Abstract
Aim and methods
The impact of medical comorbidity on the efficacy and tolerability of duloxetine in elderly patients with major depressive disorder (MDD) was investigated in this study. Data were obtained from a multicentre, randomised, double-blind, placebo-controlled study in 311 patients with MDD aged 65–89. The primary outcome measure was a prespecified composite cognitive score based on four cognitive tests: (i) Verbal Learning and Recall Test; (ii) Symbol Digit Substitution Test; (iii) 2-Digit Cancellation Test and (iv) Letter-Number Sequencing Test. Secondary measures included the Geriatric Depression Scale (GDS), 17-Item Hamilton Depression Scale (HAMD17), Clinical Global Impression-Severity (CGI-S) Scale, Visual Analogue Scale (VAS) for pain and 36-Item Short Form Health Survey (SF-36). Tolerability measures included adverse events reported as the reason for discontinuation and treatment-emergent adverse events (TEAEs). The consistency of the effect of duloxetine vs. placebo comparing patients with and without medical comorbidity (vascular disease, diabetes, arthritis or any of these) was investigated.
Results
Overall, duloxetine 60 mg/day demonstrated significantly greater improvement compared with placebo for the composite cognitive score, GDS and HAMD17 total scores, CGI-Severity, HAMD17 response and remission rates, and some of the SF-36 and VAS measures. There were few significant treatment-by-comorbidity subgroup interactions for these efficacy variables, or for adverse events reported as the reason for discontinuation and common TEAEs.
Conclusions
The present analyses suggested that the efficacy of duloxetine on cognition and depression in elderly patients, and its tolerability, were not largely affected by the comorbidity status. These results further support the use of duloxetine in elderly patients with MDD.
Disclosures
Dr Wise and Dr Iosifescu have served in consultant/advisory roles for this clinical trial. Dr Iosifescu has received honoraria from Eli Lilly and Company. Neither author has received financial support from Eli Lilly and Company in the form of research funding in the past 2 years. Dr Sheridan has no financial relationship with Eli Lilly and Company. At the time this study was completed, Dr Raskin, Dr Wiltse and Dr Xu were employees of and owned stock in Eli Lilly and Company.
What's known
Analyses of primary efficacy data from this elderly study have shown that duloxetine 60 mg/day improved cognitive function. Duloxetine 60 mg/day also produced significant improvements vs. placebo in the Geriatric Depression Scale and HAMD17 total scores, as well as some pain measures.
What's new
The present report investigates the impact of medical comorbidity on the efficacy of duloxetine in the treatment of depression, improvement of cognition, as well as quality of life and its tolerability in elderly patients with major depressive disorder.
Introduction
Major depressive disorder (MDD) is common in elderly patients, with an estimated prevalence of about 3% (1). It is often associated with physical disability and a high mortality rate (2,3). Elderly patients are more predisposed to depression than younger patients because of concurrent medical disorders, chronic pain, sadness secondary to life-cycle issues and social isolation (4). These conditions impair the quality of life in a number of ways, including social and vocational functioning, and emotional and physical well-being.
A large 4-year prospective study suggested that approximately 25% of patients ≥ 65 years with chronic medical illness suffer from depressive symptomatology (5). Substantial evidence supports the increased prevalence of depression in several chronic medical illnesses, including various forms of vascular disease (cardiovascular, cerebrovascular or peripheral vascular), diabetes mellitus and arthritis. Specifically, patients with cardiovascular disease, diabetes mellitus or arthritis appear to have approximately 2–3 times the risk for depression (6–8).
Conversely, depression tends to worsen comorbid medical illnesses and may lead to increased mortality. Depression in patients following myocardial infarction (MI) is a risk factor for mortality at 6 months of hospitalisation (9) and a significant predictor of 18-month post-MI cardiac mortality (10). Another study reported that depressive symptoms among women with HIV are associated with HIV disease progression (11). It has been found from the Systolic Hypertension in the Elderly Programme that depressive symptoms represent a significant risk factor for stroke, MI and death (12). Similarly, patients with depression and comorbid diabetes mellitus have higher risk for a poor adherence to diet and medication regimens, greater functional impairment and higher healthcare costs than their non-depressed counterparts (13).
Comorbidity of depression and medical illness in elderly patients leads to increased morbidity and mortality, as well as higher healthcare costs (14). Therefore, there is a need for a safe, well-tolerated and effective antidepressant in elderly patients with MDD, particularly in those suffering from MDD with chronic medical illness.
Duloxetine hydrochloride is an antidepressant that inhibits both serotonin (5-HT) and norepinephrine (NE) reuptake (15). The dual-acting mechanism of duloxetine makes it particularly interesting in the treatment of depression with cognitive impairment, as imbalance or deficiency in either 5-HT or NE systems has been found to contribute to cognitive deficits (16,17). Duloxetine has been shown to treat depression effectively in the elderly population on the basis of pooled subgroup analyses of two previous duloxetine clinical trials in general populations (18). To confirm the efficacy of duloxetine in elderly patients with MDD, a multicentre, parallel, double-blind, placebo-controlled study of 311 elderly patients ≥ 65 years with MDD was conducted (19).
Analyses of primary efficacy data from this elderly patient study have shown that duloxetine 60 mg/day improved cognitive function, as evidenced by significantly greater improvement in the composite cognitive score vs. placebo. Duloxetine 60 mg/day also showed significant improvements vs. placebo in the Geriatric Depression Scale (GDS) and 17-Item Hamilton Depression Scale (HAMD17) total scores, as well as some pain measures (19). The present report investigates the impact of medical comorbidity on the efficacy of duloxetine in cognition, depression and quality of life and its tolerability in elderly patients with MDD.
Methods
Study design
This multicentre, randomised, double-blind, placebo-controlled study was conducted in elderly patients with MDD. After a 1-week screening phase, all patients entered a 1-week, double-blind, placebo phase before being randomised to duloxetine 60 mg/day (n = 207) or placebo (n = 104) for 8 weeks. This was followed by a 1-week, double-blind, discontinuation phase in which the dosage of duloxetine was tapered to 30 mg/day for 4 days. Patients, investigators and all other personnel involved in the conduct of the study were blinded to individual treatment assignments for the duration of the study.
Patients
All patients in this study were ≥ 65 years of age and met diagnostic criteria for MDD as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (20). The diagnosis was confirmed by the Mini International Neuropsychiatric Interview (21), a standardised diagnostic interview based on DSM-IV criteria. Baseline disease severity was defined by patients’ scores on the HAMD17 (22). Patients were required to have: HAMD17 total score ≥ 18 at visits 1 and 2, a Mini Mental State Examination (MMSE) (23) score ≥ 20 with or without mild dementia and at least one previous episode of major depression. Patients with a MMSE score of 20–23 were categorised as having mild dementia, while those with a score of ≥ 24 were categorised as having no dementia. Administration of study drug and conduct of the study were in accordance with the Declaration of Helsinki. Each patient provided written informed consent prior to any study procedures.
Patients were excluded for the following reasons: current primary axis I diagnosis other than MDD or mild dementia (including dysthymia or psychotic depression); previous diagnosis of psychotic disorder; organic mental disorder, moderate-to-severe dementia or mental retardation diagnosis; serious or unstable medical illness, psychological condition or clinically significant laboratory abnormality that, in the opinion of the investigator, would compromise participation in this study or be likely to lead to hospitalisation during the course of the study; or ALT, AST or GGT > 1.5 times upper limit of normal, based on Eli Lilly and Company's reference ranges (24).
Efficacy and tolerability measures
The primary efficacy measure was a prespecified composite cognitive score based on four cognitive tests: (i) Verbal Learning and Recall Test (VLRT), adapted from the Rey Auditory Verbal Learning Test (25,26); (ii) Symbol Digit Substitution Test (SDST); (iii) 2-Digit Cancellation Test (2DCT) and (iv) Letter-Number Sequencing Test (LNST). These particular tests were selected because they assess aspects of cognition shown previously to be most impaired in patients with depression, specifically verbal learning and memory, attention, executive function and working memory (27,28). Moreover, each of these tests has been used extensively in clinical psychopharmacology.
Secondary measures included the GDS and HAMD17 total scores, HAMD17 response and remission, Visual Analogue Scale (VAS) for pain, Clinical Global Impression-Severity (CGI-S), and the 36-Item Short Form Health Survey (SF-36). HAMD17 response was defined as a ≥ 50% decrease in the HAMD17 total score from baseline to end-point. Remission was defined as a HAMD17 total score of ≤ 7 at end-point. Tolerability measures included adverse events (AEs) reported as the reason for discontinuation and treatment-emergent adverse events (TEAEs).
The composite cognitive score, ranging from 0 to 51, was defined as the sum of: (i) the average number of words recalled on the three learning trials of the VLRT (score 0–15) and the number of words recalled on the delayed recall test of the VLRT (score 0–15); (ii) the fraction of all possible targets correct on the SDST (number correct divided by 133) multiplied by 7 (score 0–7); (iii) the number of targets hit, minus the number incorrect, minus the number of times the patients had to be reminded of the task, divided by the possible number correct (40) on the 2DCT, multiplied by 7 (score 0–7, set to 0 if negative) and (iv) the total score on the LNST (0–21) divided by 3 (score 0–7).
The GDS scale was developed as a basic screening measure for elderly patients with MDD (29,30). The VAS quantitatively measures overall pain, headache, back pain, shoulder pain, pain interference with daily activities and time in pain while awake (31).
Cognition measures and the SF-36 were recorded once prior to randomisation and at the last visit of the acute treatment phase. The GDS, HAMD17, CGI-Severity and VAS were recorded once prior to randomisation and at every visit of the acute treatment phase.
Description of comorbidity
The medical comorbidity group included patients with one or any of the following three categories of illnesses: vascular (cardiovascular, cerebrovascular or peripheral vascular) disease, diabetes mellitus or arthritis. Medical comorbidities were recorded at baseline to indicate the presence of any of the above illness(es). Indications for comorbidity were based on physical examination, previous diagnosis or patient report.
Statistical analysis
All analyses were conducted on an intent-to-treat basis, which is an analysis of the groups to which patients are randomly assigned, even if the patient did not take the assigned treatment, did not receive the correct treatment or otherwise did not follow the protocol. Some analyses were defined a priori in the protocol; others were post hoc. The term ‘significant’ for treatment comparisons indicates statistical significance (two-sided p ≤ 0.05). No adjustments for multiple comparisons were made. Throughout this manuscript, ‘mean’ refers to the raw mean unless the least-squares (LS) mean is specified.
Unless otherwise specified, ‘baseline’ refers to the last non-missing prerandomisation observation and ‘end-point’ refers to the last non-missing observation during the treatment phase. Total scores (e.g. composite cognitive score, GDS and HAMD17 total scores) were considered to be missing if any of the item scores were missing.
Changes in continuous efficacy variables from baseline to end-point, overall and within subgroups, were analysed using a fixed-effects analysis of covariance (ANCOVA) model that included terms for treatment, investigator and baseline score. This is referred to as a mean change (MC) analysis. Categorical measures, overall and within subgroups, were analysed using Fisher's exact test.
The consistency of the effect of duloxetine compared with placebo in comorbidity subgroups, as described in the protocol, was investigated for continuous variables by adding the subgroup and treatment-by-subgroup interaction terms to the ANCOVA model. Corresponding consistency analyses for rates of HAMD17 response and remission were performed using a logistic regression model that included terms for treatment, subgroup and treatment-by-subgroup. The consistency for rates of AEs reported as the reason for discontinuation and for TEAEs in patients with comorbidity and without comorbidity were performed using the Breslow-Day test, which assesses the significance of treatment differences in incidence rates between subgroups. Interaction effects were tested at a 0.10 significance level.
TEAEs were defined as events that first occurred or worsened postrandomisation during the treatment phase when compared with the maximum prerandomisation severity. AEs were reported using preferred terms from the Medical Dictionary for Regulatory Activities (MedDRA, MMSO, Reston, VA, USA), version 7.0.
As specified in the protocol, 200 duloxetine- and 100 placebo-treated patients provided 80% power to detect an effect size (difference between MCs in the composite cognitive score divided by the common standard deviation) of 0.35, using a 5%, two-sided significance level and assuming data were available for analyses in 95% of patients. This reflects the power of the study with regard to the primary analysis.
Results
Patient characteristics
Baseline patient characteristics are summarised in Table 1. Overall, 55.3% of the patients had arthritis, 14.8% had diabetes, 36.0% had vascular comorbidity and 74.9% of patients had at least one of these comorbidities. As expected, mean age was higher in patients with vascular comorbidity vs. without vascular comorbidity and VAS overall pain severity was higher in patients with arthritis vs. without arthritis. Furthermore, patients with vascular comorbidity had more previous episodes of MDD and longer duration of the current episode of MDD; patients with diabetes had higher mean weight, which is clinically relevant and confirms the validity of the sample. It is worth noting that SF-36 physical component summary scores were lower in patients from all subgroups with medical comorbidities, suggesting that a presence of medical comorbidity is associated with worsened patient-reported physical well-being. Most of these characteristics for specific comorbidity also were reflected in the comparisons between those with or without any comorbidity.
Table 1.
Baseline patient characteristics by comorbidity
| Any | Arthritis | Diabetes | Vascular | |||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | No | Yes | |
| (n = 78) | (n = 233) | (n = 139) | (n = 172) | (n = 265) | (n = 46) | (n = 199) | (n = 112) | |
| Placebo: | 26 | 78 | 49 | 55 | 93 | 11 | 60 | 44 |
| Duloxetine: | 52 | 155 | 90 | 117 | 172 | 35 | 139 | 68 |
| Characteristics | ||||||||
| Gender, female, n (%) | 35 (44.9) | 150 (64.4) | 63 (45.3) | 122 (70.9) | 164 (61.9) | 21 (45.7) | 118 (59.3) | 67 (59.8) |
| Age, years, mean (SD) | 71.3 (5.3) | 73.4 (5.7) | 72.7 (5.6) | 73.0 (5.8) | 72.9 (5.8) | 73.0 (5.1) | 72.2 (5.7) | 74.1 (5.5) |
| Age range, years | 65–88 | 65–89 | 65–88 | 65–89 | 65–89 | 65–85 | 65–89 | 65–86 |
| Weight, kg, mean (SD) | 79.1 (17.4) | 80.4 (18.4) | 80.2 (16.1) | 80.0 (19.6) | 78.8 (18.0) | 87.5 (17.5) | 78.9 (18.0) | 82.1 (18.2) |
| Ethnicity, n (%) | ||||||||
| Caucasian | 60 (76.9) | 183 (78.5) | 105 (75.5) | 138 (80.2) | 210 (79.2) | 33 (71.7) | 154 (77.4) | 89 (79.5) |
| Hispanic | 13 (16.7) | 35 (15.0) | 25 (18.0) | 23 (13.4) | 37 (14.0) | 11 (23.9) | 35 (17.6) | 13 (11.6) |
| African descent | 3 (3.8) | 14 (6.0) | 6 (4.3) | 11 (6.4) | 15 (5.7) | 2 (4.3) | 8 (4.0) | 9 (8.0) |
| Western Asian | 1 (1.3) | 0 (0.0) | 1 (0.7) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 1 (0.5) | 0 (0.0) |
| Other | 1 (1.3) | 1 (0.4) | 2 (1.4) | 0 (0.0) | 2 (0.8) | 0 (0.0) | 1 (0.5) | 1 (0.9) |
| Psychiatric profile, mean (SD)* | ||||||||
| Composite Cognitive Score | 23.9 (8.0) | 22.5 (6.6) | 22.6 (7.3) | 23.0 (6.7) | 23.2 (7.1) | 21.1 (6.2) | 23.4 (7.5) | 21.9 (5.8) |
| HAMD17 total (visit 1) | 22.5 (3.4) | 22.2 (3.8) | 22.2 (3.6) | 22.3 (3.8) | 22.3 (3.7) | 21.9 (3.8) | 22.5 (3.8) | 21.7 (3.4) |
| HAMD17 total (randomisation) | 19.2 (5.0) | 18.7 (4.6) | 19.0 (4.7) | 18.7 (4.7) | 18.8 (4.7) | 19.4 (4.7) | 18.9 (5.0) | 18.7 (4.2) |
| GDS total | 17.5 (7.5) | 17.7 (7.0) | 17.4 (7.1) | 17.9 (7.1) | 17.6 (7.2) | 17.9 (6.8) | 18.1 (7.1) | 16.8 (7.1) |
| Duration of current episode, weeks | 59.8 (89.3) | 54.3 (85.3) | 61.8 (101.5) | 50.7 (71.4) | 56.9 (88.6) | 48.8 (71.6) | 50.3 (70.0) | 65.2 (108.7) |
| Number of previous episodes | 4.5 (6.3) | 5.8 (16.4) | 5.9 (17.7) | 5.1 (11.2) | 5.3 (13.9) | 6.4 (17.8) | 4.7 (9.9) | 6.7 (20.3) |
| VAS overall pain severity | 21.9 (21.6) | 34.4 (27.6) | 22.5 (21.7) | 38.3 (28.3) | 30.6 (26.3) | 35.0 (28.9) | 32.9 (27.7) | 28.3 (24.7) |
| SF-36 physical component | 46.0 (11.2) | 40.0 (10.3) | 44.6 (11.1) | 39.0 (9.8) | 42.3 (10.8) | 37.3 (9.9) | 42.5 (10.7) | 39.9 (10.9) |
| SF-36 mental component | 31.6 (12.3) | 32.4 (10.5) | 32.3 (12.0) | 32.2 (10.1) | 32.1 (10.8) | 33.0 (12.0) | 31.7 (11.1) | 33.1 (10.8) |
Total sample sizes for variables shown range from 304 to 311.
Efficacy in cognitive measures (primary)
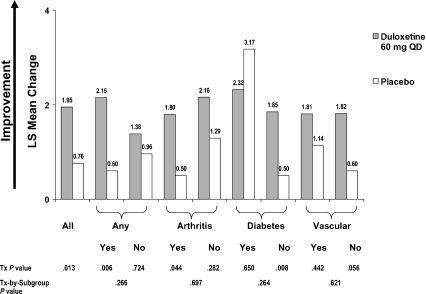
Figure 1 presents the MC from baseline in the composite cognitive score for all randomised patients and by subgroups based on presence or absence of the comorbidities. Duloxetine significantly improved cognitive performance compared with placebo in all randomised patients (1.95 vs. 0.76, p = 0.013). Duloxetine-treated patients showed significantly greater improvement compared with placebo-treated patients in the composite cognitive score for patients having any of the three medical comorbidities (2.15 vs. 0.60; p = 0.006). A significant difference did not occur for patients without a medical comorbidity (duloxetine, 1.38; placebo, 0.96; p = 0.724). However, there were no statistically significant treatment-by-comorbidity interactions for any comorbidity (p = 0.266) or for any of the individual comorbidities.
Figure 1.
Effects of duloxetine compared with placebo on composite cognitive score: mean change analysis in all randomised patients and in baseline comorbidity subgroups
Efficacy in depression measures
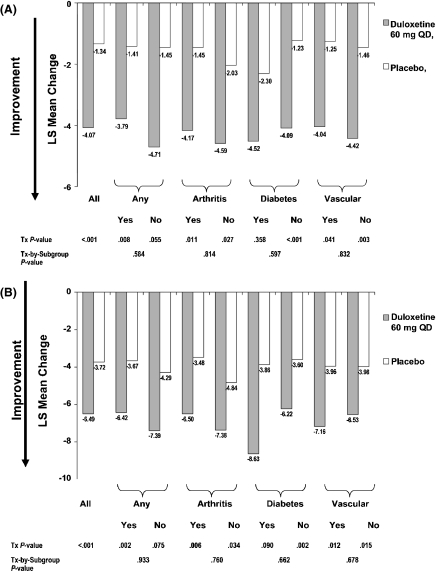
Results from the MC analyses of the depression efficacy measures are shown in Figure 2A,B. Patients treated with duloxetine had significantly greater improvement in both GDS and HAMD17 total scores than did patients treated with placebo. There were no statistically significant treatment-by-comorbidity interactions for either GDS or HAMD17 total scores. Moreover, CGI-S findings were consistent with findings for GDS and HAMD17 total scores (data not shown).
Figure 2.
(A) Effects of duloxetine compared with placebo on GDS total score: mean change analysis in all randomised patients and in baseline comorbidity subgroups. (B) Effects of duloxetine compared with placebo on HAMD17 total score: mean change analysis in all randomised patients and in baseline comorbidity subgroups
Duloxetine-treated patients had significantly greater rates of response (37.3% vs. 18.6%, p < 0.001) and remission (27.4% vs. 14.7%, p = 0.014) compared with placebo-treated patients. There were no statistically significant treatment-by-comorbidity interactions for either response or remission rate, suggesting that comorbidity status did not affect the treatment effect of duloxetine in achieving response or remission.
Efficacy in pain measures
In all randomised patients, there was a significantly greater improvement in VAS for back pain (−5.72 vs. 1.41, p = 0.008) and time in pain while awake (−6.36 vs. 0.99, p = 0.028) for duloxetine compared with placebo. The treatment difference for other pain measures, including overall pain, headache, shoulder pain and pain interference with daily activities, was not statistically significant. There were no statistically significant treatment-by-comorbidity interactions for headache and shoulder pain. However, there were statistically significant treatment-by-comorbidity interactions: overall pain for the arthritis (interaction, p = 0.037; with arthritis, duloxetine, −7.97; placebo, 1.29, p = 0.052; without arthritis, duloxetine, −1.27; placebo, −6.13, p = 0.241) and vascular (interaction, p = 0.077; with vascular disease, duloxetine, 1.81; placebo, 11.59, p = 0.059; without vascular disease, duloxetine, −7.79; placebo, −7.13, p = 0.868) comorbidities; interference with daily activities (interaction, p = 0.057; with arthritis, duloxetine, −4.85; placebo, 3.52, p = 0.067; without arthritis, duloxetine, −1.53; placebo, −6.75, p = 0.198) and back pain (interaction, p = 0.001; with arthritis, duloxetine, −8.79; placebo, 5.96, p < 0.001; without arthritis, duloxetine, −2.08; placebo, −6.64, p = 0.227) for arthritis comorbidity; and time in pain while awake for vascular comorbidity (interaction, p = 0.090; with vascular disease, duloxetine, −2.05; placebo, 10.01, p = 0.048; without vascular disease, duloxetine, −8.24; placebo, −5.30, p = 0.477). To each of these five significant interactions, duloxetine was more effective compared with placebo in patients with the comorbidity when compared with those without the comorbidity.
Efficacy in SF-36 improvement
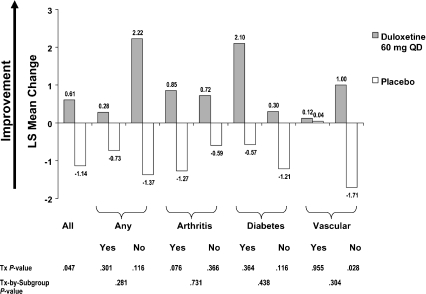
In all randomised patients, there was a significantly greater improvement with duloxetine treatment compared with placebo in the SF-36 physical component summary (0.61 vs. −1.14, p = 0.047), but not for the SF-36 mental component summary (8.33 vs. 6.18, p = 0.117). There were no statistically significant treatment-by-comorbidity interactions for the SF-36 physical component summary (Figure 3). However, there was a significant treatment-by-comorbidity interaction for the SF-36 mental component summary with respect to vascular comorbidity (p =0.077); a statistically significant benefit of duloxetine over placebo was shown for patients with vascular comorbidity (8.91 vs. 3.04, p = 0.015), but not for those without vascular comorbidity (8.17 vs. 7.85, p = 0.864). In addition, there was a significant treatment-by-comorbidity interaction (p = 0.044) for physical functioning for vascular comorbidity (with vascular disease, duloxetine, 1.96; placebo, 4.66, p = 0.519; without vascular disease, duloxetine, 4.76; placebo, −3.60, p = 0.005) and for social functioning (p = 0.016) for arthritis comorbidity (with arthritis, duloxetine, 16.58; placebo, 4.64, p = 0.004; without arthritis, duloxetine, 11.62; placebo, 15.44, p = 0.432).
Figure 3.
Effects of duloxetine compared with placebo on SF-36 Physical Component Summary: mean change analysis in all randomised patients and in baseline comorbidity subgroups
Tolerability
Tolerability was analysed in all randomised patients and by any comorbidity subgroups. Overall discontinuation rates for any reason did not significantly differ between the duloxetine and placebo groups (21.7% vs. 23.1%, p = 0.775). However, the rate of discontinuation caused by lack of efficacy was significantly greater among placebo-treated patients than duloxetine-treated patients (9.6% vs. 2.9%; p = 0.026). The incidence of discontinuation caused by AEs was similar in both treatment groups (9.7% vs. 8.7%, p = 0.839). Nausea was the only AE leading to discontinuation that occurred at a statistically significantly different rate in duloxetine-than in placebo-treated patients, and the rate of discontinuation caused by this AE was lower for duloxetine than for placebo (0.5% vs. 3.8%, p = 0.044). There were no significant treatment-by-comorbidity interactions for incidences of discontinuation because of an AE.
TEAEs for which the incidence among duloxetine-treated patients was at least 5.0% and twice the placebo rate are presented in Table 2. The incidence of at least one TEAE was similar for duloxetine and placebo (70.0% vs. 64.4%, p = 0.367) in overall patients; there was a statistically significant treatment-by-comorbidity interaction (p = 0.030) for any comorbidity. For patients with any comorbidity, the incidences were similar for the two treatment groups, but for patients with no comorbidity, the incidence was higher for duloxetine than for placebo (76.9% vs. 50.0%; p = 0.022). Of these TEAEs, those occurring significantly more frequently for duloxetine than for placebo in overall patients were dry mouth (14.5% vs. 1.9%; p < 0.001), nausea (12.6% vs. 3.8%; p = 0.014) and diarrhoea (8.2% vs. 1.9%; p = 0.042). There was no statistically significant treatment-by-comorbidity interaction for the incidence of any of the common TEAEs.
Table 2.
Treatment-emergent adverse events, overall and by any comorbidity subgroup
| Placebo | Duloxetine 60 mg QD | ||||||
|---|---|---|---|---|---|---|---|
| Event (≥ 5% and twice rate of placebo overall) | Comorbidity stratum | N | n (%) | N | n (%) | Treatment p-value* | Treatment-by-subgroup p-value† |
| Patients with ≥ 1 TEAEs | Overall | 104 | 67 (64.4) | 207 | 145 (70.0) | 0.367 | – |
| Yes | 78 | 54 (69.2) | 155 | 105 (67.7) | 0.882 | 0.030 | |
| No | 26 | 13 (50.0) | 52 | 40 (76.9) | 0.022 | ||
| Dry mouth | Overall | 104 | 2 (1.9) | 207 | 30 (14.5) | < 0.001 | – |
| Yes | 78 | 2 (2.6) | 155 | 23 (14.8) | 0.003 | 0.440 | |
| No | 26 | 0 (0.0) | 52 | 7 (13.5) | 0.088 | ||
| Nausea | Overall | 104 | 4 (3.8) | 207 | 26 (12.6) | 0.014 | – |
| Yes | 78 | 3 (3.8) | 155 | 19 (12.3) | 0.055 | 0.933 | |
| No | 26 | 1 (3.8) | 52 | 7 (13.5) | 0.257 | ||
| Constipation | Overall | 104 | 5 (4.8) | 207 | 21 (10.1) | 0.131 | – |
| Yes | 78 | 4 (5.1) | 155 | 12 (7.7) | 0.588 | 0.311 | |
| No | 26 | 1 (3.8) | 52 | 9 (17.3) | 0.151 | ||
| Dizziness | Overall | 104 | 3 (2.9) | 207 | 17 (8.2) | 0.087 | – |
| Yes | 78 | 3 (3.8) | 155 | 13 (8.4) | 0.275 | 0.345 | |
| No | 26 | 0 (0.0) | 52 | 4 (7.7) | 0.295 | ||
| Diarrhoea | Overall | 104 | 2 (1.9) | 207 | 17 (8.2) | 0.042 | – |
| Yes | 78 | 2 (2.6) | 155 | 12 (7.7) | 0.150 | 0.366 | |
| No | 26 | 0 (0.0) | 52 | 5 (9.6) | 0.163 | ||
| Fatigue | Overall | 104 | 3 (2.9) | 207 | 13 (6.3) | 0.279 | – |
| Yes | 78 | 1 (1.3) | 155 | 10 (6.5) | 0.105 | 0.135 | |
| No | 26 | 2 (7.7) | 52 | 3 (5.8) | 1.000 | ||
| Somnolence | Overall | 104 | 1 (1.0) | 207 | 11 (5.3) | 0.067 | – |
| Yes | 78 | 1 (1.3) | 155 | 7 (4.5) | 0.274 | 0.455 | |
| No | 26 | 0 (0.0) | 52 | 4 (7.7) | 0.295 | ||
Significant (p ≤ 0.05) within-stratum treatment comparison p-values are bolded if the treatment-by-subgroup p-value is statistically significant (p ≤ 0.10).
Significant (p ≤ 0.10) treatment-by-subgroup p-values are bolded.
Discussion
These results support the efficacy and tolerability of duloxetine in the treatment of elderly patients with MDD with or without common comorbid medical conditions. Overall, duloxetine-treated patients demonstrated significantly greater improvement compared with placebo-treated patients on outcome measures that included the composite cognitive score, depression severity measures and several of the SF-36 and VAS measures. Few significant treatment-by-comorbidity subgroup interactions occurred for these efficacy variables, or for AEs reported as the reason for discontinuation or common TEAEs, suggesting that the efficacy and tolerability of duloxetine in the treatment of depression in elderly patients were not largely affected by comorbidity status. Specifically, the results regarding the impact of comorbid medical illness on treatment outcomes in MDD were consistent regardless of whether the outcomes were measured with the GDS, the HAMD17 or CGI-S.
This study reaffirms the high prevalence of medical comorbidity in patients with late-life depression because nearly 75% of patients participating in the study met criteria for the presence of medical comorbidity. This proportion may be an underestimate, as patients with unstable acute medical illness were excluded from the study. Moreover, we measured only three common medical comorbidities; a higher prevalence of comorbidities likely would have been measured if more medical illnesses were recorded. Most of the findings in patient baseline characteristics were as expected; for instance, mean age was higher in patients with vascular comorbidity and VAS overall pain severity was higher in patients with arthritis. The fact that patients with vascular comorbidity had more previous episodes and longer duration of current episode of MDD may be attributed to certain vascular-related factors contributing to depression, including brain damage from infarcts or microvascular effects (6). Such factors are in line with the notion that physical illness and depression tend to have mutually worsening effects.
Although cognitive deficits in patients with MDD, especially the elderly patients, have been demonstrated in many studies (27), antidepressant drugs do not routinely improve cognition in such patients (32,33). Follow-up studies of patients treated for MDD have shown that some patients demonstrate poor performance on cognitive tests even after treatment (34). In the present study, duloxetine-treated patients demonstrated similar improvement on the composite cognitive score with or without a comorbid illness. However, even though the treatment-by-subgroup interaction was not significant, patients with any of the three comorbid illnesses and treated with duloxetine showed a significantly greater improvement on the composite cognitive score compared with the placebo group. In the subgroup without a comorbid illness, duloxetine did not show significantly greater improvement than did placebo. Although the explanation for this lesser improvement in the group without a comorbid illness is unknown, it could be an artifact as a result of the relatively small number of patients in that subgroup. Nevertheless, the augmenting effect of duloxetine on 5-HT and NE activity may make it particularly beneficial in treating the cognitive deficits associated with depression as imbalance or deficiency in either 5-HT or NE neurotransmission has been found to contribute to cognitive deficits (16,17). Treatment of depression with tricyclics, such as imipramine, has been found to improve cognitive function despite the detrimental anticholinergic effects (35).
The comorbidity of pain and depression in elderly patients is of special significance when considering the high prevalence of pain in this age group (36,37). A direct relationship between pain severity and depression has been identified in elderly patients with chronic pain (37). In the present analyses, duloxetine demonstrated statistically significant improvements in two of the six pain measures: the VAS for back pain and time in pain while awake. These findings suggest that duloxetine may be effective in relieving pain caused by chronic conditions, including arthritis and vascular disease. Duloxetine has been shown to be effective in the management of diabetic peripheral neuropathic pain (38,39) and in improving painful physical symptoms independent of improvement of depression severity in younger MDD populations (40). However, patients enrolled in this study were not selected specifically for pain and the pain reported was generally not severe. It is possible that more or less robust efficacy for duloxetine might be observed in patients with depression who have a higher baseline pain severity.
The present results are consistent with findings in acute treatment studies of antidepressants, including SSRIs, in medically ill patients with depression (41–45). A review of 18 studies in mostly non-elderly patients with depression and having at least one physical disorder found that antidepressant treatment was significantly better than placebo in improving depressive symptoms (42). In an 8-week study, older patients treated with sertraline had similar responses on the HAMD17 total score, CGI-S and CGI-I whether they had a comorbid medical illness or not (41). In a study by Small et al. (45), geriatric patients with major depression and with none, 1, 2, 3, 4 or ≥ 5 chronic physical illnesses were treated with fluoxetine 20 mg/day or placebo for 6 weeks. The analyses showed that the number of chronic illnesses did not have an influence on treatment outcomes. One outcome of interest from that study was the finding that patients with a greater number of illnesses had a greater response to fluoxetine, whereas the opposite was found with the placebo group, although the total number of patients was somewhat small in the subgroup with no chronic illnesses (N = 73). A similar finding was found with elderly patients with vascular illnesses treated with sertraline (44). In a pooled analysis of two studies, patients were placed into three groups: with hypertension, with vascular illness and no hypertension, and no hypertension or cardiovascular illness. The patients in the first two groups showed a consistently greater percentage of responders on the HAMD and CGI-I compared with the third group.
In the present study, the absolute response and remission rates were relatively low, which may be attributed to the short 8-week study duration, as well as the fixed dosing schedule. Nevertheless, the relative advantages of duloxetine in response and remission rates were convincing, as evidenced by the fact that they were twice the placebo rates and the differences were statistically significant. In general, depression trials in the elderly patients are more difficult to show positive efficacy results for active treatments over placebo than do trials in the general population (46). In a recent placebo-controlled study of fluoxetine and venlafaxine in patients ≥ 65 years with MDD, there were no significant differences among the three treatment groups in the change of HAMD21, MADRS or CGI scores, and the difference in the proportion of remitters at the last on-therapy visit was not statistically significant (47). Similarly, in a group of community-dwelling elderly patients ≥ 75 years with depression, citalopram was not more effective than placebo for the treatment of depression (48). However, in two larger studies in patients ≥ 60 years with depression, sertraline or fluoxetine was more effective than placebo (41,49). The remission rates in these studies were generally low, and the differences between treatment groups were small (20–35% for active treatments, 18–33% for placebo).
Duloxetine was well tolerated in patients with medical comorbidity as well as in patients with no medical comorbidity. More importantly, common TEAEs and discontinuation caused by AEs were comparable to those seen in younger, more general populations receiving duloxetine (50). This is also in line with what has been found with the SSRI sertraline (41). A study of patients with late-life depression and having vascular disease, diabetes mellitus or arthritis actually had a lower percentage of patients experiencing the most common TEAEs than did patients with no comorbid illnesses (41). In addition, the percentage of patients discontinuing for any reason, or specifically for AEs, were also lower in the comorbid illness group. In the pooled analysis by Krishnan et al. (44), there were also no differences between the groups with comorbid illness compared with the group with no comorbid illness in rates of TEAEs and in discontinuation caused by TEAEs.
A number of limitations should be kept in mind when evaluating the results of this study. First, the study excluded patients with acute and unstable medical conditions that are common among elderly populations. The 8-week study duration of the trial may be relatively short for a study in elderly patients. Roose and Sackeim (51) have suggested that a minimum of 12-week duration may be necessary to identify response or remission. Patients had limited-dose flexibility during the study, which may not be typical of clinical practice for an elderly population. Comorbid medical conditions could also be based on medical histories and patient report rather than a diagnosis by a physician during the physical examination. Finally, the number of patients was relatively small in the diabetes group.
In conclusion, results from this placebo-controlled trial support the hypothesis that duloxetine is effective and well tolerated in treating elderly patients with MDD. The duloxetine vs. placebo treatment effect on cognition, depression and quality-of-life in elderly patients with MDD was not largely affected by the presence or absence of one or more of the three medical comorbidities (vascular disease, diabetes and arthritis) that frequently occur in the elderly population.
Acknowledgments
The authors thank the Duloxetine Product Team, the many patients for their voluntary participation in this clinical trial and the principal investigators. The authors also thank the following staff at Eli Lilly and Company: Wenqi You, MS, and Barry Brolley, MS, for assistance with statistical analyses; Durisala Desaiah, PhD, Daniel Walker, PhD and Elizabeth Agostinelli, MA, for critical review of the manuscript and Sharie Sipowicz, BSc, for formatting assistance.
Funding
This work was sponsored by Eli Lilly and Company and Boehringer-Ingelheim GmbH. They provided the financial support for the study design, patient recruitment, data collection for this clinical trial and data analysis for this manuscript.
Author contributions
All authors accept full responsibility for the conduct of this trial, were given full access to all data from the trial, and participated in the decision to publish the data and preparation of this manuscript.
References
- 1.NIH consensus conference. Diagnosis and treatment of depression in late life. JAMA. 1992;268:1018–24. [PubMed] [Google Scholar]
- 2.Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psychiatry. 2000;57:601–7. doi: 10.1001/archpsyc.57.6.601. [DOI] [PubMed] [Google Scholar]
- 3.Conwell Y, Duberstein PR, Cox C, Herrmann JH, Forbes NT, Caine ED. Relationships of age and axis I diagnoses in victims of completed suicide: a psychological autopsy study. Am J Psychiatry. 1996;153:1001–8. doi: 10.1176/ajp.153.8.1001. [DOI] [PubMed] [Google Scholar]
- 4.Palsson S, Skoog I. The epidemiology of affective disorders in the elderly: a review. Int Clin Psychopharmacol. 1997;12(Suppl. 7):S3–13. doi: 10.1097/00004850-199712007-00002. [DOI] [PubMed] [Google Scholar]
- 5.Unutzer J, Patrick DL, Simon G, et al. Depressive symptoms and the cost of health services in HMO patients aged 65 years and older. A 4-year prospective study. JAMA. 1997;277:1618–23. doi: 10.1001/jama.1997.03540440052032. [DOI] [PubMed] [Google Scholar]
- 6.Ziegelstein RC. Depression in patients recovering from a myocardial infarction. JAMA. 2001;286:1621–7. doi: 10.1001/jama.286.13.1621. [DOI] [PubMed] [Google Scholar]
- 7.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–78. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 8.Dickens C, McGowan L, Clark-Carter D, Creed F. Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosom Med. 2002;64:52–60. doi: 10.1097/00006842-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Frasure-Smith N, Lesperance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA. 1993;270:1819–25. [PubMed] [Google Scholar]
- 10.Frasure-Smith N, Lesperance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91:999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- 11.Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285:1466–74. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 12.Wassertheil-Smoller S, Applegate WB, Berge K, et al. Change in depression as a precursor of cardiovascular events. SHEP Cooperative Research Group (Systoloc Hypertension in the elderly) Arch Intern Med. 1996;156:553–61. [PubMed] [Google Scholar]
- 13.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278–85. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- 14.Katon W, Ciechanowski P. Impact of major depression on chronic medical illness. J Psychosom Res. 2002;53:859–63. doi: 10.1016/s0022-3999(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 15.Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG, et al. Comparative affinity of duloxetine and venlafaxine for serotonin and norepinephrine transporters in vitro and in vivo, human serotonin receptor subtypes, and other neuronal receptors. Neuropsychopharmacology. 2001;25:871–80. doi: 10.1016/S0893-133X(01)00298-6. [DOI] [PubMed] [Google Scholar]
- 16.Ressler KJ, Nemeroff CB. Role of norepinephrine in the pathophysiology of neuropsychiatric disorders. CNS Spectr. 2001;6:670. doi: 10.1017/s1092852900001358. [DOI] [PubMed] [Google Scholar]
- 17.Reneman L, Lavalaye J, Schmand B, et al. Cortical serotonin transporter density and verbal memory in individuals who stopped using 3,4-methylenedioxymethamphetamine (MDMA or ‘ecstasy’): preliminary findings. Arch Gen Psychiatry. 2001;58:901–6. doi: 10.1001/archpsyc.58.10.901. [DOI] [PubMed] [Google Scholar]
- 18.Nelson JC, Wohlreich MM, Mallinckrodt CH, Detke MJ, Watkin JG, Kennedy JS. Duloxetine for the treatment of major depressive disorder in older patients. Am J Geriatr Psychiatry. 2005;13:227–35. doi: 10.1176/appi.ajgp.13.3.227. [DOI] [PubMed] [Google Scholar]
- 19.Raskin J, Wilse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164:900–9. doi: 10.1176/ajp.2007.164.6.900. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 21.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl. 20):22–33. [PubMed] [Google Scholar]
- 22.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Thompson WL, Brunelle RL, Enas GG, Simpson PJ. Routine laboratory tests in clinical trials: interpretation of results. J Clin Res Drug Dev. 1987;1:95–119. [Google Scholar]
- 25.Lezak M. Neuropsychological Assessment. New York: Oxford; 1995. pp. 438–45. [Google Scholar]
- 26.REY A. L'examen psychologique dans les cas d'encephalopathie traumatique [French] Arch Psychol. 1941;28:286–340. [Google Scholar]
- 27.Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull. 1995;117:285–305. doi: 10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- 28.Cohen RM, Weingartner H, Smallberg SA, Pickar D, Murphy DL. Effort and cognition in depression. Arch Gen Psychiatry. 1982;39:593–7. doi: 10.1001/archpsyc.1982.04290050061012. [DOI] [PubMed] [Google Scholar]
- 29.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 30.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press; 1986. pp. 165–73. [Google Scholar]
- 31.DeLoach LJ, Higgins MS, Caplan AB, Stiff JL. The visual analog scale in the immediate postoperative period: intrasubject variability and correlation with a numeric scale. Anesth Analg. 1998;86:102–6. doi: 10.1097/00000539-199801000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949–54. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- 33.Nebes RD, Pollock BG, Houck PR, et al. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res. 2003;37:99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien J, Ames D, Chiu E, Schweitzer I, Desmond P, Tress B. Severe deep white matter lesions and outcome in elderly patients with major depressive disorder: follow up study. BMJ. 1998;317:982–4. doi: 10.1136/bmj.317.7164.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sternberg DE, Jarvik ME. Memory functions in depression. Arch Gen Psychiatry. 1976;33:219–24. doi: 10.1001/archpsyc.1976.01770020055009. [DOI] [PubMed] [Google Scholar]
- 36.Andersson HI, Ejlertsson G, Leden I, Rosenberg C. Chronic pain in a geographically defined general population: studies of differences in age, gender, social class, and pain localization. Clin J Pain. 1993;9:174–82. doi: 10.1097/00002508-199309000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Turk DC, Okifuji A, Scharff L. Chronic pain and depression: role of perceived impact and perceived control in different age cohorts. Pain. 1995;61:93–101. doi: 10.1016/0304-3959(94)00167-D. [DOI] [PubMed] [Google Scholar]
- 38.Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005;6:346–56. doi: 10.1111/j.1526-4637.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 39.Wernicke JF, Pritchett YL, D'Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;24:1411–20. doi: 10.1212/01.wnl.0000240225.04000.1a. [DOI] [PubMed] [Google Scholar]
- 40.Brannan SK, Mallinckrodt CH, Brown EB, Wohlreich MM, Watkin JG, Schatzberg AF. Duloxetine 60 mg once-daily in the treatment of painful physical symptoms in patients with major depressive disorder. J Psychiatr Res. 2005;39:43–53. doi: 10.1016/j.jpsychires.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Sheikh JI, Cassidy EL, Doraiswamy PM, et al. Efficacy, safety, and tolerability of sertraline in patients with late-life depression and comorbid medical illness. J Am Geriatr Soc. 2004;52:86–92. doi: 10.1111/j.1532-5415.2004.52015.x. [DOI] [PubMed] [Google Scholar]
- 42.Gill D, Hatcher S. A systematic review of the treatment of depression with antidepressant drugs in patients who also have a physical illness. J Psychosom Res. 1999;47:131–43. doi: 10.1016/s0022-3999(99)00020-3. [DOI] [PubMed] [Google Scholar]
- 43.Evans M, Hammond M, Wilson K, Lye M, Copeland J. Treatment of depression in the elderly: effect of physical illness on response. Int J Geriatr Psychiatry. 1997;12:1189–94. [PubMed] [Google Scholar]
- 44.Krishnan KR, Doraiswamy PM, Clary CM. Clinical and treatment response characteristics of late-life depression associated with vascular disease: a pooled analysis of two multicenter trials with sertraline. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:347–61. doi: 10.1016/s0278-5846(00)00168-8. [DOI] [PubMed] [Google Scholar]
- 45.Small GW, Birkett M, Meyers BS, Koran LM, Bystritsky A, Nemeroff CB. Impact of physical illness on quality of life and antidepressant response in geriatric major depression. Fluoxetine Collaborative Study Group. J Am Geriatr Soc. 1996;44:1220–5. doi: 10.1111/j.1532-5415.1996.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 46.Walsh BT, Sysko R. Placebo control groups in trials of major depressive disorder among older patients. J Clin Psychopharmacol. 2005;25(4 Suppl. 1):S29–33. doi: 10.1097/01.jcp.0000162810.76947.bf. [DOI] [PubMed] [Google Scholar]
- 47.Schatzberg A, Roose S. A double-blind, placebo-controlled study of venlafaxine and fluoxetine in geriatric outpatients with major depression. Am J Geriatr Psychiatry. 2006;14:361–70. doi: 10.1097/01.JGP.0000194645.70869.3b. [DOI] [PubMed] [Google Scholar]
- 48.Roose SP, Sackeim HA, Krishnan KR, et al. Antidepressant pharmacotherapy in the treatment of depression in the very old: a randomized, placebo-controlled trial. Am J Psychiatry. 2004;161:2050–9. doi: 10.1176/appi.ajp.161.11.2050. [DOI] [PubMed] [Google Scholar]
- 49.Tollefson GD, Bosomworth JC, Heiligenstein JH, Potvin JH, Holman S. A double-blind, placebo-controlled clinical trial of fluoxetine in geriatric patients with major depression. The Fluoxetine Collaborative Study Group. Int Psychogeriatr. 1995;7:89–104. doi: 10.1017/s1041610295001888. [DOI] [PubMed] [Google Scholar]
- 50.Goldstein DJ, Lu Y, Detke MJ, Hudson JI, Demitrack MA. Effects of duloxetine on painful physical symptoms associated with depression. Psychosomatics. 2004;45:17–28. doi: 10.1176/appi.psy.45.1.17. [DOI] [PubMed] [Google Scholar]
- 51.Roose SP, Sackeim HA. Clinical trials in late-life depression: revisited. Am J Geriatr Psychiatry. 2002;10:503–5. [PubMed] [Google Scholar]