Abstract
Objective: To assess the effect of angiotensin II type 1 (AT1) receptor antagonist losartan on myocardium connexin43 (Cx43) gap junction (GJ) expression in spontaneously hypertensive rats (SHRs) and investigate possible mechanisms. Methods: Sixteen 9-week-old male SHRs and 8 age-matched male Wistar-Kyoto (WKY) rats were included in this study. SHRs were randomly divided into two groups to receive losartan at 30 mg/(kg·d) by oral gavage once daily for 8 weeks (SHR-L) or vehicle (0.9% saline) to act as controls (SHR-V); WKY rats receiving vehicle for 8 weeks served as normotensive controls. At the end of the experiment, rats were sacrificed and the hearts were removed. Expressions of Cx43 and nuclear factor-kappaB p65 (NF-κB p65) proteins in all three groups were observed and further investigations on the effect of angiotensin II type 1 receptor antagonist losartan (30 mg/(kg·d), 8 weeks) on Cx43 expression were conducted with Western blot and immunohistochemistry. NF-κB p65 protein in nuclear extracts was determined by Western blot. Results: Left ventricular (LV) hypertrophy was prominent in SHRs, Cx43 and NF-κB p65 protein expressions were obviously upregulated and Cx43 distribution was dispersed over the cell surface. Treatment with losarton reduced the over-expressions of Cx43 and NF-κB p65 in LV myocardium. The distribution of Cx43 gap junction also became much regular and confined to intercalated disk after losartan treatment. Conclusion: Cx43 level was upregulated in LV myocardium of SHR during early stage of hypertrophy. Angiotensin II type 1 receptor antagonist losartan prevented Cx43 gap junction remodeling in hypertrophied left ventricles, possibly through the NF-κB pathway.
Keywords: Connexin43 (Cx43), Left ventricular (LV) hypertrophy, Angiotensin II, Nuclear factor-kappaB p65 (NF-κB p65), Gap junction (GJ)
INTRODUCTION
Cardiac hypertrophy is a fundamental process of adaptation to an increased workload due to hemodynamic overload (Mondry and Svynghedauw, 1995). Development of cardiac hypertrophy is initially beneficial; however, chronic hypertrophy is associated with a significant increase in risks of heart failure, dilated cardiomyopathy, ischemic heart disease, and sudden death (Vakili et al., 2001). Hypertension-related cardiac changes were documented in spontaneously hypertensive rats (SHRs). In SHRs, left ventricular (LV) hypertrophy resembles the changes observed in patients with hypertension, and antihypertensive drugs may protect the heart from LV hypertrophy. Angiotensin receptor blockers (ARBs) have increasingly become part of the first line of treatment against hypertensive diseases, and losartan was shown to improve cardiovascular morbidity and mortality in patients with isolated systolic hypertension and LV hypertrophy (Dahlof et al., 2002).
Cardiac myocytes are connected with each other electrically and metabolically through gap junction (GJ) channels composed of connexins. Many studies have described alterations in the abundance or subcellular localization of connexin proteins under a variety of pathological conditions, including hypertrophy, heart failure, ischemic heart diseases, and atrial fibrillation (Severs et al., 2004). Connexin43 (Cx43) is expressed most abundantly in the heart. During the process of cardiac hypertrophic remodeling, Cx43 GJs also display altered expressions responding to a variety of regulatory processes. Some researches demonstrated that angiotensin II could activate the expression of Cx43 in the myocardial cells of rats in vitro (Dodge et al., 1998). Recent studies showed that nuclear factor-kappaB (NF-κB) plays a necessary role for myocyte hypertrophy in vitro and in vivo (Gupta et al., 2002; Hirotani et al., 2002; Purcell et al., 2001). The present study aimed at exploring the change of Cx43 expression in LV myocardium of SHR during early stage of hypertrophy, and investigating the effect of losartan on Cx43 expression and possible mechanisms of GJ expression.
MATERIALS AND METHODS
Animal models
All animal studies were approved by local veterinary authorities. Sixteen 9-week-old male SHRs (210~220 g) and 8 age-matched male Wistar-Kyoto (WKY) rats (175~200 g), purchased from Medical Laboratory Animal Center (Grade II, Certificate No. 2003-0003, Shanghai, China), were used in this study. They were housed under constant ambient temperature and humidity and in a light-dark cycle of 12 h. SHRs were randomly divided into two groups to receive losartan at 30 mg/(kg·d) by oral gavage once daily for 8 weeks (SHR-L) or vehicle (0.9% (w/v) saline) as controls (SHR-V); WKY rats receiving vehicle for 8 weeks served as normotensive controls (WKY-V). Each group consisted of 8 rats. Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.). At the end of the experiment, rats were sacrificed and the hearts were removed.
Determination of plasma brain natriuretic peptide (BNP) levels
At the end of the experiment, blood samples were collected from carotid artery and plasma BNP concentrations were measured by using an ELISA diagnostic kit (ADR Co., USA).
Measurement of blood pressure (BP)
Systolic BP was measured at the beginning, 2nd, 4th and 8th weeks of the experiment when all animals were in conscious conditions. Ambient temperature was maintained at 30 °C. The animals were acclimated to the restraining cages and tail-cuff apparatus before BP was determined. The standard non-invasive tail-cuff method was used.
Determination of LV hypertrophy by pathological analysis
The left ventricle (including interventricular septum) samples were weighed after the right and left atria and right ventricular free wall were dissected. The LV mass index was calculated by dividing the LV weight by the body weight in each animal. For histopathological investigation, a transmural specimen of the LV myocardium obtained from the free wall at the medium of the left ventricle was fixed in 4% (v/v) paraformaldehyde in phosphate buffered saline (PBS), pH 7.4, and postfixed overnight at 4 °C in the same fixative. Tissue blocks were rinsed for 1 h with PBS and dehydrated through a series of increasing concentrations of ethanol. After dehydration, they were cleared with chloroform and xylene, and embedded in paraffin. Part of each block was sectioned and stained with hematoxylin-eosin (HE) to assess gross cardiac microanatomy.
Determination of Cx43 expression by immunohistochemistry
Part of each sample embedded in paraffin was sectioned into 5-μm-thick slides. The sections were deparaffinized, and endogenous peroxidase activity was quenched by incubation with 0.3% (v/v) hydrogen peroxide in methanol for 10 min at room temperature. The sections were then incubated with anti-Cx43 monoclonal antibody (Zymed Laboratories Inc., South San Francisco, CA, USA) at a dilution of 1:200 in blocking buffer (2% (w/v) bovine serum albumin in PBS) at room temperature overnight. After being washed with PBS, the sections were incubated with a secondary antibody labeled with peroxidase (Histofine simple-stain kit, Nichivei, Japan) for 30 min. Finally, the sections were visualized with 3.3′-diamionbenzidine and hydrogen, and counterstained with hematoxylin.
Determination of Cx43 and NF-κB p65 expressions by Western blot analysis
Total proteins were extracted from the LV tissues homogenates. Nuclear proteins were extracted by using NE-PER nuclear extraction reagents (Pierce Biotechnology, Rockford, IL, USA). The protein concentrations were determined by the Lowry method. Equal amounts of proteins were loaded on each lane and resolved on a 12% (w/v) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred electrically to nitrocellulose membranes. The blots were blocked with tris-buffered saline (TBS) containing 5% (w/v) dry milk and 0.05% (v/v) Tween-20, and probed with monoclonal anti-mouse Cx43 antibody (Zymed Laboratories Inc., South San Francisco, CA, USA) at 1:2000 dilution or with polyclonal anti-rabbit NF-κB p65 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:500 dilution overnight at 4 °C. Subsequently, blots were incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse antibodies (Beijing Zhongshan Biotechnology Ltd., China) at 1:5000 dilution for 1 h at 37 °C. The blots were incubated for 1 min in enhanced chemiluminescent solution (ECL) (Beit Haemek Ltd., Kibbutz Beit Haemek, Israel) and exposed to X-ray film. The intensity of bands was quantified by densitometry and normalized by glyceraldehyde phosphate dehydrogenase (GAPDH).
Statistical analysis
Data were presented as mean±SD. For multiple comparisons we used one-way analysis of variance (ANOVA), followed by Scheffe’s test. P-values less than 0.05 were considered as statistically significant. All data were analyzed by using SPSS 11.5 statistical package (SPSS Inc., Chicago, Illinois, USA).
RESULTS
Changes of systolic blood pressure (SBP)
SBP was significantly elevated in SHRs aged 9 weeks, and SHR-L and SHR-V groups showed similar high SBP at the beginning of the experiment compared with WKY-V group (P<0.001). In both SHR-L and SHR-V groups, SBP increased constantly over the course, while the one in WKY-V group remained unchanged. After treatment with losartan, SBP decreased significantly in SHR-L group compared with SHR-V group (P<0.001), and became even lower than that in WKY-V group at the end of the experiment (Table 1).
Table 1.
Changes of systolic blood pressure (SBP) during the experiment
| Group | SBP (mmHg) |
|||
| Base line | At 2nd week | At 4th week | At 8th week | |
| WKY-V | 131±11 | 133±10 | 134±12 | 137±9 |
| SHR-V | 172±9** | 180±8** | 186±12** | 195±17** |
| SHR-L | 174±8** | 130±5## | 134±17## | 126±9## |
Data were presented as mean±SD, n=7~8.
P<0.001 vs WKY-V group;
P<0.001 vs SHR-V group
LV hypertrophy and plasma BNP levels of different groups
By the end of the experiment, ratios of the left ventricular weight (LVW) to body weight (BW) were similar in SHR-L and WKY-V groups, but increased significantly in SHR-V group compared with SHR-L group (P<0.001). Plasma BNP level in SHR-V group was significantly higher than those in WKY-V and SHR-L groups (P<0.001) (Table 2).
Table 2.
Influence of losartan on left ventricular (LV) hypertrophy and plasma BNP level
| Group | N | BW (g) | LVW (g) | LVW/BW (mg/g) | BNP (ng/L) |
| WKY-V | 7 | 242.86±17.99 | 0.62±0.07 | 2.58±0.32 | 18±5 |
| SHR-V | 7 | 267.14±29.70 | 0.95±0.16** | 3.58±0.48** | 53±17 ** |
| SHR-L | 8 | 272.50±20.18 | 0.64±0.06## | 2.34±0.10## | 19±9## |
Data were presented as mean±SD. BW: Body weight; LVW: Left ventricular weight; BNP: Brain natriuretic peptide.
P<0.001 vs WKY-V group;
P<0.001 vs SHR-V group
Determination of LV hypertrophy by pathological analysis
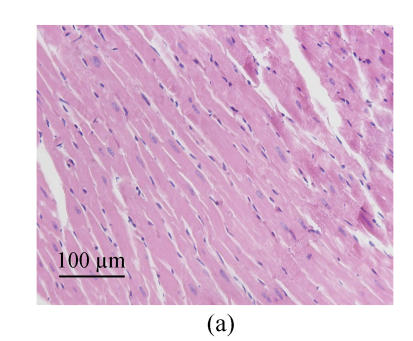
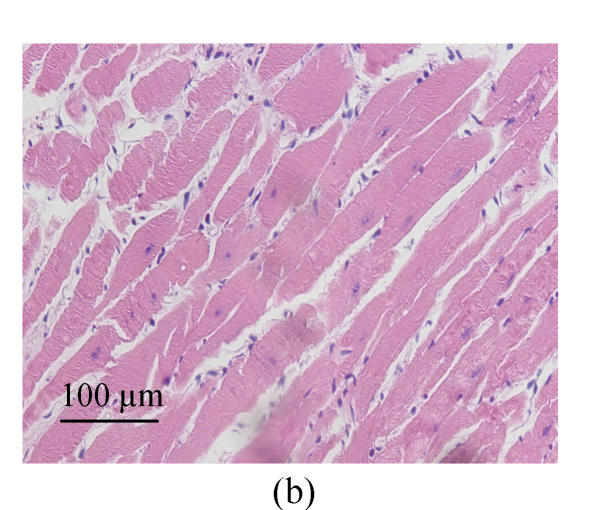
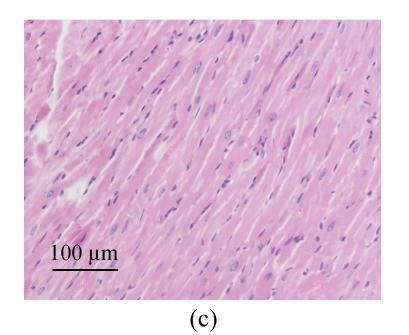
We estimated cell hypertrophy on HE stained sections. Increased sizes of cardiocytes were observed in SHR-V group (Fig.1b) in comparison with those in normotensive WKY-V group (Fig.1a). This phenomenon was countered by SHR-L group (Fig.1c). Myofibril disarray was also observed in LV tissue sections in SHR-V group. However, after treatment with losartan, LV cardiocytes arranged more regularly than untreated group.
Fig. 1.
Pathological changes of left ventricular cardiocytes in three groups (HE stain). (a) In WKY-V group, cardiocytes with normal size were regular; (b) In SHR-V group, the increased sizes of cardiocytes and myofiber disarray were prominent; (c) In SHR-L group, ventricular cardiocytes with decreased size after treatment with 30 mg/(kg·d) losartan were more regular than those in untreated group
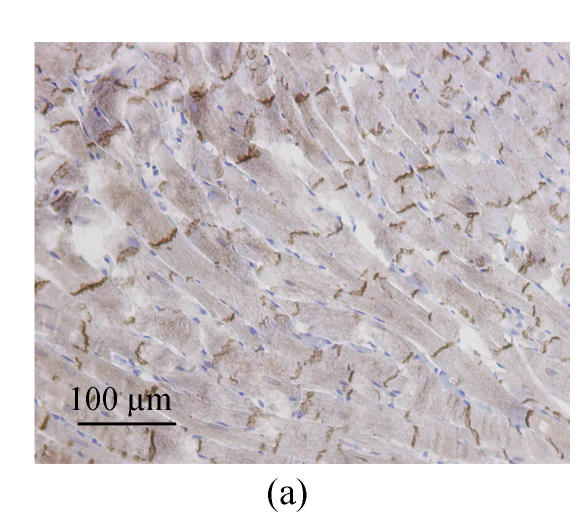
Determination of Cx43 expression by immunohistochemistry
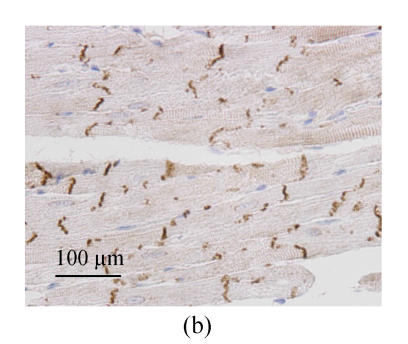
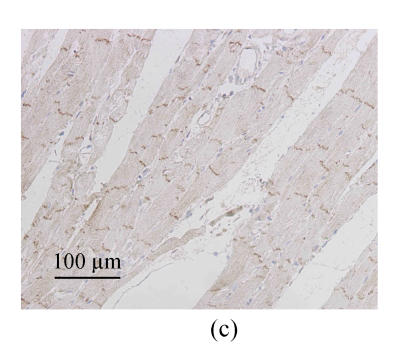
Immunohistochemistry analysis showed the distributions of immunolabeled Cx43 GJ in longitudinally sectioned LV myocardium. In WKY-V group, the Cx43 GJs were visualized as highly organized brown labels clustered at the intercalated disks running across the longitudinal axis (Fig.2a). The Cx43 staining patterns of SHR-V group differed markedly from the controls. In SHR-V group, the gap junctional labeling was no longer confined to the intercalated disks but showed varying degrees of dispersion over the cell surface (Fig.2b). A parallel myofibril arrangement was generally preserved in the hypertrophied myocardium, but constituent myocytes showed more complex and irregular configurations than control myocardium. In contrast, the Cx43 labeling pattern in SHR-L group was largely confined to the intercalated disk structure running transversely to the myocardial fiber orientation (Fig.2c).
Fig. 2.
Changes of spatial organization of Cx43 in LV myocardium determined by immunohistochemistry. (a) In WKY-V group, the distribution of Cx43 was homogeneous in normal ventricular myocardium; (b) In SHR-V group, the distribution of Cx43 was heterogeneous; (c) In SHR-L group, the distribution of Cx43 was much regular after treatment with 30 mg/(kg·d) losartan
Determination of Cx43 and NF-κB p65 expressions by Western blot analysis
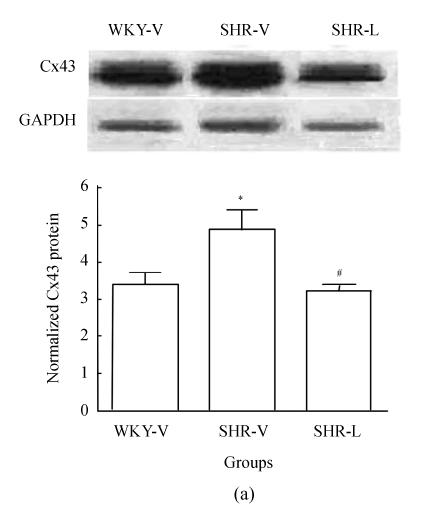
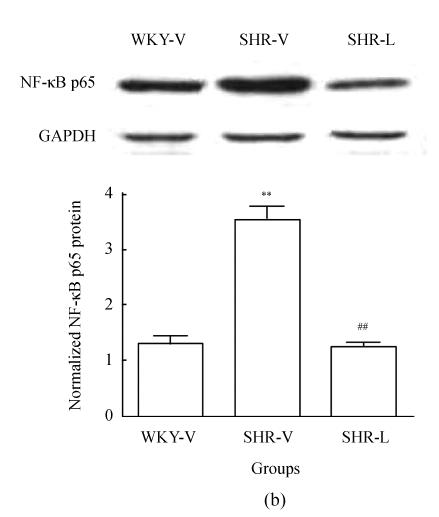
To determine whether the total amount of Cx43 protein changed after losartan treatment, Western blot was carried out in all samples. The Cx43 antibody recognized one band migrating at 43 kDa on immunoblots from LV tissue homogenates (Fig.3a). Densitometric quantification revealed that Cx43 expression in SHR-V group significantly increased (P<0.05). After treatment with losartan, Cx43 protein in SHR-L group significantly decreased (P<0.05). We also examined the NF-κB p65 protein level in the nucleus. Immunoblotting of nuclear extracts with an anti-NF-κB p65 antibody recognized one band migrating at 65 kDa (Fig.3b) and showed low expression of NF-κB p65 protein in WKY-V and SHR-L groups, whereas a strong expression was observed in SHR-V group (P<0.001).
Fig. 3.
Western blot analysis of Cx43 and NF-κB p65 in three groups. (a) Representative Cx43 immunoblot (upper panel) and relative Cx43 protein levels (lower panel, mean±SD); (b) Representative NF-κB p65 immunoblot (upper panel) and relative NF-κB p65 protein levels (lower panel, mean±SD)
* P<0.05 vs WKY-V group, # P<0.05 vs SHR-V group; ** P<0.001 vs WKY-V group; ## P<0.001 vs SHR-V group
DISCUSSION
In the present study, we found that by the end of the experiment, SBP in SHR-V group increased progressively, and LV hypertrophy was prominent, indicating the increases of plasma BNP level and LVW/BW ratio and the pathological changes of cardiomyocytes. SBP of WKY-V group remained unchanged during the experiment and there was no evidence of LV hypertrophy in WKY-V rats. The protein level of Cx43 expression increased significantly in hypertrophied LV myocardium of SHRs. Immunohistochemistry analysis showed that LV hypertrophy in SHRs was associated with disorganization of GJ distribution. In hypertrophied myocytes, punctuate Cx43 immunolabeling showed varying degrees of dispersion over the cell surface and formed side-to-side contacts of cardiomyocytes. The protein level of NF-κB p65 increased significantly in SHRs. After an 8-week treatment of losartan, SBP decreased significantly in SHR-L group. The LV hypertrophy was alleviated, which was demonstrated by decreased plasma BNP level and LVW/BW ratio and the pathological changes of cardiomyocytes. The Cx43 protein expression decreased significantly after losartan treatment, accompanied by decreased NF-κB p65 level. Cx43 immunoblot distributed regularly and mostly in intercalated disks in SHR-L group, indicating that losartan prevented Cx43 GJ remodeling in hypertrophic ventricular myocardium of SHRs.
GJs, assembled by connexins, form the cell-to-cell pathways for propagation of precisely orchestrated patterns of current flow that govern the regular rhythm of the healthy heart. Alterations of GJ organization and connexin expression are now well established as a consistent feature of human heart disease, in which there is an arrhythmic tendency. These alterations may take the form of structural remodeling, involving disturbances in the distribution of GJs and/or alteration of the amount or type of connexin(s) expressed. In the diseased ventricles, the most consistent quantitative alteration involves heterogenous reduction in Cx43 expression. It was reported that endothelial Cx43 and Cx37 expressions were reduced in N(ω)-nitro-L-arginine methyl ester (L-NAME)-induced hypertensive rats (Yeh et al., 2006). Changes of GJs in penile cavernous smooth muscle cells of SHRs had also been investigated recently (Jiang et al., 2006). A variety of changes in Cx43 GJ expression have been reported in previous studies on the hypertrophied heart. The overall amount of Cx43 protein was reported to be reduced in human patients with severe aortic stenosis (Peters et al., 1993a), but to be elevated in the early phase of hypertrophy in guinea pigs with renovascular hypertension (Peters et al., 1993b). On the other hand, in rats with the pressure overloaded hearts induced by monocrotaline treatment or by aortic banding, the cellular content of Cx43 was found to be unchanged as compared with that in control hearts (Uzzaman et al., 2000). One possible explanation of such contradictory results may include the use of different species, sampling periods, and models in which hypertrophy may develop at different rates. The study of Cx43 expression in LV tissues of SHRs is rare. A key finding of the present study is that LV myocardium Cx43 expression increased significantly in SHRs during early stage of hypertrophy.
Apart from the upregulation of Cx43, the SHR-V group was also characterized by Cx43 lateralization. Although it is not yet convincingly proven whether laterally dispersed Cx43 may contribute to a pro-arrhythmogenic substrate, several experimental studies have shown that lateralization of GJs is associated with alterations of anisotropic conduction properties of different cardiac tissues (Litchenberg et al., 2000; Polontchouk et al., 2001), including the ventricular myocardium (Uzzaman et al., 2000). While awaiting additional studies to examine whether lateral Cx43 may influence the electrophysiological properties of the hypertrophied myocardium in human patients, we found that angiotensin II type 1 (AT1) receptor antagonist losartan regressed the disorganization of Cx43 GJ remodeling, perhaps through which we could partly explain beneficial effects of ARB on ventricular hypertrophy.
Changes of GJ expression in the hypertrophied heart are a complex process involving perturbations of connexin gene expression and connexin protein synthesis and degradation, as well as the rearrangements in spatial distribution of the protein (Severs, 1999; Saffitz et al., 1999). The specific mechanisms that initiate the remodeling are poorly understood, but likely involve activation of signal transduction pathways triggered by chemical or humoral mediators of hypertrophy. It has been shown that angiotensin II, endothelin 1, vascular endothelial growth factor and transforming growth factor-β are important mediators of stretch-induced upregulation of Cx43 expression (Pimentel et al., 2002; Shyu et al., 2001). The effects of angiotensin II on Cx43 of vascular smooth muscle cells (VSMCs) in rabbit arteriosclerosis were investigated, indicating treatment with angiotensin-converting enzyme inhibitors (ACEIs) and AT1 antagonists could inhibit the expression of Cx43 mRNA and proliferation of VSMCs (Cai et al., 2006). Polontchouk et al.(2002) reported that after administrating angiotensin II to neonatal rat myocardial cells for 24 h, Cx43 increased by approximately 50% and led to multiplicative electric coupling. Some researchers showed that AT1 antagonist could inhibit the upregulation of Cx43 in rat ventricular myocytes induced by periodic mechanical stretching (Shyu et al., 2001). Emdad et al.(2001) investigated the prevention of losartan on GJ remodeling in hypertrophied left ventricles of aortic-banded rats, suggesting that tissue renin-angiotensin system plays an important role in the remodeling of GJ in ventricular myocytes under pathological condition. In our study, by specifically blocking AT1 with losartan, we found that both GJ remodeling and LV hypertrophy in SHR are substantially reduced, suggesting that angiotensin II may contribute to GJ remodeling directly or indirectly, which was in accordance with Emdad et al.(2001)’s results.
The role of NF-κB in hypertrophic growth of terminally differentiated cells has remained, until very recently, uncertain. However, sufficient evidence now exits in support of the contention that NF-κB plays a necessary role for myocyte hypertrophy, at least downstream of G-protein-coupled receptor agonists, such as catecholamines, angiotensin II and endothelin 1. Endothelin 1 and angiotensin II can selectively modulate the specific expression of cardiac Cx43 through signal transduction pathways, including extracellular signal-regulated kinases (ERKs) and p38-MAPK (mitogen-activated protein kinase) (Polontchouk et al., 2002). These MAPKs are well-known second messengers that transduce the signals from different extracellular stimuli, and NF-κB is downstream of MAPKs signal transduction pathways. No study to date has investigated the relationship between NF-κB and GJ expressions. We found that Cx43 GJ remodeling in hypertrophied LV myocardium changed to AT1 receptor antagonist losartan, and was in accordance with decreased NF-κB p65 expression, suggesting angiotensin II mediated GJ remodeling through NF-κB pathway. However, it needs further study to demonstrate the relationship between GJ expression and NF-κB pathway.
In summary, the present study demonstrates that long-term administration of losartan regressed LV hypertrophy and GJ remodeling. Whatever mechanisms, these findings have identified Cx43-mediated intercellular communication as a new potential therapeutic target in LV hypertrophy and may contribute to the benefits of losartan treatment on the hearts of SHRs.
References
- 1.Cai W, Ruan LM, Wang YN, Chen JZ. Effects of angiotensin II on connexin43 of VSMCs in arteriosclerosis. J Zhejiang Univ Sci B. 2006;7(8):648–653. doi: 10.1631/jzus.2006.B0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomized trial against atenolol. Lancet. 2002;359(9311):995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 3.Dodge SM, Beardslee MA, Darrow BJ, Green KG, Beyer EC, Saffitz JE. Effects of angiotensin II on expression of the gap junction channel protein connexin43 in neonatal rat ventricular myocytes. J Am Coll Cardiol. 1998;32(3):800–807. doi: 10.1016/S0735-1097(98)00317-9. [DOI] [PubMed] [Google Scholar]
- 4.Emdad L, Uzzaman M, Takagshi Y, Honjo H, Uchida T, Severs NJ, Kodama I, Murata Y. Gap junction remodeling in hypertrophied left ventricles of aortic-banded rats: prevention by angiotensin II type 1 receptor blockade. J Mol Cell Cardiol. 2001;33(2):219–231. doi: 10.1006/jmcc.2000.1293. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S, Purcell NH, Lin A, Sen S. Activation of nuclear factor-kappaB is necessary for myotrophin-induced cardiac hypertrophy. J Cell Biol. 2002;159(6):1019–1028. doi: 10.1083/jcb.200207149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirotani S, Otsu K, Nishida K, Higuchi Y, Morita T, Nakayama H, Yamaguchi O, Mano T, Matsumura Y, Ueno H. Involvement of nuclear factor-kappaB and apoptosis signal-regulating kinase 1 in G-protein-coupled receptor agonist-induced cardiomyote hypertrophy. Circulation. 2002;105(4):509–515. doi: 10.1161/hc0402.102863. [DOI] [PubMed] [Google Scholar]
- 7.Jiang YG, Jiang R, Jin J, Wang HP, Chen JH. Changes of gap junction in penile cavernous smooth muscle cells of hypertensive rats. Zhonghua Nan Ke Xue. 2006;12(11):1010–1013. (in Chinese) [PubMed] [Google Scholar]
- 8.Litchenberg WH, Norman LW, Holwell AK, Martin KL, Hewett KW, Gourdie RG. The rate and anisotrophy of impulse propagation in the postnatal terminal crest are correlated with remodeling of Cx43 gap junction pattern. Cardiovasc Res. 2000;45(2):379–387. doi: 10.1016/S0008-6363(99)00363-6. [DOI] [PubMed] [Google Scholar]
- 9.Mondry A, Svynghedauw B. Biological adaptation of the myocardium to chronic mechanical overload. Molecular determinants of the autonomic nervous system. Eur Heart J. 1995;16(Suppl. 1):64–73. doi: 10.1093/eurheartj/16.suppl_i.64. [DOI] [PubMed] [Google Scholar]
- 10.Peters NS, Green CR, Poole-Wilson PA, Severs NJ. Reduced content of connexin43 gap junctions in ventricular myocardium from hypertrophied and ischemic human hearts. Circulation. 1993;88(3):864–875. doi: 10.1161/01.cir.88.3.864. [DOI] [PubMed] [Google Scholar]
- 11.Peters NS, del Monte F, MacLeod KT, Green CR, Poole-Wilson PA, Severs NJ. Increased cardiac myocyte gap-junctional membrane early in renovascular hypertension. Am J Cardiol. 1993;21:59A. (Abstracts) [Google Scholar]
- 12.Pimentel RC, Yamada KA, Klebr AG, Saffitz JE. Autocrine regulation of myocyte Cx43 expression by VEGF. Circ Res. 2002;90(6):671–677. doi: 10.1161/01.RES.0000014823.75393.4D. [DOI] [PubMed] [Google Scholar]
- 13.Polontchouk L, Haefliger JA, Ebelt B, Schaefer T, Stuhlmann D, Mehlhorn U, Kuhn-Regnier F, de Vivie ER, Dhein S. Effects of chronic atrial fibrillation on gap junction distribution in human and rat atria. J Am Coll Cardiol. 2001;38(3):883–891. doi: 10.1016/S0735-1097(01)01443-7. [DOI] [PubMed] [Google Scholar]
- 14.Polontchouk L, Ebelt B, Jackeis M, Dhein S. Chronic effect of endothelin 1 and angiotensin II on gap junctions and intercellular communication in cardiac cells. FASEB J. 2002;16(1):87–89. doi: 10.1096/fj.01-0381fje. [DOI] [PubMed] [Google Scholar]
- 15.Purcell NH, Tang G, Yu C, Mercurio F, Di Donato JA, Lin A. Activation of NF-kappaB is required for hypertrophic growth of primary rat neonatal ventricular cardiomyocytes. Proc Natl Acad Sci USA. 2001;98(12):6668–6673. doi: 10.1073/pnas.111155798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saffitz JE, Schuessler RB, Yamada KA. Mechanisms of remodeling of gap junction distributions and the development of anatomic substrates of arrhythmias. Cardiovasc Res. 1999;42(2):309–317. doi: 10.1016/S0008-6363(99)00023-1. [DOI] [PubMed] [Google Scholar]
- 17.Severs NJ. Cardiovascular disease. Novartis Found Symp. 1999;219:188–211. doi: 10.1002/9780470515587.ch12. [DOI] [PubMed] [Google Scholar]
- 18.Severs NJ, Coppen SR, Dupont E, Yeh HI, Ko YS, Matsushita T. Gap junction alterations in human cardiac disease. Cardio Res. 2004;62(2):368–377. doi: 10.1016/j.cardiores.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Shyu KG, Chen CC, Wang BW, Kuan P. Angiotensin II receptor antagonist blocks the expression of connexin43 induced by cyclical mechanical stretch in cultured neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2001;33(4):691–698. doi: 10.1006/jmcc.2000.1333. [DOI] [PubMed] [Google Scholar]
- 20.Uzzaman M, Honji H, Takagishi Y, Emad L, Anthoy I, Magee AI, Severs NJ, Kodama I. Remodelling of gap junctional coupling of rats with monocrotaline-induced pulmonary hypertension. Circ Res. 2000;86(8):871–878. doi: 10.1161/01.res.86.8.871. [DOI] [PubMed] [Google Scholar]
- 21.Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J. 2001;141(3):334–341. doi: 10.1067/mhj.2001.113218. [DOI] [PubMed] [Google Scholar]
- 22.Yeh HI, Lee PY, Su CH, Tian TY, Ko YS, Tsai CH. Reduced expression of endothelial connexins 43 and 37 in hypertensive rats is rectified after 7-day carvedilol treatment. Am J Hypertens. 2006;19(2):129–135. doi: 10.1016/j.amjhyper.2005.08.020. [DOI] [PubMed] [Google Scholar]