Abstract
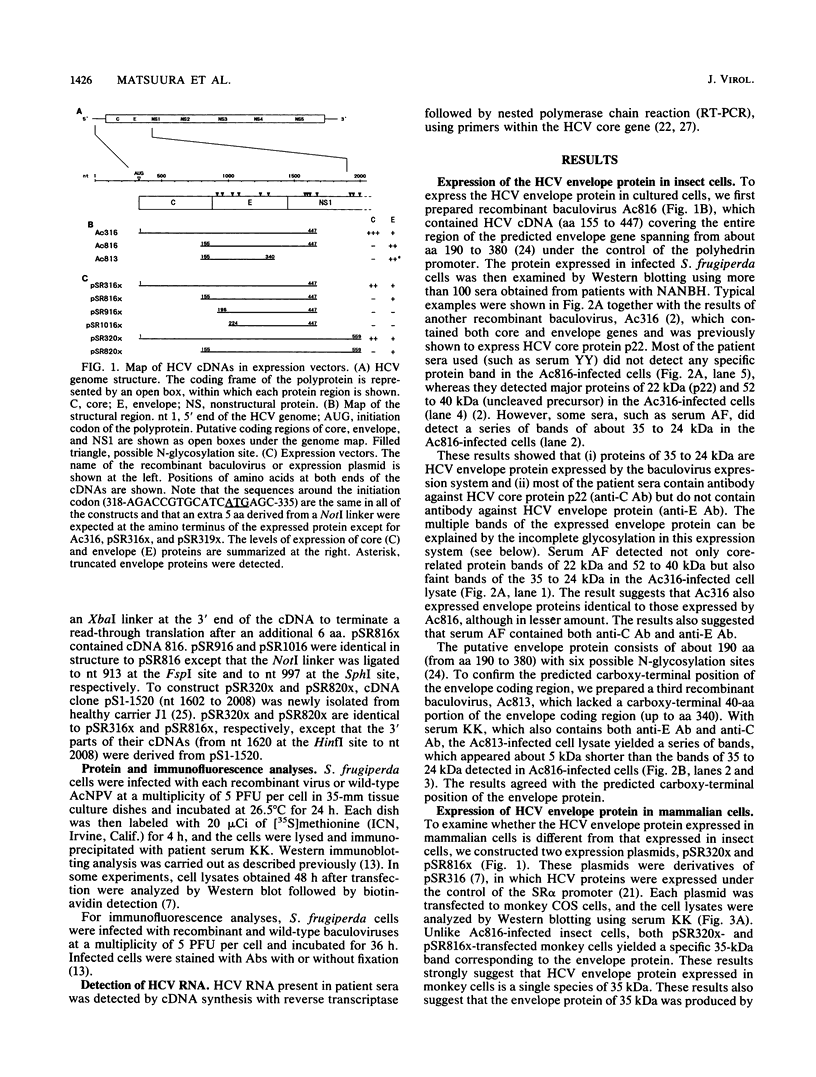
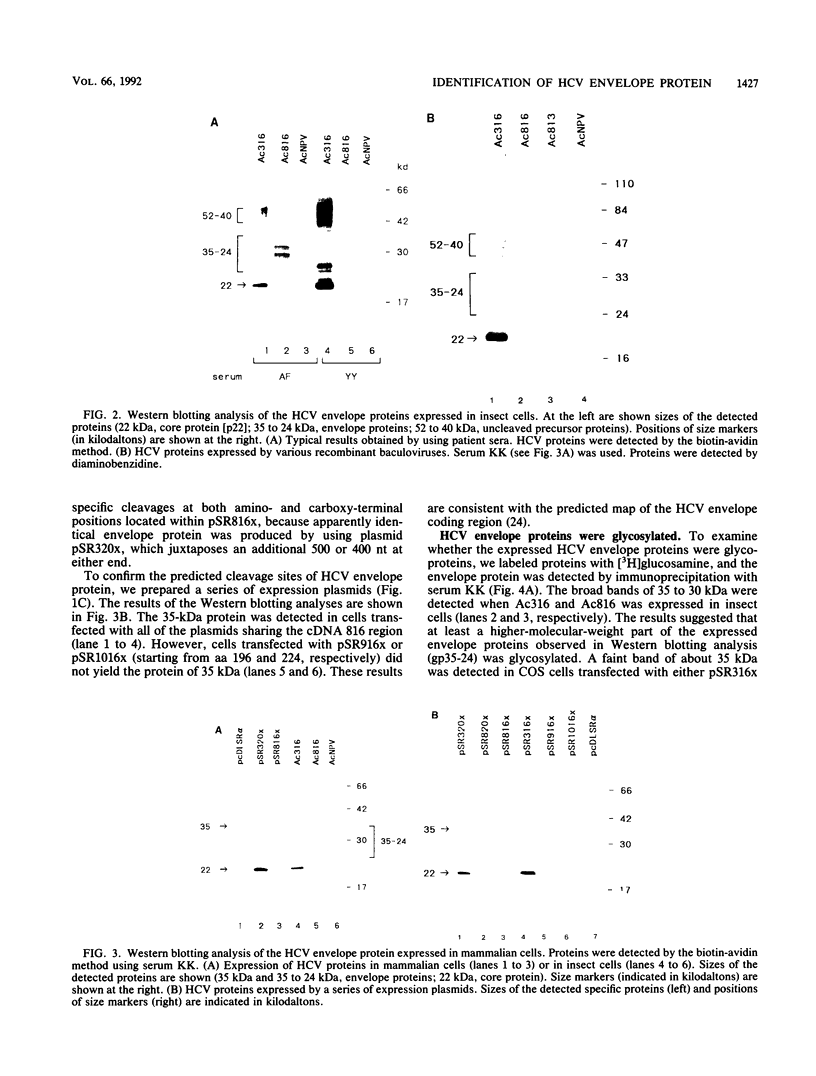
The putative envelope protein of hepatitis C virus (HCV) was expressed in insect cells by using a baculovirus expression vector and in monkey COS cells under the control of exogenous promoters. The expressed envelope proteins, identified by immunoblot analysis using sera from patients with chronic HCV infection, were a series of glycoproteins of 35 to 24 kDa (gp35-24) in insect cells and a single species of glycoprotein of 35 kDa (gp35) in monkey cells. The size difference of these proteins was due to the different degrees of glycosylation. The envelope proteins expressed in these cells were produced by common specific cleavage from the precursor protein, and cleavage positions of the envelope protein were mapped at about amino acids 190 and 380. The gp35-24 proteins expressed in insect cells were used for detection of antibody against HCV envelope protein in patient sera. The results showed that (i) the antibody is detected in 2 to 17% of various patients with hepatitis C, (ii) three patients were apparently cured after acquiring the antienvelope antibody, and (iii) in sera of patients with more than a 20-year history of infection, the antibody sometimes coexisted with HCV. These results suggest that the antienvelope antibody is neutralizing only in limited number of patients with hepatitis C.
Full text
PDF

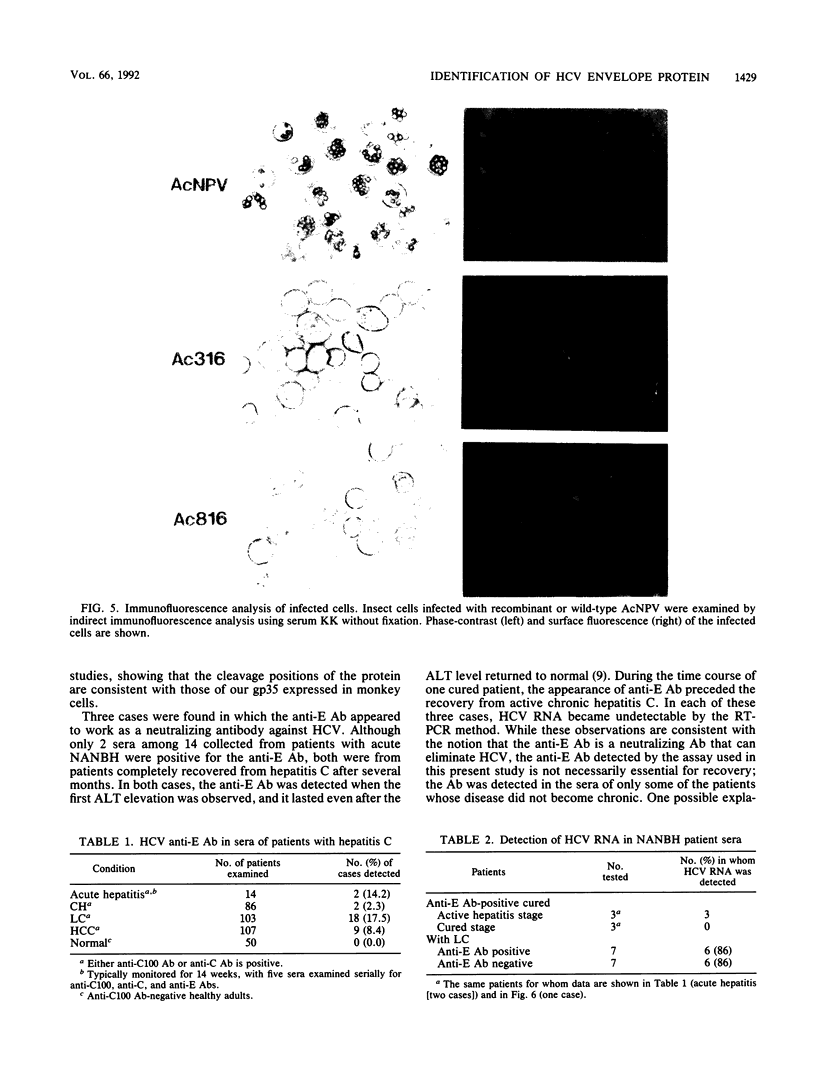
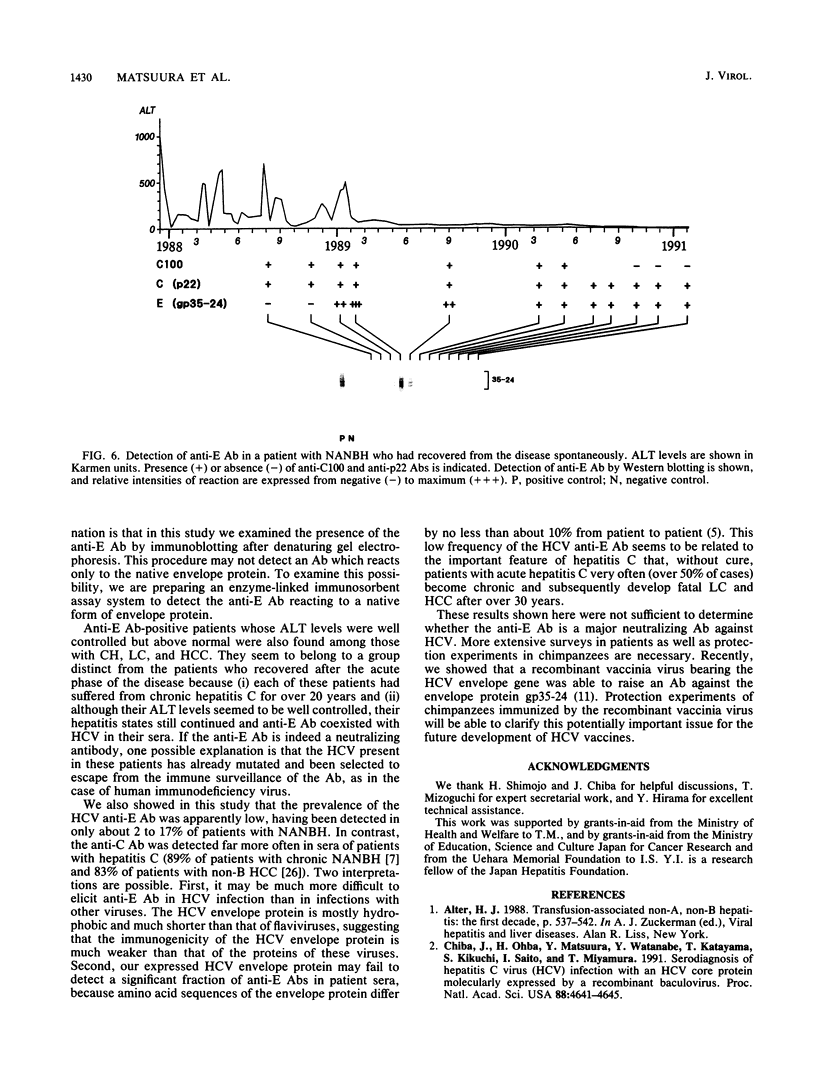

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chiba J., Ohba H., Matsuura Y., Watanabe Y., Katayama T., Kikuchi S., Saito I., Miyamura T. Serodiagnosis of hepatitis C virus (HCV) infection with an HCV core protein molecularly expressed by a recombinant baculovirus. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4641–4645. doi: 10.1073/pnas.88.11.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Richman K. H., Han J. H., Berger K., Lee C., Dong C., Gallegos C., Coit D., Medina-Selby R., Barr P. J. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou W. H., Yoneyama T., Takeuchi K., Harada H., Saito I., Miyamura T. Discrimination of hepatitis C virus in liver tissues from different patients with hepatocellular carcinomas by direct nucleotide sequencing of amplified cDNA of the viral genome. J Clin Microbiol. 1991 Dec;29(12):2860–2864. doi: 10.1128/jcm.29.12.2860-2864.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Harada S., Watanabe Y., Takeuchi K., Suzuki T., Katayama T., Takebe Y., Saito I., Miyamura T. Expression of processed core protein of hepatitis C virus in mammalian cells. J Virol. 1991 Jun;65(6):3015–3021. doi: 10.1128/jvi.65.6.3015-3021.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijikata M., Kato N., Ootsuyama Y., Nakagawa M., Shimotohno K. Gene mapping of the putative structural region of the hepatitis C virus genome by in vitro processing analysis. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5547–5551. doi: 10.1073/pnas.88.13.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Hijikata M., Ootsuyama Y., Nakagawa M., Ohkoshi S., Sugimura T., Shimotohno K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Miyamoto M., Sato T., Morita C., Yasui K. Characterization of Japanese encephalitis virus envelope protein expressed by recombinant baculoviruses. Virology. 1989 Dec;173(2):674–682. doi: 10.1016/0042-6822(89)90580-1. [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Possee R. D., Overton H. A., Bishop D. H. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J Gen Virol. 1987 May;68(Pt 5):1233–1250. doi: 10.1099/0022-1317-68-5-1233. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Purcell R. H. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviviruses as well as members of two plant virus supergroups. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2057–2061. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamura T., Saito I., Katayama T., Kikuchi S., Tateda A., Houghton M., Choo Q. L., Kuo G. Detection of antibody against antigen expressed by molecularly cloned hepatitis C virus cDNA: application to diagnosis and blood screening for posttransfusion hepatitis. Proc Natl Acad Sci U S A. 1990 Feb;87(3):983–987. doi: 10.1073/pnas.87.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Yotsumoto S., Tanaka T., Yoshizawa H., Tsuda F., Miyakawa Y., Mayumi M. The 5'-terminal sequence of the hepatitis C virus genome. Jpn J Exp Med. 1990 Jun;60(3):167–177. [PubMed] [Google Scholar]
- Saito I., Miyamura T., Ohbayashi A., Harada H., Katayama T., Kikuchi S., Watanabe Y., Koi S., Onji M., Ohta Y. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6547–6549. doi: 10.1073/pnas.87.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito I., Stark G. R. Charomids: cosmid vectors for efficient cloning and mapping of large or small restriction fragments. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8664–8668. doi: 10.1073/pnas.83.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamizawa A., Mori C., Fuke I., Manabe S., Murakami S., Fujita J., Onishi E., Andoh T., Yoshida I., Okayama H. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol. 1991 Mar;65(3):1105–1113. doi: 10.1128/jvi.65.3.1105-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K., Boonmar S., Kubo Y., Katayama T., Harada H., Ohbayashi A., Choo Q. L., Kuo G., Houghton M., Saito I. Hepatitis C viral cDNA clones isolated from a healthy carrier donor implicated in post-transfusion non-A, non-B hepatitis. Gene. 1990 Jul 16;91(2):287–291. doi: 10.1016/0378-1119(90)90102-w. [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Kubo Y., Boonmar S., Watanabe Y., Katayama T., Choo Q. L., Kuo G., Houghton M., Saito I., Miyamura T. Nucleotide sequence of core and envelope genes of the hepatitis C virus genome derived directly from human healthy carriers. Nucleic Acids Res. 1990 Aug 11;18(15):4626–4626. doi: 10.1093/nar/18.15.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K., Kubo Y., Boonmar S., Watanabe Y., Katayama T., Choo Q. L., Kuo G., Houghton M., Saito I., Miyamura T. The putative nucleocapsid and envelope protein genes of hepatitis C virus determined by comparison of the nucleotide sequences of two isolates derived from an experimentally infected chimpanzee and healthy human carriers. J Gen Virol. 1990 Dec;71(Pt 12):3027–3033. doi: 10.1099/0022-1317-71-12-3027. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Harada S., Saito I., Miyamura T. Prevalence of antibody against the core protein of hepatitis C virus in patients with hepatocellular carcinoma. Int J Cancer. 1991 May 30;48(3):340–343. doi: 10.1002/ijc.2910480305. [DOI] [PubMed] [Google Scholar]
- Yuasa T., Ishikawa G., Manabe S., Sekiguchi S., Takeuchi K., Miyamura T. The particle size of hepatitis C virus estimated by filtration through microporous regenerated cellulose fibre. J Gen Virol. 1991 Aug;72(Pt 8):2021–2024. doi: 10.1099/0022-1317-72-8-2021. [DOI] [PubMed] [Google Scholar]