Summary
Objective
To evaluate the specific components of setting up a simple multicentre clinical study four years after the new UK law on clinical trials was implemented in 2004.
Design
Timelines associated with activating a randomized multicentre trial in lung cancer patients using an investigational medicinal product (statins) were prospectively recorded.
Setting
84 trial centres in the UK.
Main outcome measures
The time taken to go through the three stages necessary to activate a trial at a centre was examined: that is, the time from when Site Specific Information was electronically transferred to a participating centre until local research ethics committee (LREC) or research and development (R&D) approvals were obtained, and a signed Clinical Trials Site Agreement (CTSA) was received.
Results
It took at least six months to obtain LREC approval in 21% of centres and R&D approval in 52% of centres. Twelve centres (14%) took at least 12 months to obtain R&D approval. 31% of centres took at least three months to return a signed CTSA. Although 52% of centres took at least six months to be activated, 13% were able to complete all three stages in two months or less.
Conclusions
While some centres can activate trials relatively quickly, there is considerable variation the time taken to set up a trial, much of which is due to the delay in obtaining R&D approval. This is having a major adverse effect on UK health research. There is a national need to streamline the process for considering multi-centre non-commercial clinical trials, in particular, having fixed timelines for R&D assessment. Without this, the costs of trials will increase because of extended duration, and the time to answer a research question and alter clinical practice will be significantly prolonged.
Introduction
Clinical trials in the UK are now more common than ever, particularly with the establishment of networks such as the National Cancer Research Network and the Clinical Research Networks, which encourage and facilitate the development and conduct of trials in cancer and other disorders. However, the introduction of the EU Clinical Trials Directive in 2001 and its transposition into UK law in 20041 has complicated the way in which trials are conducted, largely because of additional layers of bureaucracy related to, for example, increased pharmacovigilance, and securing and processing sponsorship. While commercially-sponsored trials have the financial resources to cope with these changes, academic-led trials are still suffering four years after these new laws were implemented.
Previously, researchers have commented on a specific aspect of trial set-up, for example, ethics approval or research governance.2–6 We undertook a prospective study to quantify the timelines associated with all three stages necessary to set up a trial at a centre. The main purpose of this article is to provide this information using a simple trial of an investigational medicinal product. Furthermore, this work was done four years after the UK law came into force, so any learning effect should be small. Our results should stimulate discussion and encourage the Department of Health to streamline the trial set-up process and resolve the problems we have identified.
Methods
From our unit, we chose a simple placebo-controlled trial with many potential recruiting centres (84 from England, Scotland and Wales). National Research Ethics Service (NRES) approval was given on 20 July 2006. The trial aims to examine survival in 1300 patients with lung cancer, randomized to receive a statin or placebo every day for up to two years; both are provided free of charge. All patients receive usual care (standard first-line chemotherapy) and the only extra mandatory investigations associated with the trial are a single CT scan between the third and fourth cycles of chemotherapy and two blood measurements in the baseline blood sample (although many centres do these tests anyway). In the UK, each potential centre must go through three formal administrative steps before it can recruit patients: local research ethics committee (LREC) approval, approval from the Research and Development (R&D) unit and signing a Clinical Trials Site Agreement (CTSA).
LRECs use information contained in the NRES application, allowing each centre to assess the safety and suitability of the trial for patients from that centre. The information used by LRECs, called Site Specific Information (SSI), enabling a Site Specific Assessment, is transferred electronically to the centre via the NRES website. The purpose of the R&D assessment is to evaluate the trial design and feasibility for that particular centre, including any financial costs that may arise because of the trial. The CTSA is an agreement that must be signed between the centre, the principal investigator at that recruiting centre and the sponsor of the trial (University College London in our case). The agreement sets out the responsibility of that centre when conducting the trial and was only introduced because of the EU Directive, which specified that all trials must have a named sponsor.
We recorded four time periods, measured from the point at which the SSI was electronically transferred to the centre until:
Notification of LREC (ie Site Specific Assessment) approval had been received by the coordinating centre
Notification of R&D approval had been received by the coordinating centre
All three stages (LREC, R&D and signed CTSA) have been passed
We also measured the time from when the CTSA is submitted (usually after site approval) until it has been signed and returned to the coordinating centre.
In three centres, the date of the SSI preceded the date of NRES approval; for those centres, the NRES approval date was taken to be the start point. The site approval letter is sent directly to the chief investigator, who notifies the coordinating centre within a few days.
Results
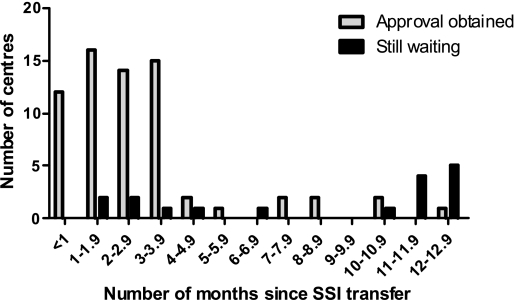
Figure 1 shows the distribution of the time it took to get LREC approval in those centres in which this had already been obtained as of 27 March 2008 (n=67) and those where approval had not yet been given (n=17). Twenty-one percent (18/84) of centres have taken at least six months to give approval. Six centres (7%) had taken 12 months to consider the application.
Figure 1.
The number of months taken to obtain LREC approval (site-specific assessment) in centres where approval has been given or where we were still waiting for a response (as of 27 March 2008)
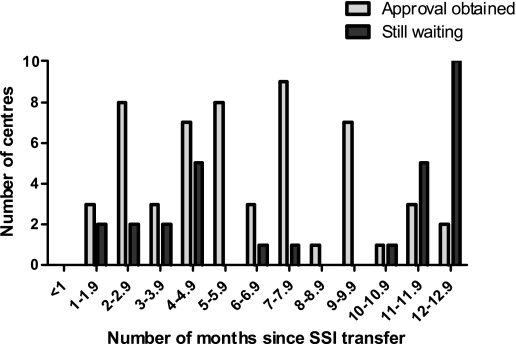
Figure 2 shows the distribution of the time it took to get R&D approval (obtained from55 centres but still outstanding from 29). 52% (44/84) of centres have taken at least six months. Twelve centres (14%) have taken at least 12 months. Sometimes R&D assessment is not undertaken until LREC approval, so we also examined the length of time between these two processes. There was a gap of at least three months in nearly half of the centres (27/55), though this could have been a parallel process. The gap was greater than six months in seven centres (13%).
Figure 2.
The number of months taken to obtain R&D approval, in centres where approval has been given or where we were still waiting for a response (as of 27 March 2008)
Among the 66 centres to which the CTSA had been sent, 39% (24/66) had taken more than three months to return a signed copy. However, 23% (15/66) took less than a month.
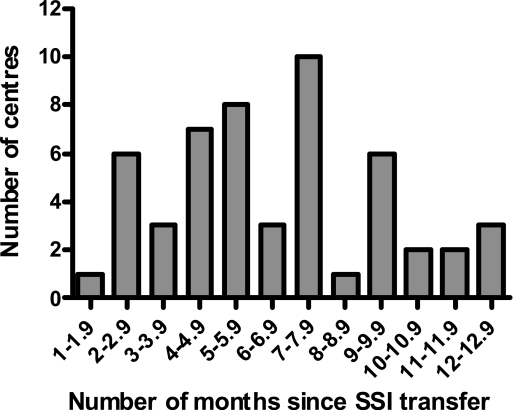
Figure 3 shows the distribution of the time taken to get LREC and R&D approvals and a signed CTSA for the 52 centres which had all three. 52% (27/52) took at least six months. However, it was noted that 13% of centres (7/52) were able to consider and approve the applications in two months or less.
Figure 3.
The number of months taken to get LREC and R&D approvals and a signed CTSA in 29 centres in which we had all three (as of 27 March 2008)
Discussion
Principal findings
The trial used here is based on giving a commonly used drug (a statin) to patients with a common cancer (lung). It has a simple design with few, if any, extra service costs to a centre. It was therefore expected that it would be relatively quick to set up nationally, particularly since the study was ‘badged’ by the National Cancer Research Network, allowing the use of their staff and resources. While some centres were able to activate the trial quickly, others took several months despite contacting them. R&D approval accounted for the longest delay. The wide range in set-up time demonstrates that some Trusts can process clinical trial applications more efficiently than others.
It is of great concern that so many centres can take as long as 12 months or more to obtain R&D approval. This is an extraordinarily long time and the reasons for the delay are not entirely clear. There could be a lag between the SSI form being electronically transferred to the centre and the research nurse/clinical trials administrator, who is responsible for the trial, informing R&D; this could be due to staff shortages or sickness. There may be other trials in set-up that have a higher priority or more funding. The R&D department may itself have staff shortages or lack other resources. While some centres have a clinical trials administrator who does much of the paperwork, other centres only have a research nurse who may already be overstretched with setting up or conducting other trials.
The delay in obtaining a signed CTSA could also have several causes. Many trusts do not have a legal department to deal with contracts and agreements, Foundation Trusts can be particularly wary of these documents, and there is a lack of guidance and training on this issue from the Department of Health. Furthermore, centres outside of England (such as Scotland) sometimes have difficulty in accepting the terms and conditions of a CTSA that is based on English law. Where an investigational medicinal product is involved the pharmacy also has to approve the trial and they may have internal issues over drug supply and handling that need to be resolved before they sign off the CTSA.
Strengths and limitations
A key strength of our study is that we systematically recorded the important dates associated with trial set-up in a prospective manner. Because this information was collected in ‘real time’ our results should be representative of the delays seen in many other clinical trials. Indeed, where the trial design is more complex, it may take longer for centres to assess the application. A limitation of this report is that it is based on only one study. However, our experience with this particular trial is not unique: we have had similar experiences with other clinical trials in our unit and elsewhere, as well as with non-investigational medicinal product trials. The problems with setting up trials are now recognized in most research departments. Another limitation is that we did not systematically record the specific reasons for any delays, but it may have been difficult to get accurateinformation on this from centres.
Comparison with other similar studies
Previously published articles have examined similar aspects of setting up research studies in the UK. One example was a simple study involving a telephone interview with a health-care manager, where applications for approval were made to 316 primary care organizations.2 Six months later, 13% had not yet given approval. Another research group reported data on the time taken to receive R&D approval for four clinical trials in neurology4 where 7% of centres took at least six months to give R&D approval, a similar outcome to this study. Other researchers have provided views on the research approval processes from their own experiences.3,5,7,8 To our knowledge, our paper is the first to examine and quantify each of the three necessary components of setting up a clinical trial in the UK, including obtaining a signed CTSA.
Implications for clinicians and policymakers
The data presented here are those associated with the timelines under the control of a participating centre. There are other stages to go through, such as recruitment of staff; preparing and obtaining multicentre research ethics approval, which usually takes three to five months; obtaining Clinical Trials Authorization if the trial involves an investigational medicinal product; registering the centre and participating clinicians with the Medicines and Health Regulatory Agency (MHRA); establishing processes for drug supply and delivery (if appropriate); and conducting site initiation visits. The result is that it can easily take a year or more from when a grant is awarded until enrolment of the first patient: 13 months in our trial.
Participating centres, the Department of Health and funders should be aware of several implications of the long delay in setting up studies:
First, the delay extends the duration of a trial, thereby increasing its cost. Employing a cancer trial coordinator for an extra year might cost about £40,000 (salary plus additional costs – national insurance, pension, etc.), so for every ten trials that need to be extended by a year the increase in cost is £400,000. This could fund at least one new cancer trial. Because there is a fixed amount of funding available, either trials have to recruit at a faster rate once they open or the total number of new trials that can be funded needs to be reduced to accommodate the increased life of ongoing trials.
Second, it is important for topical academic questions to be answered speedily if the UK academic community is to be competitive internationally. The process of designing a study, obtaining agreement for drug supply from the pharmaceutical industry and submitting the trial for funding can rarely be achieved in less than 12–18 months. A further delay of almost a year to recruit the first patient could make the UK less attractive. In an environment where new therapeutic cancer agents appear rapidly, enthusiasm for a trial may wane as newer products emerge. At hospital sites, academic studies with limited funding through the National Cancer Research Network and the as-yet-uncertain stream from NHS R&D have to compete with the more clearly defined commercial trial funding.
Third, and perhaps most importantly, once a trial proposal has been developed, peer reviewed and approved the research community has an ethical obligation to obtain the trial results and, where appropriate, alter clinical practice as soon as possible.
Possible solutions
The whole system of trial set-up could and should be streamlined for multi-centre trials that have already gone through a national approval process. The simplest solution would be to do away with the local LREC and R&D committees for these particular studies. The requirement for LREC review seems pointless because the trial has already been evaluated and approved by a national ethics committee. The only additional requirement would be a national R&D committee, which could be included with the NRES process. An alternative option would be to merge the local LREC and R&D committees when considering multi-centre studies or ensure they meet simultaneously, but with strict deadlines imposed – say 45 days. However, there is little incentive for Trusts to do this because their resources are limited; the situation is only likely to improve when research moves higher up the Trust's agenda and real money, or the absence of it, becomes an issue as the new R&D funding arrangements become clearer. The recently revised system for LREC and R&D approval involves the same application form being used by both local committees,9 but there are still two separate stages in each centre. There are plans to reduce the number of forms completed by researchers (which is beneficial),10,11 but each centre is still expected to review each application. But it is the time to approval in a centre that needs to be greatly shortened. Unless deadlines are imposed on the R&D process it is unlikely that the trial set-up time will be materially reduced.
We have also shown that obtaining a signed CTSA took several months in several centres. What is needed is a single, simple strategy for processing a CTSA for non-commercial studies within the UK. Although a national generic agreement for non-commercial research is being considered by the UK Clinical Research Collaboration, it is not known when it might be implemented.12,13
Conclusions
We demonstrate that there is a need to reduce the time taken by centres to give approval of non-commercial multi-centre trials. The wide variation in set-up time demonstrates that some Trusts have well-organized mechanisms in place, but for many this does not seem to be the case. Many Trusts have limited resources (some with reduced budgets and staff redundancies), further strengthening the case for streamlining the process. Ideally this would be through a central system in the same way that multi-centre ethics are assessed. However, implementing strict timeframes for centre assessment would also be effective. If we can achieve this we will be able to continue to capitalize on our expertise in many diseases. However, failure to change the current process in the UK will impair our ability to perform clinical trials in a timely fashion. And without such changes, new treatments or preventive strategies which save lives or reduce morbidity could take much longer to be evaluated and adopted into clinical practice.
Footnotes
DECLARATIONS —
Competing interests None declared
Funding The study was sponsored by University College London
Ethical approval Not applicable
Guarantor AH
Contributorship AH and HF had the idea for the study. HF collected and recorded the data, which were analysed by AH. MS is the chief investigator for the trial used in this paper. All authors were involved in writing the paper and gave final approval
Acknowledgements
We are grateful for comments from Kate Law, Julie Hearn and Daljit Kaur (Cancer Research UK), Nicole Gower (CRUK and UCL Cancer Trials Centre) and Michael Cullen and Lindsay James (Lungstar Trial Management group)
References
- 1.Medicines for Human Use (Clinical Trials) Regulations 2004. Available at http://www.opsi.gov.uk/si/si2004/20041031.htm. [PubMed]
- 2.Kielmann T, Tierney A, Porteous R, Huby G, Sheikh A, Pinnock H. The Department of Health's research governance framework remains an impediment to multi-centre studies: findings from a national descriptive study. J R Soc Med. 2007;100:234–8. doi: 10.1258/jrsm.100.5.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheard L, Tompkins CNE, Wright NMJ, Adams CE. Non-commercial clinical trials of a medicinal product: can they survive the current process of research approvals in the UK? J Med Ethics. 2006;32:430–4. doi: 10.1136/jme.2005.015180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salman RA, Brock TM, Dennis MS, Sandercock PAG, White PM, Warlow C. Research governance impediments to clinical trials: a retrospective survey. J R Soc Med. 2007;100:101–4. doi: 10.1258/jrsm.100.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamrozik K. Research ethics paperwork: what is the plot we seem to have lost? BMJ. 2004;329:286–7. doi: 10.1136/bmj.329.7460.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hearn J, Sullivan R. The impact of the Clinical Trials Directive on the cost and conduct of non-commercial cancer trials in the UK. Eur J Cancer. 2007;43:8–13. doi: 10.1016/j.ejca.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Elwyn G, Seagrove A, Thorne K, Cheung W-Y. Ethics and research governance in a multicentre study: add 150 days to your study protocol. BMJ. 2005;330:847. doi: 10.1136/bmj.330.7495.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galbraith N, Hawley C, De-Souza V. Research governance. Research governance approval is putting people off research. BMJ. 2006;332:238. doi: 10.1136/bmj.332.7535.238-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. http://www.rdforum.nhs.uk/docs/Bulletin_Dec06.pdf
- 10. http://www.rdforum.nhs.uk/docs/Bulletin_Aug07.pdf
- 11. http://www.rdforum.nhs.uk/iras.htm
- 12. http://www.rdforum.nhs.uk/docs/newsletters/news280906.htm
- 13. http://www.rdforum.nhs.uk/docs/newsletters/news311006.htm