Abstract
PURPOSE
To examine the acquisition of lytic activity and interferon gamma (IFN-γ) production by herpes simplex virus (HSV) type 1–specific CD8+ cytotoxic T-lymphocyte precursors (HSV-CTLps) after exposure to in vitro HSV-1–infected fibroblasts derived from the immunoprivileged cornea (HSV-cFb) or nonprivileged skin (HSV-sFb) or to in vitro HSV-1–infected splenocytes (HSV-Spls) obtained from noninfected mice.
METHODS
Chromium release assays were used to assess HSV-CTL cytotoxicity, and flow cytometry was used to assess intracellular granzyme (Gr) B content and lytic granule exocytosis through surface CD107a expression. In addition, the BLT esterase assay was used to assess functional GrA release. [3H]-Thymidine incorporation and total CD8+ cell numbers, as assessed by flow cytometry, were used to assess CTLp proliferation. ELISA and intracellular flow cytometric analysis were used to assess CTL IFN-γ production and release.
RESULTS
HSV-cFb, HSV-sFb, and HSV-Spl individually induced strong cytotoxic and IFN-γ responses by HSV-CTL. Simultaneous exposure to HSV-Spl and HSV-cFb virtually abrogated the cytotoxic response while enhancing IFN-γ production by HSV-CTL. In contrast, exposure to HSV-sFb, in conjunction with HSV-Spl, did not alter the cytotoxic or IFN-γ response of HSV-CTL compared with stimulation with either cell type alone. Abrogation of the cytotoxic response after simultaneous exposure to HSV-Spl and HSV-cFb was associated with reduced production, storage, or both of GrA and GrB but with unimpaired lytic granule release.
CONCLUSIONS
These findings suggest that an interesting regulatory circuit protects the cornea from the potentially damaging effects of CD8+ T-cell cytotoxic function while maintaining their ability to control virus replication through enhanced production of the antiviral cytokine IFN-γ.
The cornea, like other tissues that compose the visual axis, enjoys a degree of immune privilege. For instance, histo-incompatible corneal grafts are usually accepted.1 When such grafts are rejected, a CD8+ cytotoxic T lymphocyte (CTL) response is not thought to have contributed.2 It is also well documented that an inoculum of DBA-2 mastocytoma cells (P815), which is rapidly rejected by BALB/c mice when administered subcutaneously, grows progressively in the immunoprivileged environment of the anterior chamber of the eye.3,4 CD8+ CTL precursors (CTLps) infiltrate these intraocular tumors but fail to differentiate into effector cells.5 A similar phenomenon has been demonstrated in the mouse brain,6 which is also considered an immunoprivileged site. Together these studies suggest that something within the immunoprivileged microenvironments of the cornea, ocular anterior chamber, and central nervous system may discourage the acquisition of cytotoxic function by CTLp.
CD8+ CTLs have the potential to inhibit herpes simplex virus (HSV) type 1 replication by lysing infected cells and by producing antiviral cytokines, such as INF-γ and tumor necrosis factor (TNF)-α. Localized HSV-1 infection of the mouse results in the activation, expansion, and arming of HSV-1–specific CTLps in draining lymph nodes (DLNs).7–11 However, direct ex vivo cytolytic function is difficult to detect from DLN-derived CD8+ T cells,12 most likely because of low HSV-CTLp frequency, the requirement for further expansion and arming at the peripheral infection site, and rapid emigration of activated CTL effectors from the DLN to the spleen and the site of infection.13 In vitro culture, with or without antigen, of DLN cells leads to rapid expansion and differentiation of CTLps, allowing the detection of cytolytic function.10 Furthermore, the acquisition of cytotoxic effector function has been correlated with cell cycle progression in murine14 and human15 CD8+ T cells.
We have observed that HSV-1 corneal infection results in the expansion of HSV-CTLps in cervical and submandibular lymph nodes that drain the ocular surface.7 Although CD8+ T cells can infiltrate and protect HSV-1–infected skin16,17 and sensory ganglia,18–20 with most strains of HSV-1 they are sparsely present in the infected cornea.21–24 Therefore, we propose that the cornea might represent another immunoprivileged environment in which CTL proliferation and, therefore, further cytolytic arming are discouraged. Our results establish that corneal cells can participate in an interesting regulatory circuit that selectively blocks an event in CD8+ T-cell cytolytic arming while enhancing IFN-γ production.
MATERIALS AND METHODS
HSV-1 Corneal Infection
Female A/J mice (Jackson Laboratories, Bar Harbor, ME), 6 to 8 weeks old, were anesthetized by intraperitoneal injection of 2.0 mg ketamine hydrochloride and 0.04 mg xylazine (Phoenix Scientific, St. Joseph, MO) in 0.2 mL HBSS (BioWhittaker, Walkersville, MD). The purified RE strain of HSV-1 was prepared, and 1 × 105 plaque-forming units were applied to the scarified corneas of A/J mice, as previously described.25 All experimental animal procedures were reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Isolation of CD8+ T Cells from the DLNs of Infected Mice
At day 6 after infection, mice received intraperitoneal injections of 0.5 mg anti–CD4 monoclonal antibody (mAb; clone GK1.5; American Type Culture Collection [ATCC], Manassas, VA) to eliminate possible regulatory influences of CD4+ T cells on CTLp differentiation. The resultant cervical and submandibular DLN cells routinely contained 30% CD8+ T cells and less than 1% CD4+ T cells (CD4− DLN). In some experiments, cell suspension was further enriched for CD8+ cells by negative selection with magnetic beads coated with a rat anti–mouse major histo-compatibility complex (MHC) class II mAb. Bead preparation consisted of incubating goat anti–rat IgG–coated beads (Biomag; PerSeptive Biosystems, Framingham, MA) with rat anti–mouse MHC class II mAb (ATCC no. TIB-120; designation, M5/114.15.2) at a concentration of 1 pg antibody per 5 × 107 beads. The beads were washed and added to the cell suspension at a bead-cell ratio of 50:1. Three rounds of magnetic separation removed the bound cells. Resultant cell suspensions were routinely greater than 95% CD8+ and less than 1% CD4+ T cells, as assessed by flow cytometric analysis (CD8+ DLN).
Isolation of Corneal and Skin Fibroblasts
Fibroblasts were grown from fresh explants of corneas and skin of noninfected mice. Eyes were enucleated, and corneas were sterilely excised under a dissecting microscope. Abdominal skin was sterilized with povidone iodine (Baxter Healthcare Corp., Deerfield, IL) and shaved, and dermal biopsy samples were prepared. Both tissues were cut into small pieces and digested with 150 U/mL collagenase type 1 (Sigma-Aldrich Co., St. Louis, MO) for 45 minutes at 37°C. Tissue pieces were washed and cultured with 1 mL fibroblast growth medium (DMEM containing 10% FBS and 20 mM HEPES) at 37°C in T25 flasks. An additional 2 mL of growth medium was added to each flask at days 3 and 7 of culture. Cells were removed with trypsin and replated at day 10 (passage 1), day 12 (passage 2), and day 14 (passage 3). All cells showed typical fibroblastoid morphology after passage 1. Cells from passage 3 were used as stimulator cells and targets in cytotoxicity and flow cytometric assays.
In Vitro Restimulation of DLN Cells
Spleens were sterilely excised from noninfected mice, and single-cell suspensions were prepared as previously described.7 Spleen cells were infected with HSV-1 at a multiplicity of infection (MOI) of 3 for 1 hour, treated with mitomycin C (0.1 mg/mL) for 20 minutes, and washed 4 times before use as stimulator cells. Fibroblasts derived from the immunoprivileged cornea (cFb) or nonprivileged skin (sFb) were placed in wells of a 24-well tissue culture plate (2.5 × 105 cells/well) and incubated overnight, and confluent monolayers were infected with HSV-1 at an MOI of 0.1 to be used as stimulator cells. DLN cells (2 × 106 cells/well) were suspended in culture medium (10% FCS, 10 mM HEPES, 0.05 mM 2-mercaptoethanol [ME] in RPMI-1640 medium) as a source of CTLps. The CTLps were stimulated for 48 hours with various combinations of HSV-Spl (1 × 106 cells/well), HSV-cFb, and HSV-sFb in the wells of a 24-well tissue culture plate. Unless otherwise indicated, all cultures received 10 U/mL recombinant murine IL-2 (rmIL-2; R&D Systems, Minneapolis, MN). Where indicated, cultures received 100 µg/mL anti–IL-2 receptor (α-IL-2R) mAb (clone 7D4; ATCC). Cultures were incubated for 48 hours. Culture supernatant fluids were removed and analyzed for IFN-γ content using an ELISA assay. In vitro–restimulated DLN cells were isolated on a density gradient (Fico-lite LM; Atlanta Biologicals, Lawrenceville, GA) and were used as effector cells in standard chromium (51Cr) release cytotoxicity assays and flow cytometry–based assays. In some experiments, inserts with cell-impermeable membranes (Transwell; Corning Costar Corp., Cambridge, MA) were used to prevent direct contact between different cell populations while permitting free diffusion of soluble products.
Cytotoxicity Assay
cFbs on their third passage were used as targets for all cytotoxicity assays. Monolayers of cFbs in culture medium containing 0.5 U rmIFN-γ /mL were exposed to 200 µCi 51Cr (Perkin Elmer, Wellesley, MA) for 16 hours. cFb monolayers were then infected with HSV-1 at an MOI of 10 for 2 hours. HSV-cFb was removed with trypsin, washed three times, incubated at 37°C for 1 hour, washed again to minimize spontaneous 51Cr release, and resuspended in assay medium (5% FCS, 10 mM HEPES, 0.05 mM 2-ME in RPMI-1640 medium). Titrated numbers of effector cells and 5 × 103 51Cr-labeled targets were mixed in round-bottomed microtiter plates at various effector-to-target (E:T) ratios. Cytotoxic activity was measured in a 3.5-hour 51Cr release assay, as described previously.7 Percentage of specific lysis was calculated as (experimental release – spontaneous release)/(maximal release – spontaneous release) × 100.
IFN-γ ELISA
Wells of a 96-well enzyme immunoassay (EIA) plate were coated overnight with the capture antibody (rat mAb to mouse IFN-γ; clone R46A2) and then were washed and incubated with blocking solution (3% BSA in PBS) for 2 hours. The sample or standard was incubated in the coated wells overnight. The plate was washed and incubated with the biotinylated detection antibody (rat mAb to IFN-γ; clone XMG1.2) for 1 hour, followed by incubation with streptavidin-conjugated horseradish peroxidase for 30 minutes. Wells were washed, and the substrate, TMB-ELISA, was added. After 3 minutes, the reaction was stopped with 1 N H2SO4, and the amount of reaction product was determined spectrophotometrically. The concentration of IFN-γ in each sample was determined from a standard curve obtained with rmIFN-γ (Biosource International, Camarillo, CA).
Flow Cytometry
Cultures of DLN cells were prepared as described except that the mitomycin C–treated HSV-Spls were stained (DiI; Molecular Probes, Carlsbad, CA) according to the manufacturer’s protocol, permitting exclusion of these cells on analysis. After 48 hours of culture, cells were either stained for surface makers and intracellular granzyme (Gr) B or stimulated for another 6 hours with HSV-cFb in the presence of a protein transport inhibitor (GolgiPlug; BD Biosciences, Franklin Lakes, NJ) and stained for surface CD107a (LAMP-1, a marker of lytic granule exocytosis) and intracellular IFN-γ. Nonspecific staining was inhibited by pretreatment of cells with anti–mouse CD16/CD32 Ab (Fc III/II receptor; clone 2.4G2; BD PharMingen, San Diego, CA) before incubation with fluorochrome-conjugated antibodies for 30 minutes. The following antibodies were used for surface staining (all from BD Phar-Mingen): PE-Cy7–conjugated anti–CD8α (Ly-2; clone 53–6.7), PE-conjugated anti–CD4 (clone RM4–5), PerCP-conjugated anti–CD45 (30-F11), FITC-conjugated anti–CD107a (clone 1D4B), and their respective isotype control antibodies. Intracellular staining was performed (BD Cytofix/Cytoperm Kit; BD Biosciences) according to the manufacturer’s instructions. Antibodies for intracellular staining included APC-conjugated GrB (clone GB12) and its isotype (Caltag, Carlsbad, CA) and APC-conjugated IFN-γ (clone XMG1.2) and its isotype (BD Phar-Mingen).
[3H]-Thymidine Incorporation
Enriched CD8+ T cells were cultured for 72 hours in wells of 96-well culture plates, as described, with volumes and cell numbers adjusted for the smaller culture well surface area. Cells were pulsed with [3H]-thymidine (1 µCi/well) for the final 6 hours of culture. Cells were then harvested onto glass fiber filter strips, and [3H]-thymidine incorporation was measured with a β-scintillation counter (1450 Microbeta Wallac Trilux; Perkin Elmer, Wellesley, MA).
BLT Serine Esterase (Granzyme A) Assay
To assess functional GrA release, enriched CD8+ T cells from the specified culture conditions were washed, resuspended in 30-µL assay medium (5% FCS, 10 mM HEPES, 0.05 mM 2-ME in RPMI-1640 medium) for spontaneous release or assay medium containing phorbol 12-myristate 13-acetate (PMA) and ionomycin for experimental release, and incubated for 4 hours at 37°C. Cells were then centrifuged, and 20 µL supernatant from each condition was mixed with 180 µL Tris-buffer containing N-α-benzyloxycarbonyl-L-lysine thiobenzyl ester (BLT) and incubated at 37°C for 20 minutes. The resultant color reaction was measured spectrophotometrically at 405 nm with a microplate auto-reader (EL311; BioTek, Winooski, VT).
Statistical Analysis
Software (Prism; GraphPad, San Diego, CA) was used for all statistical analyses. Where indicated, P values were calculated using the Student t test when comparing two groups or one-way ANOVA with Bonferroni posttest when comparing more than two groups. P < 0.05 was considered significant.
RESULTS
Inhibition of HSV-CTLp Differentiation by HSV-cFbs
HSV-Spls are commonly used as stimulator cells to induce HSV-CTLp differentiation into cytotoxic effector cells in vitro. Therefore, we compared CTLp differentiation after 48 hours of restimulation with HSV-Spls or HSV-cFbs as stimulator cells. Unless otherwise indicated, all cultures received exogenous IL-2. For all experiments, the source of HSV-CTLp was DLN cells obtained 8 days after HSV-1 corneal infection. DLN cells were enriched for CD8+ T cells by depletion of CD4+ cells only (subsequently referred to as CD4− DLN) or of CD4+ and MHC class II+ cells (subsequently referred to as CD8+ DLN). HSV-cFbs were used as targets in all cytotoxicity assays.
Stimulation of CD4− DLN cells with HSV-Spls or HSV-cFbs induced a strong cytotoxic response against HSV-1–infected targets, though the response to HSV-cFbs was slightly weaker than that induced by HSV-Spls (Fig. 1A). Interestingly, simultaneous exposure of the same CD4− DLN cells to a combination of HSV-Spls and HSV-cFbs resulted in almost complete abrogation of the cytotoxic response. A similar pattern of CTL activity was observed after stimulation of CD8+ DLN cells. Stimulation of CD8+ DLN cells with HSV-Spls or HSV-cFbs individually induced a strong cytotoxic response to HSV-infected targets, whereas simultaneous stimulation with HSV-Spls and HSV-cFbs failed to induce a cytotoxic response (Fig. 1B). Because the patterns of CTL activity produced by the two CD8+-enriched DLN populations were nearly identical, the two DLN populations were used interchangeably in subsequent experiments.
FIGURE 1.
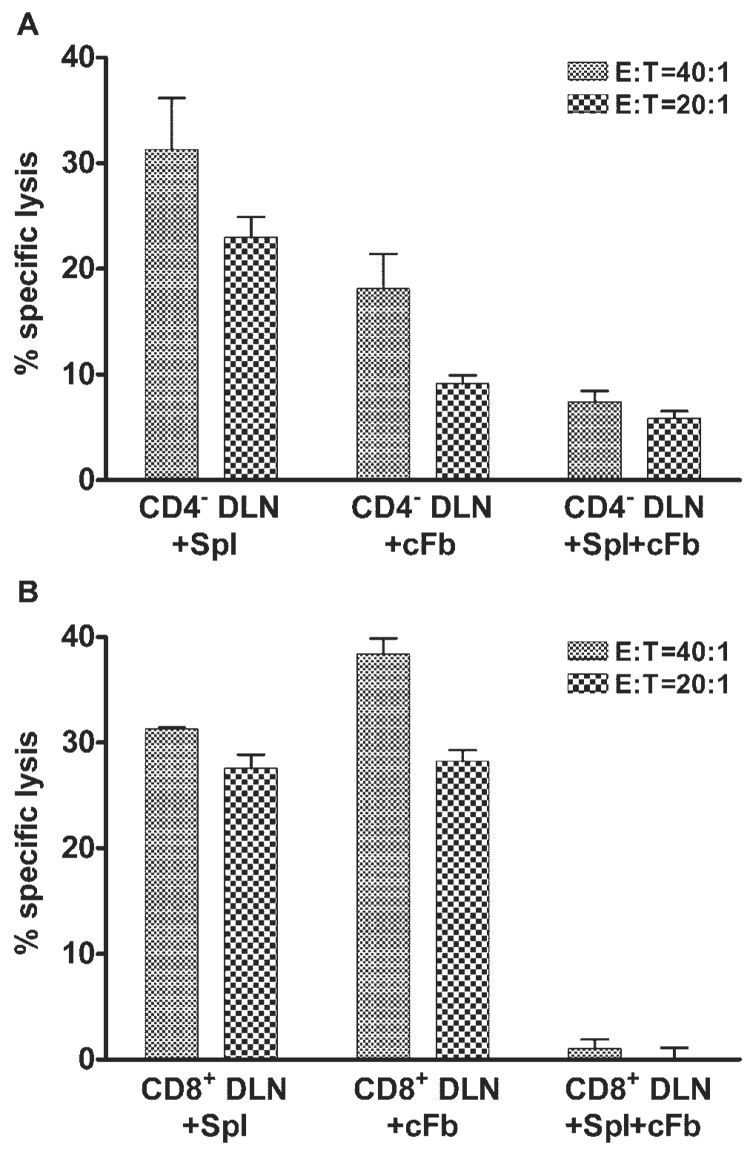
CTLp differentiation is altered by simultaneous exposure to HSV-1–infected corneal fibroblasts (cFb) and splenocytes (Spl). Eight days after HSV-1 corneal infection, DLN suspensions enriched for CD8+ T cells by CD4 depletion only (CD4− DLN; A) or by CD4 and MHC class II depletion (CD8+ DLN; B) were cultured for 48 hours with 1 × 106 HSV-1–infected Spls alone, with a monolayer of HSV-1– infected cFbs alone, or with Spls and cFbs. Cells were then removed from the cultures and exposed to 51Cr-loaded HSV-1–infected target cells. Cytotoxicity was measured in a 3.5-hour 51Cr release assay. Percentage of specific lysis against uninfected targets was less than 5% for all culture conditions at all E:T ratios. Data are presented as mean ± SEM of triplicate samples and are representative of three independent experiments with nearly identical results. (A) Statistically significant differences were obtained for the following comparisons: at E:T = 40:1, CD4− DLN+Spl+cFb versus CD4− DLN+Spl (P < 0.01); and at E:T = 20:1, CD4− DLN+Spl+cFb versus CD4− DLN+Spl or CD4− DLN+cFb (P < 0.001). (B) Statistically significant differences were obtained for the following comparisons: at E:T = 40:1, CD8+ DLN+Spl+cFb versus CD8+ DLN+Spl (P < 0.001) or CD8+ DLN+cFb (P < 0.01); and at E:T = 20:1, CD8+ DLN+Spl+cFb versus CD8+ DLN+Spl or CD8+ DLN+cFb (P < 0.001).
Inhibition of HSV-CTLp Proliferation by HSV-cFbs
Acquisition of cytotoxic function was associated with blast transformation, as assessed by FACS forward versus side scatter, and enhanced proliferation of CD8+ T cells. However, CD4− DLN cells that were simultaneously stimulated with HSV-Spls and HSV-cFbs had approximately half the number of blasting cells as those stimulated with HSV-Spls or HSV-cFbs alone (Figs. 2A, 2B). Consistent with these observations, CD8+ DLN cells incorporated approximately half as much [3H]-thymidine when exposed to both types of stimulator cells compared with those cultured with HSV-Spls alone (Fig. 2C).
FIGURE 2.
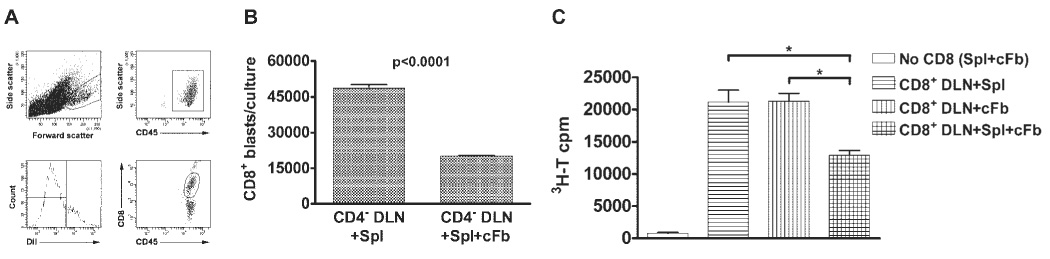
CD8+ T-cell blast transformation and proliferation are inhibited by simultaneous exposure to HSV-1–infected corneal fibroblasts (cFb) and splenocytes (Spl). Eight days after HSV-1 corneal infection, DLN suspensions enriched for CD8+ T cells by CD4 depletion only (CD4− DLN; A, B) or CD4 and MHC class II depletion (CD8+ DLN; C) were cultured with 1 × 106 HSV-1–infected DiI-labeled (A, B) or unlabeled (C) Spls alone, with a monolayer of HSV-1–infected cFbs alone, or with Spls and cFbs. (A) Cells were then removed from the cultures after 48 hours, stained for surface CD45 and CD8α, and analyzed by flow cytometry. Cells undergoing blast transformation were identified based on forward angle and side scatter, and CD45 was used to distinguish bone marrow–derived cells including CD8+ T cells. Cells derived from the DLN and Spls were differentiated based on DiI staining, and blasting DiI− CD8+ T cells were gated for further analysis. (B) The percentage of blasting DiI− CD8+ T cells was multiplied by the total number of cells retrieved from each culture to obtain the mean ± SEM absolute number of blasting DLN-derived CD8+ T cells per culture. The graph contains combined data from duplicate cultures of two independent experiments. (C) 3H-Thymidine (3H-T) incorporation into cellular DNA was measured during the last 6 hours of a 72-hour culture as a measure of cellular proliferation. Data are recorded as the mean ± SEM 3H-T cpm in triplicate cultures of each group and are representative of three independent experiments with nearly identical results. *P < 0.001.
We hypothesized that an increased rate of apoptosis might also have contributed to the reduced number of CD8+ blasts and reduced cytotoxic activity observed in cultures of CD8+ cells that were exposed to HSV-Spls and HSV-cFbs. To test this hypothesis, cultures were stained with Annexin V, which binds phosphatidylserine, a marker of early apoptosis. Low and equal frequency of CD8+ Annexin V+ apoptotic cells (less than 5%) was detected in cultures of CD8+ DLN cells exposed to HSV-Spls, HSV-cFbs, or both cell types (data not shown). Thus, differential rates of apoptosis do not account for the differences in expansion of HSV-CTL in these cultures.
HSV-cFbs Inhibit Development of Functional Lytic Granules in HSV-CTL
To identify changes in the lytic machinery of CD8+ T cells on simultaneous exposure to HSV-Spls and HSV-cFbs, we assessed intracellular GrB content by fluorescence-activated cell sorter (FACS) staining (Fig. 3A). Both the percentage (Fig. 3B) and the absolute number (FIG. 3C) of CD8+ T cells containing GrB were significantly reduced in CD4+ DLN cultures containing HSV-Spls and HSV-cFbs compared with CD4− DLN cultures containing only HSV-Spls.
FIGURE 3.
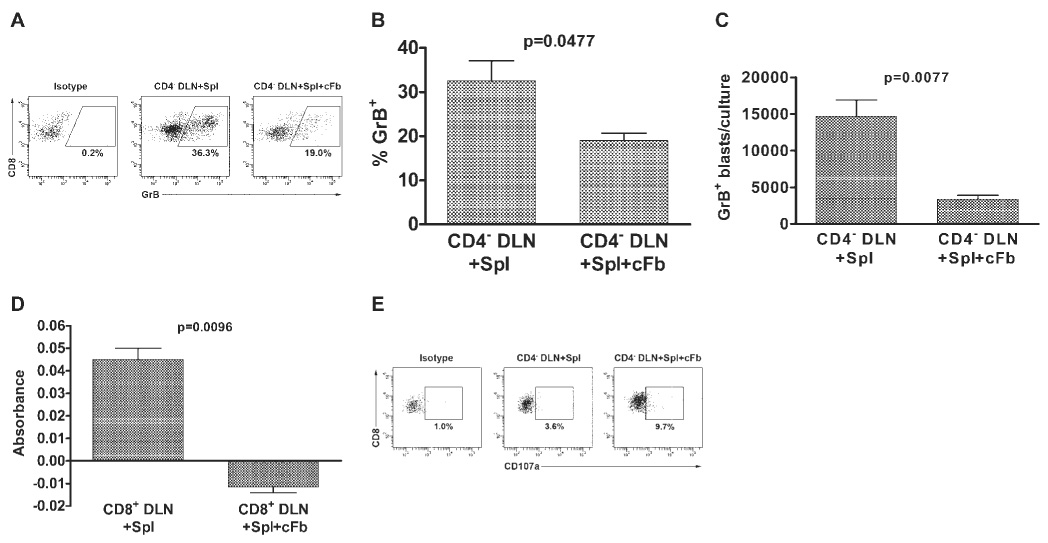
Simultaneous exposure to HSV-1–infected corneal fibroblasts (cFb) and splenocytes (Spl) inhibits cytolytic arming but not degranulation of CTLps. Eight days after HSV-1 corneal infection, DLN suspensions enriched for CD8+ T cells by CD4 depletion only (CD4− DLN; A–C, E) or CD4 and MHC class II depletion (CD8+ DLN; D) were cultured for 48 hours with 1 × 106 HSV-1–infected DiI-labeled (A–C, E) or unlabeled (D) Spls alone, with a monolayer of HSV-1–infected cFbs alone, or with Spls and cFbs. (A) Cells were then removed from the cultures, stained for surface CD8α and intracellular GrB, and analyzed by flow cytometry. Cells undergoing blast transformation were identified based on forward angle and side scatter, and CD8+ T cells derived from the DLN and Spls were differentiated based on DiI staining. Dot plots are representative of duplicate cultures from two independent experiments. The percentage of blasting DiI− CD8+ GrB+ T cells (B) was multiplied by the total number of cells retrieved from each culture to obtain the mean ± SEM absolute number of blasting DLN-derived CD8+ GrB+ T cells per culture (C). Graphs contain combined data from duplicate cultures of two independent experiments. (D) Alternatively, the cells were removed from culture and resuspended in medium alone (spontaneous release) or medium containing PMA and ionomycin (experimental release) for 4 hours. BLT esterase (GrA) activity of culture supernatants was then measured spectrophotometrically. Values represent experimental release with spontaneous release subtracted. Data are presented as the mean ± SEM of triplicate samples and are representative of three independent experiments with nearly identical results. (E) Cells were removed from the cultures and exposed to HSV-1–infected cFb targets at an E:T ratio of 5:1 in the presence of protein transport inhibitor (GolgiPlug) and anti–CD107a mAb for 6 hours. Cells were then washed, stained for surface CD8α, and analyzed by flow cytometry as in (A). Dot plots are representative of two independent experiments.
The BLT serine esterase assay was used to measure functional GrA release as further characterization of the content of CD8+ T-cell lytic granules. Compared with CD8+ DLN cells exposed to HSV-Spls alone, CD8+ DLN cells cultured with HSV-Spls and HSV-cFbs secreted significantly less, if any, functional GrA (Fig. 3D). To rule out the possibility that CD8+ T cells cultured with both types of stimulator cells may be incapable of releasing their lytic granules, we stained for the lysosomal-associated membrane protein 1 (LAMP-1 or CD107a) on the surfaces of these cells during exposure to HSV-1–infected targets in the presence of a protein transport inhibitor (Golgi-Plug; BD Biosciences). Surprisingly, a slightly higher percentage of blasting CD8+ T cells from CD4− DLN cultures that were simultaneously stimulated with HSV-Spls and HSV-cFbs were found to express CD107a compared with blasting CD8+ T cells from CD4− DLN cells cultured with HSV-Spls alone (Fig. 3E). Therefore, it appears that CD8+ T cells cultured with HSV-Spls and HSV-cFbs are capable of degranulating, but their lytic granules are deficient in granzymes known to mediate apoptosis.
HSV-cFbs Enhance HSV-CTL IFN-γ Production
Before the cytotoxicity assays were performed, supernatant fluids were removed from in vitro restimulation cultures and tested for IFN-γ content by ELISA. Although CD4− DLN cells expressed low levels of cytotoxic activity after simultaneous stimulation with HSV-Spl and HSV-cFb, supernatant fluids from these cultures exhibited significantly higher levels of IFN-γ than cultures stimulated with HSV-Spl or HSV-cFb alone (Fig. 4A). Moreover, intracellular FACS staining demonstrated that an increased percentage (Fig. 4B) and absolute number (Fig. 4C) of CD8+ T cells produced IFN-γ in CD4− DLN cultures containing HSV-Spls and HSV-cFbs.
FIGURE 4.
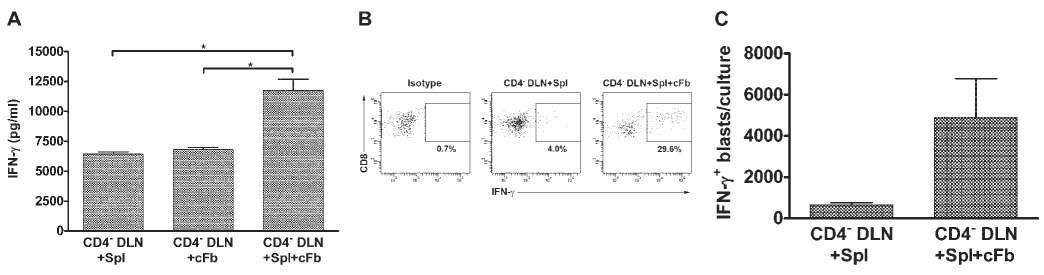
cFbs exposed to spleen cells do not inhibit IFN-γ production by CTLp. Eight days after HSV-1 corneal infection, DLN suspensions enriched for CD8+ T cells by CD4 depletion (CD4− DLN) were cultured for 48 hours with 1 × 106 HSV-1–infected unlabeled (A) or DiI-labeled (B, C) spleen cells (Spl), with a monolayer of HSV-1–infected corneal fibroblasts (cFb), or with Spls and cFbs. (A) IFN-γ production measured by ELISA of culture supernatants. Data are presented as the mean ± SEM and are representative of three independent experiments with similar response patterns. *P < 0.001. (B) Cells were removed from the cultures and exposed to HSV-1–infected cFb targets at an E:T ratio of 5:1 in the presence of GolgiPlug for 6 hours Cells were then washed, stained for surface CD8α and intracellular IFN-γ, and analyzed by flow cytometry. Cells undergoing blast transformation were identified based on forward angle and side scatter, and CD8+ T cells derived from the DLN and Spl were differentiated based on DiI staining. Dot plots are representative of two independent experiments. (C) The percentage of blasting DiI− CD8+ IFN-γ + T cells was multiplied by the total number of cells retrieved from each culture to obtain the mean ± SEM absolute number of blasting DLN-derived CD8+ IFN-γ+ T cells per culture. Graph contains combined data from duplicate cultures of two independent experiments.
Inhibition of CTLp Differentiation Requires Direct Contact with HSV-cFb but Not with HSV-Spl
Transwell cultures were prepared with different combinations of CD8+ DLN cells, HSV-Spls, and HSV-cFbs such that a cell-impermeable membrane separated particular cell types. After 48 hours of incubation, the CD8+ DLN cells were tested for lytic activity against HSV-1–infected targets. Cytotoxic activity was induced when CD8+ DLN cells were in direct contact with HSV-Spls but were separated from HSV-cFbs (Fig. 5A). In contrast, CD8+ DLN cells failed to demonstrate lytic activity when cultured in direct contact with HSV-cFbs and separated from HSV-Spls. Thus, inhibition of the differentiation of CTLps into cytotoxic effector cells appears to require direct contact with HSV-cFbs that are exposed to a soluble factor(s) produced by HSV-Spls.
FIGURE 5.
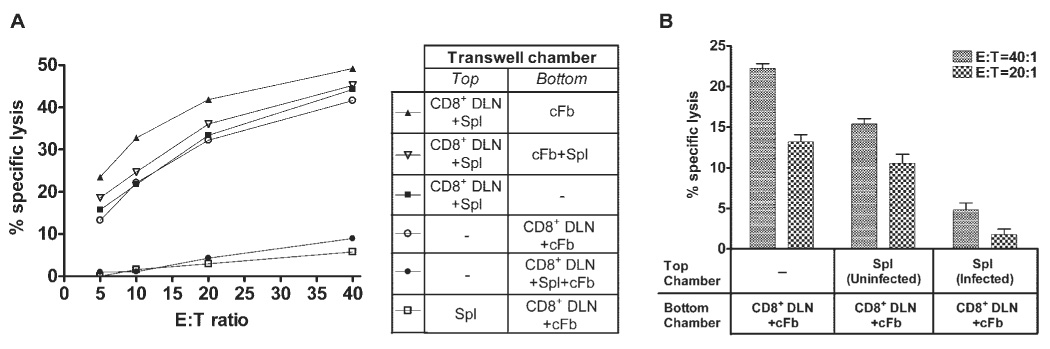
Inhibition of CTLp differentiation requires direct contact with HSV-1–infected corneal fibroblasts (cFb) and HSV-1 infection of spleen cells (Spl). (A) Eight days after HSV-1 corneal infection, DLN suspensions enriched for CD8+ T cells by CD4 and MHC class II depletion (CD8+ DLN) were cultured for 48 hours with different combinations of 1 × 106 HSV-1–infected Spls and monolayers of HSV-1–infected cFbs in Transwell cultures containing cell-impermeable membranes between the top and bottom chambers. SEM was less than 2% for all culture conditions at all E:T ratios. (B) Cultures were prepared as in (A) except that the bottom chamber invariably contained CD8+ T cells and cFbs, whereas the top chamber contained no Spls, uninfected Spls, or HSV-1–infected Spls. CD8+ T cells were then removed from the cultures and exposed to 51Cr-loaded HSV-1–infected target cells. Cytotoxicity was measured in a 3.5-hour 51Cr release assay. Percentage of specific lysis against uninfected targets was less than 5% for all culture conditions at all E/T ratios. Data are representative of three independent experiments with nearly identical results.
Inhibition of CTLp Differentiation Requires HSV-1 Infection of Spleen Cells
We next wanted to determine whether HSV-1 infection of the splenic stimulator cells was required for the observed inhibition of CTLp effector differentiation in the presence of HSV-cFbs. CD8+ DLN cells were cultured with HSV-cFbs for 48 hours in the lower wells of Transwell chambers, separated from HSV-Spls, noninfected spleen cells, or no spleen cells in the top chamber.
Differentiation of CTLps into effector CTLs capable of killing infected targets was only inhibited when the CD8+ DLN cells and HSV-cFbs were exposed to soluble factors produced by HSV-Spls (Fig. 5B). Therefore, one or more soluble products produced by HSV-1–infected, but not noninfected, spleen cells were required to confer an inhibitory phenotype on cFbs.
Inhibition of CTLp Differentiation Occurs Downstream of IL-2 Signaling
Acquisition by CTLps of cytolytic activity and IFN-γ production when stimulated with HSV-Spls or HSV-cFbs was IL-2 dependent. Failure to add exogenous IL-2 to restimulation cultures, in combination with neutralizing the effect of endogenous IL-2 by adding an antibody to the IL-2 receptor, prevented the induction of cytotoxic activity and IFN-γ production (Figs. 6A, 6B). Moreover, pretreatment of CD8+ DLN cells with exogenous IL-2 for 16 hours completely abrogated the suppression of cytotoxic activity after simultaneous stimulation with HSV-Spls and HSV-cFbs (Figs. 6C, 6D). Thus, simultaneous exposure to HSV-Spls and HSV-cFbs inhibits an event downstream of the effect of IL-2 that is differentially required for the development of cytotoxic activity, and this inhibitory event can be bypassed by previous exposure to IL-2.
FIGURE 6.
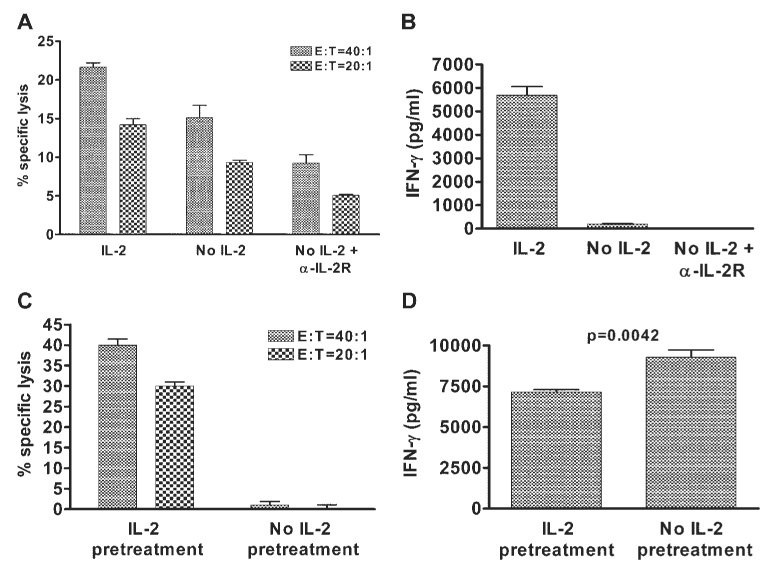
Exogenous IL-2 is required for the acquisition of CLTp cytotoxic function, and pretreatment of CTLps with IL-2 abrogates inhibition of cytotoxic function on exposure to HSV-1–infected splenocytes (Spl) and HSV-1 infected corneal fibroblasts (cFb). Eight days after HSV-1 corneal infection, DLN suspensions enriched for CD8+ T cells by CD4 and MHC class II depletion were cultured for 48 hours with 1 × 106 Spls and a monolayer cFbs. (A, B) Cultures received 10 U/mL rmIL-2, medium alone (No IL-2), or medium plus α-IL-2R mAb (No IL-2 + αIL-2R) for the duration of the culture. (A) Cells were removed from the cultures and exposed to 51Cr-loaded HSV-1–infected target cells. Cytotoxicity was measured in a 3.5-hour 51Cr release assay. Statistically significant differences (P < 0.05) were obtained between all three groups at both E:T ratios. Percentage of specific lysis from control cultures that received medium only plus an isotype control antibody was nearly identical with that of cultures that received medium only (not shown). (B) Before cytotoxicity assays, culture supernatants were tested for IFN-γ content by ELISA. (C, D) CTLps were pretreated with 10 U/mL IL-2 or medium only for 16 hours before restimulation with HSV-Spls and HSV-cFbs and were analyzed. Percentage of specific lysis against uninfected targets was less than 5% for all culture conditions at all E:T ratios. Data are presented as the mean ± SEM of triplicate samples and are representative of at least two independent experiments with nearly identical results.
HSV-Spls Do Not Confer an Inhibitory Phenotype on HSV-sFbs
To determine whether the inhibition of CTLp differentiation is a property shared by fibroblasts derived from tissues that are not immunoprivileged, HSV-sFbs were used alone or in combination with HSV-Spls to stimulate CTLp differentiation. CD8+ DLN cells exhibited only slightly decreased levels of cytotoxic activity when stimulated with HSV-sFbs alone or with a combination of HSV-sFbs and HSV-Spls compared with stimulation with HSV-Spls alone (Fig. 7). Thus, the capacity of cFbs to inhibit CTLp differentiation is not a universal property of fibroblasts from all tissues.
FIGURE 7.
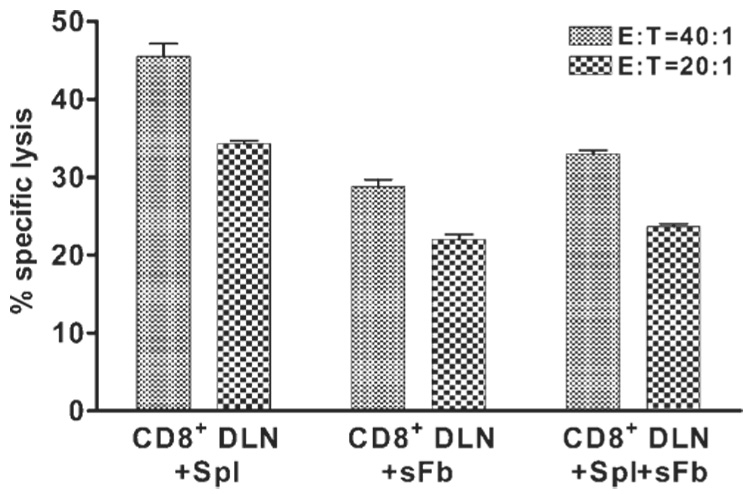
HSV-1–infected skin fibroblasts (sFbs) do not inhibit CTLp acquisition of cytotoxic function. Eight days after HSV-1 corneal infection, DLN suspensions enriched for CD8+ T cells by CD4 and MHC class II depletion (CD8+ DLN) were cultured for 48 hours with 1 × 106 HSV-1–infected spleen cells (Spl) alone, with a monolayer of sFb alone, or with Spls and sFbs. Cells were then removed from the cultures and exposed to 51Cr-loaded HSV-1–infected target cells. Cytotoxicity was measured in a 3.5-hour 51Cr release assay. Percentage of specific lysis against uninfected targets was less than 5% for all culture conditions at all E:T ratios. Data are presented as the mean ± SEM of triplicate samples and are representative of three independent experiments with nearly identical results. Statistically significant differences were obtained when comparing CD8+ DLN+Spl versus either CD8+ DLN+Spl+sFb or CD8+ DLN+sFb (P < 0.001) at both E:T ratios.
DISCUSSION
CD8+ T cells use two types of effector mechanisms to provide protection against viral infections: cytotoxicity mediated by lytic granule exocytosis or death receptor interactions and production of antiviral cytokines, such as IFN-γ and TNF-α. Given that virus-specific CD8+ T cells can recognize viral peptides expressed on infected cells before progeny virus is produced,26 they can limit through cytokines or eliminate through cytotoxicity the source of virus and establish a cyto-kine-induced antiviral state in surrounding cells. However, cytotoxicity could lead to a permanent loss of tissue function if the infected cell is vital to the functioning of the tissue or cannot be regenerated. Corneal function requires tissue clarity, which in turn is dependent on the appropriate conformation of the extracellular matrix that composes the corneal stroma.27 Proper corneal architecture is maintained by corneal keratocytes that produce the extracellular matrix of the corneal stroma and by corneal endothelial cells that control corneal hydration.28 Destruction of keratocytes and their replacement by myofibroblasts leads to scar tissue formation; destroyed corneal endothelial cells are not replaced. Thus, destruction of these corneal cells by CD8+ T cells would only be desirable when viral damage to the cell is irreversible. Tissues such as the cornea would benefit from a mechanism to selectively stimulate virus-specific CD8+ T cells to produce antiviral cytokines while actively inhibiting their cytotoxic function.
Certain immunoprivileged tissues, such as the uveal tract of the eye5 and the brain,6 have acquired the capacity to inhibit CTLp differentiation into cytotoxic cells. This study describes a novel regulatory circuit in which cFbs, in the proper inflammatory setting, can selectively inhibit the proliferation and lytic granule–mediated cytotoxic function of HSV-1–specific CD8+ T cells while stimulating their production of the antiviral cytokine IFN-γ.
By 8 days after corneal infection, the HSV-1–specific CD8+ T-cell response peaks in the DLN, but most of these cells are CTLps that exhibit minimal cytotoxic activity against HSV-1– infected targets directly ex vivo. Stimulation of HSV-1–specific CTLps with HSV-1–infected splenocytes in vitro resulted in their blast transformation, proliferation, and production and storage of cytotoxic effector molecules, such as GrA, GrB, and perforin, in lytic granules.29 This may reflect events that occur when CTLps infiltrate sites of HSV-1 infection. Interference with the expansion of HSV-1–specific CD8+ T cells or the loading or directed release of their lytic granules could all result in impaired cytotoxic function.
Our current findings demonstrate that HSV-cFbs are sensitive to the lytic activity of HSV-1–specific CTLs and that HSV-cFbs and HSV-Spls can individually stimulate CTLp differentiation into effector cells capable of cytotoxic function and IFN-γ production. Thus, HSV-cFbs and HSV-Spls appear to efficiently process and present viral antigens in the context of MHC class I for recognition by the CD8+ T-cell receptor. However, when HSV-CTLps are exposed simultaneously to HSV-cFbs and HSV-Spls, they fail to acquire cytotoxic function, though their IFN-γ production is actually increased. The reason for the increased IFN-γ production in response to the combined stimulator cells was not clear because the numbers of both types of stimulator cells were titrated to provide optimal stimulation. It is conceivable that increased IFN-γ production resulted from prolonged exposure to infected targets because of the failure of CD8+ T cells to acquire lytic activity.
The proliferation and blast transformation of DLN CD8+ T cells were reduced by 50% when stimulated with HSV-cFbs and HSV-Spls compared with stimulation with either cell type alone, but this did not reflect a failure to expand HSV-specific CTLs as the absolute number of IFN-γ–producing blast cells, and the number of cells that released lytic granules was actually increased in these cultures. Apparently, the reduced proliferation and blast transformation resulted from reduced bystander activation of HSV-nonspecific CD8+ T cells. Because HSV-specific CTLs expanded normally and released lytic granules when exposed simultaneously to HSV-cFbs and HSV-Spls, we tentatively concluded that the reduced cytotoxic function in these cultures resulted from failure to produce or store cytotoxic effector molecules in lytic granules.
In agreement with this hypothesis is the flow analysis revealing significantly reduced production or storage of the lytic granule component GrB in CD8+ T cells stimulated with HSV-Spls and HSV-cFbs compared with those stimulated with HSV-Spls alone. Although the lack of an antibody to mouse GrA precluded direct analysis of GrA storage in CD8+ T cells, their ability to release functional GrA (BLT esterase assay) was completely abrogated by simultaneous exposure to HSV-Spls and HSV-cFbs. Because their ability to release lytic granules (CD107a assay) was not compromised, this observation strongly suggested that simultaneous exposure to HSV-Spls and HSV-cFbs inhibited the production and/or storage of GrA and GrB in lytic granules of HSV-specific CTL. Given that GrA and GrB are the lytic granule components primarily responsible for inducing apoptosis in target cells, these findings suggest that the lack of cytotoxic activity of CD8+ T cells after simultaneous exposure to HSV-Spls and HSV-cFbs resulted from a failure in the development of functional lytic granules.
The mechanism(s) by which CTLp differentiation into cytotoxic effector cells is inhibited during combined exposure to HSV-cFbs and HSV-Spls is unclear. Our data establish that inhibition requires direct contact with HSV-cFbs but not with HSV-Spls. Thus, cFbs acquire the capacity to differentially regulate the acquisition of lytic function only when exposed to a soluble factor produced by HSV-1–infected, but not noninfected, splenocytes. Characterization of the cellular source(s) of the soluble factor is ongoing, but preliminary data suggest that it is enriched in plastic adherent cells (our unpublished observation). Because macrophages are present in the normal cornea and more infiltrate after HSV-1 infection, the possible involvement of macrophages in this regulatory circuit is suspected. The requirement for direct contact between CD8+ T cells and cFbs suggests the involvement of a cell-surface molecule that is expressed on cFbs when exposed to a soluble splenocyte-derived factor. Interestingly, sFbs do not acquire this inhibitory phenotype when exposed to HSV-Spls, suggesting that participation in this regulatory circuit is not a universal property of fibroblasts and may be selectively expressed in the immunoprivileged cornea.
A previous report showed that CD8+ T cells require a higher density of epitope to produce IFN-γ than is required for cytotoxic function.30 Thus, the selective inhibition of cytotoxic function resulting from simultaneous exposure to HSV-cFbs and HSV-Spls is probably not caused by an overall reduction of TCR signaling. Instead, it would appear that signaling pathways leading to the production of lytic granule components and IFN-γ can be differentially regulated. The bifurcation of the signaling pathways leading to IFN-γ production and lytic function appears to be downstream of IL-2 signaling because exposure to IL-2 is required for CTLp acquisition of both effector functions. Moreover, pretreatment with IL-2 completely abrogated the inhibition of lytic activity resulting from simultaneous exposure to HSV-Spls and HSV-cFbs. Thus, the inhibited event that differentially regulates the production and storage of GrA and GrB in lytic granules, but not IFN-γ production, is downstream of IL-2 signaling. This interpretation is consistent with a study demonstrating differential gene expression for lytic granule components and IFN-γ.31 Moreover, ligation of CD8+ T-cell MHC class I–specific inhibitory receptors, such as killer-cell immunoglobulinlike receptors (KIRs) in humans and CD94/NKG2A in humans and mice, can result in the downregulation of T-cell activation and selective inhibition of lytic granule-mediated cytotoxicity.32–37 Moreover, Qa-1, the murine ligand of CD94/NKG2A, is expressed in the mouse cornea.38 The involvement of such a mechanism is under investigation.
Several ocular immunopathologies, including herpes stromal keratitis and corneal allograft rejection, appear to be mediated by immune components other than CD8+ CTLs.2,22–24 The current findings demonstrate that within a viral setting, cFbs inhibit CTLp blast transformation, proliferation, and acquisition of cytotoxic function while enhancing their production of the antiviral cytokine IFN-γ. The results of this study support the notion that under certain inflammatory conditions, cFbs can inhibit CD8+ CTLp proliferation and differentiation, thus abrogating their potentially damaging cytotoxic effects on the cornea.
Acknowledgments
Supported by National Eye Institute Grants R01 EY010359 (RLH) and P30-EY08098 (RLH); by an unrestricted research grant from Research to Prevent Blindness, Inc. (RLH); and by a grant from the Eye and Ear Foundation of Pittsburgh (RLH).
Footnotes
Disclosure: J.E. Knickelbein, None; S. Divito, None; R.L. Hendricks, None
References
- 1.Ksander BR, Sano Y, Streilein JW. Role of donor-specific cytotoxic T cells in rejection of corneal allografts in normal and high-risk eyes. Transpl Immunol. 1996;4:49–52. doi: 10.1016/s0966-3274(96)80034-7. [DOI] [PubMed] [Google Scholar]
- 2.Niederkorn JY. The immune privilege of corneal grafts. J Leukoc Biol. 2003;74:167–171. doi: 10.1189/jlb.1102543. [DOI] [PubMed] [Google Scholar]
- 3.Streilein JW, Niederkorn JY, Shadduck JA. Systemic immune unresponsiveness induced in adult mice by anterior chamber presentation of minor histocompatibility antigens. J Exp Med. 1980;152:1121–1125. doi: 10.1084/jem.152.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Streilein JW. Immune regulation and the eye: a dangerous compromise. FASEB J. 1987;1:199–208. [PubMed] [Google Scholar]
- 5.Ksander BR, Streilein JW. Failure of infiltrating precursor cytotoxic T cells to acquire direct cytotoxic function in immunologically privileged sites. J Immunol. 1990;145:2057–2063. [PubMed] [Google Scholar]
- 6.Gordon LB, Nolan SC, Cserr HF, Knopf PM, Harling-Berg CJ. Growth of P511 mastocytoma cells in BALB/c mouse brain elicits CTL response without tumor elimination: a new tumor model for regional central nervous system immunity. J Immunol. 1997;159:2399–2408. [PubMed] [Google Scholar]
- 7.Ksander BR, Hendricks RL. Cell-mediated immune tolerance to HSV-1 antigens associated with reduced susceptibility to HSV-1 corneal lesions. Invest Ophthalmol Vis Sci. 1987;28:1986–1993. [PubMed] [Google Scholar]
- 8.Prymowicz D, Moore RN, Rouse BT. Frequency of herpes simplex virus-specific helper T lymphocyte precursors in the lymph node cells of infected mice. J Immunol. 1985;134:2683–2688. [PubMed] [Google Scholar]
- 9.Rouse BT, Wagner H. Frequency of herpes simplex virus-specific cytotoxic T lymphocyte precursors in lymph node cells of infected mice. Immunology. 1984;51:57–64. [PMC free article] [PubMed] [Google Scholar]
- 10.McNally JM, Andersen HA, Chervenak R, Jennings SR. Phenotypic characteristics associated with the acquisition of HSV-specific CD8 T-lymphocyte-mediated cytolytic function in vitro. Cell Immunol. 1999;194:103–111. doi: 10.1006/cimm.1999.1498. [DOI] [PubMed] [Google Scholar]
- 11.Jones CM, Cose SC, Coles RM, et al. Herpes simplex virus type 1-specific cytotoxic T-lymphocyte arming occurs within lymph nodes draining the site of cutaneous infection. J Virol. 2000;74:2414–2419. doi: 10.1128/jvi.74.5.2414-2419.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfizenmaier K, Jung H, Starzinski-Powitz A, Rollinghoff M, Wagner H. The role of T cells in anti-herpes simplex virus immunity, I: induction of antigen-specific cytotoxic T lymphocytes. J Immunol. 1977;119:939–944. [PubMed] [Google Scholar]
- 13.Coles RM, Mueller SN, Heath WR, Carbone FR, Brooks AG. Progression of armed CTL from draining lymph node to spleen shortly after localized infection with herpes simplex virus 1. J Immunol. 2002;168:834–838. doi: 10.4049/jimmunol.168.2.834. [DOI] [PubMed] [Google Scholar]
- 14.Oehen S, Brduscha-Riem K. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J Immunol. 1998;161:5338–5346. [PubMed] [Google Scholar]
- 15.Meng Y, Harlin H, O’Keefe JP, Gajewski TF. Induction of cytotoxic granules in human memory CD8+ T cell subsets requires cell cycle progression. J Immunol. 2006;177:1981–1987. doi: 10.4049/jimmunol.177.3.1981. [DOI] [PubMed] [Google Scholar]
- 16.Larsen HS, Russell RG, Rouse BT. Recovery from lethal herpes simplex virus type 1 infection is mediated by cytotoxic T lymphocytes. Infect Immun. 1983;41:197–204. doi: 10.1128/iai.41.1.197-204.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendricks RL, Tumpey TM. Concurrent regeneration of T lymphocytes and susceptibility to HSV-1 corneal stromal disease. Curr Eye Res. 1991;10 suppl:47–53. doi: 10.3109/02713689109020357. [DOI] [PubMed] [Google Scholar]
- 18.Liu T, Tang Q, Hendricks RL. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimeld C, Whiteland JL, Nicholls SM, Easty DL, Hill TJ. Immune cell infiltration in corneas of mice with recurrent herpes simplex virus disease. J Gen Virol. 1996;77(pt 5):977–985. doi: 10.1099/0022-1317-77-5-977. [DOI] [PubMed] [Google Scholar]
- 20.Simmons A, Tscharke DC. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med. 1992;175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newell CK, Martin S, Sendele D, Mercadal CM, Rouse BT. Herpes simplex virus-induced stromal keratitis: role of T-lymphocyte subsets in immunopathology. J Virol. 1989;63:769–775. doi: 10.1128/jvi.63.2.769-775.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doymaz MZ, Rouse BT. Herpetic stromal keratitis: an immunopathologic disease mediated by CD4+ T lymphocytes. Invest Ophthalmol Vis Sci. 1992;33:2165–2173. [PubMed] [Google Scholar]
- 23.Hendricks RL, Tumpey TM. Contribution of virus and immune factors to herpes simplex virus type I-induced corneal pathology. Invest Ophthalmol Vis Sci. 1990;31:1929–1939. [PubMed] [Google Scholar]
- 24.Banerjee K, Biswas PS, Rouse BT. Elucidating the protective and pathologic T cell species in the virus-induced corneal immunoinflammatory condition herpetic stromal keratitis. J Leukoc Biol. 2005;77:24–32. doi: 10.1189/jlb.0904486. [DOI] [PubMed] [Google Scholar]
- 25.Lepisto AJ, Frank GM, Xu M, Stuart PM, Hendricks RL. CD8 T cells mediate transient herpes stromal keratitis in CD4-deficient mice. Invest Ophthalmol Vis Sci. 2006;47:3400–3409. doi: 10.1167/iovs.05-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin S, Zhu XX, Silverstein SJ, et al. Murine cytotoxic T lymphocytes specific for herpes simplex virus type 1 recognize the immediate early protein ICP4 but not ICP0. J Gen Virol. 1990;71(pt 10):2391–2399. doi: 10.1099/0022-1317-71-10-2391. [DOI] [PubMed] [Google Scholar]
- 27.Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18:529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- 28.Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res. 2003;22:359–389. doi: 10.1016/s1350-9462(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 29.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 30.Valitutti S, Muller S, Dessing M, Lanzavecchia A. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J Exp Med. 1996;183:1917–1921. doi: 10.1084/jem.183.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelso A, Costelloe EO, Johnson BJ, Groves P, Buttigieg K, Fitzpatrick DR. The genes for perforin, granzymes A-C and IFN-gamma are differentially expressed in single CD8(+) T cells during primary activation. Int Immunol. 2002;14:605–613. doi: 10.1093/intimm/dxf028. [DOI] [PubMed] [Google Scholar]
- 32.Braud VM, Allan DS, O’Callaghan CA, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 33.McMahon CW, Raulet DH. Expression and function of NK cell receptors in CD8+ T cells. Curr Opin Immunol. 2001;13:465–470. doi: 10.1016/s0952-7915(00)00242-9. [DOI] [PubMed] [Google Scholar]
- 34.Moser JM, Gibbs J, Jensen PE, Lukacher AE. CD94-NKG2A receptors regulate antiviral CD8(+) T cell responses. Nat Immunol. 2002;3:189–195. doi: 10.1038/ni757. [DOI] [PubMed] [Google Scholar]
- 35.Suvas S, Azkur AK, Rouse BT. Qa-1b and CD94-NKG2a interaction regulate cytolytic activity of herpes simplex virus-specific memory CD8+ T cells in the latently infected trigeminal ganglia. J Immunol. 2006;176:1703–1711. doi: 10.4049/jimmunol.176.3.1703. [DOI] [PubMed] [Google Scholar]
- 36.Vance RE, Kraft JR, Altman JD, Jensen PE, Raulet DH. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1(b) J Exp Med. 1998;188:1841–1848. doi: 10.1084/jem.188.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wojtasiak M, Jones CM, Sullivan LC, Winterhalter AC, Carbone FR, Brooks AG. Persistent expression of CD94/NKG2 receptors by virus-specific CD8 T cells is initiated by TCR-mediated signals. Int Immunol. 2004;16:1333–1341. doi: 10.1093/intimm/dxh136. [DOI] [PubMed] [Google Scholar]
- 38.Kim CY, Masli S, Streilein JW. Qa-1, a nonclassical MHC molecule with immunomodulatory functions, is ubiquitously expressed in the immunoprivileged anterior chamber of the eye. Ocul Immunol Inflamm. 2005;13:271–277. doi: 10.1080/09273940590951052. [DOI] [PubMed] [Google Scholar]


