Abstract
Diverse roles in cellular functions have been ascribed to nitric oxide (NO), and its involvement in induction of long-term depression in cerebellar Purkinje cells has been demonstrated. Manipulations of NO concentration or its synthesis in cerebellar tissues therefore provide a means for investigating roles of NO in cerebellar functions at both cellular and behavioral levels. We tested adaptive control of locomotion to perturbation in cats, and found that this form of motor learning was abolished by application of either an inhibitor of NO synthase or a scavenger of NO to the cerebellar cortical locomotion area. This finding supports the view that NO in the cerebellum plays a key role in motor learning.
Keywords: cerebellum, nitric oxide, synaptic plasticity, motor learning
In the cerebellum, long-term depression (LTD) is persistent reduction of transmission efficacy at synapses from parallel fibers to Purkinje cells, which occurs when the parallel fibers are activated in conjunction with climbing fibers converging onto the same Purkinje cells (1, 2). It has been reported that stimulation of the white matter of the cerebellum enhances nitric oxide (NO) concentration in the molecular layer (3), and that NO activates soluble guanylate cyclase, leading to an increase in the level of cyclic GMP (4, 5). When combined with parallel fiber stimulation, NO donors or cyclic GMP induce LTD-like phenomena in slice preparations (3, 6). LTD and these LTD-like phenomena are abolished by NG-monomethyl-l-arginine (l-NMMA), an inhibitor of NO synthase, or hemoglobin, a scavenger of NO (3, 7–9). In a recent study, when NO was photolytically released inside Purkinje cells in conjunction with postsynaptic depolarization, LTD was induced as a manner of parallel fiber activation plus depolarization (10). Thus, NO has been proposed to play an important role in signal transduction processes underlying LTD.
LTD has been thought to be a cellular basis of motor learning (1). To establish a causal relationship between LTD and actual motor learning, it is prerequisite that a blockade of a biochemical step that is unique to LTD impairs motor learning. As has been reported (11), exposure of one forelimb to a belt velocity higher than that of the other limbs during locomotion of a cat on a treadmill provides an effective means for testing motor learning. Thereby the coordination of limb movements was disturbed, and climbing fiber responses were induced at high probability in Purkinje cells of the cerebellar vermis (12). After a number of steps in the perturbed locomotion, the cats regained interlimb coordination and stability. In view of the findings that the adaptation takes place in decerebrate cats (11), that interlimb coordination is seriously impaired in spinal cats (13, 14), and that mechanical lesions or cooling of the cerebellum induces disturbances of locomotion (15–18), the cerebellum is the most likely site responsible for this adaptation. In this study, we investigated the involvement of NO in the adaptation of locomotion by direct localized application of either an inhibitor of NO synthase or a scavenger of NO to the cerebellum, and found that the adaptation was abolished thereby.
METHODS
Adult cats weighing between 3.0 and 4.5 kg were used. Under pentobarbital anesthesia (40 mg/kg), the cats were operated under sterile conditions and were decerebrated at the precollicular and premammillary level using electrocoagulation (11). There are two major reasons why decerebrate cats were preferred. One is that once the treadmill belt had begun to move, they automatically performed stable locomotion, whereas intact cats rarely exhibited. The other is that decerebrate cats have the advantage of providing greater possibilities for analytical studies, because their lower brainstem-cerebellum-spinal system is isolated from complications that may arise from the cerebrum and upper brainstem. Yet, it is generally agreed that essential locomotor mechanisms are retained in decerebrate cats (19). After a three- to four-day recovery period, decerebrate cats that were able to walk spontaneously on the floor and maintain normal body equilibrium were selected (n = 12). In each experiment, a decerebrate cat was initially anesthetized with diethyl ether for a tracheotomy and then placed on a treadmill (Fig. 1A) with its head fixed in a stereotaxic frame. Then, under deep halothane anesthesia, a partial craniotomy was made over the vermis of lobule V and VI. Within 1–1.5 hr after disruption of the anesthesia, the cat recovered and supported its body weight, spontaneously showing stepping movements. The cat showed no signs of pain or discomfort such as restlessness or avoidance. In unperturbed locomotion, all belts were driven at 36 cm/s. Perturbation was applied by driving the belt for the left forelimb at a high velocity (61 cm/s). First, the decerebrate cat was trained with three to eight repeated trials of unperturbed locomotion. Next, the perturbed locomotions were imposed. Each of these trials consisted of 30–200 successive steps with intertrial intervals of about 5 min. Step cycles and their phases during locomotion were monitored with potentiometers attached to the knee of each forelimb (Fig. 1B).
Figure 1.
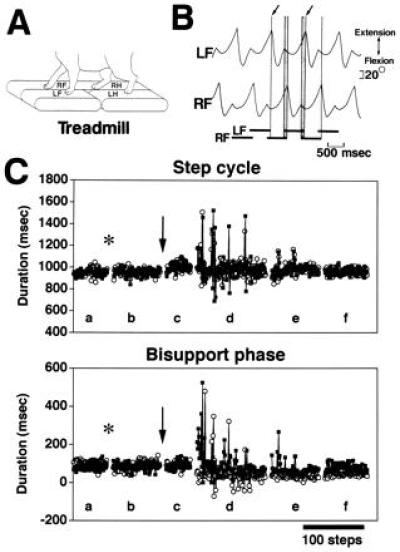
Experimental arrangement and example of adaptation to perturbed locomotion. (A) The treadmill had three moving belts, one mounted under the left forelimb (LF), another under the left hindlimb (LH) and a third under the right forelimb (RF) and right hindlimb (RH). (B) Example of angular movements detected by the potentiometers that were attached to the knee of each forelimb. Each step cycle was divided into swing and stance phases, and measured as the duration from one lift-off (arrow) to the next lift-off. Horizontal bars at the bottom show stance phase. The bisupport phases (vertical shaded bars), during which both forelimbs come into contact with the ground to switch the weight-bearing limb from the RF to the LF or vice versa, are of particular importance for smooth and stable locomotion. Scatter diagrams (Figs. 3 and 4) pairing the duration of the bisupport phase with its included step cycle of each limb at each step. (C) The adaptation to perturbed locomotion occurred in a cat injected with d-NMMA into the cerebellum. The upper diagram shows plots of the duration of the step cycle against the number of steps. Left forelimb, ▪; right forelimb, ○. The lower diagram shows plots of the duration of the bisupport phases. The phases last from LF touch-down to RF lift-off (▪) and from RF touch-down to LF lift-off (○). If the lift-off of a limb started earlier than touch-down of the contralateral limb, the value was represented as negative. Each point represents one step. Data in the left three portions of each graph (Lanes a–c) are from the successive trials of unperturbed locomotion. Data in the right three portions of each graph (Lanes d–f) are from the successive trials of perturbed locomotion. Insertion of an injection needle into the primary fissure of the cerebellum is indicated by the asterisk. Injection of d-NMMA is indicated by the arrow. The discontinuities in each graph represent about 5 min of standing with no motion on the treadmill.
After 3–5 repeated trials of unperturbed locomotion, an injection needle (0.5 mm in diameter), tilted back by 28° (dorsocaudal to ventrorostral), was inserted into the primary fissure, a deep groove separating the anterior and posterior lobes (Fig. 2A), at the median, 3 mm below the dorsal surface of the cerebellum. The oblique orifice of the injection needle faced anteriorly and the needle was connected by a Teflon tube to a 100 μl syringe. The animals subjected to injection were divided into two groups. The NO deprivation group consisted of seven cats, four injected with 15 μl of 100 μM hemoglobin dissolved in artificial cerebrospinal fluid (9), and the three others injected with a total of 30–50 μl of 1–10 mM l-NMMA into the primary fissure. The control group consisted of five cats, two injected with NG-monomethyl-d-arginine (d-NMMA) into the primary fissure, two injected with artificial cerebrospinal fluid alone, and one injected with l-NMMA into lobule VI (Fig. 2B).
Figure 2.
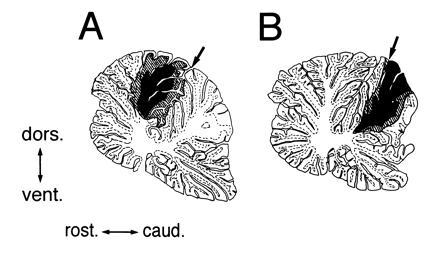
Extent of spread of the injected solutions in the cerebellum. To confirm distribution of the injected solutions in the cerebellum, at the end of the locomotion experiments on two cats, 15 μl of artificial cerebrospinal fluid containing 2% Pontamine sky blue was injected into the primary fissure (A) or a more caudal groove (B). The cats were perfused for fixation 1 hr after the injection. The extent of the diffusion of the dye is shown in a parasagittal section (right side, 0.8 mm) of the cerebellum. Arrow points to the insertion point of the injection needle through the dorsal surface. The filled area represents the region that most intensively retained the diffused dye. The hatched area delineates the maximal extent of diffusion. caud., caudal; dors., dorsal; rost., rostral; vent., ventral. (A) The diffused dye was intensively localized in the cerebellar vermis at lobule V. Mediolaterally, the dye diffused to about 2.4 mm from the midline. The dye diffused to the white matter, but not to the deep cerebellar nuclei. These observations suggest that the injected hemoglobin was localized in the vermis at lobule V, because the molecular weight of hemoglobin is much higher than that of the dye. (B) The dye was localized in the caudal part of lobule VI.
At the end of each experiment, the cat was deeply anesthetized with sodium pentobarbital, and was perfused with saline and then formalin. The brain was serially sectioned in a parasagittal plane at 100 μm thickness.
RESULTS
After the 10 mM d-NMMA (that has no inhibitory effect on NO synthase) was injected into the cerebellum of the control decerebrate cats, the adaptation took place in these cats as reported (11). Fig. 1C shows the manner of locomotive adaptation to the perturbation as a plot of the duration of the step cycle and the bisupport phase during which the body weight was supported by both forelimbs. The left three portions of each graph (lanes a–c) represent three successive trials of unperturbed locomotion. Neither the implantation of the injection needle (asterisk) nor the injection of d-NMMA (downward arrow) induced any appreciable disturbance of the locomotion. The right three portions (lanes d–f) represent three successive trials of perturbed locomotion. During the first trial of perturbed locomotion (lane d), the durations of both the step cycle and the bisupport phase of the two forelimbs exhibited marked fluctuations. These fluctuations, represented by spikelike deflections in Fig. 1C, progressively subsided, and the locomotion was stabilized from the beginning of the third trial of perturbed locomotion (lane f). Observations similar to those shown in Fig. 1C are represented in the scatter diagrams in Fig. 3. The stability of ongoing gait during unperturbed locomotion after injection of artificial cerebrospinal fluid is represented by convergence of the plotted points within a small area for both forelimbs (Fig. 3A). Subsequently, when the left forelimb alone was exposed to a belt velocity of 61 cm/s, perturbation thus induced in the ongoing locomotion is reflected by the wide dispersion of the plotted points along both the ordinate and the abscissa (Fig. 3B). The points plotted in Fig. 3C for the second trial of perturbed locomotion are also scattered, and it is notable that the bisupport phases of the left forelimb became longer, and those of the right forelimb shorter, than during unperturbed locomotion. In the third trial of perturbed locomotion, these plots were differentially converged with step cycles slightly shortened and with bisupport phases asymmetrical, suggesting that stable locomotion was achieved (Fig. 3D).
Figure 3.
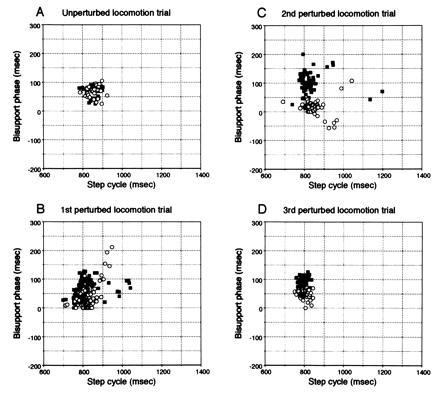
Locomotive adaptation. Scatter diagrams show variations in step cycles for LF vs. bisupport phase from LF touch-down to RF lift-off (▪), and for RF vs. bisupport phase from RF touch-down to LF lift-off (○). (A) Unperturbed locomotion trial. (B) First perturbed locomotion trial. (C) Second perturbed locomotion trial. (D) Third perturbed locomotion trial. Each point represents one step.
After injection of hemoglobin, locomotor movements during unperturbed locomotion were unaffected (Fig. 4A). However, adaptation no longer took place during successive trials of perturbed locomotion (Fig. 4 B–D). The plotted points remained widely dispersed over a large area of the scatter diagrams even for the third trial of perturbed locomotion. During the unperturbed locomotion trials conducted after these perturbed locomotion trials, locomotion was stable in the same manner as in the unperturbed locomotion trials conducted before the perturbed locomotion trials (data not shown). Similar loss of adaptation occurred in three other cats injected with hemoglobin and three cats injected with l-NMMA. Adaptation occurred normally when l-NMMA was injected into lobule VI instead of lobule V in one cat (data not shown).
Figure 4.
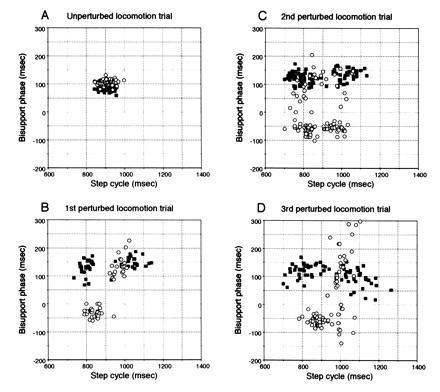
Effects of hemoglobin applied to the cerebellar vermis at lobule V on locomotive adaptation. (A) Unperturbed locomotion trial. (B) First perturbed locomotion trial. (C) Second perturbed locomotion trial. (D) Third perturbed locomotion trial.
Data obtained from five control cats and seven NO deprivation cats injected with hemoglobin or l-NMMA are summarized in Fig. 5. In the control group, variations of both the step cycle and the bisupport phase durations during successive perturbed locomotion trials exhibited significant decreases (ANOVA, P < 0.05), to smaller values than in unperturbed locomotion (δ < 1), representing the adaptation via which locomotion was stabilized and precisely controlled throughout this motor learning (Fig. 5 A and C). The cats in the NO deprivation group showed similar large variations in step cycle and bisupport phase durations to those of control cats during the first trial, but these variations did not decrease even after repeated trials (Fig. 5 B and D). No statistically significant differences were observed among trials (ANOVA, P > 0.05).
Figure 5.
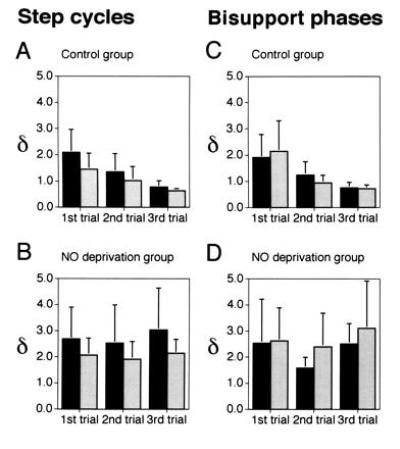
Effects of NO deprivation on the adaptation to perturbed locomotion. Ordinates, values of δ that is defined as the ratio of standard deviations of the step cycle durations (A and B) or the bisupport phase durations (C and D) in each trial of perturbed locomotion relative to those of unperturbed locomotion in the same cats. (A and C) Control group (n = 5). (B and D) NO deprivation group (n = 7). Filled columns, left forelimbs; shaded columns, right forelimbs. Bars represent SDs.
The possibility that the failure of adaptation in the NO deprivation cats is due to a performance deficit can be excluded for the following reasons. First, neither the unperturbed locomotion before nor that after perturbed locomotion was impaired in the NO deprivation cats. Second, the variations in the duration of the step cycles and bisupport phases in the first trial of perturbed locomotion, did not differ significantly between the control and NO deprivation groups (ANOVA, P > 0.05).
DISCUSSION
The cerebellum receives information through the spinocerebellar pathways about the ongoing activities both in the spinal stepping generator and at the somatosensory receptors during locomotion (19, 20). This information is conveyed by mossy fiber afferents to Purkinje cells via granule cells and their axons, i.e., parallel fibers. Purkinje cells transform the mossy fiber input signals to output signals that in turn modulate activities in descending tract neurons involved in locomotion. On the other hand, Purkinje cells in vermal lobule V receive enhanced climbing fiber signals during perturbed locomotion (12). These climbing fiber signals are expected to induce LTD at parallel fiber synapses mediating “ongoing locomotion”-related mossy fiber signals. If the mossy fiber-to-Purkinje cell signal transformation is modified by LTD at parallel fiber-Purkinje cell synapses, this will lead to changes in activities in the descending tract neurons, and thereby to adaptation in locomotion.
Close association between NO and the adaptation in locomotion is now apparent, because the adaptation to perturbed locomotion was abolished when NO was deprived from vermal lobule V. It is important to note that NO deprivation does not affect normal, unperturbed locomotion; it abolishes only adaptation. This situation is similar to that reported for adaptation of the vestibuloocular reflex in the monkey, rabbit, and goldfish (21, 22). Application of hemoglobin to the subdural space over the cerebellar flocculus did not affect dynamic characteristics of the oculomotor system; however, it abolished adaptive changes of the vestibuloocular reflex (21). Furthermore, the classically conditioned eye-blink response, in which the neural circuits essential for acquisition and expression of its learned response have been identified to be in the cerebellum (23, 24), was impaired by systemic injection of an inhibitor of NO synthase (25). These results are also consistent with the fact that NO deprivation abolishes LTD without affecting normal synaptic transmission in cerebellar synapses (3). From the present results we conclude that NO-dependent cerebellar function is critically involved in adaptive control of locomotion. Since adaptation or conditioning is a simple form of learning, our conclusion, considered together with the results of previous studies (21, 22, 25) can be expanded to motor learning in general. Because NO is indispensable for induction of LTD, our findings support the view that cerebellar LTD plays a key role in motor learning (1). Exposure of one forelimb to a higher belt velocity than that of the other limbs during locomotion on a treadmill in decerebrate cats would provide an effective means of investigating involvement of the cerebellar LTD in motor learning/memory.
Acknowledgments
We thank Dr. M. Ito for critical reading of the manuscript. We are also grateful to Dr. D. Okada and the late Dr. M. Udo for helpful comments. This work was supported in part by grants from the Toyota Physical and Chemical Research Institute.
Footnotes
Abbreviations: LTD, long-term depression; l-NMMA, NG-monomethyl-l-arginine; d-NMMA, NG-monomethyl-d-arginine.
References
- 1.Ito M. Annu Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- 2.Sakurai M. J Physiol (London) 1987;394:463–480. doi: 10.1113/jphysiol.1987.sp016881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shibuki K, Okada D. Nature (London) 1991;349:326–328. doi: 10.1038/349326a0. [DOI] [PubMed] [Google Scholar]
- 4.Southam E, Garthwaite J. Eur J Neurosci. 1991;3:379–382. doi: 10.1111/j.1460-9568.1991.tb00825.x. [DOI] [PubMed] [Google Scholar]
- 5.Schuman E M, Madison D V. Annu Rev Neurosci. 1994;17:153–183. doi: 10.1146/annurev.ne.17.030194.001101. [DOI] [PubMed] [Google Scholar]
- 6.Hartell N A. NeuroReport. 1994;5:833–836. doi: 10.1097/00001756-199403000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Crepel F, Jaillard D. NeuroReport. 1990;1:133–136. doi: 10.1097/00001756-199010000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Daniel H, Hemart N, Jaillard D, Crepel F. Eur J Neurosci. 1993;5:1079–1082. doi: 10.1111/j.1460-9568.1993.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 9.Ito M, Karachot L. NeuroReport. 1990;1:129–132. doi: 10.1097/00001756-199010000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Lev-Ram V, Makings L R, Keitz P F, Kao J P Y, Tsien R Y. Neuron. 1995;15:407–415. doi: 10.1016/0896-6273(95)90044-6. [DOI] [PubMed] [Google Scholar]
- 11.Yanagihara D, Udo M, Kondo I, Yoshida T. Neurosci Res. 1993;18:241–244. doi: 10.1016/0168-0102(93)90060-4. [DOI] [PubMed] [Google Scholar]
- 12.Yanagihara D, Udo M. Neurosci Res. 1994;19:245–248. doi: 10.1016/0168-0102(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 13.Forssberg H, Grillner S, Rossignol S. Brain Res. 1977;132:121–139. doi: 10.1016/0006-8993(77)90710-7. [DOI] [PubMed] [Google Scholar]
- 14.Eidelberg E, Story J L, Meyer B L, Nystel J. Exp Brain Res. 1980;40:241–246. doi: 10.1007/BF00237787. [DOI] [PubMed] [Google Scholar]
- 15.Chambers W W, Sprague J M. Arch Neurol Psychiatr. 1955;74:653–680. doi: 10.1001/archneurpsyc.1955.02330180071008. [DOI] [PubMed] [Google Scholar]
- 16.Udo M, Matsukawa K, Kamei H. Brain Res. 1979;160:559–564. doi: 10.1016/0006-8993(79)91087-4. [DOI] [PubMed] [Google Scholar]
- 17.Udo M, Matsukawa K, Kamei H. Brain Res. 1979;166:405–408. doi: 10.1016/0006-8993(79)90228-2. [DOI] [PubMed] [Google Scholar]
- 18.Udo M, Matsukawa K, Kamei H, Oda Y. J Neurophysiol. 1980;44:119–134. doi: 10.1152/jn.1980.44.1.119. [DOI] [PubMed] [Google Scholar]
- 19.Arshavsky Yu I, Gelfand I M, Orlovsky G N. Trends Neurosci. 1983;6:417–422. [Google Scholar]
- 20.Ito M. The Cerebellum and Neural Control. New York: Raven; 1984. [Google Scholar]
- 21.Nagao S, Ito M. NeuroReport. 1991;2:193–196. doi: 10.1097/00001756-199104000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Smith S S, McElligott J G. J Neurophysiol. 1995;74:489–494. doi: 10.1152/jn.1995.74.1.489. [DOI] [PubMed] [Google Scholar]
- 23.Krupa D J, Thompson J K, Thompson R F. Science. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- 24.Thompson R F, Krupa D J. Annu Rev Neurosci. 1994;17:519–549. doi: 10.1146/annurev.ne.17.030194.002511. [DOI] [PubMed] [Google Scholar]
- 25.Chapman P F, Atkins C M, Todd Allen M, Haley J E, Steinmetz J E. NeuroReport. 1992;3:567–570. doi: 10.1097/00001756-199207000-00005. [DOI] [PubMed] [Google Scholar]


