Abstract
Herein, solid lipid nanoparticles (SLN) were proposed as a new drug delivery system for adefovir dipivoxil (ADV). The octadecylamine-fluorescein isothiocynate (ODA-FITC) was synthesized and used as a fluorescence maker to be incorporated into SLN to investigate the time-dependent cellular uptake of SLN by HepG2.2.15. The SLN of monostearin with ODA-FITC or ADV were prepared by solvent diffusion method in an aqueous system. About 15 wt% drug entrapment efficiency (EE) and 3 wt% drug loading (DL) could be reached in SLN loading ADV. Comparing with free ADV, the inhibitory effects of ADV loaded in SLN on hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg) and hepatitis B virus (HBV) DNA levels in vitro were significantly enhanced.
Keywords: Adefovir dipivoxil (ADV), Solid lipid nanoparticles (SLN), Octadecylamine-fluorescein isothiocynate (ODA-FITC), Hepatitis B virus (HBV)
INTRODUCTION
Chronic hepatitis B virus (HBV) infection is a major global public health problem, especially in the developing countries of Asia and Africa (Jiang et al., 2007). It is believed that there are approximately 400 million chronic HBV carriers in the world, and they have a significant risk for the development of cirrhosis or hepatocellular carcinoma (Lai et al., 2003; Raney et al., 2003).
Adefovir dipivoxil (ADV) is a nucleotide analogue that has been shown to effectively reduce serum HBV DNA level and improve serum alanine aminotransferase (ALT) level as well as liver histology (Hadziyannis et al., 2003; Marcellin et al., 2003). ADV also has potent antiviral activity to effectively treat chronic HBV patients with lamivudine-resistant HBV (Perrillo et al., 2000; 2004; Westland et al., 2005). However, biochemical and virological rebounds, as well as hepatic decompensation, have been reported (Dai et al., 2007), indicating the need for novel therapeutic approaches to enhance drug target effect.
Recently, solid lipid nanoparticles (SLN) made from biodegradable solid lipids with submicron size range have attracted increasing attention in drug delivery systems. The advantages of SLN include possibility of controlled drug release and drug targeting, protection of incorporated compound against chemical degradation, no biotoxicity, and no problems with respect to large-scale production (Marengo et al., 2000; Mehnert and Mäder, 2001; Müller et al., 2000).
Fluorescence technique is a widely used method for investigating cellular uptake and in vivo distribution of nanoparticles. As reported in our previous research, the octadecylamine-fluorescein isothiocynate (ODA-FITC) as a fluorescent probe was not only used to investigate in vivo transport of SLN (Yuan et al., 2007), but also used for the cellular uptake evaluation of SLN (Yuan et al., 2008).
In this study, the chemical conjugate of ODA-FITC was incorporated into SLN for evaluating cellular uptake of SLN composed of monostearin. ADV was then chosen as a model drug, and HepG2.2.15 human hepatoblastoma cells were chosen as the model cells. The inhibitory effects of ADV mediated by SLN on HBsAg, HBeAg and HBV DNA levels were evaluated in vitro. The physicochemical properties of ADV-loaded SLN, such as particle size, zeta potential, drug entrapment efficiency and drug loading, were also examined.
MATERIALS AND METHODS
Reagents
Monostearin was supplied by Shanghai Chemical Reagent Co., Ltd., China. The surfactant, poloxamer 188, was provided by Shenyang Pharmaceutical University Jiqi Co., Ltd., China. The chemical conjugate of ODA-FITC was kindly provided by Prof. Hu’s Study Group (College of Pharmaceutical Sciences, Zhejiang University, China). Dulbecco’s modified Eagle medium (DMEM), fetal bovine serum and G418 were purchased from GIBCO BRL (USA). All other solvents were analytical or chromatographic grade.
Preparation of ADV-loaded SLN
The ADV-loaded SLN were prepared by solvent diffusion method in an aqueous system referred to our previous study (Hu et al., 2005; Yuan et al., 2007; 2008). Briefly, 2 ml ethanol completely dissolved 50 mg lipid materials with 10 mg ADV in 50 °C water bath. To prepare the fluorescent blank SLN, 7.5 mg ODA-FITC was used instead of the same amount of lipid materials without ADV. Then the obtained organic solution was quickly dispersed into 10 ml distilled water (pH 7.4) under mechanical agitate (DC-40, Hangzhou Electrical Engineering Instruments, China) at 400 r/min in ice bath for 5 min to obtain the SLN dispersion.
The pH value of above obtained SLN dispersion was adjusted to 1.20 by addition of 0.1 mol/L HCl to form the SLN aggregation that was received by centrifugation (20 000 r/min for 20 min, 3K30, Sigma, Germany). The collected SLN precipitate was then resuspended in 6 ml distilled water containing 0.1% (w/w) poloxamer 188 in water bath through ultrasonic treatment for 0.5 min, and then used for further in vitro tests.
Physicochemical properties of ADV-loaded SLN
The ADV-loaded SLN in redispersion were diluted 20 times with distilled water containing 0.1% (w/w) poloxamer 188. The volume average diameter and zeta potential of the samples were determined with Zetasizer (3000HS, Malvern Instruments, UK).
After the SLN were received by the centrifugation, the drug content in the supernatant was determined in triplicate by ultraviolet spectrophotometric method at the wavelength of 260 nm. The drug entrapment efficiency (EE) and drug loading (DL) of SLN were calculated from Eqs.(1) and (2):
| EE=(Wa−Ws)/Wa×100%, | (1) |
| DL=(Wa−Ws)/(Wa+WL−Ws)×100%, | (2) |
where W a is the weight of drug added in system; W s is the analytic weight of drug in supernatant after the centrifugation; W L is the weight of lipid added in system.
Cell culture
HepG2.2.15 cell line was kindly provided by the State Key Lab for Diagnosis and Treatment of Infectious Diseases, Zhejiang University, China. This cell line was cultured at 37 °C in DMEM supplemented with 10% (w/w) fetal bovine serum and 500 μg/ml G418 in the air containing 5% (v/v) CO2.
Cellular uptake of fluorescent blank SLN
HepG2.2.15 cells were seeded in a 24-well plate at a seeding density of 10 000 cells per well with 1 ml of growth medium each well and allowed to attach for 48 h. Cells were incubated with ODA-FITC-loaded SLN (the concentration was 100 µg/ml) for different time. The cells were then washed twice with phosphate buffered saline (PBS, pH 7.4) and directly viewed under a fluorescence microscope (OLYMPUS America, Melville, NY, USA). The fluorescence images were obtained at 20× magnification.
Virological assessment
HepG2.2.15 cells were plated. The ADV loaded in SLN or dissolved in distilled water (pH 7.4) was added with the given concentrations and incubated for 2 d or 5 d. The culture media from the wells were collected for the virological assessment. HBsAg and HBeAg productions were determined by commercial enzyme immunoassay kits (AXSYM System, Abbott, Wiesbaden, Germany). HBV DNA was quantified by a commercial real-time polymerase chain reaction (PCR) kit (PG Biotech., Shenzhen, China).
Statistical analysis
Data were expressed as means of three separate experiments, and were compared by analysis of variance (ANOVA). A P<0.05 was considered statistically significant in all cases.
RESULTS
Characteristic of ADV-loaded SLN
The ADV-loaded SLN were prepared by solvent diffusion method. The volume average diameters, polydispersity indexes and zeta potentials of resulted ADV-loaded SLN are listed in Table 1. Table 1 also indicates the EE and DL of ADV-loaded SLN. About 15 wt% EE and 3 wt% DL could be reached by the present preparation method.
Table 1.
Properties of resulted ADV loaded SLN (n=3)
|
dv |
PI | ζ (mV) | EE (%) | DL (%) | |||
| Area (%) | Mean (nm) | Width (nm) | Average (nm) | ||||
| 43.6, 56.4 | 300.7, 457.9 | 103.4, 215.5 | 389.4±166.5 | −0.371 | −30.5±2.8 | 15.32±2.58 | 3.06±0.51 |
d v, PI, ζ, EE and DL indicate the volume average diameter, polydispersity index, zeta potential, drug entrapment efficiency and drug loading, respectively
Cellular uptake of fluorescent SLN
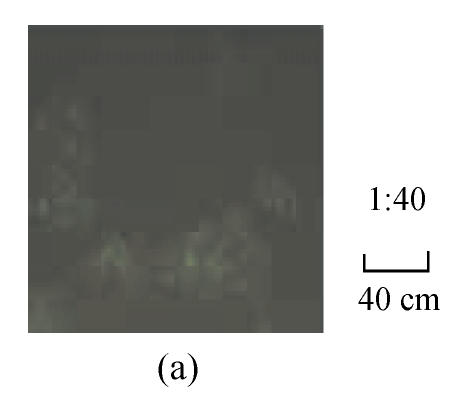
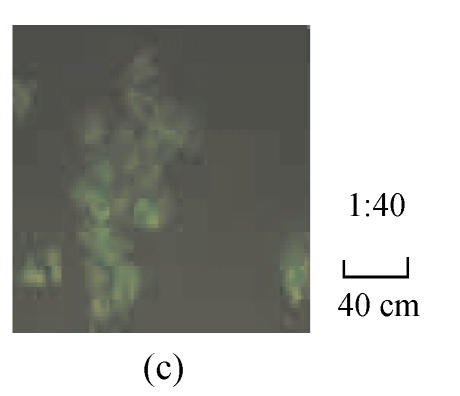
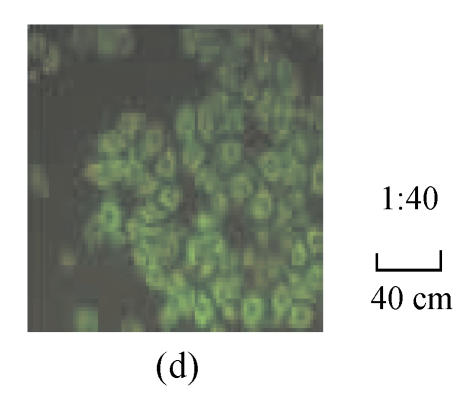
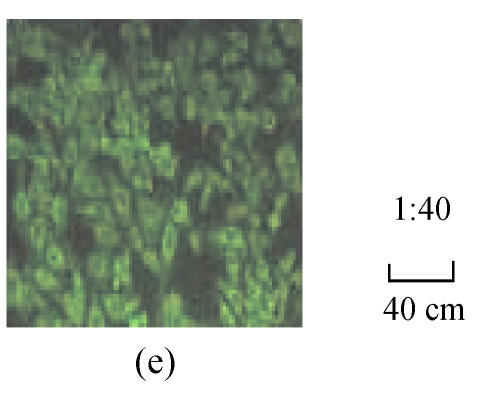
The fluorescence images in Fig.1 show that the HepG2.2.15 cells were incubated with fluorescent SLN for different incubation time at the same incubation concentration of SLN (100 µg/ml). It can be seen that the fluorescence intensity inside HepG2.2.15 increased with incubation time, which means the cellular uptake of SLN was time-dependent.
Fig. 1.
Fluorescence image after the cells were incubated with the fluorescent SLN (100 µg/ml) for different incubation time. (a) 1 h; (b) 2 h; (c) 4 h; (d) 12 h; (e) 24 h
Antiviral effect of ADV on HepG2.2.15 cells
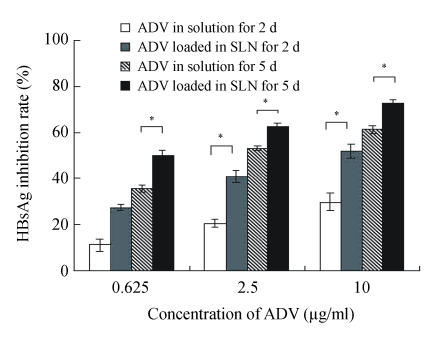
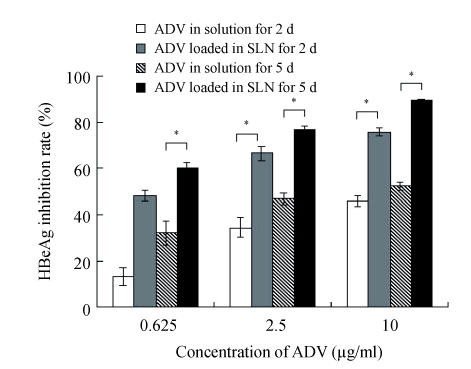
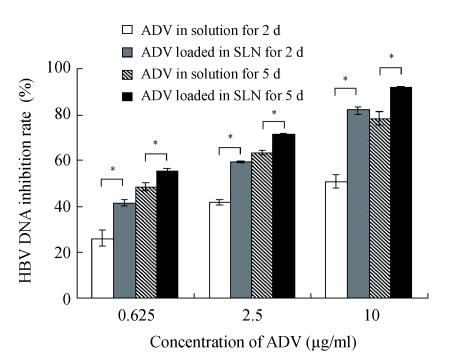
Figs.2~3 show the higher efficiency of ADV loaded in SLN for inhibiting both HBsAg and HBeAg expressions than that of free ADV in solution after incubated with HepG2.2.15 cells for both 2 d and 5 d (P<0.05). From Fig.4, it was found that the ADV loaded in SLN could more significantly inhibit the production of HBV DNA than free ADV in solution after incubated with HepG2.2.15 cells for both 2 d and 5 d (P<0.05).
Fig. 2.
Inhibitory effect of ADV on the HBsAg expression in HepG2.2.15 cells
The data represent mean±SD (n=3); * Significant difference between free ADV in solution and ADV loaded in SLN (P<0.05)
Fig. 3.
Inhibitory effect of ADV on the HBeAg expression in HepG2.2.15 cells
The data represent mean±SD (n=3); * Significant difference between free ADV in solution and ADV loaded in SLN (P<0.05)
Fig. 4.
Inhibitory effect of ADV on the HBV DNA in HepG2.2.15 cells
The data represent mean±SD (n=3); * Significant difference between free ADV in solution and ADV loaded in SLN (P<0.05)
DISCUSSION
From Fig.1, it was found that the SLN were accumulated in the cytoplasm after being uptaken by HepG2.2.15 cells. As reported (Iyer et al., 2005), the antiviral activity of ADV was manifested through inhibition of viral reverse transcriptase by the corresponding diphosphorylated metabolite, so the main action target of ADV is reverse transcriptase in the cytoplasm. Therefore, it is suitable to deliver ADV into HepG2.2.15 cells via SLN for enhancing the target effect. To our knowledge (Yuan et al., 2008), internalization of drug into cells was enhanced when the drug was incorporated into SLN, due to the membrane affinity of lipid material and nanometer particle size. From Figs.2~4, compared with free ADV in solution, ADV loaded in SLN enhanced inhibitory effect not only on HBV DNA replication but also on HBV antigen expression. It is probably because ADV-loaded SLN may improve cellular uptake of drug and the drug accumulation in target region through the controlled release profile of drug-loaded SLN, which served as a drug-container releasing the drug continuously, thus enhancing the drug effect. The data in Figs.2~4 also clearly show that the inhibitory activities of free ADV in solution and ADV loaded in SLN on HBV-induced HBsAg, HBeAg and HBV DNA productions were promoted both dose-dependently and time-dependently. Therefore, further work is needed to investigate the changes of cellular drug concentration with dose increasing and time extending, and to determine the enhancement mechanism of ADV loaded in SLN on anti-HBV activity.
References
- 1.Dai CY, Chuang WL, Hsieh MY, Lee LP, Huang JF, Hou NJ, Lin ZY, Chen SC, Hsieh MY, Wang LY, et al. Adefovir dipivoxil treatment of lamivudine-resistant chronic hepatitis B. Antiviral Res. 2007;75(2):146–151. doi: 10.1016/j.antiviral.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Hadziyannis SJ, Tassopoulos NC, Jenny Heathcote E, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Wulfsohn MS, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348(9):800–807. doi: 10.1056/NEJMoa021812. [DOI] [PubMed] [Google Scholar]
- 3.Hu FQ, Jiang SP, Du YZ, Yuan H, Ye YQ, Zeng S. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloid and Surfaces B-Biointerfaces. 2005;45(3-4):167–173. doi: 10.1016/j.colsurfb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Iyer RP, Padmanabhan S, Zhang GR, Morrey JD, Korba BE. Nucleotide analogs as novel anti-hepatitis B virus agents. Curr Opin Pharmacol. 2005;5(5):520–528. doi: 10.1016/j.coph.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Jiang JT, Xu N, Zhang XY, Wu CP. Lipids changes in liver cancer. J Zhejiang Univ Sci B. 2007;8(6):398–409. doi: 10.1631/jzus.2007.B0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003;362(9401):2089–2094. doi: 10.1016/S0140-6736(03)15108-2. [DOI] [PubMed] [Google Scholar]
- 7.Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348(9):808–816. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- 8.Marengo E, Cavalli R, Caputo O, Rodriguez L, Gasco M. Scale-up of the preparation process of solid lipid nanospheres. Int J Pharm. 2000;205(1-2):3–13. doi: 10.1016/S0378-5173(00)00471-3. [DOI] [PubMed] [Google Scholar]
- 9.Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47(2-3):165–196. doi: 10.1016/S0169-409X(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 10.Müller R, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–177. doi: 10.1016/S0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 11.Perrillo R, Schiff E, Yoshida E, Statler A, Hirsch K, Wright T, Gutfreund K, Lamy P, Murray A. Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology. 2000;32(1):129–134. doi: 10.1053/jhep.2000.8626. [DOI] [PubMed] [Google Scholar]
- 12.Perrillo R, Hann HW, Mutimer D, Willems B, Leung N, Lee WM, Moorat A, Gardner S, Woessner M, Bourne E, et al. Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology. 2004;126(1):81–90. doi: 10.1053/j.gastro.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 13.Raney AK, Hamatake RK, Hong Z. Agents in clinical development for the treatment of chronic hepatitis B. Expert Opin Investig Drugs. 2003;12(8):1281–1295. doi: 10.1517/13543784.12.8.1281. [DOI] [PubMed] [Google Scholar]
- 14.Westland CE, Yang H, Delaney IV WE, Wulfsohn M, Lama N, Gibbs CS, Miller MD, Fry J, Brosgart CL, Schiff ER, et al. Activity of adefovir dipivoxil against all patterns of lamivudine-resistant hepatitis B viruses in patients. J Viral Hepat. 2005;12(1):67–73. doi: 10.1111/j.1365-2893.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 15.Yuan H, Chen J, Du YZ, Hu FQ, Zeng S. Studies on oral absorption of stearic acid SLN by a novel fluorometric method. Colloid and Surfaces B-Biointerfaces. 2007;58(2):157–164. doi: 10.1016/j.colsurfb.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Yuan H, Miao J, Du YZ, You J, Hu FQ, Zeng S. Cellular uptake of solid lipid nanoparticles and cytotoxicity of encapsulated paclitaxel in A549 cancer cells. Int J Pharm. 2008;348(1-2):137–145. doi: 10.1016/j.ijpharm.2007.07.012. [DOI] [PubMed] [Google Scholar]