, Zhou QL, Coleman KA, Chouinard M, Boese Q, Czech MP. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci USA. 2003;100:7569–7574. doi: 10.1073/pnas.1332633100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- Chong-Kopera H, Inoki K, Li Y, Zhu T, Garcia-Gonzalo FR, Rosa JL, Guan KL. TSC1 stabilizes TSC2 by inhibiting the interaction between TSC2 and the HERC1 ubiquitin ligase. J Biol Chem. 2006;281:8313–8316. doi: 10.1074/jbc.C500451200. [DOI] [PubMed] [Google Scholar]
- Thorens B. Glucose transporters in the regulation of intestinal, renal, and liver glucose fluxes. Am J Physiol. 1996;270:G541–G553. doi: 10.1152/ajpgi.1996.270.4.G541. [DOI] [PubMed] [Google Scholar]
- Arbiser JL, Brat D, Hunter S, D'Armiento J, Henske EP, Arbiser ZK, Bai X, Goldberg G, Cohen C, Weiss SW. Tuberous sclerosis-associated lesions of the kidney, brain, and skin are angiogenic neoplasms. J Am Acad Dermatol. 2002;46:376–380. doi: 10.1067/mjd.2002.120530. [DOI] [PubMed] [Google Scholar]
- Bissler JJ, Kingswood JC. Renal angiomyolipomata. Kidney Int. 2004;66:924–934. doi: 10.1111/j.1523-1755.2004.00838.x. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Krebs M, Brunmair B, Brehm A, Artwohl M, Szendroedi J, Nowotny P, Roth E, Furnsinn C, Promintzer M, Anderwald C, Bischof M, Roden M. The mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes. 2007;56:1600–1607. doi: 10.2337/db06-1016. [DOI] [PubMed] [Google Scholar]
- Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- Del Bufalo D, Ciuffreda L, Trisciuoglio D, Desideri M, Cognetti F, Zupi G, Milella M. Antiangiogenic potential of the mammalian target of rapamycin inhibitor temsirolimus. Cancer Res. 2006;66:5549–5554. doi: 10.1158/0008-5472.CAN-05-2825. [DOI] [PubMed] [Google Scholar]
- Semela D, Piguet AC, Kolev M, Schmitter K, Hlushchuk R, Djonov V, Stoupis C, Dufour JF. Vascular remodeling and antitumoral effects of mTOR inhibition in a rat model of hepatocellular carcinoma. J Hepatol. 2007;46:840–848. doi: 10.1016/j.jhep.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, Loda M, Lane HA, Sellers WR. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- Szelies B, Herholz K, Heiss WD, Rackl A, Pawlik G, Wagner R, Ilsen HW, Wienhard K. Hypometabolic cortical lesions in tuberous sclerosis with epilepsy: demonstration by positron emission tomography. J Comput Assist Tomogr. 1983;7:946–953. doi: 10.1097/00004728-198312000-00002. [DOI] [PubMed] [Google Scholar]
- Rintahaka PJ, Chugani HT. Clinical role of positron emission tomography in children with tuberous sclerosis complex. J Child Neurol. 1997;12:42–52. doi: 10.1177/088307389701200107. [DOI] [PubMed] [Google Scholar]
- Crino PB. Molecular pathogenesis of tuber formation in tuberous sclerosis complex. J Child Neurol. 2004;19:716–725. doi: 10.1177/08830738040190091301. [DOI] [PubMed] [Google Scholar]
- Jansen FE, Notenboom RG, Nellist M, Goedbloed MA, Halley DJ, de Graan PN, van Nieuwenhuizen O. Differential localization of hamartin and tuberin and increased S6 phosphorylation in a tuber. Neurology. 2004;63:1293–1295. doi: 10.1212/01.wnl.0000140702.08902.d7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.