Abstract
Rationale: In a clinical trial by the Acute Respiratory Distress Syndrome Network (ARDSNet), mechanical ventilation with tidal volumes of 6 ml/kg decreased mortality from acute lung injury. However, interpretations of these results generated controversy and it was unclear if this trial would change usual-care practices.
Objectives: First, to determine if clinical practices at ARDSNet hospitals changed after the tidal volume trial. Second, to determine if tidal volume and plateau pressure (Pplat) within 48 hours before randomization affected hospital mortality in patients subsequently managed with 6 ml/kg predicted body weight (PBW).
Methods: We used preenrollment data from 2,451 patients enrolled in six trials (1996–2005) to describe changes in tidal volume over time. We used logistic regression to determine if preenrollment tidal volume or Pplat affected mortality.
Measurements and Main Results: Median preenrollment tidal volume decreased from 10.3 ml/kg PBW (range, 4.3–17.1) during the tidal volume trial (1996–1999) to 7.3 ml/kg PBW (range, 3.9–16.2) after its completion (P < 0.001). Preenrollment tidal volume was not associated with mortality (P = 0.566). The odds of death increased multiplicatively with each cm H2O of preenrollment Pplat (P < 0.001) (e.g., the odds of death was 1.37 times greater when preenrollment Pplat increased by 10 cm H2O).
Conclusions: Physicians used lower tidal volumes after publication of the tidal volume trial. Preenrollment Pplat was strongly associated with mortality, and may reflect disease severity independent of tidal volume. Pplat measured early in the course of acute lung injury, after accounting for tidal volume, is a respiratory system–specific value with strong prognostic significance.
Keywords: acute lung injury, mechanical ventilation, clinician practices
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
In an ARDS Network clinical trial, mechanical ventilation with low tidal volumes decreased mortality from acute lung injury. Interpretations of these results generated controversy, and it was unclear if this trial would change clinical practice.
What This Study Adds to the Field
Substantial reduction in usual-care tidal volumes occurred at ARDS Network hospitals after the trial. Plateau pressure early in the course of acute lung injury, accounting for tidal volume, is a respiratory system–specific value that predicts mortality.
In the United States, acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) affect approximately 190,000 patients each year and are associated with 75,000 deaths (1). In May 2000, the National Institutes of Health Acute Respiratory Distress Syndrome Network (ARDSNet) published results of a randomized clinical trial that compared clinical outcomes of patients who received mechanical ventilation with a traditional or a lower tidal volume strategy. Hospital mortality was decreased and ventilator-free days were greater in patients who received the lower tidal volume strategy (2). Interpretations of these results, however, generated controversy regarding the relationship between the tidal volumes used in the trial and those used in usual-care practices (3). Moreover, early reports documented either no change or a modest change in tidal volume shortly after publication of the tidal volume trial (4–7). Therefore, it was unclear if the results of the tidal volume trial would change usual-care clinical practice at ARDSNet hospitals. To address this question, we used data from the preenrollment period in patients who were enrolled in ARDSNet clinical trials. We assume that preenrollment tidal volume reflects usual-care clinical practices at ARDSNet hospitals. Although clinical practices at ARDSNet hospitals may not represent those at other hospitals in the United States, we reasoned that changes in clinical practices were unlikely to occur at other hospitals if they did not change at ARDSNet hospitals.
In a previous analysis of ARDSNet data between March 1996 and March 1999, preenrollment tidal volumes were highly variable (8). Therefore, our secondary objective was to determine if exposure of less than 48 hours to higher tidal volume affected hospital mortality in patients who were subsequently managed with lower tidal volume after enrollment in ARDSNet clinical trials. Data were available in patients who were enrolled within 36 (2, 9–11) to 48 hours (12, 13) after the onset of ALI/ARDS, but not in patients who died in that time period or in patients who were mechanically ventilated for longer than 36 to 48 hours after onset of ALI/ARDS. We reported preliminary results in an abstract (14).
METHODS
Description of Studies
Since 1996, the ARDSNet has conducted seven multicenter clinical trials (2, 9–13, 15). Patients were eligible for enrollment if they were intubated, received mechanical ventilation, and met American–European Consensus Conference criteria for ALI/ARDS (16). ARDSNet trials required enrollment within 36 (2, 9–11) to 48 hours (12, 13) when ALI/ARDS criteria were first met. Patients who were mechanically ventilated for longer than 36 to 48 hours after the onset of ALI/ARDS were excluded. The ARDSNet recorded physician-prescribed ventilator settings before randomization in six trials. After enrollment, tidal volume was controlled according to protocol rules; therefore, preenrollment tidal volumes were in use for less than 36 (2, 9–11) to 48 hours (12, 13) according to the eligibility criteria of each clinical trial.
Definitions
We defined volume-targeted ventilation when assist-control, synchronized-intermittent mechanical ventilation, with or without pressure support, or pressure-regulated volume control was used. We defined pressure-targeted ventilation when pressure control, pressure assist-control, inverse ratio ventilation, or pressure support was used.
Between March 1996 and March 1999, patients received either a traditional tidal volume strategy (goal Vt of 12 ml/kg predicted body weight [PBW], with plateau pressure [Pplat] limited to 50 cm H2O) or a lower tidal volume strategy (goal Vt of 6 ml/kg PBW with Pplat limited to 30 cm H2O) (2). After the results of the tidal volume trial were publicly announced in March 1999, all patients were subsequently managed with the 6-ml/kg PBW tidal volume protocol. We defined the period during the tidal volume trial as March 1996 to March 1999. We defined the period after completion of the tidal volume trial as April 1999 to October 2005. We stratified preenrollment tidal volumes into 6.5 ml/kg PBW or less, 6.51–12 ml/kg PBW, and greater than 12 ml/kg PBW. We used 6.5 ml/kg PBW as the lower cutoff because this cutoff was chosen by the ARDSNet when evaluating compliance with the 6-ml/kg PBW tidal volume protocol.
We calculated static respiratory system compliance as Vt/(Pplat − positive end-expiratory pressure).
Biostatistical Methods
Our first objective was to determine if mechanical ventilation practices at ARDSNet hospitals changed after the tidal volume trial. Our second objective was to determine if a higher tidal volume and Pplat during the 48 hours preceding randomization (i.e., preenrollment) affected hospital mortality in patients subsequently managed with 6 ml/kg PBW.
The primary outcome for our first objective was preenrollment tidal volume. Secondary outcomes at preenrollment included modes of ventilation, Pplat, and positive end-expiratory pressure (PEEP). We used the Cochran-Armitage statistic (17) to assess for trends in proportions over time. We used a χ2 test to compare medians (18). We used a χ2 test to compare multiple correlation coefficients (18). We used a t test to compare means and linear regression to compare slopes.
The outcome of our second objective was hospital mortality. We used a subset of patients managed with the 6-ml/kg PBW tidal volume protocol after enrollment. We excluded patients managed with the 12-ml/kg PBW tidal volume protocol after enrollment because these patients had greater hospital mortality than those managed with the 6-ml/kg PBW tidal volume protocol (2).
We approached our analysis in steps. First, we conducted an exploratory analysis to investigate the shape of the relationship between each predictor and the log odds of death. Second, we used logistic regression to model the effects of preenrollment tidal volume and Pplat on hospital mortality after controlling for age, APACHE (Acute Physiology and Chronic Health Evaluation) III score, partial pressure of carbon dioxide (PaCO2), length of hospital stay before onset of ALI/ARDS, calendar year, sex (males were the reference category), ethnicity (whites were the reference category), PaO2/FiO2, and PEEP. With the exception of preenrollment tidal volume, the relationship between continuous predictors and the log odds of death was closely linear. We used a natural cubic regression spline (19) to model the nonlinear shape of the relationship between preenrollment tidal volume and the log odds of death. Third, we used the Hosmer-Lemeshow test to determine goodness-of-fit (20), and evaluated residuals to identify outliers (20).
RESULTS
Study Sample
The ARDSNet recorded preenrollment ventilator management data on 2,451 subjects with ALI/ARDS in six clinical trials between 1996 and 2005. In the 48-hour period before enrollment, 2,112 subjects (86%) were managed with volume-targeted ventilation, 315 (13%) with pressure-targeted ventilation, and 10 (<1%) with other modes of ventilation. We could not adequately determine the preenrollment mode of ventilation in 14 subjects (<1%). Of the 2,112 subjects who received volume-targeted ventilation before enrollment, 353 (17%) received the 12-ml/kg PBW tidal volume protocol after enrollment; 164 (8%) did not have a recorded tidal volume or PBW; 364 (17%) did not have a recorded Pplat; 146 (7%) did not have a recorded FiO2, PaO2, PaCO2, PEEP, or APACHE III score; 58 (3%) did not meet the hypoxemic criterion for ALI/ARDS in the arterial blood gas that immediately preceded enrollment; and one subject did not have a hospital admission date. We excluded data for one subject who had a preenrollment tidal volume of 1.5 ml/kg PBW. Fifty-nine percent (1,249/2,112) of patients with ALI/ARDS who received volume-targeted ventilation before enrollment were managed with the ARDS low–tidal volume protocol after enrollment and had complete data for regression analysis.
Modes of Ventilation
Of 2,437 subjects with a recorded mode of ventilation before enrollment between 1996 and 2005, 1,720 (71%) were managed with the assist-control mode (Figure 1A). The proportion of subjects managed with assist-control increased from 61% in 1996 to 82% in 2005. During the tidal volume trial, there was no change in the proportion of patients managed with assist-control over time (Cochran-Armitage statistic = 1.9; P = 0.168). After completion of the trial, assist-control was used with increasing frequency over time (Cochran-Armitage statistic = 16.7; P < 0.001). The proportion of patients managed with the synchronized-intermittent mandatory ventilation mode decreased over time, from 22% in 1996 to 3% in 2005 (Figure 1A). The proportion of patients managed with pressure control ventilation also decreased, from 14% in 1996 to 6% in 2005. After completion of the tidal volume trial, both synchronized-intermittent mandatory ventilation (Cochran-Armitage statistic = 28.0; P < 0.001) and pressure control ventilation (Cochran-Armitage statistic = 8.5; P = 0.004) were used less frequently over time.
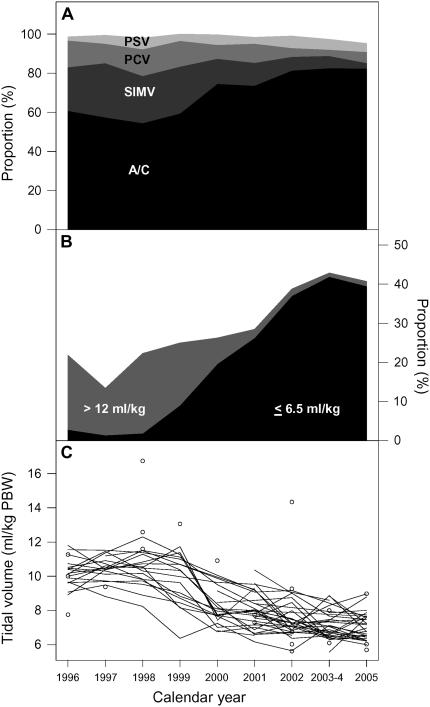
Figure 1.
Serial trends in preenrollment modes of ventilation and in preenrollment tidal volumes at ARDS (Acute Respiratory Distress Syndrome) Network hospitals, 1996–2005. (A) Preenrollment ventilator modes used over time. Each shade of gray represents a different ventilator mode (A/C = assist-control, SIMV = synchronized-intermittent mandatory ventilation, PCV = pressure support ventilation, and PSV = pressure support ventilation. Other modes of ventilation are not included in this panel). The x axis represents calendar year from 1996 to 2005, and the y axis represents the proportion for each ventilator mode. (B) Categories of preenrollment tidal volumes of greater than 12 ml/kg of predicted body weight (PBW) and 6.5 ml/kg PBW or less. This panel shows the proportion of patients with preenrollment tidal volumes of greater than 12 ml/kg of PBW and less than or equal to 6.5 ml/kg PBW. Data for patients with preenrollment tidal volumes of 6.51–12 ml/kg PBW are not shown, but the sum of proportions for each year adds up to 100%. (C) Mean preenrollment tidal volume over time by hospital where each line represents data from one hospital. The circles represent tidal volume of hospitals that only contributed one patient in a particular year.
Trends in Volume-targeted Ventilation
Of the 2,112 patients who received volume-targeted ventilation before enrollment, 1,948 had a recorded preenrollment tidal volume in milliliters per kilogram of PBW. Of these, 578 had a preenrollment tidal volume before completion of the trial (March 1996 to March 1999) and 1,370 had a preenrollment tidal volume after completion of the trial (April 1999 to October 2005). Patients with ALI/ARDS received lower preenrollment tidal volumes over time at ARDSNet hospitals. When stratified by categories of preenrollment tidal volume, 17% (100/578) of patients received preenrollment tidal volume greater than 12 ml/kg PBW and 2% (12/578) received preenrollment tidal volume of 6.5 ml/kg PBW or less during the tidal volume trial (Figure 1B). In contrast, after completion of the trial, 3% (37/1,370) of patients received a preenrollment tidal volume greater than 12 ml/kg PBW (χ2 statistic = 130.3; P < 0.001) and 32% (432/1,370) received a preenrollment tidal volume of 6.5 ml/kg PBW or less (χ2 statistic = 198.7; P < 0.001). In 2005, only two patients (1%) received preenrollment tidal volumes greater than 12 ml/kg PBW, whereas 40% (70/177) received tidal volumes of 6.5 ml/kg PBW or less. The decrease in preenrollment tidal volume over time was consistent across all participating hospitals (Figure 1C).
Median preenrollment tidal volume decreased from 10.3 ml/kg PBW (interquartile range [IQR], 9.2–11.5) during the tidal volume trial to 7.3 ml/kg PBW (IQR, 6.3–8.6) after completion of the trial (Figure 2A; median test; P < 0.001). By 2005, median preenrollment tidal volume had decreased to 6.8 ml/kg PBW (IQR, 6.1–7.7). The rate of decrease in preenrollment tidal volume was also different over time. During the tidal volume trial, mean preenrollment tidal volume did not change significantly between March 1996 and March 1999 (t-statistic = 0.45; P = 0.627). After completion of the tidal volume trial, however, patients were managed with a consistently lower mean preenrollment tidal volume over time (t-statistic = −9.7; P < 0.001).
Figure 2.
Serial trends in preenrollment tidal volumes and plateau pressure, and hospital mortality at ARDS Network hospitals, 1996–2005. Multipanel graph shows the effects of the tidal volume trial on preenrollment tidal volumes, preenrollment inspiratory plateau pressures, and hospital mortality. (A) The time series of preenrollment tidal volumes represented by box plots for each year. The notches represent approximate 95% confidence intervals for the median tidal volume. (B) The proportion of patients who had preenrollment plateau pressure (Pplat) greater than 30 cm H2O. (C) The proportion of patients who died in the hospital in the subset of patients who received the lower tidal volume protocol (Vt goal of 6 ml/kg of predicted weight with Pplat limited to 30 cm H2O).
Of 2,112 patients who were managed with volume-targeted ventilation before enrollment, 1,748 had a recorded preenrollment Pplat. Of these, 624 had a recorded preenrollment Pplat before completion of the tidal volume trial (March 1996 to March 1999) and 1,124 had a recorded preenrollment Pplat after completion of the trial (April 1999 to October 2005). Mean preenrollment Pplat was 27.7 cm H2O (SD, 7.5), and decreased from 29.2 cm H2O in 1996 to 26.3 cm H2O in 2005. The proportion of patients with preenrollment Pplat greater than 30 cm H2O decreased from 43% (267/624) before completion of the tidal volume trial to 24% (271/1,124) after completion of the trial (Figure 2B; χ2 statistic = 64.8; P < 0.001). Of note, the average APACHE III score increased from 82.8 before completion of the tidal volume trial (March 1996 to March 1999) to 94.1 after completion of the trial (April 1999 to October 2005). There was low correlation between preenrollment Pplat and APACHE III score (Pearson correlation coefficient of 0.08).
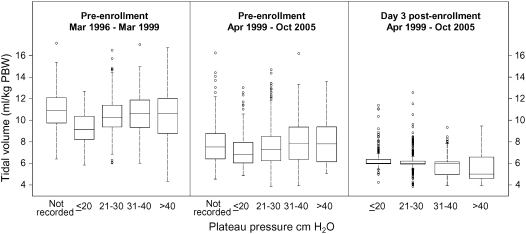
Physicians used lower preenrollment tidal volumes after completion of the tidal volume trial, but there was no relationship between preenrollment Pplat and preenrollment tidal volume (Figure 3). Furthermore, there was no difference in mean preenrollment tidal volume between patients who did not have a recorded preenrollment Pplat and those with a recorded Pplat (t test; P = 0.502). Correlations between preenrollment Pplat and preenrollment tidal volume during and after the completion of the tidal volume trial were 0.16 and 0.13, respectively. At Day 3 after enrollment, the correlation between Pplat and tidal volume was −0.19, opposite in direction when compared with the preenrollment correlations (χ2 statistic with 2 degrees of freedom = 66.2; P < 0.001).
Figure 3.
Relationship between plateau pressure and tidal volume, 1996–2005. Box plots of tidal volume stratified by time period (during and after publication of the tidal volume trial, and Day 3 after enrollment) and by a range of plateau pressures. Baseline tidal volumes decreased after completion of the tidal volume trial in 1999. However, there was no relationship of preenrollment tidal volumes to plateau pressure either during or after completion of the trial. On Day 3 after enrollment in ARDS Network trials, there was an inverse relationship between plateau pressure and tidal volume. The reason for this inverse relationship on Day 3 is that tidal volumes were decreased to 4 to 6 ml/kg of predicted body weight (PBW) according to the tidal volume protocol when plateau pressures exceeded 30 cm H2O.
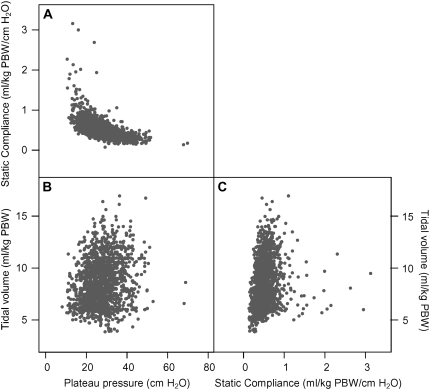
There was a strong reciprocal relationship between preenrollment Pplat and preenrollment respiratory system static compliance (Figure 4A). On the other hand, there was a less obvious relationship between preenrollment Pplat and preenrollment tidal volume (Figure 4B), and between preenrollment static respiratory system compliance and preenrollment tidal volume (Figure 4C).
Figure 4.
Scatterplots between preenrollment static respiratory system compliance, preenrollment plateau pressures, and preenrollment tidal volume. (A) Scatterplot between preenrollment plateau pressure (cm H2O) and static respiratory system compliance (ml/cm H2O by kg predicted body weight [PBW]) shows a reciprocal [compliance ∝ (1/plateau pressure)] relationship between preenrollment static respiratory system compliance and preenrollment plateau pressure. (B) Scatterplot between preenrollment tidal volume (ml/ kg PBW) and preenrollment plateau pressure. No obvious relationship is apparent. (C) Scatterplot between preenrollment tidal volume and static respiratory system compliance. Again, no obvious relationship is apparent.
PEEP
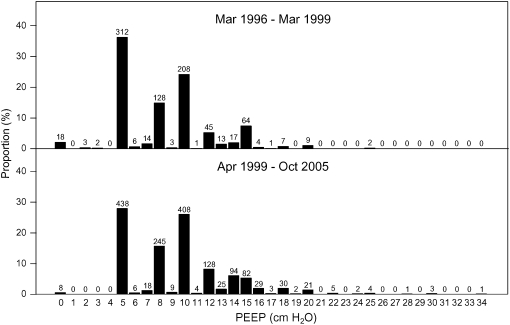
The use of PEEP changed only modestly during the 10-year (1996–2005) period. Preenrollment PEEP was available in 2,422 subjects (99%) who were managed with either volume-targeted ventilation or with pressure-targeted ventilation. Preenrollment PEEP had a bimodal distribution (Figure 5): physicians prescribed either 5 or 10 cm H2O in 56% (1,366/2,422) of patients. Median preenrollment PEEP increased from 8 cm H2O (IQR, 5–10) during the tidal volume trial to 10 cm H2O (IQR, 5–12) after completion of the trial (χ2 statistic = 62.5; P < 0.001). The proportion of patients with zero PEEP decreased from 2% (18/857) during the tidal volume trial to less than 1% (8/1,565) after completion of the tidal volume trial (χ2 statistic = 11.7; P < 0.001).
Figure 5.
Distribution of preenrollment positive end-expiratory pressure (PEEP) during and after completion of the ARDS Network tidal volume trial, 1996–2005. Numbers over bars indicate counts of patients within each level of PEEP.
Hospital Mortality
Hospital mortality was 30% (729/2,427) in patients managed with either volume-targeted ventilation or pressure-targeted ventilation before enrollment. In the subset of patients who were managed with either volume-targeted ventilation or pressure-targeted ventilation before enrollment and who received the lower tidal volume protocol after enrollment in ARDSNet trials, hospital mortality was 28% (559/2,002) (Figure 2C). In this subset of patients, hospital mortality did not vary over time (Cochran-Armitage statistic = 2.1; P = 0.279).
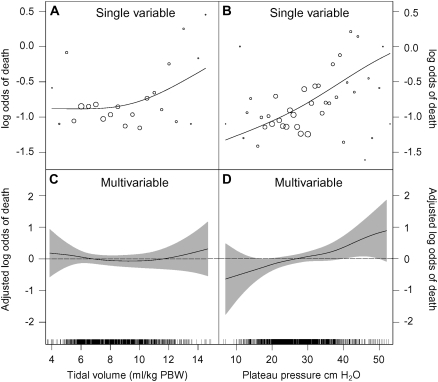
In single variable analysis, the unadjusted log odds of death was constant between preenrollment tidal volume of 3 and 10 ml/kg PBW and increased for preenrollment tidal volume greater than 10 ml/kg PBW (Figure 6A). In multivariable analysis, however, preenrollment tidal volume was not significantly associated with hospital mortality (Figure 6B; likelihood ratio test P value = 0.566). On the other hand, preenrollment Pplat was linearly related with the log odds of death in single variable (Figure 6C) and multivariable analysis (Figure 6D). The adjusted odds of death increased multiplicatively by a factor of 1.03 (odds ratio [OR], 1.03; 95% confidence interval [CI], 1.01–1.06; P = 0.011) for each cm H2O increase in preenrollment Pplat. That is, the adjusted odds of death was 1.17 times greater when preenrollment Pplat increased from 15 to 20 cm H2O, 1.37 times greater when preenrollment Pplat increased from 20 to 30 cm H2O and 1.87 times greater when preenrollment Pplat increased from 30 to 50 cm H2O.
Figure 6.
Effects of preenrollment tidal volume and preenrollment plateau pressure on hospital mortality, 1996–2005. (A) Relationship between preenrollment tidal volume and hospital mortality. (B) Relationship between preenrollment plateau pressure and hospital mortality. (A) and (B) represent the unadjusted relationships between each predictor and hospital mortality. We calculated the unadjusted log odds of death as log[(yi + 0.5)/(ni − yi + 0.5)] across small intervals of each predictor, where yi represents the number of deaths in the ith interval and ni represents the number of subjects in that same interval. The size of the circles is proportional to the square root of the number of subjects in each interval. The lines represent smoothing splines for the unadjusted relationship between each predictor and the log odds of death. (C) and (D) show results of a generalized additive logistic model of the effects of preenrollment tidal volume and preenrollment plateau pressure on hospital mortality after adjusting for age, APACHE (Acute Physiology and Chronic Health Evaluation) III score, length of hospital stay, and Hispanic ethnicity. The y axis represents the adjusted log odds of death centered on the mean of each predictor. The lines represent smoothing splines and the shaded areas correspond to 95% (±1.96 SE) confidence bands. We added frequency plots at the bases. We truncated results for plateau pressures greater than 60 cm H2O because the confidence bands were too wide beyond these values (there were only two data points with values greater than 60 cm H2O).
APACHE III score, age, and hospital stay before enrollment were significantly associated with hospital mortality, whereas sex, calendar year, PaCO2, preenrollment PEEP, and PaO2/FiO2 were not. Hispanics had a significantly higher chance of death than did whites, but other ethnic groups did not. Thus, we subsequently categorized ethnicity into Hispanics versus non-Hispanics. The Hosmer-Lemeshow goodness-of-fit test indicated that our regression model fit the data well (χ2 statistic with 8 degrees of freedom = 9.37; P = 0.312). Regression diagnostics did not reveal outliers. The most parsimonious regression model that explained hospital mortality included only five predictors: preenrollment Pplat (OR, 1.03 per each cm H2O increase; 95% CI, 1.01–1.05), APACHE III score, age, hospital days before enrollment, and Hispanic ethnicity. These five predictors were statistically significant at the 0.05 level.
DISCUSSION
This study demonstrates that physicians at ARDSNet hospitals changed how they managed patients with ALI after completion of the tidal volume trial. These changes were most apparent in preenrollment tidal volumes and modes of ventilation. Although median preenrollment (usual care) tidal volumes decreased from 10.3 ml/kg PBW in 1996 to 6.8 ml/kg PBW in 2005, there was still substantial variation in this aspect of clinical practice. Nonetheless, exposures of less than 48 hours to moderate or high tidal volumes did not increase the chance of death in patients who were subsequently managed with low tidal volumes. On the other hand, plateau pressures in the first 48 hours of ALI were strongly associated with hospital mortality.
Mechanical ventilation with lower tidal volumes has been associated with decreased hospital mortality in the ARDSNet clinical trial (2), in two smaller randomized trials (21, 22), and in uncontrolled, observational studies (23–25). In experimental models, ventilator-induced lung injury occurred within minutes to hours of exposure to mechanical ventilation with high tidal volumes and high airway pressures (26–29). Moreover, in humans with ALI, concentrations of inflammatory mediators in the bloodstream and bronchoalveolar fluid were significantly higher 1 hour after tidal volumes were raised from 5 to 12 ml/kg of PBW (30). We therefore hypothesized that exposures of less than 48 hours to higher preenrollment tidal volumes among patients who were enrolled into clinical trials increased the chance of death. Instead, we found that exposures to preenrollment higher tidal volumes for as many as 48 hours after onset of ALI did not increase the chance of death when these exposures were followed by mechanical ventilation with lower tidal volumes after enrollment. Although an explanation for this finding is not evident, we offer some potential explanations. First, subsequent ventilation with lower tidal volumes may have allowed sufficient recovery from early ventilator-induced lung injury such that mortality was not affected. Second, our analysis may have been underpowered to establish a statistically significant relationship between preenrollment tidal volume and hospital mortality. Third, some patients with high preenrollment tidal volumes may have died before enrollment or were moribund and not enrolled in clinical trials. We did not have information on patients with ALI who died within 48 hours, but in an unpublished analysis of data from academic hospitals in a recent study (1), 9% of deaths from ALI occurred within 48 hours of onset (Dr. Gordon Rubenfeld, University of Toronto, personal communication). Finally, traditional tidal volumes of 15 to 20 ml/kg of PBW or tolerance of very high plateau pressures, which by today's standards would be considered injurious, were no longer reflected in usual-care practices for enrolled patients during these trials. Of greater importance, however, is that patients at participating hospitals who are not enrolled in ARDSNet clinical trials are likely receiving, on average, lower tidal volumes than before completion of the trial, and consequently have a better chance of survival.
Earlier reports found only a modest change in physician practice in the 2-year period after publication of the tidal volume trial (7, 8). Some authors speculated that this lack of change was a consequence of the underlying controversies regarding the interpretation of the tidal volume trial (31). Our findings, however, are quite different from those reported in previous studies. There was a gradual and ultimately substantial effect on preenrollment tidal volumes at ARDSNet hospitals after completion of the tidal volume trial. Thus, clinically relevant research can affect clinical behavior. Because our results are based on data from patients enrolled into ARDSNet trials, these findings may not be generalizable to nonenrolled patients or to patients from other academic or community hospitals.
Although our analysis shows that some aspects of clinical practice have changed in response to the tidal volume trial, there are other aspects that have either met resistance to change or will take longer to change. For example, although physicians used lower tidal volumes after the tidal volume trial, these tidal volumes were the same regardless of plateau pressure. Other investigators have suggested that physicians might have titrated tidal volumes according to some marker of disease severity such as plateau pressure (32, 33), but our results are not consistent with this hypothesis. If a substantial proportion of physicians had titrated preenrollment tidal volumes according to plateau pressures, then we probably would have seen lower tidal volumes at higher plateau pressures. However, we observed the opposite relationship. That is, preenrollment physician-prescribed tidal volumes were either the same or greater at higher levels of plateau pressure. A positive slope between preenrollment Pplat and tidal volume is expected physiologically, and suggests that the majority of physicians had not adjusted tidal volumes according to plateau pressure (34). On the other hand, once on the protocol, the slope between tidal volume and Pplat was negative because tidal volumes were reduced according to the protocol when plateau pressures were greater than 30 cm H2O.
We did not use static respiratory system compliance in our analysis because of the strong relationship between static respiratory system compliance, tidal volume, and inspiratory Pplat. First, there is a strong reciprocal relationship between static respiratory system compliance and Pplat. Therefore, in a regression analysis, it may be difficult to separate the contributions of static respiratory system compliance from Pplat. Second, when adjusted for tidal volume, compliance is likely to provide similar information as Pplat. Third, compliance is a calculated value that depends on physician-prescribed tidal volume and recorded Pplat. Because pressure–volume curves are curvilinear, static respiratory system compliance varies with tidal volume size (35). Therefore, the use of calculated chord compliances may lead to inappropriate associations between static respiratory system compliance and tidal volume (34).
Although we documented that physician-prescribed tidal volumes were on average lower after the tidal volume trial than during the trial, tidal volumes remained highly variable. In addition, changes in clinician behavior were gradual. Like most advances in medicine, the use of lower tidal volumes disseminated slowly across ARDSNet hospitals (36). Some physicians may have maintained different priorities of care including maintenance of acid-base homeostasis and breathing comfort (37). Other explanations for the slow adoption of lower tidal volumes are that there was a lack of early recognition of ALI (4), and that there was uncertainty about the comparability of lower and intermediate tidal volumes, which may be easier to use. On the other hand, the proportion of physicians who prescribed tidal volumes greater than 12 ml/kg of PBW was substantially lower shortly after completion of the tidal volume trial.
Previous authors hypothesized that the effect of Pplat on hospital mortality was J-shaped, with a nadir in hospital mortality at plateau pressures between 28 and 32 cm H2O (3). Other authors suggested that there was a safe upper limit for plateau pressures of approximately 30 to 35 cm H2O (38, 39). Our results show that the relationship between preenrollment Pplat and hospital mortality (in the log odds scale) was linear, extended to plateau pressures that were below 30 cm H2O, and was statistically significant even after adjusting for preenrollment tidal volume and other baseline variables. Given that Pplat is determined by respiratory system compliance and tidal volume, and that exposures of less than 48 hours to a range of preenrollment tidal volumes did not affect hospital mortality, it follows that the relationship between preenrollment Pplat and hospital mortality may reflect severity of disease independent of the effect of tidal volume. Thus, Pplat measured early in the course of ALI, when adjusted for tidal volume, is a respiratory system–specific value that has strong prognostic significance. Our finding differs from a previous study (32) that demonstrated a linear but nonsignificant relationship between Pplat and hospital mortality after adjusting for confounders. One possibility for this difference is that the previous study, with 467 patients, was underpowered to demonstrate a statistically significant relationship. Another possibility is that the ARDSNet hospitals used a similar ventilation strategy in the postenrollment period for all patients, whereas there was substantial variability in mechanical ventilation practices across the 361 intensive care units included in the previous study. That is, interpretation of the effect of Pplat on hospital mortality may be less comparable between studies because of the lack of a uniform ventilation strategy and the lack of adjustment for tidal volume in the previous study.
Another aspect of clinical care that changed very little after completion of the tidal volume trial was the use of PEEP. Although the tidal volume trial was not designed to compare levels of PEEP, it used a systematic titration of PEEP and FiO2 according to PaO2. Our data suggest that PEEP was not adjusted precisely according to patient characteristics in the first 48 hours. In our analysis, early levels of PEEP did not predict hospital mortality. Furthermore, the proportion of patients managed with zero PEEP (⩽2%) at ARDSNet hospitals was very small and lower than the 7% reported in a previous multicenter, international study (32).
In summary, clinical practice of mechanical ventilation at ARDSNet hospitals changed after completion of the tidal volume trial, resulting in a gradual but ultimately substantial reduction in usual care tidal volumes. We were unable to detect a difference in mortality with the use of higher tidal volumes for up to 48 hours in patients who were enrolled in our clinical trials and who were subsequently managed with lower tidal volumes. Finally, we found that Pplat in the first 48 hours of ALI, after accounting for tidal volume, is a respiratory system–specific value that carries strong prognostic significance.
Acknowledgments
The authors thank Dr. Hank Fessler, Dr. David Hager, and members of the ARDS Network Publications Committee for their review of this manuscript and helpful comments.
Supported in part by a postdoctoral National Research Service Award (1F32HL090179-01) awarded to W.C. by the National Heart, Lung, and Blood Institute, National Institutes of Health. The clinical trials were supported by contracts NO1-HR-46046-64 and NO1-HR-16146-54 with the National Heart, Lung, and Blood Institute, National Institutes of Health.
These results were presented, in part, at the International Conference of the American Thoracic Society in San Francisco, California, on May 21, 2007.
Originally Published in Press as DOI: 10.1164/rccm.200709-1424OC on March 20, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693. [DOI] [PubMed] [Google Scholar]
- 2.The ARDS Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the ARDS. N Engl J Med 2000;342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 3.Eichacker PQ, Gerstenberger EP, Banks SM, Cui X, Natanson C. Meta-analysis of acute lung injury and acute respiratory distress syndrome trials testing low tidal volumes. Am J Respir Crit Care Med 2002;166:1510–1514. [DOI] [PubMed] [Google Scholar]
- 4.Rubenfeld GD, Cooper C, Carter G, Thompson BT, Hudson LD. Barriers to providing lung-protective ventilation to patients with acute lung injury. Crit Care Med 2004;32:1289–1293. [DOI] [PubMed] [Google Scholar]
- 5.Kalhan R, Mikkelsen M, Dedhiya P, Christie J, Gaughan C, Lanken PN, Finkel B, Gallop R, Fuchs BD. Underuse of lung protective ventilation: analysis of potential factors to explain physician behavior. Crit Care Med 2006;34:300–306. [DOI] [PubMed] [Google Scholar]
- 6.Weinert CR, Gross CR, Marinelli WA. Impact of randomized trial results on acute lung injury ventilator therapy in teaching hospitals. Am J Respir Crit Care Med 2003;167:1304–1309. [DOI] [PubMed] [Google Scholar]
- 7.Young MP, Manning HL, Wilson DL, Mette SA, Riker RR, Leiter JC, Liu SK, Bates JT, Parsons PE. Ventilation of patients with acute lung injury and acute respiratory distress syndrome: has new evidence changed clinical practice? Crit Care Med 2004;32:1260–1265. [DOI] [PubMed] [Google Scholar]
- 8.Thompson BT, Hayden D, Matthay MA, Brower R, Parsons PE. Clinicians' approaches to mechanical ventilation in acute lung injury and ARDS. Chest 2001;120:1622–1627. [DOI] [PubMed] [Google Scholar]
- 9.The ARDS Network. Ketoconazole for early treatment of acute lung injury and ARDS: a randomized controlled trial. JAMA 2000;283:1995–2002. [DOI] [PubMed] [Google Scholar]
- 10.The ARDS Network. Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and ARDS. Crit Care Med 2002;30:1–6. [DOI] [PubMed] [Google Scholar]
- 11.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the ARDS. N Engl J Med 2004;351:327–336. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 2006;354:2213–2224. [DOI] [PubMed] [Google Scholar]
- 13.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354:2564–2575. [DOI] [PubMed] [Google Scholar]
- 14.Checkley W, Brower RG, Korpak A, Thompson BT. Effects of early management of mechanical ventilation (MV) on hospital mortality in patients with acute lung injury. Am J Respir Crit Care Med 2007;175:A245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006;354:1671–1684. [DOI] [PubMed] [Google Scholar]
- 16.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818–824. [DOI] [PubMed] [Google Scholar]
- 17.Berry G, Armitage P. Statistical methods in medical research, 3rd ed. London: Blackwell Publishing; 1994. p. 403.
- 18.Zar J. Biostatistical analysis, 2nd ed. Englewood Cliffs, NJ: Prentice Hall; 1985. pp. 145, 315.
- 19.Hastie T, Tibshirani RJ. Generalized additive models. London: Chapman & Hall; 1995. p. 95. [DOI] [PubMed]
- 20.Agresti A. Categorical data analysis, 2nd ed. Hoboken, NJ: John Wiley & Sons; 2002. pp. 168, 175, 219.
- 21.Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998;338:347–354. [DOI] [PubMed] [Google Scholar]
- 22.Villar J, Kacmarek RM, Perez-Mendez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med 2006;34:1311–1318. [DOI] [PubMed] [Google Scholar]
- 23.Kallet RH, Jasmer RM, Pittet JF, Tang JF, Campbell AR, Dicker R, Hemphill C, Luce JM. Clinical implementation of the ARDS Network protocol is associated with reduced hospital mortality compared with historical controls. Crit Care Med 2005;33:925–929. [DOI] [PubMed] [Google Scholar]
- 24.Jardin F, Fellahi JL, Beauchet A, Vieillard-Baron A, Loubieres Y, Page B. Improved prognosis of acute respiratory distress syndrome 15 years on. Intensive Care Med 1999;25:936–941. [DOI] [PubMed] [Google Scholar]
- 25.Hickling KG, Walsh J, Henderson S, Jackson R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med 1994;22:1568–1578. [DOI] [PubMed] [Google Scholar]
- 26.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures: protection by positive end-expiratory pressure. Am Rev Respir Dis 1974;110:556–565. [DOI] [PubMed] [Google Scholar]
- 27.Parker JC, Hernandez LA, Longenecker GL, Peevy K, Johnson W. Lung edema caused by high peak inspiratory pressures in dogs: role of increased microvascular filtration pressure and permeability. Am Rev Respir Dis 1990;142:321–328. [DOI] [PubMed] [Google Scholar]
- 28.Dreyfuss D, Basset G, Soler P, Saumon G. Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am Rev Respir Dis 1985;132:880–884. [DOI] [PubMed] [Google Scholar]
- 29.Kolobow T, Moretti MP, Fumagalli R, Mascheroni D, Prato P, Chen V, Joris M. Severe impairment in lung function induced by high peak airway pressure during mechanical ventilation: an experimental study. Am Rev Respir Dis 1987;135:312–315. [DOI] [PubMed] [Google Scholar]
- 30.Stuber F, Wrigge H, Schroeder S, Wetegrove S, Zinserling J, Hoeft A, Putensen C. Kinetic and reversibility of mechanical ventilation-associated pulmonary and systemic inflammatory response in patients with acute lung injury. Intensive Care Med 2002;28:834–841. [DOI] [PubMed] [Google Scholar]
- 31.Villar J, Perez-Mendez L, Aguirre-Jaime A, Kacmarek RM. Why are physicians so skeptical about positive randomized controlled clinical trials in critical care medicine? Intensive Care Med 2005;31:196–204. [DOI] [PubMed] [Google Scholar]
- 32.Ferguson ND, Frutos-Vivar F, Esteban A, Anzueto A, Alia I, Brower RG, Stewart TE, Apezteguia C, Gonzalez M, Soto L, et al.; Mechanical Ventilation International Study Group. Airway pressures, tidal volumes, and mortality in patients with acute respiratory distress syndrome. Crit Care Med 2005;33:21–30. [DOI] [PubMed] [Google Scholar]
- 33.Deans KJ, Minneci PC, Suffredini AF, Danner RL, Hoffman WD, Ciu X, Klein HG, Schechter AN, Banks SM, Eichacker PQ, et al. Randomization in clinical trials of titrated therapies: unintended consequences of using fixed treatment protocols. Crit Care Med 2007;35:1509–1516. [DOI] [PubMed] [Google Scholar]
- 34.Brower R, Thompson BT; ARDS Network Investigators. Tidal volumes in acute respiratory distress syndrome: one size does not fit all. Crit Care Med 2006;34:263–264. [DOI] [PubMed] [Google Scholar]
- 35.Suter P, Fairley HB, Isenberg MD. Effect of tidal volume and positive end-expiratory pressure on compliance during mechanical ventilation. Chest 1978;73:158–162. [DOI] [PubMed] [Google Scholar]
- 36.Berwick DM. Disseminating innovations in health care. JAMA 2003;289:1969–1975. [DOI] [PubMed] [Google Scholar]
- 37.West JB. The physiological challenges of the 1952 Copenhagen poliomyelitis epidemic and a renaissance in clinical respiratory physiology. J Appl Physiol 2005;99:424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tobin MJ. Culmination of an era in research on the acute respiratory distress syndrome. N Engl J Med 2000;342:1360–1361. [DOI] [PubMed] [Google Scholar]
- 39.Slutsky AS. Mechanical ventilation. American College of Chest Physicians' Consensus Conference. Chest 1993;104:1833–1859. [DOI] [PubMed] [Google Scholar]