Abstract
Maximal bile acid secretory rates and expression of bile acid transporters in liver and ileum are increased in lactation, possibly to facilitate increased enterohepatic recirculation of bile acids. We determined changes in the size and composition of the bile acid pool and key enzymes of the bile acid synthetic pathway [cholesterol 7α-hydroxylase (Cyp7a1), sterol 27-hydroxylase (Cyp27a1), and sterol 12α-hydroxylase (Cyp8b1)] in lactating rats relative to female virgin controls. The bile acid pool increased 1.9 to 2.5-fold [postpartum (PP) days 10, 14, and 19–23], compared with controls. A 1.5-fold increase in cholic acids and a 14 to 20% decrease in muricholic acids in lactation significantly increased the hydrophobicity index. In contrast, the hepatic concentration of bile acids and small heterodimer partner mRNA were unchanged in lactation. A 2.8-fold increase in Cyp7a1 mRNA expression at 16 h (10 h of light) demonstrated a shift in the diurnal rhythm at day 10 PP; Cyp7a1 protein expression and cholesterol 7α-hydroxylase activity were significantly increased at this time and remained elevated at day 14 PP but decreased to control levels by day 21 PP. There was an overall decrease in Cyp27a1 mRNA expression and a 20% decrease in Cyp27a1 protein expression, but there was no change in Cyp8b1 mRNA or protein expression at day 10 PP. The increase in Cyp7a1 expression PP provides a mechanism for the increase in the bile acid pool.
Keywords: postpartum, circadian rhythm, lipid absorption, Cyp7a1, Cyp27a1, Cyp8b1
Bile acids are secreted by the hepatocyte into the proximal small intestine where they serve to promote absorption of dietary lipids, lipid-soluble vitamins, and cholesterol. Lactation is characterized by an increased energy requirement (four- to fivefold) (10) and food intake (7, 10) to maintain nourishment for the mother and supply maternal milk for the suckling young. Early studies showed that, during lactation, bile flow, bile acid synthesis and secretion rates, bile acid pool size (3, 16), and biliary excretion of lipophilic xenobiotics (16, 28) are all increased. Consistent with these findings, our laboratory showed that the expression of Na+/taurocholate cotransporting polypeptide (Ntcp), bile salt export pump (Bsep/Abcb11), and ileal apical sodium-dependent bile acid transporter (Asbt) are significantly increased in the dam during lactation (6, 21–23, 27). Despite these findings, the activity of the rate-limiting enzyme of bile acid synthesis, cholesterol 7α-hydroxylase (Cyp7a1), was shown to be significantly lower in postpartum (PP) vs. control female rats (38).
On the basis of the reported increase in the bile acid pool size (3), despite decreased Cyp7a1 activity (38), we examined the size, composition, and the hydrophobicity index of the bile acid pool. We also measured expression of Cyp7a1, sterol 27-hydroxylase (Cyp27a1), and sterol 12α-hydroxylase (Cyp8b1) throughout the light-dark cycle during early, midlactation, and late lactation. Finally, we measured expression of the farnesoid × receptor (FXR) target gene, small heterodimer partner (SHP), as an indicator of hepatic FXR activation. These studies demonstrated that lactation induced a phase shift in the diurnal rhythm of Cyp7a1 and increased its expression and activity, changes that correlate with a markedly increased bile acid pool size and increased hydrophobicity of the pool. However, hepatic bile acid concentrations and SHP expression were not changed, most likely due to the increased expression of Bsep and bile acid secretory maximum in lactating rats (5, 22).
Materials and Methods
Chemicals and reagents
TRIzol Reagent and SuperScript III First-Strand Synthesis System for RT-PCR were purchased from Invitrogen Life Technologies (Carlsbad, CA), LightCycler (version 3.5) and LightCycler DNA Master SYBR Green I from Roche Diagnostics (Indianapolis, IN), and RNeasy Mini kit from Qiagen (Valencia, CA). Goat α-human Cyp7a1 (N-17), donkey α-goat horseradish peroxidase (HRP), and donkey α-rabbit HRP antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit α-mouse Cyp27a1 antibody was a generous gift from Dr. David Russell (University of Texas Southwestern Medical Center, Dallas, TX). Reagents used for the measurement of Cyp7a1 hydroxylase activity were from Sigma-Aldrich (St. Louis, MO). ECL-Plus Reagent was purchased from Amersham Pharmacia Biotech (Piscataway, NJ). Kodak Biomax MR Film was used for Western blot analysis. HPLC analysis was performed with the Waters (Milford, MA) 1525 Binary HPLC Pump, 717 plus autosampler, and 2487 dual λ absorbance detector; C-18 reverse-phase Symmetry, 4.6 mm × 250 mm (bile acid pool size and composition), 4.6 mm × 150 mm (Cyp7a1 hydroxylase assay); Breeze software (Alpharetta, GA); and nonsterile Millex-HV syringe-driven filter units, 0.45 μm, from Millipore (Billerica, MA).
Animal care
Female Sprague-Dawley rats (Harlan Industries, Indianapolis, IN) were maintained in a temperature-controlled environment on a 12-h light-dark cycle (6 AM lights on/6 PM lights off). Animals had free access to Teklad Global Diet 2018 (Harlan Laboratories, Cincinnati, OH) and water. For determination of Cyp7a1, Cyp27a1, and Cyp8b1 expression, female virgin rats (control group) and PP animals were euthanized at 4 h (10 h of dark), 10 h (4 h of light), 16 h (10 h of light), and 22 h (4 h of dark). Pregnant rats were killed at day 20 of gestation (G-20), and PP animals were killed at early lactation (days 3 and 6), midlactation (days 10–14), or late lactation (days 19–23, before weaning). For all experiments, litter size was culled within 24 h of birth to 8–11 pups. All protocols involving animals were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Kentucky.
Real-time PCR analysis
Real-time PCR was performed on a Roche LightCycler with a SYBR Green kit by using cDNA synthesized from total RNA isolated with TRIzol Reagent and further purified with the Qiagen RNeasy Mini kit. Serial dilutions of cDNA template (0.1, 0.01, 0.001, and 0.001) were amplified, and the cycle number vs. the log of the fluorescence measurement at the threshold was plotted to generate a standard curve for semiquantitation of mRNA expression. Primers used for real-time PCR are listed in Table 1. The conditions used for amplification are the following: denaturation for 30 s at 94°C and 40 cycles of 94°C for 0 s; 55°C for 30 s (Cyp7a1), 60°C for 20 s (Cyp8b1), 57°C for 25 s (SHP), 56°C for 20 s (18S); and 72°C for 30 s (Cyp7a1 and 18S), 25 s (SHP), 20 s (Cyp8b1). The conditions for 18S amplification were adapted from Schmittgen and Zakrajsek (37).
Table 1. Primers used for real-time PCR.
| 18S forward | 5′-GTA ACC CGT TGA ACC CCA TT-3′ |
| 18S reverse | 5′-CCA TCC AAT CGG TAG TAG CG-3′ |
| Cyp7a1 forward | 5′-TGC CTT CTG TTA CCG AGT GAT GTT-3′ |
| Cyp7a1 reverse | 5′-ACC GGC AGG TCA TTC AGT TGC ACT-3′ |
| Cyp27a1 forward | 5′-ATG TGG CCA ATC TTC TCT ACC-3′ |
| Cyp27a1 reverse | 5′-GGG AAG GAA AGT GAC ATA GAC-3′ |
| Cyp8b1 forward | 5′-GGC TGG CTT CCT GAG CTT ATT′3′ |
| Cyp8b1 reverse | 5′-ACT TCC TGA ACA GCT CAT CGG-3′ |
| SHP forward | 5′-CTT GCT AGA GGA ACC CAA CAG TGG T-3′ |
| SHP reverse | 5′-AAC ACT GTA TGC AAA CCG AGG A-3′ |
Cyp7a1, cholesterol 7α-hydroxylase; Cyp27a1, sterol 27-hydroxylase; Cyp8b1, sterol 12α-hydroxylase; SHP, small heterodimer partner.
Measurement of the bile acid pool size
Control female rats and rats at day 20 gestation and at 6, 10, 14, and 19–23 days PP were killed and liver and small intestine (including contents) removed and weighed. Bile acids were quantitated by HPLC with the use of a method modified from Rossi et al. (36) and Turley et al. (40) that measures the bile acids present in the liver and small intestine. Briefly, liver and small intestine were homogenized in 100% ethanol (12.5 ml/g tissue), and 6 μmol/50 ml ethanol of 12 mM glycodeoxycholate was added as an internal standard. The samples were heated overnight at 60°C and then centrifuged at 1,700 g for 15 min. A 10-ml portion of the resulting supernatant was taken to dryness under a stream of N2, and the residue containing bile acids was resuspended in 1 ml of 75% methanol. The samples were centrifuged at 14,000 g for 10 min, the supernatant filtered using a nonsterile Millex-HV syringe-driven filter unit (0.45 μm), and taken to dryness under a stream of N2. Bile acids were resuspended in 1 ml of 75% methanol and centrifuged at 14,000 g for 3 min. An aliquot of the sample was assayed by HPLC in a mobile phase containing 10 mM potassium phosphate, pH 5.6 in 75% methanol. The bile acids were detected by UV absorbance at 200 nm following separation on a C-18 reverse-phase Symmetry 4.6 mm × 250 mm column. The retention time of the bile acids was compared with a series of bile acid standards to identify individual bile acids. The peak area of each bile acid was quantitated with Breeze HPLC software. The hydrophobicity index and bile composition were calculated by quantitation of individual HPLC peaks as previously described by Heuman (12) and bile acids expressed as mole percentage of the bile acid pool.
Isolation of mitochondria and microsomes
Mitochondria were harvested from livers of control and day 10 PP rats in isolation buffer (0.25 M sucrose, 0.5 mM EDTA, and 10 mM potassium phosphate, pH 7.4) as previously described (32). Briefly, liver homogenates were centrifuged at 600 g for 10 min, and the resulting supernatant was removed and centrifuged at 7,500 g for 20 min. The resulting mitochondrial pellet was washed twice with isolation buffer and resuspended with 0.1 M Tris · HCl, pH 7.7. For microsomes, the supernatant from the 7,500 g spin was centrifuged at 100,000 g for 1 h, the pellet rinsed, and centrifuged again at 100,000 g for 1 h. The final pellet was resuspended in 0.1 M potassium phosphate buffer, pH 7.4. Mitochondrial and microsomal preparations were aliquoted and stored at −70°C. The protein concentrations were measured as described (24).
Western blot analysis
Microsomal (Cyp7a1, 50 μg; Cyp8b1, 40 μg) or mitochondrial (Cyp27a1, 50 μg) proteins were separated on an 8% Tris-glycine gel and electrophoretically transferred to nitrocellulose membranes. Ponceau red staining was performed for each membrane to ensure equal loading in each lane. Membranes were blocked overnight at 4°C in a 5% nonfat dry milk Tris-buffered saline-Tween (25 mM Tris, pH 7.4, 150 mM NaCl, and 0.1% Tween-20) solution. Membranes were immunoblotted with goat-α-human Cyp7a1 N-17 (1:100), goat-α-human Cyp8b1 P-18 (1:100), or rabbit-α-mouse Cyp27a1 (1:500) antibody for 2 h at room temperature followed by incubation with 1:10,000 α-goat HRP or α-rabbit HRP antibody. After the membranes were washed with TBS-Tween solution, immunoreactive bands were visualized with enhanced chemiluminescence detection system (ECL-Plus Reagent) and exposed to film for 5 min.
Measurement of Cyp7a1 activity
Cholesterol hydroxylase activity was determined as described (13). Microsomal proteins (1 mg) were incubated at 37°C with a potassium phosphate buffer, pH 7.4, containing 50 mM NaF, 5 mM DTT, 1 mM EDTA, 20% glycerol, and 0.015% Chaps. The hydroxylase reactions (with the use of endogenous cholesterol as the substrate) were initiated upon the addition of an NADPH regenerating system and incubated for 20 min at 37°C. The reaction was stopped by the addition of 20% sodium cholate containing 2.5 nmol 7β-hydroxycholesterol (internal recovery standard). The 7α-hydroxycholesterol generated in the first reaction was converted to 7α-hydroxy-4-cholesten-3-one by the addition of 0.1% cholesterol oxidase in 10 mM potassium phosphate buffer, pH 7.4, 1 mM DTT, and 20% glycerol to the reactions and incubation at 37°C for another 20 min. The reactions were terminated upon the addition of 95% ethanol. The metabolites were extracted three times with petroleum ether and separated by HPLC analysis with a C-18 reverse-phase Symmetry column (4.6 mm × 150 mm). A standard curve for the formation of 7α-hydroxy-4-cholesten-3-one or 7β-hydroxy-4-cholesten-3-one was generated by adding a series of dilutions of either 7α-hydroxycholesterol or 7β-hydroxycholesterol in the cholesterol oxidase reaction. The picomoles per minute per miligram of product formed was determined by plotting the area under the curve against that of the standard curve and corrected by the percent recovery of the internal standard.
Statistical analysis
All data are expressed as the means ± SD for n = 3 to 8 animals per group. Statistical analysis was performed with Student's t-test, one-way ANOVA followed by Tukey's multiple comparison test, or two-way ANOVA test followed by Bonferroni's test with GraphPad Prism 4.0 software (San Diego, CA) as indicated in figure legends.
Results
Changes in bile acid pool size and composition during lactation
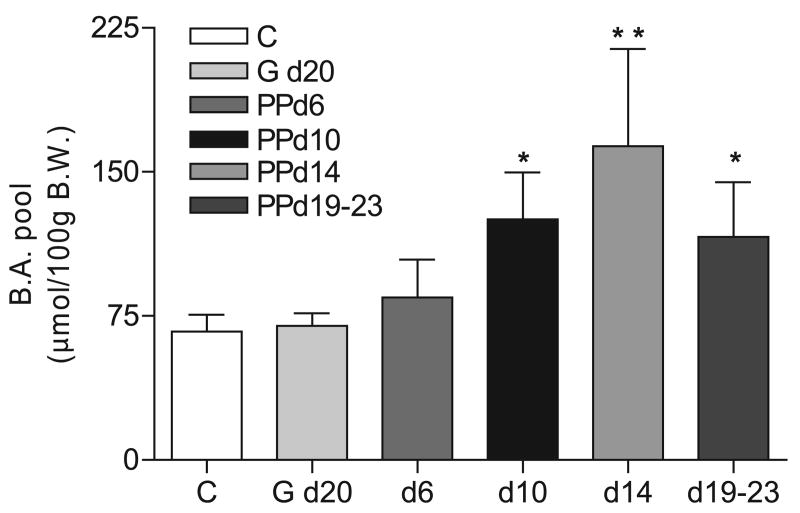
Earlier studies in rats used the bile drainage technique to demonstrate an increase in the bile acid pool size and increased rates of synthesis, secretion, and proportion of cholic acids during lactation (3, 15). We expanded upon these findings by measuring the bile acid pool size and composition for control, pregnant (G-20), and lactating (PP days 6, 10, 14, and 19–23) rats. The bile acid pool size was not different between control and G-20 or control and PP day 6 rats (Fig. 1). Significant increases in the bile acid pool size from control rats were found in middle and late lactation rats (1.9-fold increase, PP day 10; 2.4-fold increase, PP day 14; 1.7-fold increase, PP days 19–23; Fig. 1). The increase in the bile acid pool size was not due to an increase in body or liver weight since these values were similarly increased in G-20 and lactating animals (Table 2). The weight of the small intestine (including contents) was not significantly increased at G-20 but was increased twofold at PP day 6 and threefold at PP days 10–23 (Table 2).
Fig. 1.
The bile acid (BA) pool size is significantly increased in lactating rats. Bile acids were harvested from control female rats (C) (n = 8), rats at day 20 of gestation (G d20) (n = 4), and at days 6 (n = 4), 10 (n = 3), 14 (n = 6), and 19–23 (n = 4) of lactation (postpartum, PP). The bile acids were measured by HPLC analysis. Shown are the means ± SD. *P < 0.05 and **P < 0.001 vs. control female rats, determined by one-way ANOVA, followed by Bonferroni's multiple comparison post hoc test. d, day; BW, body weight.
Table 2. Body, liver, and small intestine weights in control, G-20, PP days 6, 10, 14, 19–23 rats.
| Body Weight, g | Liver Weight, g | Intestine Weight, g | |
|---|---|---|---|
| Control | 236.6±16.7 | 6.8±0.9 | 7.7±0.1 |
| G-20 | 354.5±3.5‡ | 12.1±0.5* | 10.6±0.8 |
| PP day 6 | 266.0±0.7 | 13.3±0.7† | 16.5±0.8† |
| PP day 10 | 319.0±5.6† | 15.5±0.4‡ | 24.8±2.3‡ |
| PP day 14 | 301.5±4.5† | 13.1±0.1† | 27.2±2.2‡ |
| PP day 19–23 | 321.3±20.3‡ | 15.1±1.3‡ | 21.6±1.8‡ |
All data are expressed as means ± SD.
P < 0.05.
P < 0.01.
P < 0.001 vs. control. PP, postpartum; G-20, day 20 of gestation.
We measured the hepatic bile acid concentration in control and PP days 8–12 animals to determine whether the increased pool size was reflected in the liver. SHP mRNA expression was not different in control and lactating rats (645 ± 120, n = 4 and 633 ± 386.9, n = 4, respectively). The lack of change in SHP mRNA is consistent with unchanged hepatic bile acid concentration between the two groups, whether expressed as micromoles per gram of liver (control, 2.5 ± 0.69, n = 4; PP, 2.2 ± 0.67, n = 4) or as micromole per 100 g body wt (control, 8.2 ± 2.7, n = 4; PP, 10.2 ± 3.8, n = 4). These data are also in agreement with increased hepatic bile acid transport in lactating animals as previously shown by increased Ntcp and Bsep expression in early and middle lactation (6).
The composition of the bile acid pool changed significantly in lactating rats. The mole percentage of cholic acids increased ∼1.5-fold at PP day 14 and PP days 19–23 (Table 3). Muricholic acids decreased 14% at PP day 10, 17% at PP day 14, and 20% at PP days 19–23 (Table 3). These decreases in the muricholic acids, which are primarily the tauro-muricholic acids, resulted in a small decrease in the proportion of taurine-conjugated bile acids and a corresponding increase in glycine-conjugated bile acids. However, there was no difference in the mole percentage of chenodeoxycholic acids between mid- and late lactation animals relative to control animals. Consistent with the increase in the proportion of cholic acids and a decrease in the proportion of muricholic acids, the hydrophobicity index was significantly elevated by midlactation in rats and remained elevated until late lactation, before pups were weaned.
Table 3. Hydrophobicity index and composition of bile in middle and late lactating rats.
| Control | PP day 10 | PP day 14 | PP day 19–23 | |
|---|---|---|---|---|
| Hydrophobicity index | −0.48±0.01 | −0.38±0.04* | −0.38±0.01* | −0.31±0.02‡ |
| Bile composition (mole%) | ||||
| Cholic acids | 20.1±1.4 | 26.4±3.3 | 31.2±1.3‡ | 31.7±1.3‡ |
| Chenodeoxycholic acids | 3.6±0.3 | 8.4±0.7 | 6.1±1.2 | 6.9±1.3 |
| Muricholic acids | 74.9±0.6 | 64.6±3.4† | 62.0±0.7‡ | 59.9±2.2‡ |
All data are expressed as means ± SD. Bile acids were extracted from control female rats and PP rats at days 10, 14, and 19–23. Bile acids are expressed as a percentage of the total recovered (mole %). Cholyl conjugates: tauroursodeoxycholic acid, taurocholic acid, glycocholic acid, and taurodeoxycholic acid. Chenodeoxycholic acids: taurochenodeoxycholic acid and glychochenodeoxycholic acid. Muricholic acids: tauro-α and β-muricholic acids and glyco-β muricholic acid.
P < 0.05.
P < 0.01.
P < 0.001 vs. control rats as determined by one-way ANOVA, followed by Bonferroni's multiple comparisons test.
Diurnal expression of Cyp7a1
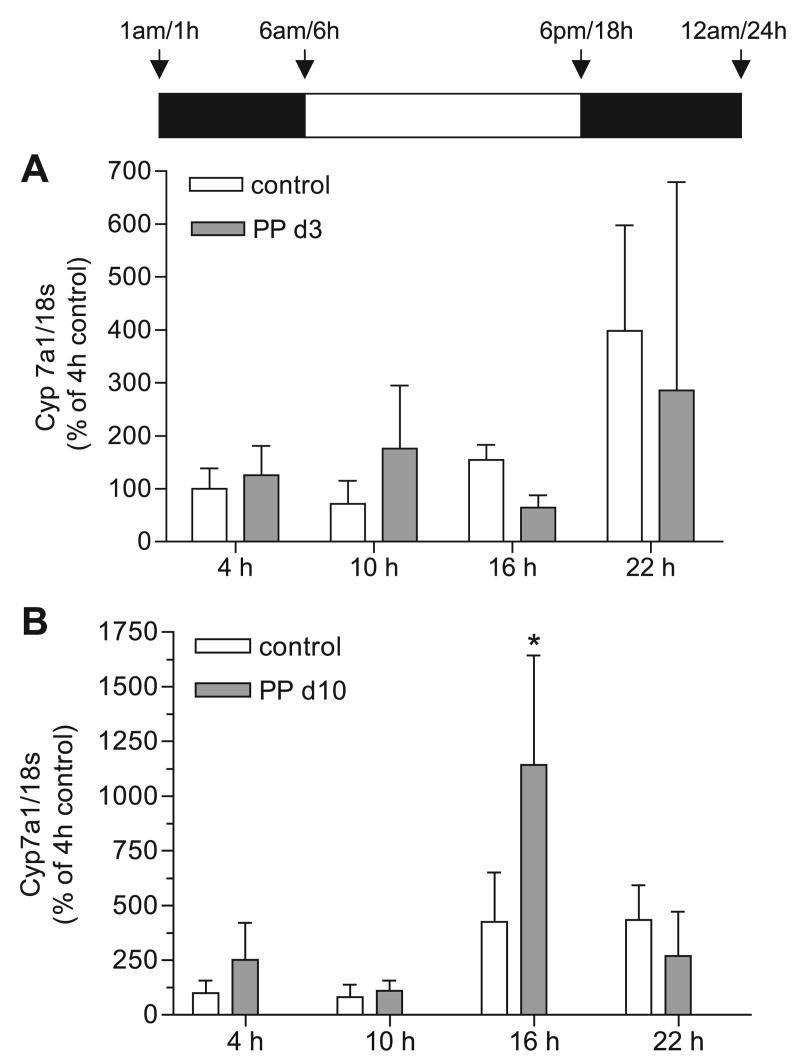
Cyp7a1 mRNA, protein, and enzyme activity are maximal in nonlactating rodents during the dark cycle when food consumption, activity, and glucocorticoid levels are maximal (29). To determine whether the circadian rhythms of bile acid synthesis were different in lactating rats, we measured Cyp7a1 mRNA expression from total RNA of control and lactating dams (PP day 3 and day 10) at 4 (10 h of dark), 10 (4 h of light), 16 (10 h of light), and 22 (4 h of dark) h. Cyp7a1 mRNA expression was not significantly different at any of the time points measured between control and PP day 3 rats (Fig. 2A). A clear diurnal shift of Cyp7a1 mRNA expression was present in PP day 10 rats (interaction, P < 0.0006; lactation effect, P < 0.03; time effect, P < 0.0001) (Fig. 2B). Cyp7a1 mRNA expression was increased 2.8-fold in the lactating group at 16 h (P < 0.001) but remained unchanged at 4, 10, and 22 h (Fig. 2B). These data indicate that Cyp7a1 mRNA expression was increased during midlactation and represented a phase shift in the diurnal cycle.
Fig. 2.
Expression of cholesterol 7α-hydroxylase (Cyp7a1) mRNA at early and midlactation. Real-time PCR was performed in duplicate on cDNA synthesized from liver total RNA from control female rats (open bars) and PP rats (shaded bars) at 4, 10, 16, and 22 h for n = 3–6 animals per group. Each bar represents the mean ± SD. A: there were no differences between control and PP day 3 rats. B: expression of Cyp7a1 mRNA was increased at 16 h in PP day 10 rats. *Significant increase in Cyp7a1 mRNA expression at 16 h in PP day 10 vs. control group (P < 0.001); significant interaction of the data (P < 0.0006) with a significant effect of time (P < 0.0001) and lactation (P < 0.03) was determined by 2-way ANOVA and Bonferroni post hoc test.
Since the expression of Cyp7a1 mRNA was elevated at 16 h (10 h of light) in day 10 PP rats, we measured the protein expression and enzymatic activity of Cyp7a1 at days 10 and 11 PP at 16 h. As shown in Fig. 3A, protein expression of Cyp7a1 was increased ∼ 1.9-fold relative to control animals (P < 0.005). Cyp7a1 hydroxylase activity was also elevated twofold in PP day 10/11 animals (P < 0.01) (Fig. 3D), consistent with the initial increase in the bile acid pool size at this time during lactation. Since protein expression directly correlated with enzymatic activity, we also measured Cyp7a1 protein expression at days 14 and 21 PP to determine whether Cyp7a1 remained elevated throughout lactation. We found that Cyp7a1 protein expression remained elevated at day 14 PP, albeit to a lesser extent (14% increase, P < 0.05, Fig. 3B) than at day 10 PP and declined to control levels by day 21 PP (Fig. 3C). Therefore, maximal Cyp7a1 expression correlated with the first increases in the bile acid pool and gradually declined throughout the remainder of lactation, possibly due to the activation of the negative feedback of the hydrophobic bile acids in the latter stages of lactation.
Fig. 3.
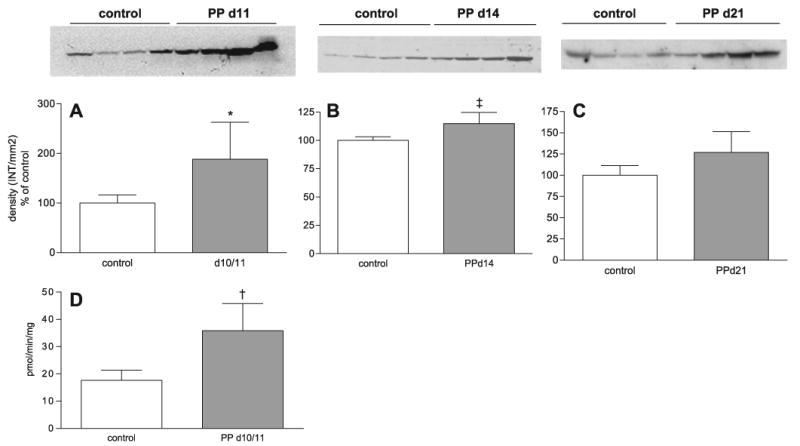
Cyp7a1 protein and hydroxylase activity are increased at 16 h in PP day 10/11 in 14 rats. Microsomal proteins were harvested from control or PP day 10/11, 14, and 21 rats killed at 16 h. Western blot analysis of Cyp7a1 protein expression in control and PP day 10/11 (*P < 0.005, PP day 10/11 vs. control rats) (A), PP day 14 (‡P < 0.05, PP day 14 vs. control rats) (B), and PP day 21 (C) rats. D: Cyp7a1 hydroxylase activity in control and PP day 10/11 rats. †P < 0.01, PP day 10/11 vs. control rats, determined by Student's t-test. A–C: densitometric analysis of Cyp7a1 Western blots (n = 4 animals per group). A–D: each bar represents the mean ± SD. For Western blot analysis, Ponceau red staining was performed for each membrane to ensure equal loading in each lane. INT, intensity.
Diurnal expression of Cyp27a1 in control and lactating rats
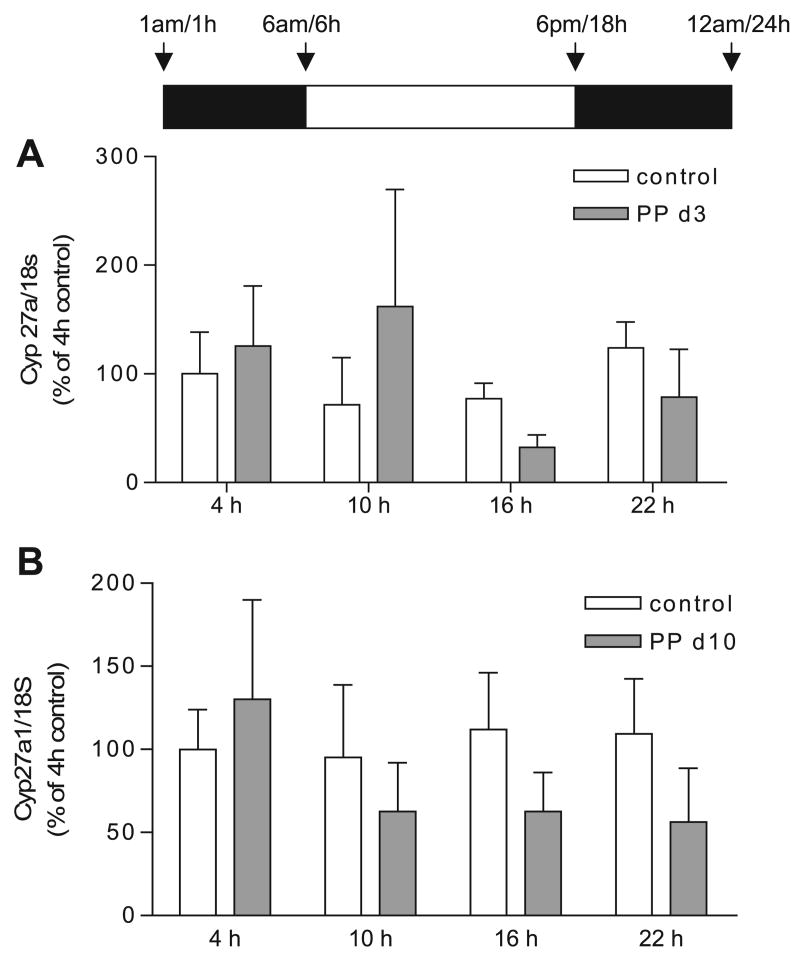
Cyp27a1 can contribute significantly to the bile acid pool when Cyp7a1 expression is repressed in primary rat hepatocytes and in Cyp7a1−/− mice (2, 44). Also, Cyp27a1 has a circadian rhythm similar to Cyp7a1, but this is not as dramatic due to the longer half-life of Cyp27a1 mRNA (39, 43). To determine whether increases in Cyp27a1 occurred in a manner similar to Cyp7a1, we measured Cyp27a1 mRNA expression from total RNA of control and lactating dams (PP days 3 and 10) at 4 (10 h of dark), 10 (4 h of light), 16 (10 h of light), and 22 (4 h of dark) h. There was no significant change in Cyp27a1 mRNA expression between control and lactating animals on PP day 3 (Fig. 4A). At midlactation (PP day 10), there was a decrease at 10, 16, and 22 h in Cyp27a1 mRNA expression (Fig. 4B, lactation effect, P < 0.03, two-way ANOVA). In addition, there were 20% (P < 0.05, Fig. 5A) and 7% decreases (P < 0.05, Fig. 5B) in Cyp27a1 protein expression at PP days 10 and 14 at 16 h (10 h of light). These data indicate that increased Cyp27a1 expression is not required for an increase in the bile acid pool during lactation and could explain the decreases shown in the muricholic acids (Table 3) since the pathways of muricholic acid synthesis are still unclear.
Fig. 4.
Expression of sterol 27-hydroxylase (Cyp27a1) mRNA during early and midlactation. Real-time PCR was performed in duplicate on cDNA synthesized from liver total RNA from control (open bars) or PP rats (shaded bars) at 4, 10, 16, or 22 h, n = 5–8 animals per group. Each bar represents the mean ± SD. A: Cyp27a1 was not different between control and PP day 3 rats, determined by two-way ANOVA. B: Cyp27a1 mRNA expression was decreased in PP day 10 rats. P < 0.03 effect of lactation determined by two-way ANOVA. No significant difference was found between control and the lactating animals at the time points measured (Bonferroni post hoc test).
Fig. 5.
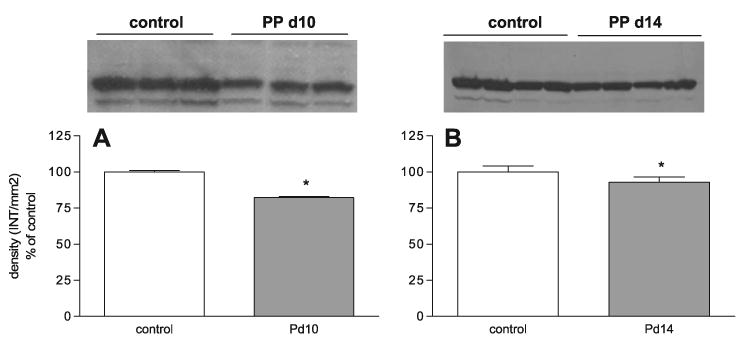
The expression of Cyp27a1 protein during PP days 10 and 14. Western blot analysis of Cyp27a1 protein expression in control and PP day 10 (A) and 14 (B) rats. Densitometric analysis of Cyp27a1 Western blots (n = 3–4 animals). Each bar represents the mean ± SD of Cyp27a1 density (INT/mm2). *P < 0.05 vs. control rats as determined by Student's t-test. For Western blot analysis, Ponceau red staining was performed for each membrane to ensure equal loading in each lane.
Diurnal expression of Cyp8b1 in control and lactating rats
Cyp8b1 is a microsomal enzyme that is required for the synthesis of cholic acid; lack of Cyp8b1 results in a complete loss of cholic acid in the bile acid pool, whereas overexpression of Cyp8b1 results in an increase of cholic acid synthesis and the cholic acid-chenodeoxycholic acid ratio (30). To address whether an increase of Cyp8b1 expression might contribute to the increase in the hydrophobicity of the bile acid pool or whether a phase shift of expression occurred during lactation, we measured Cyp8b1 mRNA and protein expression of control or PP day 10 lactating dams at 4 (10 h of dark), 10 (4 h of light), 16 (10 h of light), and 22 (4 h of dark) h. We did not detect a significant change in Cyp8b1 mRNA or protein at any of the time points measured (Fig. 6, A–B).
Fig. 6.
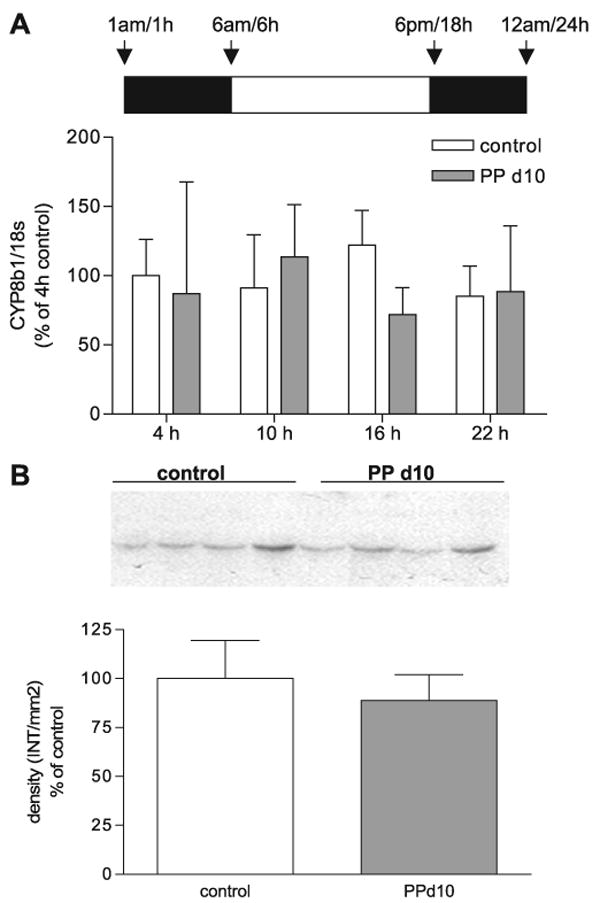
Expression of sterol 12α-hydroxylase (Cyp8b1) mRNA and protein in control and midlactation rats. A: real-time PCR was performed in duplicate on cDNA synthesized from liver total RNA from control female rats (open bars) and PP day 10 rats (shaded bars) at 4, 10, 16, and 22 h. There were no differences between control and PP day 10 rats. B: Western blot analysis of Cyp8b1 protein expression in control and PP day 10 rats. Densitometric analysis of Cyp8b1 Western blots of Cyp8b1 density (INT/mm2). Each bar represents the mean ± SD of n = 3–8 (A) or n = 4 (B) animals per group. There were no significant differences found between control and PP day 10 rats. For Western blot analysis, Ponceau red staining was performed for each membrane to ensure equal loading in each lane.
Discussion
This study demonstrates dynamic changes in bile acid synthesis in lactating rats. The size and hydrophobicity of the bile acid pool were significantly increased in mid- to late lactation animals, accompanied by an increased expression and activity of Cyp7a1 that occurred at 16 h (10 h of light) rather than 22 h (4 h of dark). Our data imply alternative regulatory mechanisms of Cyp7a1 expression in lactating rats that result in both a phase shift and an increase in Cyp7a1 expression, despite an increased bile acid pool. Several such mechanisms can be postulated based on our understanding of the regulation of Cyp7a1 and the changes that occur in lactation. First, in control rats, Cyp7a1 has a diurnal rhythm (29) that coincides with maximal food consumption during the dark cycle. Food consumption during the day (light cycle) is increased from ∼14% in control rats to 30% during lactation (42). Therefore, it is likely that altered food consumption patterns contributed to a phase shift in Cyp7a1 expression in lactating animals and may explain the findings of others showing decreased Cyp7a1 activity during the dark cycle in midlactation (38). Also, serum insulin concentrations are lower in day 12–15 PP rats (33), which may lead to altered insulin-mediated negative regulation of Cyp7a1 mRNA expression. The Cyp7a1 promoter contains diurnal binding protein response elements that are critical for its diurnal rhythm (17). Furthermore, SHP, HNF4-α, and PGC1-α, transcription factors and coactivators key to the regulation of Cyp7a1 transcription, also have a diurnal rhythm in the liver (20, 30, 35). We are presently characterizing the diurnal expression and binding of these factors to Cyp7a1 response elements in lactation relative to control animals. Second, we show that hepatic bile acid concentrations and SHP mRNA expression are unchanged in lactating rats at PP day 10, the time of the initial significant increase in the bile acid pool. This supports our previous findings of an increase in the secretory maximum for taurocholate in midlactation rats and a prolactin-induced increase in Bsep expression (6), which likely prevents bile acid-activated FXR/SHP-mediated negative feedback regulation of Cyp7a1 by minimizing the intracellular hepatic concentrations of bile acids. It is also important to note that the increased size of the bile acid pool and bile acid synthesis described here are consistent with the early findings of Moltz and colleagues (3, 15), who used the bile drainage methodology described by Mok et al. (26).
Recent studies have correlated negative energy balance associated with lactation to decreased serum leptin concentrations in lactating rodents (42). Leptin is a hormone secreted by adipocytes that serves as an appetite suppressant and increases energy expenditure (1, 8, 11, 45). In rats, adipocyte leptin mRNA expression and serum leptin concentrations, as well as food intake, are maximal in the dark cycle (42). However, lactating rats have decreased serum leptin concentrations and adipose leptin mRNA throughout the light-dark cycle (38). Prolactin, the major hormone of lactation required for milk protein synthesis, prevents leptin secretion from mouse (19) and rat (4) adipocytes. However, although removal of the suckling stimulus leads to rapid restoration of leptin levels, it is the loss of milk production (and presumably the decreased energy demand), not the suckling stimulus itself, that returns leptin to nonlactating levels (5). These data imply that factors in addition to prolactin influence leptin levels in lactation. Importantly, infusion of leptin by either the intraperitoneal (ob/ob mouse) route or intracerebroventricular (Sprague-Dawley rat) route decreases Cyp7a1 mRNA and protein (25) and enzymatic activity (25, 41). Leptin treatment in ob/ob mice over the course of 28 days significantly decreased the bile acid pool and the hydrophobicity of the bile acid pool, as well as the intestinal absorption of cholesterol (14). The hypoleptinemic state of lactating animals thus provides one likely mechanism for the increase in Cyp7a1 expression and subsequent increase in the hydrophobicity and size of the bile acid pool. However, the hyperphagic effects of lactation, coupled with the accompanying changes in the diurnal rhythm, are also likely important in regulating changes in Cyp7a1 expression and the size and composition of the bile acid pool. These changes are likely to promote absorption of lipids and lipid-soluble nutrients and thus compensate for the net negative energy balance in lactating rats.
Lactating animals showed an overall decrease in Cyp27a1 mRNA and a 20% decrease in Cyp27a1 protein expression at PP day 10 (Fig. 3, B–D), which is the time point when a significant increase in the bile acid pool was first detected. Cyp27a1 catalyzes the first step in the shortening of the cholesterol side chain, the first reaction in the alternative pathway of bile acid synthesis. Like Cyp7a1, Cyp27a1 expression has a diurnal rhythm (43) and is negatively regulated by hydrophobic bile acids (34, 39), but Cyp27a1 mRNA expression has a longer half-life than Cyp7a1 (39) so that the transcriptional regulation of Cyp27a1 and Cyp7a1 does not always occur in tandem. However, it is not clear why Cyp27a1 expression is decreased, whereas that of Cyp7a1 is increased, but this may provide a mechanism to alter composition of the bile acid pool.
Cyp8b1 mRNA and protein expression remained unchanged in PP day 10 rats (Fig. 6, A–B). Although Cyp8b1 expression is essential for the production of cholic acids, and its overexpression results in an increase in the hydrophobicity of the bile acid pool (18, 31), it is not the rate-limiting enzyme in the bile acid synthetic pathway. The present data clearly indicate that the enzymatic capacity of Cyp8b1 is sufficient to 12α-hydroxylate the increased amounts of bile acid intermediates that result from the twofold increase in Cyp7a1 activity. We have shown that Asbt expression and activity are increased at midlactation (27); the resulting increased reclamation of intestinal bile acids, together with increased Cyp7a1 activity, can apparently provide enough bile acid intermediates to increase the proportion of cholic acids in the bile acid pool during lactation.
Much of our understanding of the regulation of bile acid synthesis derives from disease models (e.g., bile duct ligation studies), bile acid or cholesterol feeding studies, and knockout mouse models. The lactating dam provides an important and novel model of adaptive responses that derive from a physiologically increased energy demand. Changes related to bile acid homeostasis begin soon after lactation. Within 48–72 h of lactation, the suckling stimulus causes marked increases in plasma prolactin that acts at the liver prolactin receptor to activate the Jak2/Stat5 signal transduction pathway and increase transcription of Ntcp, the hepatocyte basolateral transporter that mediates sodium-dependent uptake of bile salts (9). Although the precise mechanism is not known, expression of Bsep also increases in response to prolactin (6). The increase in Asbt does not occur until midlactation, and this increase is not induced by administration of prolactin (27). However, it is clear that this is a coordinated response such that, by midlactation, the increased synthesis of bile acids occurs subsequent to (Ntcp, Bsep) or together (Asbt) with increased expression of the bile acid transporters necessary to ensure their efficient enterohepatic recirculation, with no hepatotoxicity and concomitantly increased absorption of lipids. Decreased fecal loss via increased reclamation of bile acids likely provides an additional mechanism for the increase in the bile acid pool size during lactation. Asbt expression remains elevated from midlactation (when Cyp7a1 expression is increased) and remains elevated at late lactation (when Cyp7a1 expression has decreased to control levels). This indicates that increased bile acid reclamation is essential to the increased bile acid pool size as lactation progresses and Cyp7a1 levels return to that of the control rats. Further work is needed to understand how these responses to lactation are coordinated and whether other components essential for efficient lipid absorption and hepatic processing are involved.
In summary, the present study describes significant increases in the size and hydrophobicity of the bile acid pool that correlate with increased expression of Cyp7a1 mRNA, protein, and enzymatic activity in lactating rats. Our data also demonstrate that the initial rise in Cyp7a1 activity and the total bile acid pool size does not result in an increase in hepatic bile acid concentrations and activation of SHP. Whereas increases in the bile acid pool size by feeding studies have demonstrated repression of Cyp7a1, the prolactin-mediated increases in Ntcp and Bsep expression in lactation prevent intracellular accumulation of bile acids and enable expansion of the bile acid pool. We postulate that the expanded bile acid pool is essential for the increased lipid absorption needed for meeting nutrient demands of the dam and the pups.
Acknowledgments
We thank Dr. David Russell for the generous gift of Cyp27a1 antibody.
Grants
This work was supported by National Institutes of Health Grants DK-48873 and DK-56626 (to D. E. Cohen) and DK-46923 (to M. Vore).
References
- 1.Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 2.Arnon R, Yoshimura T, Reiss A, Budai K, Lefkowitch JH, Javitt NB. Cholesterol 7-hydroxylase knockout mouse: a model for monohydroxy bile acid-related neonatal cholestasis. Gastroenterology. 1998;115:1223–1228. doi: 10.1016/s0016-5085(98)70094-0. [DOI] [PubMed] [Google Scholar]
- 3.Bolt MJ, Lee TM, Moltz H. Altered bile acid physiology during lactation in the rat. Proc Soc Exp Biol Med. 1984;176:164–167. doi: 10.3181/00379727-176-41857. [DOI] [PubMed] [Google Scholar]
- 4.Brandebourg TD, Bown JL, Ben-Jonathan N. Prolactin upregulates its receptors and inhibits lipolysis and leptin release in male rat adipose tissue. Biochem Biophys Res Commun. 2007;357:408–413. doi: 10.1016/j.bbrc.2007.03.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brogan RS, Mitchell SE, Trayhurn P, Smith MS. Suppression of leptin during lactation: contribution of the suckling stimulus versus milk production. Endocrinology. 1999;140:2621–2627. doi: 10.1210/endo.140.6.6802. [DOI] [PubMed] [Google Scholar]
- 6.Cao J, Huang L, Liu Y, Hoffman T, Stieger B, Meier PJ, Vore M. Differential regulation of hepatic bile salt and organic anion transporters in pregnant and postpartum rats and the role of prolactin. Hepatology. 2001;33:140–147. doi: 10.1053/jhep.2001.20895. [DOI] [PubMed] [Google Scholar]
- 7.Cripps AW, Williams VJ. The effect of pregnancy and lactation on food intake, gastrointestinal anatomy and the absorptive capacity of the small intestine in the albino rat. Br J Nutr. 1975;33:17–32. doi: 10.1079/bjn19750005. [DOI] [PubMed] [Google Scholar]
- 8.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 9.Ganguly TC, O'Brien ML, Karpen SJ, Hyde JF, Suchy FJ, Vore M. Regulation of the rat liver sodium-dependent bile acid cotransporter gene by prolactin. Mediation of transcriptional activation by Stat5. J Clin Invest. 1997;99:2906–2914. doi: 10.1172/JCI119485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond KA. Adaptation of the maternal intestine during lactation. J Mammary Gland Biol Neoplasia. 1997;2:243–252. doi: 10.1023/a:1026332304435. [DOI] [PubMed] [Google Scholar]
- 11.Havel PJ. Role of adipose tissue in body-weight regulation: mechanisms regulating leptin production and energy balance. Proc Nutr Soc. 2000;59:359–371. doi: 10.1017/s0029665100000410. [DOI] [PubMed] [Google Scholar]
- 12.Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989;30:719–730. [PubMed] [Google Scholar]
- 13.Hylemon PB, Studer EJ, Pandak WM, Heuman DM, Vlahcevic ZR, Chiang JY. Simultaneous measurement of cholesterol 7 alpha-hydroxylase activity by reverse-phase high-performance liquid chromatography using both endogenous and exogenous [4-14C]cholesterol as substrate. Anal Biochem. 1989;182:212–216. doi: 10.1016/0003-2697(89)90581-2. [DOI] [PubMed] [Google Scholar]
- 14.Hyogo H, Roy S, Paigen B, Cohen DE. Leptin promotes biliary cholesterol elimination during weight loss in ob/ob mice by regulating the enterohepatic circulation of bile salts. J Biol Chem. 2002;277:34117–34124. doi: 10.1074/jbc.M203912200. [DOI] [PubMed] [Google Scholar]
- 15.Kilpatrick SJ, Bolt M, Moltz H. The maternal pheromone and bile acids in the lactating rat. Pharmacol Biochem Behav. 1980;12:555–558. doi: 10.1016/0091-3057(80)90188-4. [DOI] [PubMed] [Google Scholar]
- 16.Klaassen CD, Strom SC. Comparison of biliary excretory function and bile composition in male, female, and lactating female rats. Drug Metab Dispos. 1978;6:120–124. [PubMed] [Google Scholar]
- 17.Lee YH, Alberta JA, Gonzalez FJ, Waxman DJ. Multiple, functional DBP sites on the promoter of the cholesterol 7 alpha-hydroxylase P450 gene, CYP7. Proposed role in diurnal regulation of liver gene expression. J Biol Chem. 1994;269:14681–14689. [PubMed] [Google Scholar]
- 18.Li-Hawkins J, Gafvels M, Olin M, Lund EG, Andersson U, Schuster G, Bjorkhem I, Russell DW, Eggertsen G. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J Clin Invest. 2002;110:1191–1200. doi: 10.1172/JCI16309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling C, Billig H. PRL receptor-mediated effects in female mouse adipocytes: PRL induces suppressors of cytokine signaling expression and suppresses insulin-induced leptin production in adipocytes in vitro. Endocrinology. 2001;142:4880–4890. doi: 10.1210/endo.142.11.8514. [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Ganguly T, Hyde JF, Vore M. Prolactin increases mRNA encoding Na+-TC cotransport polypeptide and hepatic Na+-TC cotransport. Am J Physiol Gastrointest Liver Physiol. 1995;268:G11–G17. doi: 10.1152/ajpgi.1995.268.1.G11. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Hyde JF, Vore M. Prolactin regulates maternal bile secretory function post partum. J Pharmacol Exp Ther. 1992;261:560–566. [PubMed] [Google Scholar]
- 23.Liu Y, Suchy FJ, Silverman JA, Vore M. Prolactin increases ATP-dependent taurocholate transport in canalicular plasma membrane from rat liver. Am J Physiol Gastrointest Liver Physiol. 1997;272:G46–G53. doi: 10.1152/ajpgi.1997.272.1.G46. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Lundasen T, Liao W, Angelin B, Rudling M. Leptin induces the hepatic high density lipoprotein receptor scavenger receptor B type I (SR-BI) but not cholesterol 7alpha-hydroxylase (Cyp7a1) in leptin-deficient (ob/ob) mice. J Biol Chem. 2003;278:43224–43228. doi: 10.1074/jbc.M302645200. [DOI] [PubMed] [Google Scholar]
- 26.Mok HY, Perry PM, Dowling RH. The control of bile acid pool size: effect of jejunal resection and phenobarbitone on bile acid metabolism in the rat. Gut. 1974;15:247–253. [PMC free article] [PubMed] [Google Scholar]
- 27.Mottino AD, Hoffman T, Dawson PA, Luquita MG, Monti JA, Sanchez Pozzi EJ, Catania VA, Cao J, Vore M. Increased expression of ileal apical sodium-dependent bile acid transporter in postpartum rats. Am J Physiol Gastrointest Liver Physiol. 2002;282:G41–G50. doi: 10.1152/ajpgi.00309.2001. [DOI] [PubMed] [Google Scholar]
- 28.Muraca M, Leyten R, Fevery J. Conjugation and maximal biliary excretion of bilirubin in the rat during pregnancy and lactation and during estroprogestogen treatment. Hepatology. 1984;4:633–638. doi: 10.1002/hep.1840040411. [DOI] [PubMed] [Google Scholar]
- 29.Noshiro M, Nishimoto M, Okuda K. Rat liver cholesterol 7 alpha-hydroxylase. Pretranslational regulation for circadian rhythm. J Biol Chem. 1990;265:10036–10041. [PubMed] [Google Scholar]
- 30.Oiwa A, Kakizawa T, Miyamoto T, Yamashita K, Jiang W, Takeda T, Suzuki S, Hashizume K. Synergistic regulation of the mouse orphan nuclear receptor SHP gene promoter by CLOCK-BMAL1 and LRH-1. Biochem Biophys Res Commun. 2007;353:895–901. doi: 10.1016/j.bbrc.2006.12.131. [DOI] [PubMed] [Google Scholar]
- 31.Pandak WM, Bohdan P, Franklund C, Mallonee DH, Eggertsen G, Bjorkhem I, Gil G, Vlahcevic ZR, Hylemon PB. Expression of sterol 12alpha-hydroxylase alters bile acid pool composition in primary rat hepatocytes and in vivo. Gastroenterology. 2001;120:1801–1809. doi: 10.1053/gast.2001.24833. [DOI] [PubMed] [Google Scholar]
- 32.Petrack B, Latario BJ. Synthesis of 27-hydroxycholesterol in rat liver mitochondria: HPLC assay and marked activation by exogenous cholesterol. J Lipid Res. 1993;34:643–649. [PubMed] [Google Scholar]
- 33.Pickavance L, Tadayyon M, Williams G, Vernon RG. Lactation suppresses diurnal rhythm of serum leptin. Biochem Biophys Res Commun. 1998;248:196–199. doi: 10.1006/bbrc.1998.8934. [DOI] [PubMed] [Google Scholar]
- 34.Rao YP, Vlahcevic ZR, Stravitz RT, Mallonee DH, Mullick J, Avadhani NG, Hylemon PB. Down-regulation of the rat hepatic sterol 27-hydroxylase gene by bile acids in transfected primary hepatocytes: possible role of hepatic nuclear factor 1alpha. J Steroid Biochem Mol Biol. 1999;70:1–14. doi: 10.1016/s0960-0760(99)00099-0. [DOI] [PubMed] [Google Scholar]
- 35.Reddy AB, Maywood ES, Karp NA, King VM, Inoue Y, Gonzalez FJ, Lilley KS, Kyriacou CP, Hastings MH. Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology. 2007;45:1478–1488. doi: 10.1002/hep.21571. [DOI] [PubMed] [Google Scholar]
- 36.Rossi SS, Converse JL, Hofmann AF. High pressure liquid chromatographic analysis of conjugated bile acids in human bile: simultaneous resolution of sulfated and unsulfated lithocholyl amidates and the common conjugated bile acids. J Lipid Res. 1987;28:589–595. [PubMed] [Google Scholar]
- 37.Schmittgen TD, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods. 2000;46:69–81. doi: 10.1016/s0165-022x(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 38.Smith JL, Lear SR, Forte TM, Ko W, Massimi M, Erickson SK. Effect of pregnancy and lactation on lipoprotein and cholesterol metabolism in the rat. J Lipid Res. 1998;39:2237–2249. [PubMed] [Google Scholar]
- 39.Stravitz RT, Vlahcevic ZR, Russell TL, Heizer ML, Avadhani NG, Hylemon PB. Regulation of sterol 27-hydroxylase and an alternative pathway of bile acid biosynthesis in primary cultures of rat hepatocytes. J Steroid Biochem Mol Biol. 1996;57:337–347. doi: 10.1016/0960-0760(95)00282-0. [DOI] [PubMed] [Google Scholar]
- 40.Turley SD, Schwarz M, Spady DK, Dietschy JM. Gender-related differences in bile acid and sterol metabolism in outbred CD-1 mice fed low- and high-cholesterol diets. Hepatology. 1998;28:1088–1094. doi: 10.1002/hep.510280425. [DOI] [PubMed] [Google Scholar]
- 41.Vanpatten S, Karkanias GB, Rossetti L, Cohen DE. Intracerebroventricular leptin regulates hepatic cholesterol metabolism. Biochem J. 2004;379:229–233. doi: 10.1042/BJ20040134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vernon RG, Denis RG, Sorensen A, Williams G. Leptin and the adaptations of lactation in rodents and ruminants. Horm Metab Res. 2002;34:678–685. doi: 10.1055/s-2002-38258. [DOI] [PubMed] [Google Scholar]
- 43.Vlahcevic ZR, Jairath SK, Heuman DM, Stravitz RT, Hylemon PB, Avadhani NG, Pandak WM. Transcriptional regulation of hepatic sterol 27-hydroxylase by bile acids. Am J Physiol Gastrointest Liver Physiol. 1996;270:G646–G652. doi: 10.1152/ajpgi.1996.270.4.G646. [DOI] [PubMed] [Google Scholar]
- 44.Vlahcevic ZR, Stravitz RT, Heuman DM, Hylemon PB, Pandak WM. Quantitative estimations of the contribution of different bile acid pathways to total bile acid synthesis in the rat. Gastroenterology. 1997;113:1949–1957. doi: 10.1016/s0016-5085(97)70015-5. [DOI] [PubMed] [Google Scholar]
- 45.Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiol Behav. 2001;74:683–701. doi: 10.1016/s0031-9384(01)00612-6. [DOI] [PubMed] [Google Scholar]