Abstract
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear receptor superfamily. The activation of PPARs by their specific ligands is regarded as one of the promising strategies to inhibit cancer cell growth. However, recent clinical trials targeting several common cancers showed no beneficial effect when PPAR ligands are used as a monotherapy. Retinoid X receptors (RXRs), which play a critical role in normal cell proliferation as a master regulator for nuclear receptors, preferentially form heterodimers with PPARs. A malfunction of RXRα due to phosphorylation by the Ras/MAPK signaling pathway is associated with the development of certain types of human malignancies. The activation of PPARγ/RXR heterodimer by their respective ligands synergistically inhibits cell growth, while inducing apoptosis in human colon cancer cells when the phosphorylation of RXRα was inhibited. We herein review the synergistic antitumor effects produced by the combination of the PPAR, especially PPARγ, ligands plus other agents, especially retinoids, in a variety of human cancers. We also focus on the phosphorylation of RXRα because the inhibition of RXRα phosphorylation and the restoration of its physiological function may activate PPAR/RXR heterodimer and, therefore, be a potentially effective and critical strategy for the inhibition of cancer cell growth.
1. INTRODUCTION
Peroxisome proliferator-activated receptors (PPARs) are members of a superfamily of nuclear hormone receptors comprising three isoforms, PPARα, PPARβ/δ, and PPARγ, which act as ligand-activated transcription factors. PPARs play key roles in energy homeostasis by modulating glucose and lipid metabolism and transport. Through these metabolic actions, PPARs can regulate cell proliferation, differentiation and survival [1, 2]. PPARs also control immune and inflammatory responses [3]. Because these physiological activities of PPARs are closely associated with normal cell homeostasis, the aberrant expression and function of PPARs have been observed in a variety of human malignancies. Moreover, these reports also suggest the possibility that targeting PPARs might be a critical strategy for inhibiting the development and growth of cancers. Indeed, numerous in vivo and in vitro studies have demonstrated that PPAR agonists, especially, PPARγ ligands can inhibit cell growth, cause apoptosis, and thus exert antitumor effects in various types of human malignancies [4–6]. Based on the antigrowth and prodifferentiation action of PPARs, several clinical studies have been conducted using the PPAR ligands in human cancers. However, with the exception of a small trial on liposarcomas, the clinical trials have so far indicated that the PPAR agonists may not be useful as a monotherapy for advanced malignancies [7–10].
On the other hand, recent preclinical studies show absorbing evidence that the combined treatment with PPAR ligands plus a variety of other agents can cause a synergistic effect to inhibit growth in cancer cells. For instance, we recently found that the activation of PPARγ/RXR heterodimer by their respective ligands synergistically inhibited cell growth and induced apoptosis in human colon cancer cells [11]. Therefore, the aim of this paper is to review the possibility that the combined usage of the PPAR ligands with other agents may therefore be a critical strategy for the treatment of certain types of human cancers. We also review the significance of the aberrant phosphorylation of retinoid X receptor (RXR), which is a heterodimeric partner for PPARs, as described in the next section.
2. RXRs AND PPARs
RXRs and retinoic acid receptors (RARs), both of which are composed of three subtypes (α, β, and γ), are also members of the nuclear hormone receptor superfamily. The ligands for RXRs and RARs are the retinoids, a group of structural and functional analogues of vitamin A, and the retinoids have a profound effect on such cellular activities as growth, differentiation, apoptosis, and morphogenesis primarily through binding to RXRs and/or RARs. A small portion of dietary retinoids is converted to retinoic acid (RA), which is an active metabolite of the retinoids. RXR is specific for the 9-cis RA, while RAR binds both 9-cis RA and all-trans RA (ATRA). The nuclear retinoid receptors are ligand-dependent transcription factors that bind to the retinoic acid receptor responsive element (RARE) and retinoid X receptor responsive element (RXRE), which are present in the promoter regions of retinoid responsive target genes, thereby modulating the gene expression [12, 13]. Other nuclear receptors, including PPARs, also require RXR as a heterodimeric partner in order to exert their function. After ligand binding, PPARs can regulate target gene expression by binding to the peroxisome proliferator responsive element (PPRE) in target genes as a heterodimer with RXRs (see Figure 1) [14, 15]. Therefore, RXRs play a fundamental role in controlling normal cell proliferation and metabolism and act as a master regulator of nuclear receptors. Among the retinoid receptors, RXRα is thought to be one of the most important receptors with respect to the regulation of the essential effects of cell activities.
Figure 1.
PPAR activation pathway and transcriptional regulation of target genes. After ligand binding, PPARs form heterodimers with RXR in the nucleus. The PPAR/RXR heterodimers interact with transcriptional coactivators (CoActs) and bind to sequence specific PPRE located in the target genes that control glucose and insulin homeostasis, lipid metabolism, inflammation, and cellular differentiation. L: ligand.
3. STRUCTURE OF RXRα AND SIGNIFICANCE OF RXRα PHOSPHORYLATION
RXRs have a variable N-terminal domain (A/B domain; AF-1), a highly conserved DNA-binding domain (DBD), a nonconserved hinge, and a moderately conserved C-terminus including the ligand-binding domain (LBD). Transcriptional activation is mediated by LBD, which contains four more-or-less overlapping surfaces: a ligand-binding pocket for the binding of small, lipophilic molecules, a transactivation domain (AF-2 or helix 12), a cofactor binding surface, and a dimerization surface [16]. Recent studies revealed that phosphorylation processes are critical for the transcriptional activity of RAR/RXR heterodimers. Bruck et al. [17] reported that the activation of c-Jun N-terminal kinases (JNKs) induces phosphorylation of both at three residues (serine 61, serine 75, and threonine 87) located in the N-terminal AF-1 domain and one residue (serine 265) in the Omega loop in LBD (AF-2 domain) of RXRα. The RA-induced phosphorylation of the same three residues in the AF-1 domain is required for the cooperation of RXRα with RARγ for maximal transcriptional activity [18]. The phosphorylation of RXRα in its N-terminal domain plays a role to activate a subset of RA-responsive genes and for the antiproliferative effect of RA [19]. These findings suggest that RXRα “positively” regulates the transactivation of target genes through phosphorylation [20].
On the other hand, there are some contrary reports which show the phosphorylation of RXRα to “negatively” modulate the function of its heterodimeric binding partners. Indeed, MAPK-mediated phosphorylation of the RXRα LBD impairs the transcriptional activity of RXR/RAR [21, 22] and RXR/vitamin D3 receptor (VDR) [23]. These “negative” effects of RXRα via its phosphorylation might be associated with certain types of human diseases, including cancer [20]. In the next section, we review the specific roles of the aberrant phosphorylation of RXRα in carcinogenesis, especially focusing on the development of hepatocellular carcinoma (HCC).
4. RXRα PHOSPHORYLATION AND CANCER
Abnormalities in the expression and function of retinoids and their receptors play an important role in influencing the development of various human malignancies and, therefore, might be critical targets for cancer chemoprevention and/or chemotherapy [24]. Specifically, we previously reported that hepatocarcinogenesis is accompanied by an accumulation of the phosphorylated (i.e., inactivated) form of RXRα and the inhibition of RXRα phosphorylation may thus be an effective strategy for preventing the development of HCC. Initially, we showed that the RXRα protein is anomalously phosphorylated at a specific site of the serine/threonine residues and is accumulated both in human HCC tissue as well as in HCC cell lines [22]. Phosphorylation at serine 260 of RXRα, a consensus site of mitogen-activated protein kinase (MAPK), is closely linked to its retarded degradation, low transcriptional activity, and the promotion of cancer cell growth, and the abrogation of phosphorylation by MAPK-specific inhibitors restores the degradation of RXRα in a ligand-dependent manner [22, 25]. In addition, the phosphorylated form of RXRα (p-RXRα) is also resistant to ubiquitination and subsequent proteasome-mediated breakdown in both human HCC tissues and a human HCC cell line, whereas RXRα protein is unphosphorylated and highly ubiquitinated in the normal liver and in nonproliferating hepatocyte cultures [26]. The phosphorylation of RXRα abolishes its ability to form heterodimers with RARβ, thus resulting in the loss of cell growth control, resistance to retinoids, and the acceleration of cancer development [27]. These findings suggest that the accumulation of p-RXRα (i.e., nonfunctional RXRα), which can escape from the proteasome-mediated degradation system, may interfere with the function of normal RXRα in a dominant-negative manner, thereby playing a critical role in the development of HCC (see Figure 2) [28].
Figure 2.
A schematic representation of RXRα phosphorylation in HCC cells. In normal hepatocytes, when the ligand (retinoid) binds to and activates RXRα, the receptor becomes able to heterodimerize with other nuclear receptors, including RAR, and then activates the expression of the target genes, which may regulate normal cell proliferation and differentiation, by binding to the specific responsive element. In HCC cells, the Ras/MAPK pathway is highly activated and phosphorylates RXRα at serine residues, thus impairing the functions of the receptor. Therefore, the accumulated p-RXRα interferes with the remaining normal RXRα, presumably, in a dominant negative manner, thereby playing a critical role in the development of HCC. L: ligand. Ub: ubiquitin.
In addition to HCC, a malfunction of RXRα due to a posttranslational modification by phosphorylation is also associated with the development of other types of human malignancies. We recently reported that RXRα protein is highly phosphorylated and also accumulated in human colon cancer tissue samples as well as human colon cancer cell lines, while the levels of expression of p-RXRα do not increase in normal colonic epithelial cells; RXRα protein is phosphorylated in 75% of colorectal cancer tissues when compared with corresponding normal colon epithelial tissues [11]. Similar results have also been observed in human pancreatic cancer (manuscript in preparation). Moreover, Kanemura et al. [29] reported the abnormal phosphorylation of RXRα protein to play a role in the enhancement of cell proliferation, while producing an antiapoptotic effect, and also presumably acquiring RA-resistance in HL-60R human leukemia cells. In addition to these malignancies, full-length RXRα is anomalously phosphorylated and accumulated in leiomyoma when compared to myometrial cells and this is associated with a resistance to ligand-mediated ubiquitination and a delay in the receptor proteolytic degradation [30].
What are the precise mechanisms by which phosphorylation of RXRα loses its transcriptional activity? Recent studies indicate that the phosphorylation of RXRα can regulate the function of its heterodimeric binding partners. For instance, Solomon et al. [23] reported that phosphorylation of RXRα at serine 260, which is located in the Omega loop of the LBD, results in the attenuation of ligand-dependent transactivation by RXR/VDR complex in human keratinocytes, thus resulting in the induction of malignant transformation. The residues located in the AF-2 domain are also phosphorylated in response to stress agents, including JNKs and MKK4/SEK1 [21, 31], among which serine 265 located in the Omega loop [31], and these phosphorylations are closely linked to inhibit the transcription of RA target genes. The phosphorylation of RXRα at serine 260 is also associated with retinoid resistance [22, 27]. Therefore, these findings indicate that RXRα phosphorylation, which occurs at specific residues located in the Omega loop of the LBD, is apparently associated with a malfunction in the retinoid-dependent signaling pathway. The Omega loop, located between helices H1 and H3, is a very flexible and dynamic region that moves substantially during the conformational rearrangement that accompanies ligand binding to the LBD [32]. It has therefore been proposed that phosphorylation of the residues in this loop might alter the dynamics of this region and create conformational changes within the LBD, thus disrupting the interactions with coactivators and therefore inhibiting the activation of RA-responsive genes [17, 33].
5. PHOSPHORYLATED RXRα IS A CRITICAL TARGET FOR CANCER TREATMENT
The above findings support the possibility that the inhibition of RXRα phosphorylation and the restoration of its physiological function as a master regulator of nuclear receptors must be an effective strategy for controlling cell growth in various types of human cancers. It has been shown that the new synthetic retinoid, acyclic retinoid (ACR, NIK-333: Kowa Pharmaceutical Company Ltd., Tokyo, Japan), which was originally developed as an agonist for both RXR and RAR [34, 35], can restore the function of RXRα by inactivating the Ras-Erk signaling system and thereby inhibiting RXRα phosphorylation [25]. Practically, this agent has demonstrated several beneficial effects in experimental studies both in vivo and in vitro. For instance, ACR inhibited chemically induced hepatocarcinogenesis in rats as well as spontaneously occurring hepatoma in mice [36]. This agent also inhibited growth and induced apoptosis in human HCCderived cells [37–42]. Similar growth inhibitory effects are also observed in other types of human cancer cells, such as squamous cell carcinoma or leukemia cells [43, 44].
In addition, we also confirmed the chemopreventive effect of ACR on recurrent and second primary HCCs in patients who received anticancer treatment for an initial HCC in a double-blind and placebocontrolled clinical study. Namely, the oral administration of ACR for 12 months significantly reducedthe incidence of posttherapeutic recurrence of HCC and improved the survival rate in patients who underwent potentially curative treatments, without causing any severe adverse effects [45–47]. These findings suggest that ACR is a promising agent for the chemoprevention of HCC and that p-RXRα may be a critical target for the chemoprevention and/or treatment of some types of human cancers, including HCC, which show the accumulation of p-RXRα protein.
6. SYNERGY BETWEEN PPARγ LIGANDS AND RETINOIDS IN CANCER
Since RXR forms a permissive heterodimeric complex with PPAR, and the activation of PPARγ exerts antigrowth effects in cancer cells [4–6], it seems to be reasonable that the combination of RXR and PPARγ agonists may offer new therapeutic strategies for various types of human malignancies. Firstly, Tontonoz et al. [15] reported that the combined use of PPARγ and RXRα specific ligands is able to trigger terminal differentiation of primary human liposarcoma cells in vitro. This result suggests that the combination of these ligands may be a useful therapy for the treatment of liposarcoma [15]. Beneficial effects for the combined treatment with PPAR ligands plus retinoids are extensively reported in preclinical studies of the hematologic malignancies [48–51]. Therefore, the combination of PPARγ ligand with RXR agonist or RAR agonist can enhance the differentiating and growth-inhibitory effects in human leukemia cells [48]. The combination of PPARγ ligand, ciglitazone, and ATRA synergistically reduces the cell growth rates and cell cycle arrest at the G1 phase in HL-60 human leukemia cells, and this is associated with synergistic upregulation of PTEN expression [49]. The combination of 9-cis RA and PPARγ ligand shows significant synergistic effects for the induction of apoptosis in multiple myeloma cells [50]. These reports suggest that the combination of PPARγ ligands plus retinoids holds promise as a novel therapy for some types of hematologic malignancies by activating the transcription of target genes that control apoptosis and differentiation in these malignant cells.
In addition to the hematologic malignancies, a number of preclinical studies indicate the preferable effects by the combination of PPAR ligands plus retinoids on the inhibition of cell growth in solid malignancies, especially in breast caner [52–55]. For instance, Rubin et al. [55] showed that a combination of ligands for PPARγ and RXR inhibits breast aromatase expression induced by tumor-derived factors. Because aromatase activates estrogen biosynthesis, the combination of these ligands may be able to find utility in thetreatment of estrogen-dependent carcinogenesis, such as breast cancer and endometrial cancer [56]. The combination of RXR ligand with ciglitazone also cooperatively inhibits the growth of breast cancer and lung cancer cells by activating the RARE promoter activity and inducing RARβ, which plays a critical role in mediating the growth-inhibitory effects of retinoids in various cancer cells [57]. In addition, the synergistic or cooperative effect of RXR and PPARγ agonists for growth inhibition and apoptosis induction is found in colon cancer cells [11, 58]. The detailed effects of PPARγ ligands plus retinoids to inhibit growth in colorectal cancer cells are discussed in the next section.
What are the molecular mechanisms by which the combination of PPARγ ligands and retinoids synergistically induce anticancer effects? Yang et al. [59] reported that the PPARγ and RXR ligands have been shown to differentially recruit subsets of transcriptional coactivators (i.e., p160 by RXR and DRIP205 by PPARγ) to the receptor complex, thus leading to an enhanced transcriptional activation and cellular effects. The transcriptional activity of PPRE is additively induced by treatment with a PPARγ activator plus 9-cis RA, and RXRα accumulation, by inhibiting its degradation due to the proteasome system, therefore contributes to the enhancement of PPARγ/RXR activation [60]. The transactivation of the PPRE by PPARγ/RXR heterodimer enhances the expression of the glutathione S-transferase gene, which is responsible for the cellular metabolism as well as the detoxification of several xenobiotics and carcinogenic compounds [61]. The findings of these reports suggest that the accumulation of the unphosphorylated form (i.e., functional form) of RXRα activates the transcriptional activity of PPRE and thereby enhances the expression of important target genes. The significance of the restoration of RXRα by inhibiting its aberrant phosphorylation is reported in the studies using the cell lines of HCC [22, 28], leukemia [29], and colon cancer [11], as discussed below.
7. SYNERGY BETWEEN PPARγ LIGANDS AND RETINOIDS IN COLORECTAL CANCER
Among the PPAR targeting therapies, the activation of PPARγ by its ligand is regarded as a potentially useful strategy for the chemoprevention and/or treatment of colorectal cancer because many in vivo and in vitro preclinical studies have demonstrated that PPARγ ligands can inhibit cell growth, cause apoptosis, and thus exert antitumor effects in this malignancy [5, 62–65]. As a result, there has been considerable interest in utilizing the combination of ligands for PPARγ and RXR for the prevention and treatment of colorectal cancer. In fact, it has been reported that in human colon cancer cells the combination of the RXR and PPARγ agonists produces greater efficacy in growth inhibition than either single agent alone, and this is associated with a cooperative reduction in the levels of cyclooxygenase-2 (COX-2) expression and prostaglandin E2 (PGE2) synthesis [58]. The simultaneous exposure of HT-29 human colon cancer cells to ciglitazone and 9-cis RA results in an increased apoptotic effect and greater inhibition of COX-2 expression, in comparison to cells treated with either ciglitazone or 9-cis RA alone [66]. We recently reported that the combination of 9-cis RA and ciglitazone causes a synergistic inhibition in the growth of human colon cancer Caco2 cells, which express high levels of p-RXRα protein, and this is associated with the induction of apoptosis and inhibition in the expression of both COX-2 and c-Jun proteins and mRNAs. The combination of these agents has a synergistic effect in increasing the PPRE activity and decreasing the AP-1 activity. However, we should emphasize that these preferable effects are observed when the phosphorylation of RXRα protein is inhibited [11]. Therefore, the inhibition of the phosphorylation of this protein appears to play a critical role in inducing the synergistic growth inhibitory effect in colon cancer cells.
The above findings indicate that the activation of the RXR/PPARγ heterodimer by their specific ligands can decrease the expression of COX-2, which is one of the main mediators in the inflammatory signaling pathway. This seems to be significant because COX-2 plays a critical role in the development of colorectal cancer and might, therefore, be an important molecular target for colorectal cancer prevention and treatment [67]. Recent studies have revealed 2, 4, 6-trinitrobenzene sulfonic acid-induced colitis to be significantly reduced after the administration of both PPARγ and RXR agonists, and this beneficial effect is reflected by the reduction in the NF-κB DNA binding activity in the colon [68]. The inhibition of the β-catenin mediated pathway, which promotes the development of colon cancer and is stimulated by COX-2 as well as PGE2 [69, 70], by nonsteroidal anti-inflammatory drugs, requires a high-level expression of RXRα and PPARγ [71]. Therefore, the activation of the RXR/PPARγ heterodimer by the coadministration of their ligands is clinically useful for the prevention and/or treatment of colon cancer as well as colonic inflammation [72], due to their synergistic effects on the COX-2/PGE2 axis.
8. SYNERGY BETWEEN PPARs LIGAND AND THE OTHER DRUGS EXCEPT FOR RETINOIDS IN CANCER
In addition to the retinoids, the synergistic effects of PPARγ ligands with other agents have also been reported by many investigators. Girnun et al. [73] found that agonist activation of PPARγ synergistically increases the growth-inhibitory effect of the platinum-based drugs cisplatin and carboplatin in several different types of cancers in both in vivo and in vitro studies. This synergy is associated with the reduction of multiple members of the metallothionein gene family expression, which play a role in the resistance of certain cancers to platinum-based drugs [74] by PPARγ [73]. The synergistic or enhancing effects induced by the combination of PPAR ligands plus other conventional chemotherapeutic agents to inhibit cell growth are also reported in several types of cancer cells [75–77]. In addition, it is also reported that the histone deacetylase (HDAC) inhibitors have a synergistic effect with the thiazolidinediones in the activation of PPARγ target genes [78]. In studies using cancer cells, the combination treatment using the PPARγ agonist pioglitazone and the HDAC inhibitor valproic acid has been reported to be more efficient at inhibiting prostate tumor growth than each individual therapy alone [79]. An enhanced growth inhibition is observed when neuroblastoma cells are treated with a PPARγ ligand and a HDAC inhibitor, thus suggesting that a combination therapy to treat neuroblastoma might prove more effective than using either agent alone [80]. These findings suggest that a combination therapy using PPARγ agonists and HDAC inhibitors might therefore be potentially effective for the treatment of some types of human malignancies.
Recently, molecular-targeted therapy is attractive as a new effective strategy to inhibit the growth of cancer cells, and therefore, the combination therapy using such specific molecular-targeting agents plus PPAR ligands may become an important regimen in near future. For instance, the proteasome inhibitor bortezomib, which can inhibit the NF-κB activity, augments the antiproliferative effects of the PPARγ agonist rosiglitazone in human melanoma cells [81]. The dual ligand specific for PPARα/γ synergistically enhanced the antiproliferative and proapoptotic effect of imatinib, a specific inhibitor of BCR-ABL tyrosine kinase, in Philadelphia chromosome-positive lymphocytic leukemia and chronic myelogenous leukemia blast crisis cell lines [82, 83]. The growth inhibitory effects by gefitinib, an EGFR tyrosine kinase inhibitor, on the human lung cancer cell line are potentiated by the treatment with PPARγ ligand, and this is associated with an increase in the expression of PTEN, but a reduction in the expression of p-Akt [84]. It is interesting to note that the activation of PPARγ by its ligand causes a dramatic inhibition of the tyrosine phosphorylation of HER2 and HER3 receptors, the other member for the EGFR family of receptor tyrosine kinases (RTKs), in human breast cancer cells [85]. The PPARγ ligand also blocks phosphorylation of other member of RTKs, such as IGF-1R, thereby suppressing the proliferation of breast cancer cell lines [85]. These findings may explain the mechanisms in regard to precisely how the PPARγ ligands can enhance the effects of specific RTK inhibitors, although some other molecules may also play a role.
9. IS PPAR PHOSPHORYLATION ASSOCIATED WITH CANCER?
As mentioned above, a malfunction of RXRα due to phosphorylation is associated with cancer cell growth and retinoid resistance [11, 22, 27, 29]. However, a question which arises here is whether the phosphorylation of PPARs also plays a role in carcinogenesis and/or resistance to the PPAR ligands. Recent studies have shown that PPARs are phosphoproteins, and their transcriptional activity is affected by several kinases, including ERK/MAPK, both in a ligand-dependent and/or -independent manner [86]. Although the significance of the PPARs phosphorylation in cancer has not been clarified, at least in PPARγ, the transcriptional activity of this receptor is inhibited by phosphorylation [87–89]. Extracellular signals that activate intracellular phosphorylation pathways can influence the degradation process of PPARγ [89, 90]. These reports may suggest that as well as RXRs [22, 27], the phosphorylation-mediated inhibition of transcriptional activity of PPARs is associated with cancer [20]. Hedvat et al. [91] conducted an interesting study, reporting that the activation of PPARγ is sustained by the presence of HER-kinase inhibitor, suggesting that HER-kinase and its downstream ERK/MAPK pathway phosphorylate PPARγ and, therefore, abrogate the effects of PPARγ activity through degradation of this nuclear receptor. In this study, the inhibition of HER-kinase activity was sufficient to inhibit PPARγ protein degradation [91]. These findings suggest that, in future studies, the combination of PPARγ/RXR ligands plus a specific agent which targets the RTKs and/or Ras/MAPK signaling pathway may therefore become a promising strategy to inhibit the growth of cancer cells by inhibiting the phosphorylation of PPARs/RXRs.
10. CONCLUSION
The combined use of two or more agents is often advantageous since it may permit to lower the clinical dosages, thereby decreasing the overall toxicity, and thus providing the potential for synergistic effects between agents. In this review, we made an attempt to show the synergism between PPARγ agonists and other agents (see Figure 3). Among such preferable candidates, retinoids seem to be the best partner of PPARγ ligands in order to exert synergistic antitumor effects. However, phosphorylation of RXRα represses the PPARγ/RXR-dependent anticancer effects. In some cases, the inhibition of PPAR phosphorylation may also support the antitumor function of these nuclear receptors. In addition, we should keep in mind the fact that the encouraging results obtained from the combined use of PPARγ agonists plus other agents have been exclusively reported in preclinical studies, and PPAR agonist monotherapy did not achieve a significant result for advanced malignancies in clinical trials, except for a small trial on liposarcomas [7–10]. Therefore, it might be expected that some combination with other agents may lead to breakthrough in the clinical application of PPARγ agonists for chemoprevention and/or treatment of malignancies.
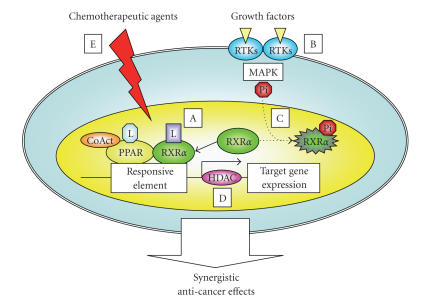
Figure 3.
A hypothetical schematic representation of the synergistic anticancer effects of the combination of PPAR ligands plus other agents. When PPARs are activated by ligand binding, they are able to heterodimerize with RXR and activate the target gene expression by binding to the PPRE element. Therefore, the retinoids which bind to RXR may be the most preferable partner for the PPAR agonists (A). However, in some types of cancers, the MAPK pathway phosphorylates RXRα, and the accumulated nonfunctional p-RXRα interferes with the function of the remaining normal RXRα, thereby promoting the growth of cancer cells. The activation of RTKs by their specific ligands (growth factors) can play a critical role in the stimulation of the MAPK pathway. Therefore, the agents which target the activation of RTKs (B) and/or the MAPK pathway (C) restore the function of RXRα as a master regulator of nuclear receptors in cancer cells and this will support the synergistic growth inhibition by PPAR and RXR ligands in cancer cells. The HDACs enforce a tight chromatin structure and thereby repress the transcription of target genes controlled by PPAR/RXR. Therefore, the combination of a PPAR agonist plus an HDAC inhibitor is more efficient to inhibit the growth of cancer cells (D). Finally, the conventional chemotherapeutic agents also cause synergistic or enhancing effects to inhibit cancer cell growth by the combination of PPAR ligands (E). L: ligand.
In conclusion, the combination treatment using the PPARγ agonists and other agents might be an effective and promising strategy for chemoprevention and/or treatment of various types of cancers. Future studies will be necessary to improve the anticancer efficacy of PPARγ agonist plus retinoids by combining with appropriate specific kinase inhibitors.
ACKNOWLEDGMENT
This work was supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan (No. 18790457 to M. S. and No. 17015016 to H. M.).
ABBREVIATIONS
- PPAR:
Peroxisome proliferator-activated receptor
- PPRE:
Peroxisome proliferator responsive element
- RXR:
Retinoid X receptor
- RAR:
Retinoic acid receptor
- p-RXRα:
Phosphorylated RXRα
- RXRE:
Retinoid X receptor responsive element
- RARE:
Retinoic acid receptor responsive element
- DBD:
DNA-binding domain
- LBD:
Ligand-binding domain
- VDR:
Vitamin D3 receptor
- RA:
Retinoic acid
- ACR:
Acyclic retinoid
- ATRA:
All-trans retinoic acid
- MAPK:
Mitogen-activated protein kinase
- ERK:
Extracellular signal-related kinase
- JNK:
c-Jun N-terminal kinase
- HCC:
Hepatocellular carcinoma
- COX-2:
Cyclooxygenase-2
- Akt:
Protein kinase B
- AP-1:
Activator protein-1
- NF-κB:
Nuclear factor-κB
- PGE2:
Prostaglandin E2
- RTK:
Receptor tyrosine kinase
- HDAC:
Histone deacetylase
- HER:
Human epidermal growth factor receptor.
References
- 1.Lefebvre P, Chinetti G, Fruchart J-C, Staels B. Sorting out the roles of PPARα in energy metabolism and vascular homeostasis. Journal of Clinical Investigation. 2006;116(3):571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemberger T, Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: a nuclear receptor signaling pathway in lipid physiology. Annual Review of Cell and Developmental Biology. 1996;12:335–363. doi: 10.1146/annurev.cellbio.12.1.335. [DOI] [PubMed] [Google Scholar]
- 3.Chinetti G, Fruchart J-C, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflammation Research. 2000;49(10):497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- 4.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nature Reviews Cancer. 2004;4(1):61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 5.Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor γ agonists. Lancet Oncology. 2004;5(7):419–429. doi: 10.1016/S1470-2045(04)01509-8. [DOI] [PubMed] [Google Scholar]
- 6.Sertznig P, Seifert M, Tilgen W, Reichrath J. Present concepts and future outlook: function of peroxisome proliferator-activated receptors (PPARs) for pathogenesis, progression, and therapy of cancer. Journal of Cellular Physiology. 2007;212(1):1–12. doi: 10.1002/jcp.20998. [DOI] [PubMed] [Google Scholar]
- 7.Demetri GD, Fletcher CDM, Mueller E, et al. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-γ ligand troglitazone in patients with liposarcoma. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(7):3951–3956. doi: 10.1073/pnas.96.7.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burstein HJ, Demetri GD, Mueller E, Sarraf P, Spiegelman BM, Winer EP. Use of the peroxisome proliferator-activated receptor (PPAR) γ ligand troglitazone as treatment for refractory breast cancer: a phase II study. Breast Cancer Research and Treatment. 2003;79(3):391–397. doi: 10.1023/a:1024038127156. [DOI] [PubMed] [Google Scholar]
- 9.Kulke MH, Demetri GD, Sharpless NE, et al. A phase II study of troglitazone, an activator of the PPARγ receptor, in patients with chemotherapy-resistant metastatic colorectal cancer. Cancer Journal. 2002;8(5):395–399. doi: 10.1097/00130404-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Smith MR, Manola J, Kaufman DS, et al. Rosiglitazone versus placebo for men with prostate carcinoma and a rising serum prostate-specific antigen level after radical prostatectomy and/or radiation therapy. Cancer. 2004;101(7):1569–1574. doi: 10.1002/cncr.20493. [DOI] [PubMed] [Google Scholar]
- 11.Yamazaki K, Shimizu M, Okuno M, et al. Synergistic effects of RXRα and PPARγ ligands to inhibit growth in human colon cancer cells—phosphorylated RXRα is a critical target for colon cancer management. Gut. 2007;56(11):1557–1563. doi: 10.1136/gut.2007.129858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambon P. A decade of molecular biology of retinoic acid receptors. The FASEB Journal. 1996;10(9):940–954. [PubMed] [Google Scholar]
- 13.Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358(6389):771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tontonoz P, Singer S, Forman BM, et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor γ and the retinoid X receptor. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(1):237–241. doi: 10.1073/pnas.94.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szanto A, Narkar V, Shen Q, Uray IP, Davies PJA, Nagy L. Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death & Differentiation. 2004;11(supplement 2):S126–S143. doi: 10.1038/sj.cdd.4401533. [DOI] [PubMed] [Google Scholar]
- 17.Bruck N, Bastien J, Bour G, et al. Phosphorylation of the retinoid X receptor at the omega loop, modulates the expression of retinoic-acid-target genes with a promoter context specificity. Cellular Signalling. 2005;17(10):1229–1239. doi: 10.1016/j.cellsig.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Gianní M, Tarrade A, Nigro EA, Garattini E, Rochette-Egly C. The AF-1 and AF-2 domains of RARγ2 and RXRα cooperate for triggering the transactivation and the degradation of RARγ2/RXRα heterodimers. Journal of Biological Chemistry. 2003;278(36):34458–34466. doi: 10.1074/jbc.M304952200. [DOI] [PubMed] [Google Scholar]
- 19.Bastien J, Adam-Stitah S, Plassat JL, Chambon P, Rochette-Egly C. The phosphorylation site located in the A region of retinoic X receptor α is required for the antiproliferative effect of retinoic acid (RA) and the activation of RA target genes in F9 cells. Journal of Biological Chemistry. 2002;277(32):28683–28689. doi: 10.1074/jbc.M203623200. [DOI] [PubMed] [Google Scholar]
- 20.Rochette-Egly C. Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cellular Signalling. 2003;15(4):355–366. doi: 10.1016/s0898-6568(02)00115-8. [DOI] [PubMed] [Google Scholar]
- 21.Lee H-Y, Suh Y-A, Robinson MJ, et al. Stress pathway activation induces phosphorylation of retinoid X receptor. Journal of Biological Chemistry. 2000;275(41):32193–32199. doi: 10.1074/jbc.M005490200. [DOI] [PubMed] [Google Scholar]
- 22.Matsushima-Nishiwaki R, Okuno M, Adachi S, et al. Phosphorylation of retinoid X receptor α at serine 260 impairs its metabolism and function in human hepatocellular carcinoma. Cancer Research. 2001;61(20):7675–7682. [PubMed] [Google Scholar]
- 23.Solomon C, White JH, Kremer R. Mitogen-activated protein kinase inhibits 1,25-dihydroxyvitamin D3- dependent signal transduction by phosphorylating human retinoid X receptor α . Journal of Clinical Investigation. 1999;103(12):1729–1735. doi: 10.1172/JCI6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nature Reviews Cancer. 2001;1(3):181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- 25.Matsushima-Nishiwaki R, Okuno M, Takano Y, Kojima S, Friedman SL, Moriwaki H. Molecular mechanism for growth suppression of human hepatocellular carcinoma cells by acyclic retinoid. Carcinogenesis. 2003;24(8):1353–1359. doi: 10.1093/carcin/bgg090. [DOI] [PubMed] [Google Scholar]
- 26.Adachi S, Okuno M, Matsushima-Nishiwaki R, et al. Phosphorylation of retinoid X receptor suppresses its ubiquitination in human hepatocellular carcinoma. Hepatology. 2002;35(2):332–340. doi: 10.1053/jhep.2002.31164. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimura K, Muto Y, Shimizu M, et al. Phosphorylated retinoid X receptor α loses its heterodimeric activity with retinoic acid receptor β . Cancer Science. 2007;98(12):1868–1874. doi: 10.1111/j.1349-7006.2007.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moriwaki H, Shimizu M, Okuno M, Nishiwaki-Matsushima R. Chemoprevention of liver carcinogenesis with retinoids: basic and clinical aspects. Hepatology Research. 2007;37(supplement 2):S299–S302. doi: 10.1111/j.1872-034X.2007.00201.x. [DOI] [PubMed] [Google Scholar]
- 29.Kanemura N, Tsurumi H, Okuno M, Matsushima-Nishiwaki R, Shimizu M, Moriwaki H. Retinoid X receptor α is highly phosphorylated in retinoic acid-resistant HL-60R cells and the combination of 9-cis retinoic acid plus MEK inhibitor induces apoptosis in the cells. Leukemia Research. 2008;32(6):884–892. doi: 10.1016/j.leukres.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Lattuada D, Viganó P, Mangioni S, et al. Accumulation of retinoid X receptor-α in uterine leiomyomas is associated with a delayed ligand-dependent proteasome-mediated degradation and an alteration of its transcriptional activity. Molecular Endocrinology. 2007;21(3):602–612. doi: 10.1210/me.2006-0206. [DOI] [PubMed] [Google Scholar]
- 31.Adam-Stitah S, Penna L, Chambon P, Rochette-Egly C. Hyperphosphorylation of the retinoid X receptor α by activated c-Jun NH2-terminal kinases. Journal of Biological Chemistry. 1999;274(27):18932–18941. doi: 10.1074/jbc.274.27.18932. [DOI] [PubMed] [Google Scholar]
- 32.Egea PF, Mitschler A, Rochel N, Ruff M, Chambon P, Moras D. Crystal structure of the human RXRα ligand-binding domain bound to its natural ligand: 9-cis retinoic acid. The EMBO Journal. 2000;19(11):2592–2601. doi: 10.1093/emboj/19.11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macoritto M, Nguyen-Yamamoto L, Huang DC, et al. Phosphorylation of the human retinoid X receptor α at serine 260 impairs coactivator(s) recruitment and induces hormone resistance to multiple ligands. The Journal of Biological Chemistry. 2008;283(8):4943–4956. doi: 10.1074/jbc.M707517200. [DOI] [PubMed] [Google Scholar]
- 34.Yamada Y, Shidoji Y, Fukutomi Y, et al. Positive and negative regulations of albumin gene expression by retinoids in human hepatoma cell lines. Molecular Carcinogenesis. 1994;10(3):151–158. doi: 10.1002/mc.2940100306. [DOI] [PubMed] [Google Scholar]
- 35.Araki H, Shidoji Y, Yamada Y, Moriwaki H, Muto Y. Retinoid agonist activities of synthetic geranyl geranoic acid derivatives. Biochemical and Biophysical Research Communications. 1995;209(1):66–72. doi: 10.1006/bbrc.1995.1471. [DOI] [PubMed] [Google Scholar]
- 36.Muto Y, Moriwaki H. Antitumor activity of vitamin A and its derivatives. Journal of the National Cancer Institute. 1984;73(6):1389–1393. [PubMed] [Google Scholar]
- 37.Nakamura N, Shidoji Y, Yamada Y, Hatakeyama H, Moriwaki H, Muto Y. Induction of apoptosis by acyclic retinoid in the human hepatoma-derived cell line, HuH-7. Biochemical and Biophysical Research Communications. 1995;207(1):382–388. doi: 10.1006/bbrc.1995.1199. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura N, Shidoji Y, Moriwaki H, Muto Y. Apoptosis in human hepatoma cell line induced by 4,5-didehydro geranylgeranoic acid (acyclic retinoid) via down-regulation of transforming growth factor-α . Biochemical and Biophysical Research Communications. 1996;219(1):100–104. doi: 10.1006/bbrc.1996.0188. [DOI] [PubMed] [Google Scholar]
- 39.Suzui M, Masuda M, Lim JTE, Albanese C, Pestell RG, Weinstein IB. Growth inhibition of human hepatoma cells by acyclic retinoid is associated with induction of p21CIP1 and inhibition of expression of cyclin D1. Cancer Research. 2002;62(14):3997–4006. [PubMed] [Google Scholar]
- 40.Suzui M, Shimizu M, Masuda M, Lim JTE, Yoshimi N, Weinstein IB. Acyclic retinoid activates retinoic acid receptor β and induces transcriptional activation of p21CIP1 in HepG2 human hepatoma cells. Molecular Cancer Therapeutics. 2004;3(3):309–316. [PubMed] [Google Scholar]
- 41.Shimizu M, Suzui M, Deguchi A, et al. Synergistic effects of acyclic retinoid and OSI-461 on growth inhibition and gene expression in human hepatoma cells. Clinical Cancer Research. 2004;10(19):6710–6721. doi: 10.1158/1078-0432.CCR-04-0659. [DOI] [PubMed] [Google Scholar]
- 42.Kanamori T, Shimizu M, Okuno M, et al. Synergistic growth inhibition by acyclic retinoid and vitamin K2 in human hepatocellular carcinoma cells. Cancer Science. 2007;98(3):431–437. doi: 10.1111/j.1349-7006.2006.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimizu M, Suzui M, Deguchi A, Lim JTE, Weinstein IB. Effects of acyclic retinoid on growth, cell cycle control, epidermal growth factor receptor signaling, and gene expression in human squamous cell carcinoma cells. Clinical Cancer Research. 2004;10(3):1130–1140. doi: 10.1158/1078-0432.ccr-0714-3. [DOI] [PubMed] [Google Scholar]
- 44.Tsurumi H, Tojo A, Takahashi T, et al. Differentiation induction of human promyelocytic leukemia cells by acyclic retinoid (polyprenoic acid) International Journal of Hematology. 1993;59(1):9–15. [PubMed] [Google Scholar]
- 45.Muto Y, Moriwaki H, Ninomiya M, et al. Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. The New England Journal of Medicine. 1996;334(24):1561–1568. doi: 10.1056/NEJM199606133342402. [DOI] [PubMed] [Google Scholar]
- 46.Muto Y, Moriwaki H, Saito A. Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma. The New England Journal of Medicine. 1999;340(13):1046–1047. doi: 10.1056/NEJM199904013401315. [DOI] [PubMed] [Google Scholar]
- 47.Takai K, Okuno M, Yasuda I, et al. Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma: updated analysis of the long-term follow-up data. Intervirology. 2005;48(1):39–45. doi: 10.1159/000082093. [DOI] [PubMed] [Google Scholar]
- 48.Konopleva M, Elstner E, McQueen TJ, et al. Peroxisome proliferator-activated receptor γ and retinoid X receptor ligands are potent inducers of differentiation and apoptosis in leukemias. Molecular Cancer Therapeutics. 2004;3(10):1249–1262. [PubMed] [Google Scholar]
- 49.Lee Y-R, Yu H-N, Noh E-M, et al. Peroxisome proliferator-activated receptor γ and retinoic acid receptor synergistically up-regulate the tumor suppressor PTEN in human promyeloid leukemia cells. International Journal of Hematology. 2007;85(3):231–237. doi: 10.1532/IJH97.A30615. [DOI] [PubMed] [Google Scholar]
- 50.Ray DM, Bernstein SH, Phipps RP. Human multiple myeloma cells express peroxisome proliferator-activated receptor γ and undergo apoptosis upon exposure to PPARγ ligands. Clinical Immunology. 2004;113(2):203–213. doi: 10.1016/j.clim.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Hirase N, Yanase T, Mu Y, et al. Thiazolidinedione induces apoptosis and monocytic differentiation in the promyelocytic leukemia cell line HL60. Oncology. 1999;57(supplement 2):17–26. doi: 10.1159/000055271. [DOI] [PubMed] [Google Scholar]
- 52.Crowe DL, Chandraratna RA. A retinoid X receptor (RXR)-selective retinoid reveals that RXR-α is potentially a therapeutic target in breast cancer cell lines, and that it potentiates antiproliferative and apoptotic responses to peroxisome proliferator-activated receptor ligands. Breast Cancer Research. 2004;6(5):R546–R555. doi: 10.1186/bcr913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mehta RG, Williamson E, Patel MK, Koeffler HP. A ligand of peroxisome proliferator-activated receptor γ, retinoids, and prevention of preneoplastic mammary lesions. Journal of the National Cancer Institute. 2000;92(5):418–423. doi: 10.1093/jnci/92.5.418. [DOI] [PubMed] [Google Scholar]
- 54.Elstner E, Williamson EA, Zang C, et al. Novel therapeutic approach: ligands for PPARγ and retinoid receptors induce apoptosis in bcl-2-positive human breast cancer cells. Breast Cancer Research and Treatment. 2002;74(2):155–165. doi: 10.1023/a:1016114026769. [DOI] [PubMed] [Google Scholar]
- 55.Rubin GL, Duong JH, Clyne CD, et al. Ligands for the peroxisomal proliferator-activated receptor γ and the retinoid X receptor inhibit aromatase cytochrome P450 (CYP19) expression mediated by promoter II in human breast adipose. Endocrinology. 2002;143(8):2863–2871. doi: 10.1210/endo.143.8.8932. [DOI] [PubMed] [Google Scholar]
- 56.Saidi SA, Holland CM, Charnock-Jones DS, Smith SK. In vitro and in vivo effects of the PPAR-alpha agonists fenofibrate and retinoic acid in endometrial cancer. Molecular Cancer. 2006;5, article 13:1–14. doi: 10.1186/1476-4598-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.James SY, Lin F, Kolluri SK, Dawson MI, Zhang X-K. Regulation of retinoic acid receptor β expression by peroxisome proliferator-activated receptor γ ligands in cancer cells. Cancer Research. 2003;63(13):3531–3538. [PubMed] [Google Scholar]
- 58.Cesario RM, Stone J, Yen W-C, Bissonnette RP, Lamph WW. Differentiation and growth inhibition mediated via the RXR:PPARγ heterodimer in colon cancer. Cancer Letters. 2006;240(2):225–233. doi: 10.1016/j.canlet.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 59.Yang W, Rachez C, Freedman LP. Discrete roles for peroxisome proliferator-activated receptor γ and retinoid X receptor in recruiting nuclear receptor coactivators. Molecular and Cellular Biology. 2000;20(21):8008–8017. doi: 10.1128/mcb.20.21.8008-8017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsao W-C, Wu H-M, Chi K-H, Chang Y-H, Lin W-W. Proteasome inhibitors induce peroxisome proliferator-activated receptor transactivation through RXR accumulation and a protein kinase C-dependent pathway. Experimental Cell Research. 2005;304(1):234–243. doi: 10.1016/j.yexcr.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 61.Park EY, Cho IJ, Kim SG. Transactivation of the PPAR-responsive enhancer module in chemopreventive glutathione S-transferase gene by the peroxisome proliferator-activated receptor-γ and retinoid X receptor heterodimer. Cancer Research. 2004;64(10):3701–3713. doi: 10.1158/0008-5472.CAN-03-3924. [DOI] [PubMed] [Google Scholar]
- 62.Sarraf P, Mueller E, Jones D, et al. Differentiation and reversal of malignant changes in colon cancer through PPARγ . Nature Medicine. 1998;4(9):1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 63.Kitamura S, Miyazaki Y, Shinomura Y, Kondo S, Kanayama S, Matsuzawa Y. Peroxisome proliferator-activated receptor γ induces growth arrest and differentiation markers of human colon cancer cells. Japanese Journal of Cancer Research. 1999;90(1):75–80. doi: 10.1111/j.1349-7006.1999.tb00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shimada T, Kojima K, Yoshiura K, Hiraishi H, Terano A. Characteristics of the peroxisome proliferator activated receptor γ (PPARγ) ligand induced apoptosis in colon cancer cells. Gut. 2002;50(5):658–664. doi: 10.1136/gut.50.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka T, Kohno H, Yoshitani S-I, et al. Ligands for peroxisome proliferator-activated receptors α and γ inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer Research. 2001;61(6):2424–2428. [PubMed] [Google Scholar]
- 66.Yang W-L, Frucht H. Activation of the PPAR pathway induces apoptosis and COX-2 inhibition in HT-29 human colon cancer cells. Carcinogenesis. 2001;22(9):1379–1383. doi: 10.1093/carcin/22.9.1379. [DOI] [PubMed] [Google Scholar]
- 67.Gupta RA, DuBois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nature Reviews Cancer. 2001;1(1):11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 68.Desreumaux P, Dubuquoy L, Nutten S, et al. Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator-activated receptor γ (PPARγ) heterodimer: a basis for new therapeutic strategies. Journal of Experimental Medicine. 2001;193(7):827–838. doi: 10.1084/jem.193.7.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castellone MD, Teramoto H, Gutkind JS. Cyclooxygenase-2 and colorectal cancer chemoprevention: the β-catenin connection. Cancer Research. 2006;66(23):11085–11088. doi: 10.1158/0008-5472.CAN-06-2233. [DOI] [PubMed] [Google Scholar]
- 70.Eisinger AL, Prescott SM, Jones DA, Stafforini DM. The role of cyclooxygenase-2 and prostaglandins in colon cancer. Prostaglandins & Other Lipid Mediators. 2007;82(1–4):147–154. doi: 10.1016/j.prostaglandins.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 71.Lu D, Cottam HB, Corr M, Carson DA. Repression of β-catenin function in malignant cells by nonsteroidal antiinflammatory drugs. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(51):18567–18571. doi: 10.1073/pnas.0509316102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dubuquoy L, Dharancy S, Nutten S, Pettersson S, Auwerx J, Desreumaux P. Role of peroxisome proliferator-activated receptor γ and retinoid X receptor heterodimer in hepatogastroenterological diseases. Lancet. 2002;360(9343):1410–1418. doi: 10.1016/S0140-6736(02)11395-X. [DOI] [PubMed] [Google Scholar]
- 73.Girnun GD, Naseri E, Vafai SB, et al. Synergy between PPARγ ligands and platinum-based drugs in cancer. Cancer Cell. 2007;11(5):395–406. doi: 10.1016/j.ccr.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Akiyama S-I, Chen Z-S, Sumizawa T, Furukawa T. Resistance to cisplatin. Anti-Cancer Drug Design. 1999;14(2):143–151. [PubMed] [Google Scholar]
- 75.Fulzele SV, Chatterjee A, Shaik MS, Jackson T, Ichite N, Singh M. 15-deoxy-Δ12,14-prostaglandin J2 enhances docetaxel anti-tumor activity against A549 and H460 non-small-cell lung cancer cell lines and xenograft tumors. Anti-Cancer Drugs. 2007;18(1):65–78. doi: 10.1097/CAD.0b013e3280101006. [DOI] [PubMed] [Google Scholar]
- 76.Copland JA, Marlow LA, Kurakata S, et al. Novel high-affinity PPARγ agonist alone and in combination with paclitaxel inhibits human anaplastic thyroid carcinoma tumor growth via p21WAF1/CIP1. Oncogene. 2006;25(16):2304–2317. doi: 10.1038/sj.onc.1209267. [DOI] [PubMed] [Google Scholar]
- 77.Budman DR, Calabro A. Studies of synergistic and antagonistic combinations of conventional cytotoxic agents with the multiple eicosanoid pathway modulator LY 293111. Anti-Cancer Drugs. 2004;15(9):877–881. doi: 10.1097/00001813-200410000-00008. [DOI] [PubMed] [Google Scholar]
- 78.Fajas L, Egler V, Reiter R, et al. The retinoblastoma-histone deacetylase 3 complex inhibits PPARγ and adipocyte differentiation. Developmental Cell. 2002;3(6):903–910. doi: 10.1016/s1534-5807(02)00360-x. [DOI] [PubMed] [Google Scholar]
- 79.Annicotte J-S, Iankova I, Miard S, et al. Peroxisome proliferator-activated receptor γ regulates E-cadherin expression and inhibits growth and invasion of prostate cancer. Molecular and Cellular Biology. 2006;26(20):7561–7574. doi: 10.1128/MCB.00605-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Emmans VC, Rodway HA, Hunt AN, Lillycrop KA. Regulation of cellular processes by PPARγ ligands in neuroblastoma cells is modulated by the level of retinoblastoma protein expression. Biochemical Society Transactions. 2004;32(5):840–842. doi: 10.1042/BST0320840. [DOI] [PubMed] [Google Scholar]
- 81.Freudlsperger C, Thies A, Pfüller U, Schumacher U. The proteasome inhibitor bortezomib augments anti-proliferative effects of mistletoe lectin-I and the PPAR-γ agonist rosiglitazone in human melanoma cells. Anticancer Research. 2007;27(1):207–213. [PubMed] [Google Scholar]
- 82.Liu H, Zang C, Fenner MH, et al. Growth inhibition and apoptosis in human Philadelphia chromosome-positive lymphoblastic leukemia cell lines by treatment with the dual PPARα/γ ligand TZD18. Blood. 2006;107(9):3683–3692. doi: 10.1182/blood-2005-05-2103. [DOI] [PubMed] [Google Scholar]
- 83.Zang C, Liu H, Waechter M, et al. Dual PPARα/γ ligand TZD18 either alone or in combination with imatinib inhibits proliferation and induces apoptosis of human CML cell lines. Cell Cycle. 2006;5(19):2237–2243. doi: 10.4161/cc.5.19.3259. [DOI] [PubMed] [Google Scholar]
- 84.Lee SY, Hur GY, Jung KH, et al. PPAR-γ agonist increase gefitinib's antitumor activity through PTEN expression. Lung Cancer. 2006;51(3):297–301. doi: 10.1016/j.lungcan.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 85.Pignatelli M, Cortés-Canteli M, Lai C, Santos A, Perez-Castillo A. The peroxisome proliferator-activated receptor γ is an inhibitor of ErbBs activity in human breast cancer cells. Journal of Cell Science. 2001;114(22):4117–4126. doi: 10.1242/jcs.114.22.4117. [DOI] [PubMed] [Google Scholar]
- 86.Burns KA, Vanden Heuvel JP. Modulation of PPAR activity via phosphorylation. Biochimica et Biophysica Acta. 2007;1771(8):952–960. doi: 10.1016/j.bbalip.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor γ is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. Journal of Biological Chemistry. 1997;272(8):5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- 88.Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ . Science. 1996;274(5295):2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 89.Camp HS, Tafuri SR. Regulation of peroxisome proliferator-activated receptor γ activity by mitogen-activated protein kinase. Journal of Biological Chemistry. 1997;272(16):10811–10816. doi: 10.1074/jbc.272.16.10811. [DOI] [PubMed] [Google Scholar]
- 90.Floyd ZE, Stephens JM. Interferon-γ-mediated activation and ubiquitin-proteasome-dependent degradation of PPARγ in adipocytes. Journal of Biological Chemistry. 2002;277(6):4062–4068. doi: 10.1074/jbc.M108473200. [DOI] [PubMed] [Google Scholar]
- 91.Hedvat M, Jain A, Carson DA, et al. Inhibition of HER-kinase activation prevents ERK-mediated degradation of PPARγ . Cancer Cell. 2004;5(6):565–574. doi: 10.1016/j.ccr.2004.05.014. [DOI] [PubMed] [Google Scholar]