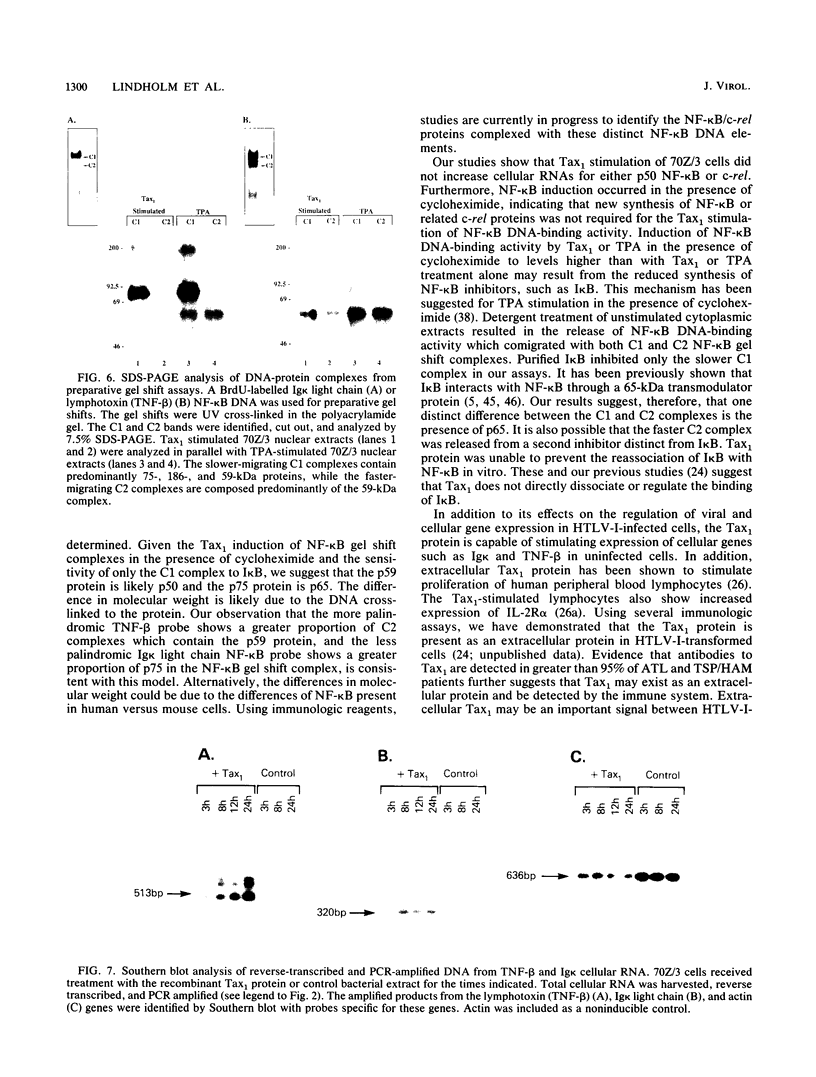
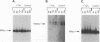Abstract
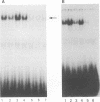
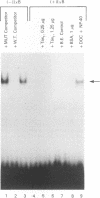
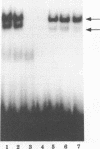
The human T-cell leukemia virus type I tax1 gene product is responsible for the increased expression of several cytokine and cellular genes that contain NF-kappa B regulatory sequences. Our laboratory has previously demonstrated that purified, extracellular Tax1 protein induced the nuclear accumulation of NF-kappa B binding activity in lymphoid cells. Since HTLV-I infection causes increased levels of lymphotoxin tumor necrosis factor-beta [TNF-beta] and immunoglobulin secretion, we have studied the interaction of NF-kappa B proteins from Tax1-stimulated cells with the TNF-beta and immunoglobulin kappa (Ig kappa) light chain genes. Tax1 induction of NF-kappa B occurred in the presence of cycloheximide, and Tax1 stimulation did not result in increased levels of NF-kappa B or c-rel RNA. These results indicate that new synthesis of NF-kappa B proteins was not required for induction of NF-kappa B-binding activity. With use of the Ig kappa NF-kappa B-binding site as a probe, two distinct NF-kappa B gel shift complexes were induced by the Tax1 protein. A slower-migrating complex, C1, was inhibited by the addition of purified I kappa B. In contrast, the faster-migrating C2 complex was not inhibited by I kappa B, but C2 was increased by detergent treatment of cytoplasmic extracts, suggesting that its binding activity was also regulated by an inhibitor. The Tax1-stimulated proteins that interacted with the NF-kappa B-binding sites in the Ig kappa and TNF-beta promoters were distinct. A 75-kDa protein preferentially associated with the Ig kappa NF-kappa B-binding site. In contrast, a 59-kDa protein associated with the TNF-beta NF-kappa B-binding site. Tax1 stimulation led to increased levels of TNF-beta and Ig kappa mRNA, as measured by reverse transcription and polymerase chain reaction analysis. These results represent the first experimental evidence that extracellular Tax1 can regulate the expression of endogenous cellular genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arluke A. The ethical thinking of animal researchers: problems and prospects. New Biol. 1991 Jan;3(1):1–2. [PubMed] [Google Scholar]
- Atchison M. L., Perry R. P. The role of the kappa enhancer and its binding factor NF-kappa B in the developmental regulation of kappa gene transcription. Cell. 1987 Jan 16;48(1):121–128. doi: 10.1016/0092-8674(87)90362-x. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. A 65-kappaD subunit of active NF-kappaB is required for inhibition of NF-kappaB by I kappaB. Genes Dev. 1989 Nov;3(11):1689–1698. doi: 10.1101/gad.3.11.1689. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa B transcription factor. Cell. 1988 Apr 22;53(2):211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A. The inducible transcription activator NF-kappa B: regulation by distinct protein subunits. Biochim Biophys Acta. 1991 Apr 16;1072(1):63–80. doi: 10.1016/0304-419x(91)90007-8. [DOI] [PubMed] [Google Scholar]
- Ballard D. W., Böhnlein E., Lowenthal J. W., Wano Y., Franza B. R., Greene W. C. HTLV-I tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science. 1988 Sep 23;241(4873):1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- Ballard D. W., Walker W. H., Doerre S., Sista P., Molitor J. A., Dixon E. P., Peffer N. J., Hannink M., Greene W. C. The v-rel oncogene encodes a kappa B enhancer binding protein that inhibits NF-kappa B function. Cell. 1990 Nov 16;63(4):803–814. doi: 10.1016/0092-8674(90)90146-6. [DOI] [PubMed] [Google Scholar]
- Böhnlein E., Lowenthal J. W., Siekevitz M., Ballard D. W., Franza B. R., Greene W. C. The same inducible nuclear proteins regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell. 1988 Jun 3;53(5):827–836. doi: 10.1016/0092-8674(88)90099-2. [DOI] [PubMed] [Google Scholar]
- Chen Y. M., Okayama A., Lee T. H., Tachibana N., Mueller N., Essex M. Sexual transmission of human T-cell leukemia virus type I associated with the presence of anti-Tax antibody. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1182–1186. doi: 10.1073/pnas.88.4.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. L., Feinberg M. B., Wolf J. B., Holbrook N. J., Wong-Staal F., Leonard W. J. Regulation of the human interleukin-2 receptor alpha chain promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell. 1987 Apr 10;49(1):47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessain A., Barin F., Vernant J. C., Gout O., Maurs L., Calender A., de Thé G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985 Aug 24;2(8452):407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Gifford A. M., Riviere L. R., Tempst P., Nolan G. P., Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990 Sep 7;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- Giam C. Z., Nerenberg M., Khoury G., Jay G. Expression of the complete human T-cell leukemia virus type I pX coding sequence as a functional protein in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7192–7196. doi: 10.1073/pnas.83.19.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985 Aug 9;229(4713):563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- Inoue J., Kerr L. D., Ransone L. J., Bengal E., Hunter T., Verma I. M. c-rel activates but v-rel suppresses transcription from kappa B sites. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3715–3719. doi: 10.1073/pnas.88.9.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Seiki M., Taniguchi T., Tsuru S., Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986 Nov;5(11):2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S., Shida H., McFarlin D. E., Fauci A. S., Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990 Nov 15;348(6298):245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- Kieran M., Blank V., Logeat F., Vandekerckhove J., Lottspeich F., Le Bail O., Urban M. B., Kourilsky P., Baeuerle P. A., Israël A. The DNA binding subunit of NF-kappa B is identical to factor KBF1 and homologous to the rel oncogene product. Cell. 1990 Sep 7;62(5):1007–1018. doi: 10.1016/0092-8674(90)90275-j. [DOI] [PubMed] [Google Scholar]
- Leung K., Nabel G. J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-kappa B-like factor. Nature. 1988 Jun 23;333(6175):776–778. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- Libermann T. A., Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990 May;10(5):2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienbaum A., Duc Dodon M., Alexandre C., Gazzolo L., Paulin D. Effect of human T-cell leukemia virus type I tax protein on activation of the human vimentin gene. J Virol. 1990 Jan;64(1):256–263. doi: 10.1128/jvi.64.1.256-263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm P. F., Marriott S. J., Gitlin S. D., Bohan C. A., Brady J. N. Induction of nuclear NF-kappa B DNA binding activity after exposure of lymphoid cells to soluble tax1 protein. New Biol. 1990 Nov;2(11):1034–1043. [PubMed] [Google Scholar]
- Lindholm P. F., Marriott S. J., Gitlin S. D., Brady J. N. Differential precipitation and zinc chelate chromatography purification of biologically active HTLV-I Tax1 expressed in E. coli. J Biochem Biophys Methods. 1991 Apr;22(3):233–241. doi: 10.1016/0165-022x(91)90071-4. [DOI] [PubMed] [Google Scholar]
- Molitor J. A., Walker W. H., Doerre S., Ballard D. W., Greene W. C. NF-kappa B: a family of inducible and differentially expressed enhancer-binding proteins in human T cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10028–10032. doi: 10.1073/pnas.87.24.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M., Nagata S. Regulatory elements responsible for inducible expression of the granulocyte colony-stimulating factor gene in macrophages. Mol Cell Biol. 1990 May;10(5):2002–2011. doi: 10.1128/mcb.10.5.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak U., Cocks B. G., Hamilton J. A. A labile repressor acts through the NFkB-like binding sites of the human urokinase gene. Nucleic Acids Res. 1991 Jun 25;19(12):3389–3393. doi: 10.1093/nar/19.12.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L., Kunkel S., Nabel G. J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul N. L., Lenardo M. J., Novak K. D., Sarr T., Tang W. L., Ruddle N. H. Lymphotoxin activation by human T-cell leukemia virus type I-infected cell lines: role for NF-kappa B. J Virol. 1990 Nov;64(11):5412–5419. doi: 10.1128/jvi.64.11.5412-5419.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessara U., Koch N. Tumor necrosis factor alpha regulates expression of the major histocompatibility complex class II-associated invariant chain by binding of an NF-kappa B-like factor to a promoter element. Mol Cell Biol. 1990 Aug;10(8):4146–4154. doi: 10.1128/mcb.10.8.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Flomenberg N., Volkman D. J., Mann D., Fauci A. S., Dupont B., Gallo R. C. Alteration of T-cell functions by infection with HTLV-I or HTLV-II. Science. 1984 Oct 26;226(4673):459–462. doi: 10.1126/science.6093248. [DOI] [PubMed] [Google Scholar]
- Pozzatti R., Vogel J., Jay G. The human T-lymphotropic virus type I tax gene can cooperate with the ras oncogene to induce neoplastic transformation of cells. Mol Cell Biol. 1990 Jan;10(1):413–417. doi: 10.1128/mcb.10.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivière Y., Blank V., Kourilsky P., Israël A. Processing of the precursor of NF-kappa B by the HIV-1 protease during acute infection. Nature. 1991 Apr 18;350(6319):625–626. doi: 10.1038/350625a0. [DOI] [PubMed] [Google Scholar]
- Ruben S. M., Dillon P. J., Schreck R., Henkel T., Chen C. H., Maher M., Baeuerle P. A., Rosen C. A. Isolation of a rel-related human cDNA that potentially encodes the 65-kD subunit of NF-kappa B. Science. 1991 Mar 22;251(5000):1490–1493. doi: 10.1126/science.2006423. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Wong-Staal F., Franchini G., Kalyanaraman V. S., Gallo R. C. Restricted expression of human T-cell leukemia--lymphoma virus (HTLV) in transformed human umbilical cord blood lymphocytes. Virology. 1983 Aug;129(1):51–64. doi: 10.1016/0042-6822(83)90395-1. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Shibuya H., Yoneyama M., Taniguchi T. Involvement of a common transcription factor in the regulated expression of IL-2 and IL-2 receptor genes. Int Immunol. 1989;1(1):43–49. doi: 10.1093/intimm/1.1.43. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Takahashi C., Yamaoka S., Nosaka T., Maki M., Hatanaka M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tendler C. L., Greenberg S. J., Blattner W. A., Manns A., Murphy E., Fleisher T., Hanchard B., Morgan O., Burton J. D., Nelson D. L. Transactivation of interleukin 2 and its receptor induces immune activation in human T-cell lymphotropic virus type I-associated myelopathy: pathogenic implications and a rationale for immunotherapy. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5218–5222. doi: 10.1073/pnas.87.13.5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson B. M., Mundy G. R., Chambers T. J. Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. J Immunol. 1987 Feb 1;138(3):775–779. [PubMed] [Google Scholar]
- Tschachler E., Robert-Guroff M., Gallo R. C., Reitz M. S., Jr Human T-lymphotropic virus I-infected T cells constitutively express lymphotoxin in vitro. Blood. 1989 Jan;73(1):194–201. [PubMed] [Google Scholar]
- Urba W. J., Longo D. L. Clinical spectrum of human retroviral-induced diseases. Cancer Res. 1985 Sep;45(9 Suppl):4637s–4643s. [PubMed] [Google Scholar]
- Urban M. B., Baeuerle P. A. The 65-kD subunit of NF-kappa B is a receptor for I kappa B and a modulator of DNA-binding specificity. Genes Dev. 1990 Nov;4(11):1975–1984. doi: 10.1101/gad.4.11.1975. [DOI] [PubMed] [Google Scholar]
- Urban M. B., Schreck R., Baeuerle P. A. NF-kappa B contacts DNA by a heterodimer of the p50 and p65 subunit. EMBO J. 1991 Jul;10(7):1817–1825. doi: 10.1002/j.1460-2075.1991.tb07707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Greene W. C., Sarin P. S., Saxinger C., Blayney D. W., Blattner W. A., Goldman C. K., Bongiovanni K., Sharrow S., Depper J. M. Functional and phenotypic comparison of human T cell leukemia/lymphoma virus positive adult T cell leukemia with human T cell leukemia/lymphoma virus negative Sézary leukemia, and their distinction using anti-Tac. Monoclonal antibody identifying the human receptor for T cell growth factor. J Clin Invest. 1984 Jun;73(6):1711–1718. doi: 10.1172/JCI111379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. E., Fang C. T., Slamon D. J., Poiesz B. J., Sandler S. G., Darr W. F., 2nd, Shulman G., McGowan E. I., Douglas D. K., Bowman R. J. Seroprevalence and epidemiological correlates of HTLV-I infection in U.S. blood donors. Science. 1988 Apr 29;240(4852):643–646. doi: 10.1126/science.2896386. [DOI] [PubMed] [Google Scholar]
- Wirth T., Baltimore D. Nuclear factor NF-kappa B can interact functionally with its cognate binding site to provide lymphoid-specific promoter function. EMBO J. 1988 Oct;7(10):3109–3113. doi: 10.1002/j.1460-2075.1988.tb03177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip M. T., Chen I. S. Modes of transformation by the human T-cell leukemia viruses. Mol Biol Med. 1990 Feb;7(1):33–44. [PubMed] [Google Scholar]
- Zabel U., Baeuerle P. A. Purified human I kappa B can rapidly dissociate the complex of the NF-kappa B transcription factor with its cognate DNA. Cell. 1990 Apr 20;61(2):255–265. doi: 10.1016/0092-8674(90)90806-p. [DOI] [PubMed] [Google Scholar]