Abstract
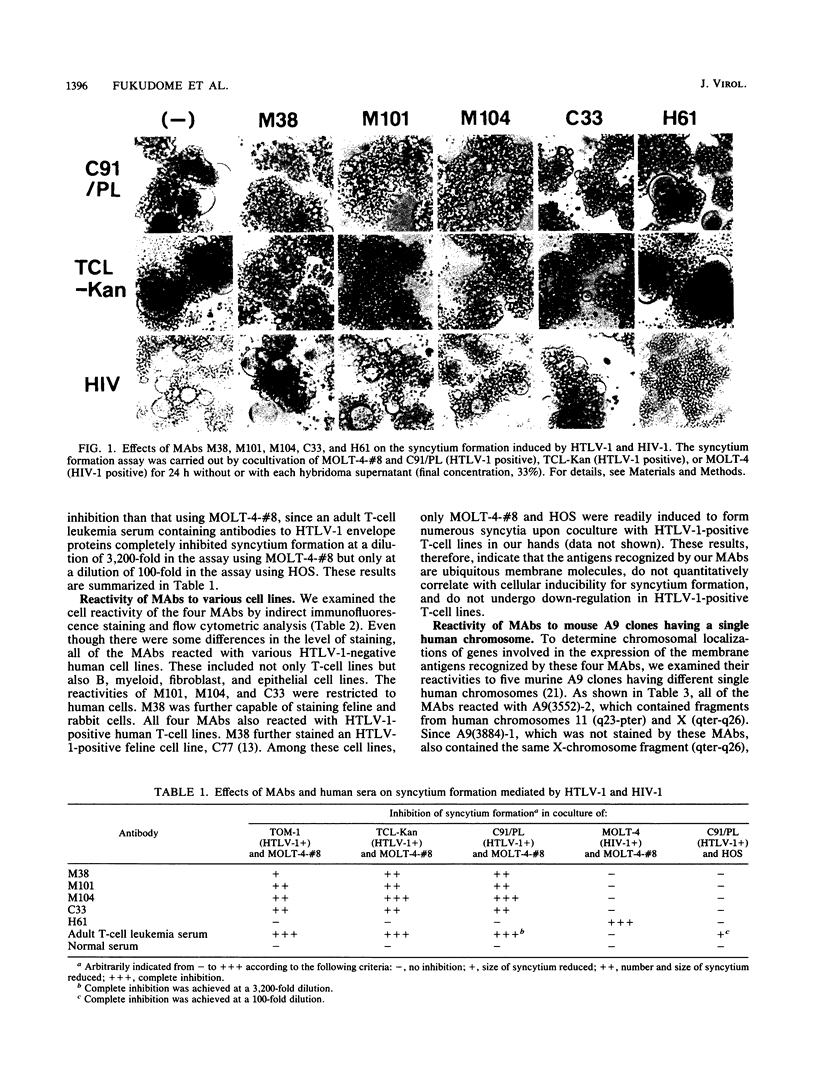
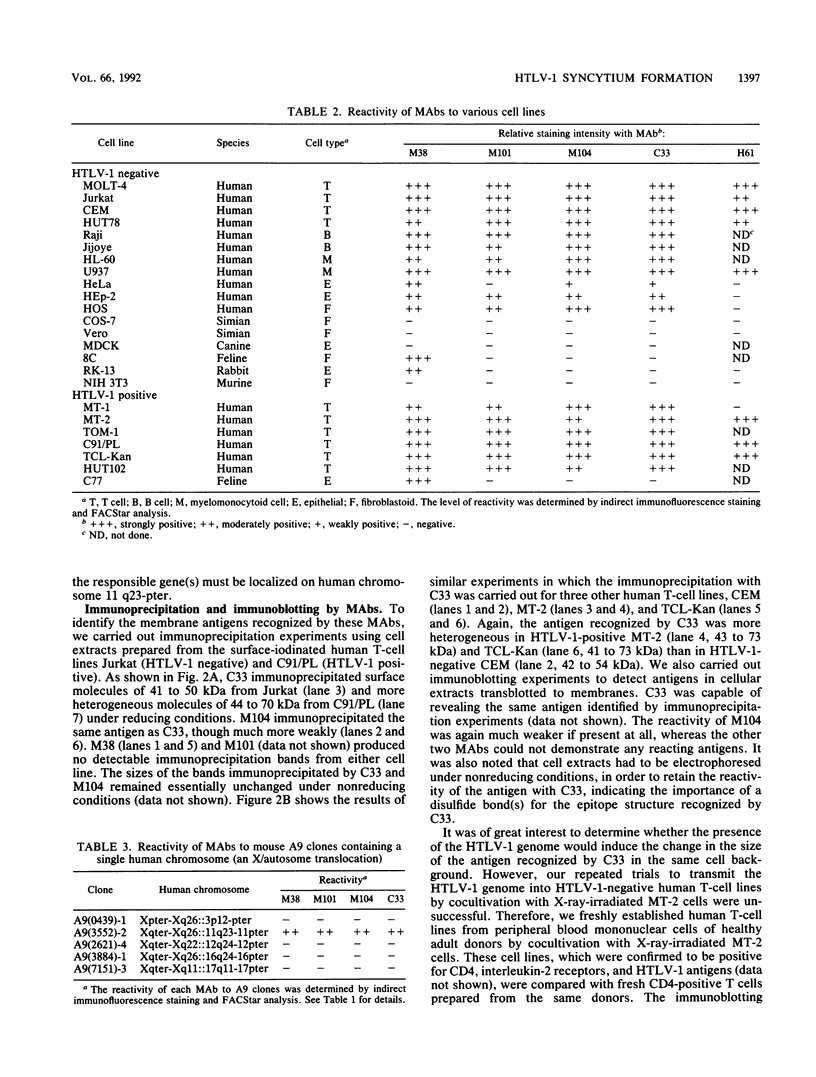
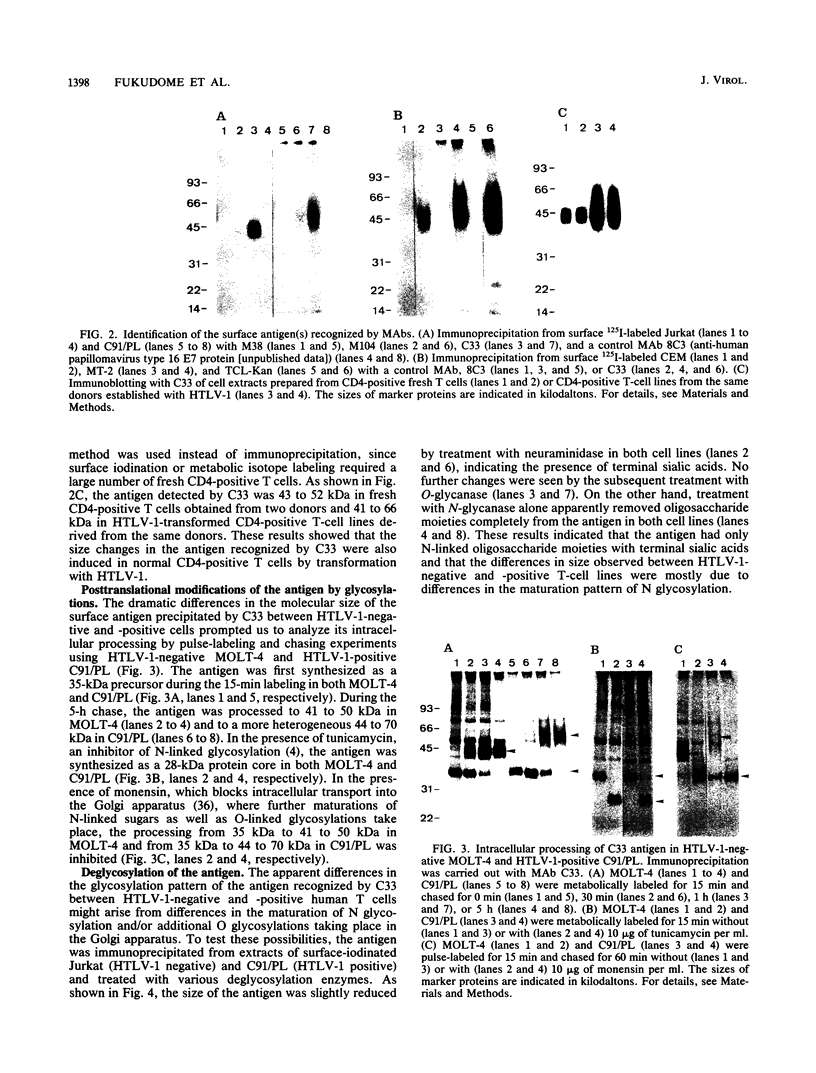
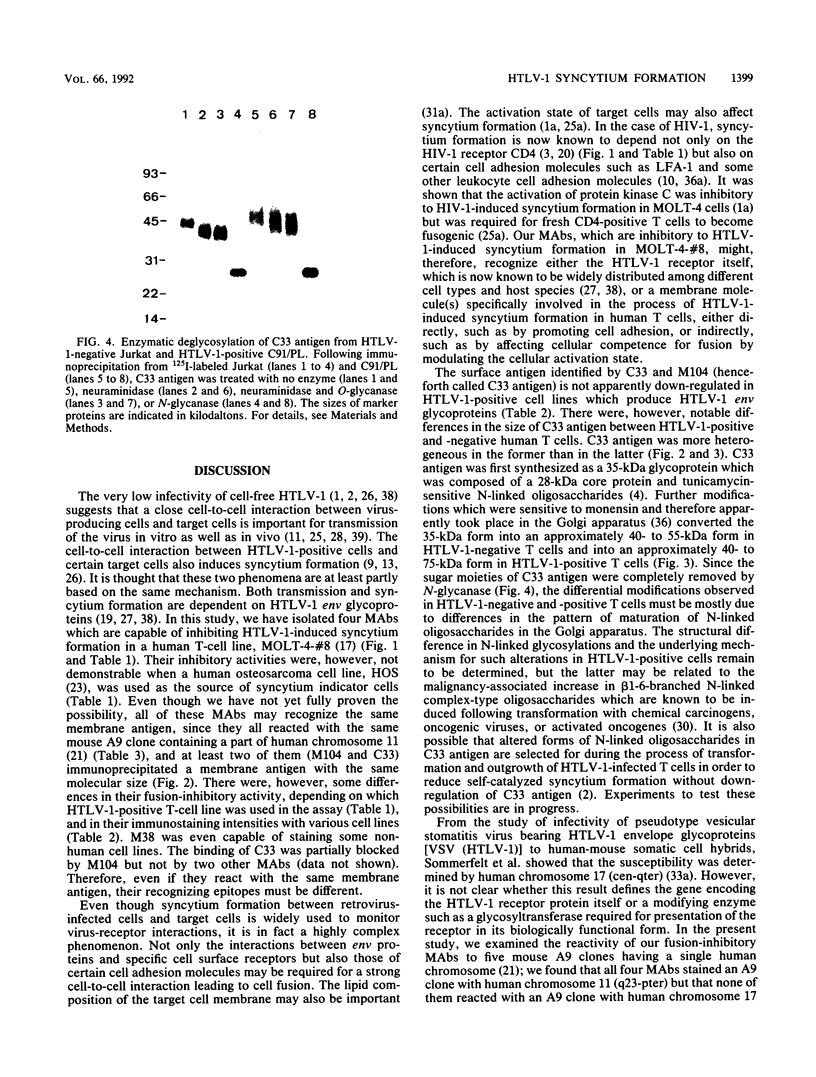
We isolated four monoclonal antibodies (MAbs), M38, M101, M104, and C33, which were capable of inhibiting syncytium formation induced in a human T-cell line, MOLT-4-#8, by coculture with human T-cell leukemia virus type 1 (HTLV-1)-positive human T-cell lines. The MAbs had, however, no inhibitory activity on syncytium formation induced in a human osteosarcoma line, HOS, by HTLV-1-positive T-cell lines. They also did not inhibit syncytium formation induced in MOLT-4-#8 by human immunodeficiency virus type 1-positive MOLT-4. All MAbs reacted with various human cell lines of lymphoid and nonlymphoid origins, including HTLV-1-positive T-cell lines. Furthermore, they all reacted with a murine A9 clone containing human chromosome 11 fragment q23-pter. Two MAbs, M104 and C33, immunoprecipitated a membrane antigen with the same molecular size. The antigen (henceforth called C33 antigen) was about 40 to 55 kDa in HTLV-1-negative Jurkat, CEM, MOLT-4, and normal peripheral blood CD4-positive human T cells and about 40 to 75 kDa in HTLV-1-positive C91/PL, TCL-Kan, MT-2, and in fresh HTLV-1-transformed CD4-positive human T-cell lines. Pulse-chase experiments revealed that C33 antigen was synthesized as a 35-kDa precursor that was then processed to 41 to 50 kDa in MOLT-4 and to 44 to 70 kDa in C91/PL. In the presence of tunicamycin, a 28-kDa protein was synthesized. The conversion from 35 kDa to 41 to 50 kDa in MOLT-4 and to 44 to 70 kDa in C91/PL was inhibited by monensin. Treatment with N-glycanase alone, but not with sialidase and O-glycanase in combination, completely removed the sugar moiety of C33 antigen from both HTLV-1-negative Jurkat and HTLV-1-positive C91/PL. Therefore, C33 antigen has only N-linked carbohydrates, the modification of which appears to be substantially altered in the presence of the HTLV-1 genome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chosa T., Yamamoto N., Tanaka Y., Koyanagi Y., Hinuma Y. Infectivity dissociated from transforming activity in a human retrovirus, adult T-cell leukemia virus. Gan. 1982 Dec;73(6):844–847. [PubMed] [Google Scholar]
- Chowdhury I. H., Koyanagi Y., Kobayashi S., Hamamoto Y., Yoshiyama H., Yoshida T., Yamamoto N. The phorbol ester TPA strongly inhibits HIV-1-induced syncytia formation but enhances virus production: possible involvement of protein kinase C pathway. Virology. 1990 May;176(1):126–132. doi: 10.1016/0042-6822(90)90237-l. [DOI] [PubMed] [Google Scholar]
- Clapham P., Nagy K., Cheingsong-Popov R., Exley M., Weiss R. A. Productive infection and cell-free transmission of human T-cell leukemia virus in a nonlymphoid cell line. Science. 1983 Dec 9;222(4628):1125–1127. doi: 10.1126/science.6316502. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- Fisher J. H., Miller Y. E., Sparkes R. S., Bateman J. B., Kimmel K. A., Carey T. E., Rodell T., Shoemaker S. A., Scoggin C. H. Wilms' tumor-aniridia association: segregation of affected chromosome in somatic cell hybrids, identification of cell surface antigen associated with deleted area, and regional mapping of c-Ha-ras-1 oncogene, insulin gene, and beta-globin gene. Somat Cell Mol Genet. 1984 Sep;10(5):455–464. doi: 10.1007/BF01534850. [DOI] [PubMed] [Google Scholar]
- Freed E. O., Myers D. J., Risser R. Characterization of the fusion domain of the human immunodeficiency virus type 1 envelope glycoprotein gp41. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4650–4654. doi: 10.1073/pnas.87.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Hattori S., Imagawa K., Shimizu F., Hashimura E., Seiki M., Yoshida M. Identification of envelope glycoprotein encoded by env gene of human T-cell leukemia virus. Gan. 1983 Dec;74(6):790–793. [PubMed] [Google Scholar]
- Hayami M., Tsujimoto H., Komuro A., Hinuma Y., Fujiwara K. Transmission of adult T-cell leukemia virus from lymphoid cells to non-lymphoid cells associated with cell membrane fusion. Gan. 1984 Feb;75(2):99–102. [PubMed] [Google Scholar]
- Hildreth J. E., Orentas R. J. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science. 1989 Jun 2;244(4908):1075–1078. doi: 10.1126/science.2543075. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y. Natural history of the retrovirus associated with a human leukemia. Bioessays. 1985 Nov;3(5):205–209. doi: 10.1002/bies.950030505. [DOI] [PubMed] [Google Scholar]
- Hoshino H., Shimoyama M., Miwa M., Sugimura T. Detection of lymphocytes producing a human retrovirus associated with adult T-cell leukemia by syncytia induction assay. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7337–7341. doi: 10.1073/pnas.80.23.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Kohno M., Nakamura M., Hinuma Y., Sugamura K. Characterization of interleukin 2-stimulated phosphorylation of 67 and 63 kDa proteins in human T-cells. Biochem J. 1987 Feb 15;242(1):211–219. doi: 10.1042/bj2420211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe M., Emanuel B. S., Givol D., Oren M., Croce C. M. Localization of gene for human p53 tumour antigen to band 17p13. Nature. 1986 Mar 6;320(6057):84–85. doi: 10.1038/320084a0. [DOI] [PubMed] [Google Scholar]
- Kikukawa-Itamura R., Harada S., Kobayashi N., Hatanaka M., Yamamoto N. Quantitative analysis of cytotoxicity induced by HTLV-I carrying cells against a human lymphoblastoid cell line, Molt-4. Leuk Res. 1987;11(4):331–338. doi: 10.1016/0145-2126(87)90177-9. [DOI] [PubMed] [Google Scholar]
- Kikukawa R., Koyanagi Y., Harada S., Kobayashi N., Hatanaka M., Yamamoto N. Differential susceptibility to the acquired immunodeficiency syndrome retrovirus in cloned cells of human leukemic T-cell line Molt-4. J Virol. 1986 Mar;57(3):1159–1162. doi: 10.1128/jvi.57.3.1159-1162.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa T., Yoshikura H., Hattori S., Seiki M., Yoshida M. Envelope proteins of human T-cell leukemia virus: expression in Escherichia coli and its application to studies of env gene functions. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6202–6206. doi: 10.1073/pnas.81.19.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Koi M., Morita H., Shimizu M., Oshimura M. Construction of mouse A9 clones containing a single human chromosome (X/autosome translocation) via micro-cell fusion. Jpn J Cancer Res. 1989 Feb;80(2):122–125. doi: 10.1111/j.1349-7006.1989.tb02278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. H., Coligan J. E., Homma T., McLane M. F., Tachibana N., Essex M. Human T-cell leukemia virus-associated membrane antigens: identity of the major antigens recognized after virus infection. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3856–3860. doi: 10.1073/pnas.81.12.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. M., Gardner M. B., Greene A. E., Bradt C., Nichols W. W., Landing B. H. Cultivation in vitro of cells derived from a human osteosarcoma. Cancer. 1971 Feb;27(2):397–402. doi: 10.1002/1097-0142(197102)27:2<397::aid-cncr2820270224>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- Miyoshi I., Yoshimoto S., Kubonishi I., Taguchi H., Shiraishi Y., Ohtsuki Y., Akagi T. Transformation of normal human cord lymphocytes by co-cultivation with a lethally irradiated human T-cell line carrying type C virus particles. Gan. 1981 Dec;72(6):997–998. [PubMed] [Google Scholar]
- Mohagheghpour N., Chakrabarti R., Stein B. S., Gowda S. D., Engleman E. G. Early activation events render T cells susceptible to HIV-1-induced syncytia formation. Role of protein kinase C. J Biol Chem. 1991 Apr 15;266(11):7233–7238. [PubMed] [Google Scholar]
- Nagy K., Clapham P., Cheingsong-Popov R., Weiss R. A. Human T-cell leukemia virus type I: induction of syncytia and inhibition by patients' sera. Int J Cancer. 1983 Sep 15;32(3):321–328. doi: 10.1002/ijc.2910320310. [DOI] [PubMed] [Google Scholar]
- Popovic M., Lange-Wantzin G., Sarin P. S., Mann D., Gallo R. C. Transformation of human umbilical cord blood T cells by human T-cell leukemia/lymphoma virus. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5402–5406. doi: 10.1073/pnas.80.17.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Reitz M. S., Jr, Sarngadharan M. G., Robert-Guroff M., Kalyanaraman V. S., Nakao Y., Miyoshi I., Minowada J., Yoshida M., Ito Y. The virus of Japanese adult T-cell leukaemia is a member of the human T-cell leukaemia virus group. Nature. 1982 Nov 4;300(5887):63–66. doi: 10.1038/300063a0. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarin P. S., Robert-Gurroff M., Kalyanaraman V. S., Mann D., Minowada J., Gallo R. C. Isolation and transmission of human retrovirus (human t-cell leukemia virus). Science. 1983 Feb 18;219(4586):856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- Robey W. G., Safai B., Oroszlan S., Arthur L. O., Gonda M. A., Gallo R. C., Fischinger P. J. Characterization of envelope and core structural gene products of HTLV-III with sera from AIDS patients. Science. 1985 May 3;228(4699):593–595. doi: 10.1126/science.2984774. [DOI] [PubMed] [Google Scholar]
- Roos D. S., Duchala C. S., Stephensen C. B., Holmes K. V., Choppin P. W. Control of virus-induced cell fusion by host cell lipid composition. Virology. 1990 Apr;175(2):345–357. doi: 10.1016/0042-6822(90)90419-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J., Yamamoto N., Hinuma Y., Hunsmann G. Sera from adult T-cell leukemia patients react with envelope and core polypeptides of adult T-cell leukemia virus. Virology. 1984 Jan 15;132(1):1–11. doi: 10.1016/0042-6822(84)90086-2. [DOI] [PubMed] [Google Scholar]
- Sommerfelt M. A., Weiss R. A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990 May;176(1):58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- Sommerfelt M. A., Williams B. P., Clapham P. R., Solomon E., Goodfellow P. N., Weiss R. A. Human T cell leukemia viruses use a receptor determined by human chromosome 17. Science. 1988 Dec 16;242(4885):1557–1559. doi: 10.1126/science.3201246. [DOI] [PubMed] [Google Scholar]
- Sugamura K., Fujii M., Kannagi M., Sakitani M., Takeuchi M., Hinuma Y. Cell surface phenotypes and expression of viral antigens of various human cell lines carrying human T-cell leukemia virus. Int J Cancer. 1984 Aug 15;34(2):221–228. doi: 10.1002/ijc.2910340213. [DOI] [PubMed] [Google Scholar]
- Sugamura K., Fujii M., Ueda S., Hinuma Y. Identification of a glycoprotein, gp21, of adult T cell leukemia virus by monoclonal antibody. J Immunol. 1984 Jun;132(6):3180–3184. [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Valentin A., Lundin K., Patarroyo M., Asjö B. The leukocyte adhesion glycoprotein CD18 participates in HIV-1-induced syncytia formation in monocytoid and T cells. J Immunol. 1990 Feb 1;144(3):934–937. [PubMed] [Google Scholar]
- Veronese F. D., DeVico A. L., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. Characterization of gp41 as the transmembrane protein coded by the HTLV-III/LAV envelope gene. Science. 1985 Sep 27;229(4720):1402–1405. doi: 10.1126/science.2994223. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Clapham P., Nagy K., Hoshino H. Envelope properties of human T-cell leukemia viruses. Curr Top Microbiol Immunol. 1985;115:235–246. doi: 10.1007/978-3-642-70113-9_15. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Okada M., Koyanagi Y., Kannagi M., Hinuma Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science. 1982 Aug 20;217(4561):737–739. doi: 10.1126/science.6980467. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]