Introduction
Today’s healthcare providers deal with multiple very complex and pressing problems including the aging population, an ever increasing proportion of patients with chronic diseases, cancer deaths, childhood and adult obesity, escalating costs, regulatory interventions, as well as increasing responsibilities for patient safety. Innovation offers very important promises to address many of these problems. In most of the developed world, academic medical centers are charged with the mandate to treat patients and advance healthcare, and as such, should be poised to play a pivotal role in the process of innovation.
In fact, there is an expectation for such innovation by the public given that interactions between academic medical centers and society rely on Federal funding for support intended to enable discovery in biomedical sciences. In 1980, the Bayh-Dole Act gave control of intellectual property generated based on inventions funded by the Federal government to universities, small businesses, and non-profit organizations. This Act further solidified the role of academic medical centers in innovation in biomedical sciences and healthcare [1]. As a result, the academic medical centers have become central to foster entrepreneurial culture by working with startup companies, pharmaceutical companies, and medical device companies to translate academic inventions into commercial products with societal impact.
The increasingly tight relationship between academic medical centers and industry has resulted in more and more defined professional and institutional conflict of interest policies. These policies have especially targeted any financial incentives held by inventors [2, 3]. As much as the public has invested to put government-funded inventions into societal usage and has mandated academic medical centers to advance healthcare, the public assumes that the innovative process has not been biased by any financial incentives and that the patient care has not been compromised as a result. Professional and institutional conflict policies have been crafted to accomplish a balance between the support of the innovative process versus protections from harm. However to date, the balance has tilted without restraint toward risk aversion for the institution and away from support of innovation.
The current study aims to identify critical steps from invention to applications, and the role of academic medical centers in this critical translation process. This study will not delve into the advantages and the disadvantages of conflict of interest policies, although they are essential to the balance between the support of the process versus an impediment to this process.
Invention to Commercialization
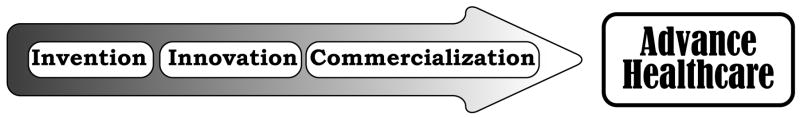
The development of a new technology, method, or drug starts with the process of invention and continues with multiple levels of innovation leading to application and commercialization (Figure 1). In a masterpiece, Professor Norbert Wiener put forth the thesis that there are at least four important conditions required for an invention to become realized as a product [4]. The first element is the creative process during which many new ideas arise. The second element relates to the availability of proper materials and techniques for the validation and execution of the idea. Wiener describes the third element as the need for effective communication between the “philosopher” and the “artisan”. Finally, the translation of an invention depends upon those people, who financially benefit from the invention by creating economic value.
Figure 1.
Steps from invention to impacting healthcare.
These general concepts remain valid today and provide an excellent roadmap to take invention through multiple layers of innovation all the way to economic impact. In order to distinguish invention from innovation, Fagerberg proposes the following description: “Invention is the first occurrence of an idea for a new product or process, while innovation is the first attempt to carry it out into practice” [5]. Although there are many equally appealing definitions of invention and innovation (Table 1), Fagerberg’s description is an effective one and the one we choose to use in this article for illustration.
Table 1.
| Invention | Innovation |
|---|---|
| An object, process, or technique which displays an element of novelty | The process of making improvements by introducing something new |
| Radical breakthrough in science or technology which extends the boundaries of human knowledge | The process of translating new ideas into tangible societal impact |
| Change that creates a new dimension of performance | |
| A creative idea that is realized |
Our interest relates to the case when the invention originates from the academic medical centers. The invention is then followed up with initial validation and application for intellectual property protection within the academic medical center. This sets the stage for multiple subsequent steps of innovation to bring the invention into clinical usage or commercialization. The innovation, thus, provides a bridge between the academia (invention) and industry (commercialization).
Innovation and Hand Off from Academic Medical Centers to Industry
The hand-off from an academic medical center to industry should occur at appropriate stages during the continuum of innovation process. Although no obvious guidelines exist to define when this hand off should take place, there are clear cases where the innovation is very complex and should be nurtured within the academic medical center prior to hand off to a commercial entity to minimize the technical risks associated with biology and particularly human biology that may not be fully appreciated by industrial colleagues or the investment community. This hand off traditionally has occurred after invention but before innovation has taken place. This model has worked well for innovations in the physical sciences such as chemistry, physics, and computer-related applications in which the underlying science is well understood quantitatively. On the other hand, in the experience of these authors, this hand off model bears much greater technical risks in human biology particularly, in the area of diagnostics.
One can make a very strong argument that academic medical centers, in order to minimize the risk of failure, should be encouraged to take the invention into the innovation space as far as possible within “incubators” within the academic medical center before hand off to industry. In this model, the technology risks have been eliminated and industry will be responsible for the business execution of the innovation for which industry is truly expert. This would inevitably reduce the risk in subsequent technology transfer to a commercial partner, and maximize the value creation and impact in advancing healthcare. This is clearly a desired outcome by all parties involved in the process, and it does fall within the mandate of the academic medical centers. In addition, the ability to validate a new technology internally and further improve its performance metrics would significantly increase the value of the invention and thus the financial benefit to the inventors and the academic medical center.
On the other hand, there are at least three major driving forces in industry to maximize the investors’ return on investment (ROI), especially in venture-backed start-ups, to assume this additional risk and to enter as early as possible in the innovative process. Sometimes this approach is appropriate and sometimes not. With respect to the three major driving forces, the first force is to minimize the cost of invention typically by investing in the new idea soon after its conception within an academic medical center. The second force is to exercise strong control over the intellectual property landscape in order to minimize potential intellectual property leak that is more likely to occur within an academic environment. This is an important factor that shapes the relationship between academia and industry even after the intellectual property is licensed by an industrial outfit from an academic medical center. The third is the relentless time pressures under which industry functions while engaged in the innovative process, especially in those companies that are backed by venture capital. While the timelines are usually flexible and treated as general guidelines in academia, a serious slippage in timelines results in significant loss of valuation in subsequent rounds of investment, or worse, failure of the company with the loss of innovation for patient care.
A hybrid alternative approach for hand off from academia to industry might be to work with technology innovation companies that are strategically positioned between the inventor and industry in order to translate emerging innovations to customers [e.g. 8]. These companies bring critical expertise in product development, manufacturing, packaging, scale up among others, and mitigate the risk of failure while increasing the value of the initial discovery. Furthermore, the development of a product is a complex process and requires expertise that is typically beyond the scope of an academic medical center. The ability to diminish the risks of failure associated with manufacturing and product development through the use of technology innovation enterprises may also enable academic medical centers to access alternative and more mature sources of funding than the venture capital. As a result, the opportunity to work with technology innovation companies promises a very rewarding partnership for academic medical centers.
Conflict of Interest Concerns
The financial conflict-of-interest of the inventor (scientist or physician) creates major concerns among academic medical centers and regulators; however, this conflict tends to be less of a concern to the consumers of healthcare. The conflict issue is covered in depth in other chapters in this special issue as well as in several recent reviews [2, 3,9, 10]. Briefly, there are two polarizing views. One view adapts a risk-free approach by prohibiting relationships between academic medical center inventors/innovators with industry because such conflicts might be viewed negatively and might compromise integrity of biomedical science and patient care. Another view accepts that real conflict is an exception and not the rule and therefore, these potential conflicts should, more appropriately, be handled with proper guidelines and actions on a case-by-case basis. This latter view assumes that policy developed upon exceptions represents poor policy. Furthermore, this latter view deals with conflict ethically and efficiently as, and if, it arises, and thus, it has the net effect of promoting innovation within academic medical centers. The benefit of mitigating important risk factors within academic medical centers through innovation and proper validation prior to hand off to industry offers much greater promise to impact in healthcare and may significantly outweigh any of its potential disadvantages.
Innovation in Academic Medical Centers
Today, innovation is the centerpiece of many successful academic centers and institutions, and it is especially blossoming in biomedical sciences across our nation and internationally. Many universities and academic medical centers seek mechanisms to devise creative structures to enable the innovation process toward economic value creation and societal impact while avoiding the academic medical centers’ policies that impede innovation. For example, a report published in 1997 concerning innovation at MIT summarized astonishingly powerful outcomes with a total of 4,000 companies founded by MIT graduates and faculty [11]. These companies employ over 1.1 million employees and net $232 billion in revenue. Much of this success was in the fields of communication, engineering, and computers. The gap between the ‘philosopher’ and the ‘artisan’ is greater in biological sciences and clinical medicine, which creates a significant divide between the academic medical centers and commercialization (or application).
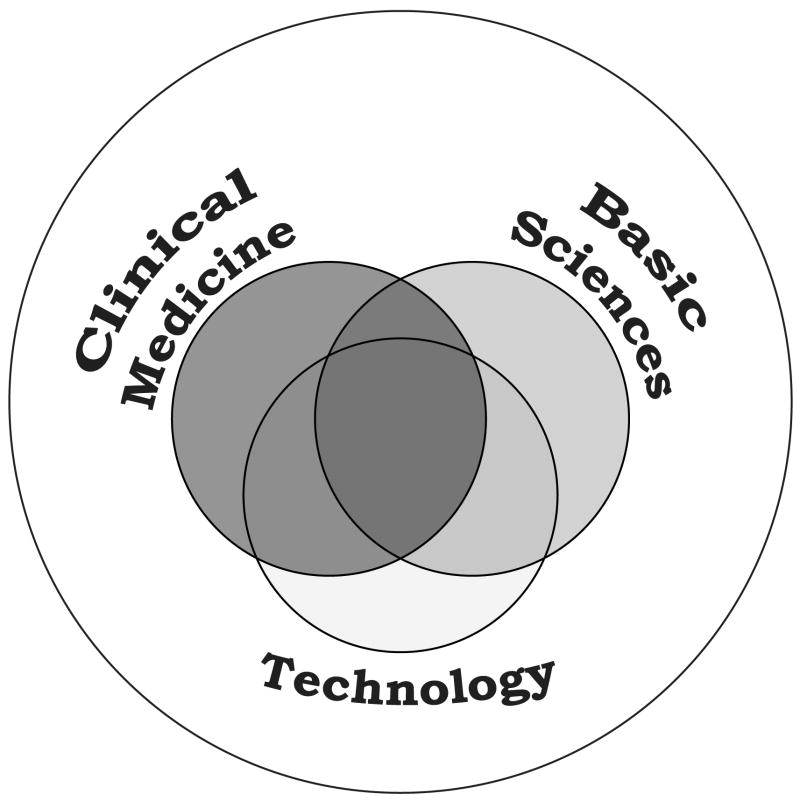
Academic medical centers are especially uniquely poised to play a leadership role in innovation in biomedical sciences. The top 10 research hospitals received approximately $1.2 billion and the top 10 medical schools approximately $3.4 billion in NIH funding in 2005 [12]. Such a strong statement from appropriations indicates that the Congress and the public expect important innovation in the area of healthcare and the effectiveness in the delivery of healthcare. In addition to excellent Federal funding, the top academic medical centers have expertise in basic biological science, technology, and clinical medicine (Figure 2), which creates a very unique and truly multi-disciplinary environment for innovation. Furthermore, the academic medical centers greatly benefit from the fact that both the patients (users) and physicians (practitioners) are located within the academic medical centers; whereas, end-users or consumers and practitioners of new technologies emerging from universities in physical sciences are outside the university. This creates an environment highly favorable for innovation at academic medical centers. Much of the validation of new technologies can be implemented internally within the academic medical center where the invention originated. This provides an excellent opportunity to reduce major risks associated with biological sciences and the complexity of human biology, and consequently minimize the gap between innovation and application.
Figure 2.
Academic medical centers have expertise in basic sciences, technology, and clinical medicine. This contrasts university-based innovation in medicine in which only basic sciences and technology are represented. The consumer must be recruited to explore opportunities to develop the innovative phase.
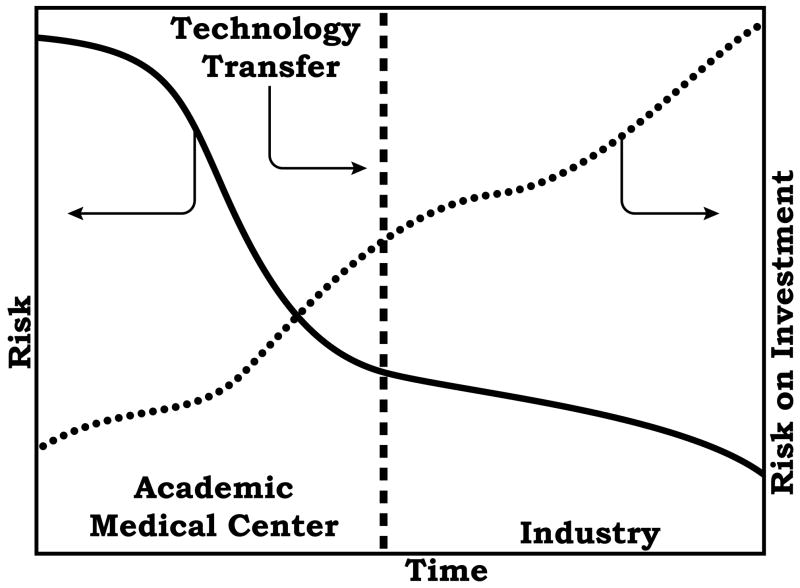
The benefits of internal validation of new ideas and technologies within the academic medical center would be at the least twofold. First, the reduction in risk correlates with higher return on investment because the value of the invention would be much higher for the investment community (Figure 3). Second, and more importantly, many potentially very good ideas and technologies fail due to premature technology transfer, and the society suffers the consequences of such a failure irrespective of whether the failure was the fault of the inventor/innovator versus their industrial colleagues. This uniqueness is bound to create concerns regarding the conflict issues and safety of patients as described in detail in other papers in this special issue. These are serious issues that must be properly managed without stifling innovation. This creates unique challenges to academic centers in the continuum of invention to application and societal impact.
Figure 3.
Relationship between risk and return on investment to the academic medical center, and the timing of technology hand off to industry. Typically, technology transfer occurs before innovation begins in which the technology transfer line (not shown in this figure) would be well to the left. When “incubators” are employed, this technology transfer line is shifted to the right reducing risk and increasing the return on investment to the academic medical center.
Outlook
The potential for the academic centers to achieve in healthcare what physical sciences have accomplished over the last century in entrepreneurship may be one of the most exciting opportunities in the 21st century. There is an ever-growing role for academic medical centers in innovation and the development of new devices, drugs, and applications. It is arguable that the innovation is essential in academic medical centers given the tremendous investment to these institutions by the public via various Federal agencies as well as private philanthropy. Moreover, the role of academic medical centers is not only to take care of patients but also to advance healthcare by bringing these innovations to the bedside. The innovation process between the invention and commercialization (application) is thus central to the mandate of academic medical centers and the innovation process needs to be properly incorporated into the intellectual and ethical fabric of academic medical centers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thursby JG, Thursby MC. University licensing and the Bayh-Dole Act. Science. 2003;301:1052. doi: 10.1126/science.1087473. [DOI] [PubMed] [Google Scholar]
- 2.Angell M. Is academic medicine for sale? N Eng J Med. 2000;342:1516–1518. doi: 10.1056/NEJM200005183422009. [DOI] [PubMed] [Google Scholar]
- 3.Stossel TP. Regulating academic-industrial research relationship - Solving problems or stifling progress? N Eng J Med. 2005;353:1060–1065. doi: 10.1056/NEJMsb051758. [DOI] [PubMed] [Google Scholar]
- 4.Wiener N. Invention. 3. MIT Press; Cambridge: Massachusetts; 1996. [Google Scholar]
- 5.Fagerberg J. Innovation: A guide to the literature. In: Fagerberg J, Mowery DC, Nelson RR, editors. The Oxford Handbook of Innovations. Oxford University Press; 2004. pp. 1–26. [Google Scholar]
- 6.Invention. [September 26, 2007];Wikipedia. http://en.wikipedia.org/wiki/Invention.
- 7.Innovation. [September 26, 2007];Wikipedia. http://en.wikipedia.org/wiki/Innovation.
- 8. [September 26, 2007];TIAX LLC. http://www.tiaxllc.com/
- 9.Loscalzo J. Entrepreneurship in the medical academy: possibilities and challenges in commercialization of research discoveries. Circulation. 2007;115:1504–1507. doi: 10.1161/CIRCULATIONAHA.107.694869. [DOI] [PubMed] [Google Scholar]
- 10.Moses H, Braunwald E, Martin JB, Their SO. Collaborating with industry –Choices for the academic medical center. N Eng J Med. 2002;347:1371–1375. doi: 10.1056/NEJMsb021319. [DOI] [PubMed] [Google Scholar]
- 11. [January 4, 2008. ];Bank Boston Economics Department. CMIT; the impact of innovation. http://web.mit.edu/newsoffice/founders.
- 12. [January 4, 2008];Awards to independent domestic hospitals: fiscal year 2005. http://grants1.nih.gov/grants/awards/trends/