Abstract
Francisella tularensis is a highly infectious, Gram-negative intra-cellular pathogen that can cause the zoonotic disease tularemia. Although the receptors critical for internalization of Francisella by macrophages are beginning to be defined, the identity of the downstream signaling pathways essential for the engulfment are not yet identified. In this study we have tested the role of Syk in the phagocytosis of Francisella. We report that Syk is activated during Francisella infection and is critical for the uptake of the organisms. Pharmacologic inhibition of Syk almost completely abrogated uptake, whereas the overexpression of Syk significantly enhanced uptake. However, Syk appears to be dispensable during initial host-pathogen contact. Further analyses of the molecular mechanism of Syk influence on Francisella uptake revealed that the MAPK Erk but not the PI3K/Akt pathway is the downstream effector of Syk. Thus, the inhibition of Erk in Syk-overexpressing cells or the inhibition of Syk in Erk-overexpressing cells led to a significant attenuation of uptake. Collectively, these data identify Syk and Erk as key players in the phagocytosis of Francisella.
Keywords: Francisella, Syk, PI3K/Akt, Erk, phagocytosis
Introduction
The Gram-negative intra-cellular pathogen Francisella tularensis is the causative agent of the zoonotic disease tularemia. Four sub-species of Francisella tularensis exist- tularensis (Type A), holarctica (Type B), novicida and mediasiatica. Francisella novicida is virulent in mice but not in humans1. However, the intra-cellular life style of F. novicida is similar to that of the highly virulent Type A strain2. Thus, F. novicida is a frequently used experimental model for tularemia in the murine system. Because the infectious dose of F. tularensis tularensis is very low and the organism can be easily aerosolized, it is currently considered a potential biological weapon1.
Francisella tularensis primarily infects immune cells such as macrophages, monocytes and neutrophils. After bacterial internalization, the Francisella-containing phagosomes fail to fuse with the lysosome and the bacteria escape into the cytosol. In the cytosol, the bacteria replicate and subsequently trigger apoptosis of the host cell3,4. The host response to Francisella infection is beginning to be delineated. Host response involves key processes such as phagocytosis, production of inflammatory mediators and generation of toxic metabolites. The molecular mechanisms leading to the production of inflammatory mediators has received a lot of attention. The activation of MAP Kinases and NFκB has been reported in Francisella infected cells5-9 and we have recently reported that in addition to the MAP Kinase pathway, the phosphatidylinositol 3 kinase (PI3K)/Akt pathway is also activated and plays a critical role in the production of inflammatory cytokines7-9. Further, activation of the PI3K/Akt pathway and the subsequent generation of inflammatory cytokines are negatively regulated by the inositol phosphatase SHIP8. Recent reports also demonstrate that activation of the inflammasome complex is mediated by Francisella that escape into the cytosol, leading to the processing and release of IL-1β10,11.
In contrast to the large body of information on host cell inflammatory response, the mechanisms underlying phagocytosis of Francisella are currently unknown. Several host cell receptors including complement receptor3 (CR3)12-14, mannose receptor14,15, class A scavenger receptor16, Toll-like receptor 217,18 and Fcγ receptors14 have been implicated in the recognition of Francisella. However, the downstream signals that orchestrate phagocytosis of Francisella have not been identified.
Syk is a tyrosine kinase that has been shown to be critical for various immune cell functions, including cytoskeletal rearrangements and phagocytosis19,20. Thus, in this study we specifically examined the role of Syk in the uptake of Francisella. Our data demonstrate that Syk is phosphorylated in Francisella-infected cells. Inhibition of Syk markedly attenuates uptake of Francisella, whereas overexpression of Syk enhances uptake. Interestingly, this Syk-dependent uptake is mediated via Erk but not through the PI3K/Akt pathway. These data suggest an indispensable role for Syk during the phagocytosis of Francisella.
Materials and Methods
Cells, antibodies and reagents
Raw 264.7 and THP-1 cells were obtained from ATCC and maintained in RPMI 1640 with 5% heat-inactivated fetal bovine serum (FBS). Antibodies specific for phospho-tyrosine, Syk, Erk and actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against pErk and pSer Akt were obtained from Cell Signaling Technology (Beverly, MA). Mouse anti-Francisella lipopolysaccharide primary antibody was from Immune-Precise Antibodies Limited (Victoria, B.C., Canada). Piceatannol, UO126 and LY294002 were purchased from Calbiochem (San Diego, CA). F. novicida U112 (JSG1819), a generous gift of Dr. John Gunn (The Ohio State University, OH) were used in all experiments. Bacteria were streaked and grown overnight on Chocolate II agar plates (Becton, Dickinson and Company, MD) at 37°C.
Cell stimulation, lysis, and Western blotting
Macrophages were infected with plate-grown F. novicida (grown on Chocolate II agar plates for 16-18 h at 37°C) as previously described8 at a multiplicity of infection (MOI) of 100. Briefly, RAW 264.7 cells were plated in 12-well or 6 well plates and allowed to adhere. F. novicida resuspended in RPMI medium containing 5% heat inactivated FBS was added to the adherent macrophages and then incubated at 37°C and 5% CO2 for the indicated time points. In parallel, the viability of bacteria was tested by plating the inoculum on Chocolate II agar plates and bacterial numbers in the inoculum were quantified using the Petroff-Hauser chamber. These data indicate that >98% of bacteria in the inoculum were viable. During the infection, cells were not washed at any point unless indicated otherwise. Where indicated, before infection, cells were incubated with specific pharmacological inhibitors for 30 minutes. Post-infection, the cell supernatant was aspirated and uninfected and infected cells were lysed in TN1 buffer (50mM Tris pH 8.0, 10mM EDTA, 10mM Na4P2O7, 10mM NaF, 1% Triton-X 100, 125mM NaCl, 10mM Na3VO4, 10μg/ml each aprotinin and leupeptin). Post-nuclear lysates were boiled in Laemmli Sample Buffer and were separated by SDS/PAGE, transferred to nitrocellulose filters, probed with the antibody of interest and developed by enhanced chemiluminescence (ECL).
Western blot data quantitation
The ECL signal was quantitated using a scanner and a densitometry program (Scion Image) as previously described7,9. To quantitate the phospho-specific signal in the activated samples, we first subtracted background, normalized the signal to the amount of actin or total target protein in the lysate, and plotted the values as arbitrary units (a.u). Statistical analysis was performed by unpaired Student's t-test. p<0.05 was considered significant.
Colony forming unit (CFU) assay
CFU assays were performed as we have previously reported8 with few modifications. Briefly, RAW 264.7 cells were infected with 100 MOI and then centrifuged at 650g for 4 minutes. Infection was allowed to occur for a total of 60 minutes, next infected cells were washed two times with sterile PBS and treated with 50 μg/ml of gentamicin for 30 min at 37°C and 5% CO2. Cells were then washed twice and subsequently lysed in 0.1% SDS for 5 minutes. Immediately, 10 fold serial dilutions were made and appropriate dilutions were plated on Chocolate II agar plates. Assays were performed in triplicate for each test group. Statistical analysis was performed by unpaired Student's t-test. p<0.05 was considered significant.
Microscopy analysis of Francisella association with macrophages
Phagocytosis of Francisella was measured by microscopy as previously described7, with a few modifications. In brief, 60 minutes post-infection, cells were washed (with PBS) and fixed in 4% paraformaldehyde for 20 minutes. The cells were washed again and one of the two sets of samples was permeabilized with 100% methanol for 10 minutes and the other set was left non-permeabilized. Immunostaining was then performed with mouse anti-F. novicida LPS antibody (diluted 1/100; Immune Precise Antibodies) and the bacteria were visualized by anti-mouse Alexa Fluor 488 secondary antibody. Bacteria binding to macrophages were counted on non-permeabilized samples whereas total number of bacteria associated (both attached and phagocytosed) with the cells were counted on methanol permeabilized samples using the X100 oil immersion objective of a BX40 Olympus fluorescence microscope. The number of bacteria phagocytosed was obtained by subtracting bacterial numbers adhering to macrophages from the total number of bacteria associated with the cell. At least 100 cells per sample were examined and three separate sets of infection were analyzed. Phagocytic and binding indices are defined as the number of bacteria phagocytosed or adherent to 100 macrophages, respectively. Statistical analysis was performed by unpaired Student's t-test. p<0.05 was considered significant.
Plasmids and transient transfection
The construct encoding Syk was a kind gift from Dr. Axel Ullrich (Max-Planck Institute of Biochemistry, Germany). GST-Erk2 was a generous gift from Dr. Mark Coggeshall (Oklahoma Medical Research Foundation, OK). RAW 264.7 cells were transfected with the appropriate plasmid DNA using the Amaxa Nucleofector apparatus (Amaxa biosystems, Germany) as previously described8. Briefly, 7 ×106 cells were resuspended in 100 μl Nucelofector Solution V and were nucelofected with 8 μg of appropriate plasmid. Immediately post- nucleofection, 500 μl of pre-warmed RPMI was added to the transfection mix before transferring to 12-well plates containing 1.5 ml pre-warmed RPMI per well. Plates were incubated for 16 hours at 37°C before infections were performed.
Results
Syk is phosphorylated during Francisella infection
The activation of Syk is accompanied by autophosphorylation, so we used tyrosine phosphorylation of Syk as a measure of its activity. To examine whether Syk is activated during Francisella infection, RAW 264.7 cells were infected with F. novicida (MOI of 100) for different time points indicated in Figure 1A and the induction of Syk phosphorylation was examined. For this, Syk was immunoprecipitated from uninfected and infected cells and Western blotting was performed using phospho-tyrosine (pY) antibody (Figure 1A, upper panel). The same membrane was reprobed with Syk antibody to ensure equal loading in all lanes (Figure 1A, lower panel). Results indicated that Syk is phosphorylated as early as 30 seconds in cells infected with Francisella. We next examined whether the phosphorylation of Syk was dose-dependent. To test this, RAW 264.7 cells were infected with 1, 10 or 100 MOI of F. novicida and phosphorylation of Syk was assessed as described above. Results shown in Figure 1B indicate that Syk phosphorylation is minimal at 1 MOI but clearly evident at 10 MOI. Further, Syk was also phosphorylated when cells were infected with F. tularensis LVS, the vaccine strain of F. tularensis (Figure 1C).
Figure 1. Syk is phosphorylated during Francisella infection. A&B.

RAW 264.7 cells were infected with 100 MOI of F. novicida for the indicated time points (A) or with indicated MOI for 1 minute (B). Syk protein was immunoprecipitated, resolved by SDS/PAGE and analyzed by Western blotting with phospho-tyrosine (pY) antibody (upper panel). The same membrane was reprobed with Syk antibody to ensure equal loading (lower panel). C. RAW 264.7 cells were infected with 100 MOI of F. novicida (FN) or F. tularensis LVS (LVS) for 1 minute and phosphorylation of Syk was examined by Western blotting. D. THP1 cells were infected with 100 MOI (FN) for indicated time points and Syk phosphorylation was assessed. These data are representative of three independent experiments. R, resting, uninfected cells.
Host responses in human and mouse cells may differ considerably during Francisella infection. Thus, to test if Syk is phosphorylated in human cells, THP1 cells (human monocytic cell line) were infected for different time points and the phosphorylation of Syk was assessed by Western blot analysis. Results shown in Figure 1D indicate that Syk is also phosphorylated in infected human monocytic cells. Collectively, these data demonstrate that Syk is activated when cells are infected with Francisella, suggesting a potential role for Syk in macrophage/monocyte response to Francisella.
Syk promotes the phagocytosis of Francisella
Having established that Syk is activated early in infection, we next examined whether Syk is required for phagocytosis of Francisella. For this, RAW 264.7 cells were incubated with DMSO (vehicle control) or piceatannol, a specific Syk inhibitor, and then infected with 100 MOI of F. novicida for 1 hour. Phagocytosis of the bacteria was assessed by colony forming unit (CFU) assay or by immunofluorescence microscopy and the results are shown in Figure 2. Inhibition of Francisella phagocytosis by the actin polymerization inhibitor cytochalasin-D served as our positive control. As shown in Figure 2A, inhibition of Syk activation significantly decreased the phagocytosis of Francisella. As expected, treatment of cells with cytochalasin-D also suppressed the engulfment of Francisella. At the concentrations used, piceatannol was not toxic to the cells as assessed by trypan blue exclusion (data not shown). To test whether Syk activation was inhibited by piceatannol, RAW 264.7 cells were treated with DMSO or piceatannol prior to infection with 100 MOI of F. novicida for 1 minute and the phosphorylation of Syk was measured as described in Figure 1. Results, shown in Figure 2B, indicate that Francisella-induced Syk phosphorylation was inhibited by pre-treatment of cells with piceatannol.
Figure 2. Syk inhibition suppresses the phagocytosis of Francisella.
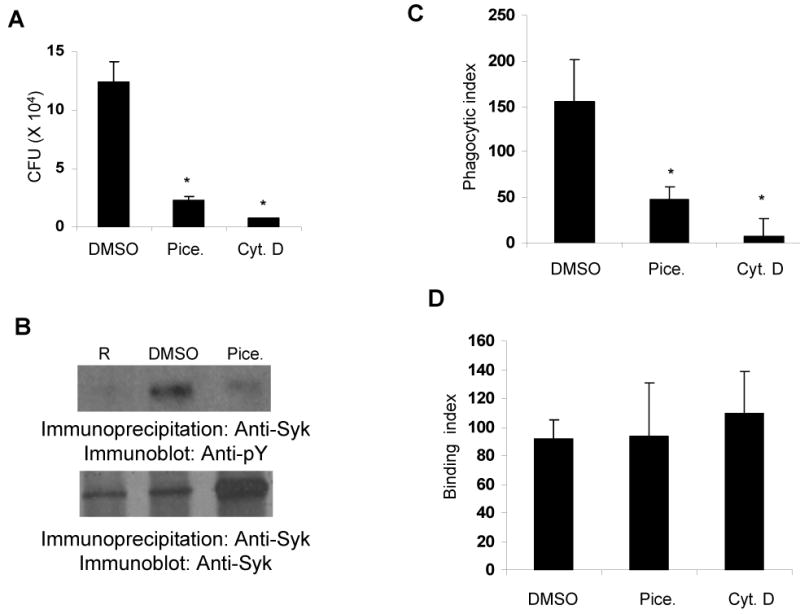
A. RAW 264.7 cells were treated with vehicle control (0.1% DMSO) or 25 μg/mL piceatannol (Pice.) or 5 μg/mL of cytochalasin-D (Cyt. D) for 30 minutes; cells were infected with 100 MOI of F. novicida for 1 hour and phagocytosis was assessed by CFU assays. B. RAW 264.7 cells were treated with vehicle control (0.1% DMSO) or 25 μg/mL piceatannol (Pice.) for 30 minutes; cells were infected with 100 MOI of F. novicida for 1 minute and phosphorylation of Syk was examined as described in the legend of Figure 1. C&D. RAW 264.7 cells were treated with 0.1 % DMSO or 25 μg/mL piceatannol (Pice.) or 5 μg/mL cytochalasin-D (Cyt. D) for 30 minutes, cells were infected with 100 MOI of F. novicida for 1 hour, phagocytosis (C) and binding (D) of the bacteria was analyzed by immunofluorescence. Phagocytic and binding indices are defined as the number of bacteria phagocytosed or adherent to 100 macrophages, respectively. Data represent mean and standard deviation of 3 independent experiments. *, p<0.05 compared to DMSO value.
As an additional approach, we tested the role of Syk in Francisella phagocytosis by immunofluorescence microscopy and the results are shown in Figure 2C and 2D. Consistent with the results obtained with CFU assays, inhibition of Syk significantly decreased Francisella phagocytosis (Figure 2C). However, the attachment of the bacteria to macrophages remained unaffected by Syk inhibition (Figure 2D).
To validate the findings obtained above with piceatannol, the role of Syk in the phagocytosis of Francisella was assessed using a genetic approach. Here, RAW 264.7 cells were transiently transfected with empty vector or a Syk encoding construct. Sixteen hours after transfection, cells were infected with 100 MOI of F. novicida for 1 hour and phagocytosis was assessed by CFU assay. The results shown in Figure 3A indicate that over-expression of Syk significantly increased the phagocytosis of Francisella. In parallel experiments, the transfectants were analyzed by Western blotting with Syk antibody to test the over-expression of Syk. The data shown in Figure 3B verify that Syk is indeed over-expressed in cells transfected with the Syk construct. Collectively, these experiments demonstrate that Syk promotes the phagocytosis of Francisella.
Figure 3. Syk overexpression enhances the phagocytosis of Francisella.

A. RAW 264.7 cells were transfected with vector alone or a Syk-encoding plasmid. 16 hours post-transfection the cells were infected with 100 MOI of F. novicida for 1 hour and phagocytosis was assessed by CFU assays. The graph represents mean and standard deviation of values obtained from 3 independent experiments (a.u., arbitrary units). *, p<0.05 compared to vector value. B. Protein-matched lysates from the transfectants were resolved by SDS/PAGE and the expression of Syk was analyzed by Western blotting with Syk antibody (upper panel). The same membrane was reprobed with Actin antibody (lower panel). These data are representative of at least three independent experiments.
Syk is upstream of the Erk and Akt pathways
Having established that Syk is essential for the engulfment of Francisella, we then examined the molecular mechanism underlying Syk-dependent phagocytosis. We have reported previously that in addition to the activation of Erk, the PI3K/Akt pathway is also activated during F. novicida infection7-9. Thus, we examined whether Syk were upstream of either the Erk pathway and/or the PI3K/Akt pathway. For this, cells were treated with vehicle control or piceatannol, infected for 15 minutes and the cell lysates were examined for the phosphorylation status of Erk and Akt by Western blotting with phospho-specific antibodies (Figures 4A and B, upper panels). The same membranes were reprobed with Actin antibody to ensure equal loading (Figures 4A and B, middle panels). The phosphorylation signals were quantitated and plotted (Figures 4A and B, lower panels). Results show a significant decrease in the phosphorylation of Erk and Akt when Syk is inhibited with piceatannol.
Figure 4. Syk is required for the activation of Erk and Akt.

A&B. RAW 264.7 cells were treated with vehicle control (0.1% DMSO) or 25 μg/mL piceatannol (Pice.) for 30 minutes; cells were infected with 100 MOI of F. novicida for 15 minutes and protein-matched lysates were resolved by SDS/PAGE and analyzed by Western blotting with indicated phospho-specific antibodies (upper panels). The membranes were reprobed with Actin antibody (middle panels). The phosphorylation signals were quantitated, normalized to actin in each lane and graphed (lower panels). C&D. Vector and Syk transfectants were infected with 100 MOI of F. novicida and phosphorylation levels of Erk and Akt in the transfectants were analyzed by Western blotting with phospho-specific antibodies (upper panels). The membranes were reprobed with Actin antibody (middle panels). Phosphorylation signals were normalized to actin content (lower panels). The graphs represent mean and standard deviation of values obtained from three independent experiments. R, resting, uninfected cells. *, p<0.05.
To test the role of Syk in the activation of Erk and PI3K/Akt using an alternate approach, cells were transfected with either an empty vector or a plasmid encoding Syk. The transfectants were subsequently infected and cell lysates were analyzed by Western blotting with phospho-specific antibodies. The results are shown in Figures 4C and D and indicate that phosphorylation of Erk and Akt is significantly enhanced in the Syk-overexpressing cells compared to cells transfected with vector alone. Taken together, these data demonstrate that Syk is upstream of and required for the activation of Erk and PI3K/Akt.
Erk but not PI3K/Akt is required for the phagocytosis of Francisella
Having established that Syk is upstream of Erk and PI3K/Akt, we next examined which of these pathways is required for the phagocytosis of Francisella. To test this, cells were incubated with vehicle control or U0126 (MEK/Erk pathway inhibitor) or LY294002 (PI3K/Akt inhibitor), infected and phagocytosis of Francisella was assessed by CFU and immunofluorescence assays. The results shown in Figures 5A and B indicate that pre-treatment of cells with the Erk but not the PI3K/Akt inhibitor significantly decreased phagocytosis of Francisella by macrophages. However the attachment of Francisella with the host macrophages was not altered (Figure 5C). This observation was consistent with the unchanged pathogen-host attachment when Syk is inhibited (Figure 2D). In parallel experiments, we tested the efficacy of Erk and PI3K/Akt inhibitors by Western blotting analysis (Figure 5D). Treatment of cells with U0126 specifically blocked the phosphorylation of Erk proteins where as incubation of cells with LY294002 inhibited the phosphorylation of Akt. Inhibition of Erk also significantly suppressed the phagocytosis of Francisella at a lower MOI, suggesting that the decrease in the phagocytosis from Erk blockade is dose independent (data not shown).
Figure 5. Erk but not PI3K/Akt is required for phagocytosis of Francisella.

A-C. RAW 264.7 cells were treated with vehicle control (0.2% DMSO) or 2.5 μM UO126 (UO) or 20 μM of LY294002 (LY) for 30 minutes; cells were infected with 100 MOI of F. novicida for 1 hour and the phagocytosis (A&B) or adherence (C) of Francisella was examined by CFU (A) and microscopy assays (B&C). The graphs represent mean and standard deviation of values obtained from 3 independent experiments. *, p<0.05 compared to DMSO value. D. RAW 264.7 cells were treated with vehicle control or inhibitors (as described above), infected for 1 hour and protein-matched lysates were analyzed by Western blotting with phospho-specific antibodies (upper and middle panels). The same membrane was reprobed with Actin antibody to ensure equal loading (lower panel). R, resting, uninfected cells.
We next examined the role of Erk in the phagocytosis of Francisella using an over-expression system. For this, cells were transfected either with vector alone or a construct encoding GST-tagged Erk2. The transfectants were infected and phagocytosis of Francisella was analyzed by CFU assays. Results shown in Figure 6A indicate that GST-Erk2 transfected cells ingest a significantly higher number of bacteria than cells transfected with vector alone. In parallel, the over-expression of GST-Erk2 protein was tested by Western blotting (Figure 6B). Collectively these data demonstrate that Erk but not PI3K/Akt is critical for the phagocytosis of Francisella by macrophages.
Figure 6. Erk overexpression enhances the phagocytosis of Francisella.

RAW 264.7 cells were transfected with vector or GST-Erk2 encoding construct. 16 hours post-tranfection the cells were infected with 100 MOI of F. novicida for 1 hour and phagocytosis was assessed by CFU assays. The graph represents mean and standard deviation of values obtained from 3 independent experiments. *, p<0.05 compared to vector value. B. Vector and GST-Erk2 transfectants were lysed, protein-matched lysates were resolved by SDS/PAGE and the expression of Erk was analyzed by Western blotting with Erk antibody (upper panel). The same membrane was reprobed with Actin antibody (lower panel). These data are representative of three independent experiments. NS, non-specific band.
Syk-dependent increase in phagocytosis of Francisella is abrogated by Erk inhibition
We next examined if the enhancement of phagocytosis due to Syk over-expression can be inhibited by blocking the downstream Syk-effector Erk. For this, cells transfected with vector alone or a plasmid encoding Syk were incubated with vehicle control, Erk inhibitor or PI3K/Akt inhibitor. The cells were subsequently infected and the phagocytosis of Francisella was examined by CFU assay. The results shown in Figure 7A indicate that the increase in phagocytosis due to Syk over-expression was suppressed Erk when the Erk pathway was inhibited but not when the PI3K/Akt pathway was inhibited. In parallel experiments, the effectiveness of the inhibitors and Syk over-expression were tested by Western blotting (Figures 7B and C).
Figure 7. Syk-dependent increase in the phagocytosis of Francisella is abrogated by Erk inhibition.

A. Vector and Syk transfectants were treated with 0.2% DMSO or 2.5 μM UO126 (UO) or 20 μM of LY294002 (LY) for 30 minutes; cells were infected with 100 MOI of F. novicida for 1 hour and phagocytosis was examined by CFU assays. The graph represents mean and standard deviation of values obtained from three independent experiments. *, p<0.05, compared to corresponding vector transfectants. B. RAW 264.7 cells were treated with vehicle control or with inhibitors (as described above), infected for 1 hour and protein-matched lysates were analyzed by Western blotting with phospho-specific antibodies (upper and middle panels). The same membrane was reprobed with Actin antibody to ensure equal loading (lower panel). R, resting, uninfected cells. C. Vector and Syk transfectants were lysed, protein-matched lysates were resolved by SDS/PAGE and the expression of Syk was analyzed by Western blotting with Syk antibody (upper panel). The same membrane was reprobed with Actin antibody (lower panel). These data are representative of three independent experiments.
The Erk-dependent increase in phagocytosis of Francisella is abrogated by Syk inhibition
Finally, we tested whether the Erk-dependent phagocytosis of Francisella is blocked if signaling through upstream Syk is inhibited. To test this, vector- or GST-Erk2-transfected cells were treated with DMSO or the Syk inhibitor piceatannol. Cells were subsequently infected and phagocytosis was assessed by CFU assays (Figure 8A). Erk2 overexpression significantly increased the phagocytosis of Francisella, but treatment of Erk2-overexpressing cells with Syk inhibitor significantly decreased the phagocytosis of Francisella to control levels. In parallel experiments, the effect of Syk inhibition on the phosphorylation status of both exogenous and endogenous Erk was monitored by Western blotting with phospho-Erk antibody. Results shown in Figure 8B indicate that piceatannol inhibited phosphorylation of both exogenous and endogenous Erk proteins. Collectively, the data obtained in this study demonstrate that Syk promotes the phagocytosis of Francisella via Erk but not through the PI3K/Akt pathway.
Figure 8. Erk-dependent increase in the phagocytosis of Francisella is abrogated by Syk inhibition.
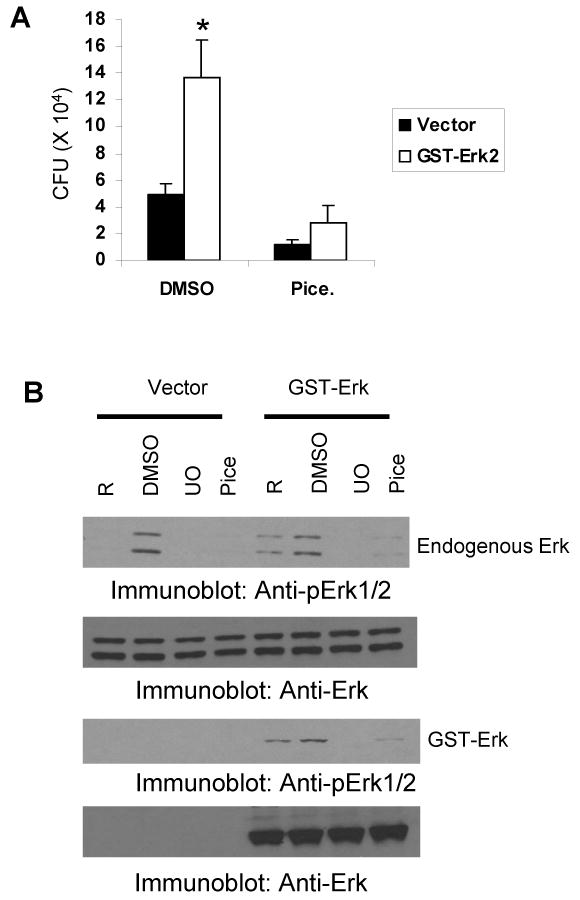
A. Vector and GST-Erk2 transfectants were treated with 0.1% DMSO or 25 μg/mL piceatannol for 30 minutes; cells were infected with 100 MOI of F. novicida for 1 hour and phagocytosis was examined by CFU assays. The graph represents mean and standard deviation of values obtained from three independent experiments. *, p<0.05, compared to corresponding vector transfectants. B. RAW 264.7 cells were treated with DMSO or piceatannol (as described above), infected for 15 minutes and protein-matched lysates were analyzed by Western blotting with phospho-Erk antibody (upper panel). The same membrane was reprobed with Erk2 antibody to test the over-expression of Erk2 (lower panel). R, resting, uninfected cells. These data are representative of 2 independent experiments.
Discussion
Phagocytosis is one of the earliest host immune responses against a pathogen. However, the mechanisms of phagocytosis differ between pathogens. The molecular mechanisms governing the uptake of Francisella are not understood. Our current study demonstrates a critical requirement for Syk activation in the engulfment of Francisella. These findings are consistent with the widely accepted role of Syk in phagocytosis of various agents such as opsonized zymozan and erythrocytes. Attesting to the importance of Syk during phagocytosis, inhibition of Syk by RNAi suppressed the reorientation of actin around the forming phagosomes in HL60 cells fed with opsonized zymosan19. Likewise, Syk-deficient macrophages were found to exhibit defective phagosomal closure19,20. However, Syk did not influence binding of either C3bi-opsonized zymosan19 nor IgG-coated erythrocytes20 to macrophages in these studies indicating that Syk is not involved in particle binding.
The requirement of Erk activation for the phagocytosis of Francisella is mechanistically consistent with other studies. For example, Kugler et al. reported that overexpression of an Erk phosphatase, MKP-1, significantly decreased the phagocytosis of Listeria monocytogenes by macrophages indicating that Erk is critical for the phagocytosis of this organism21. Further, Erk was reported to be crucial for the phagocytosis of IgG-coated erythrocytes by macrophages22. Both Erk and Syk were also found to be essential for the phagocytosis of E. coli by haemocytes of Manduca Sextata, a lepidopteran insect23. However, Erk was reported to be dispensable for the phagocytosis by monocytes and microglia suggesting that Erk requirement for phagocytosis may differ depending upon the target and the immune cell type24,25.
Although Erk is reported to be critical, its precise role in the phagocytosis of pathogenic organisms or IgG-coated erythrocytes is poorly understood. It is possible that Erk promotes phagocytosis via indirect and direct mechanisms. Though less likely, Erk may induce the transcriptional synthesis of proteins involved in the engulfment of Francisella. The more conceivable explanation for Erk-dependent phagocytosis is that Erk, similar to Syk, may modulate the actin cytoskeletal rearrangements that are critical for the uptake of Francisella. Several pieces of information support this hypothesis. First, MAPKs are reported to be important for the activation of phoshpolipaseA2 which via profilin can modulate the actin cytoskeleton26. Second, Erk2 phosphorylation is required for the polarization of the microtubule organizing center in natural killer cells27. Third, a recent study showed that leukocyte specific protein-1, an F-actin binding cytoskeletal protein, interacts with Erk2 and targets it to peripheral actin filaments28.
It is noteworthy that the uptake of Francisella is not affected by inhibition of the PI3K pathway. The activation of PI3K has been shown to be critical for phagocytosis initiated through various receptors such as FcγR29, complement receptor30 and CD4431. The lack of PI3K requirement for phagocytosis of Francisella is consistent with our recent findings that SHIP, an inositol phosphatase that negatively regulates Francisella-induced PI3K/Akt, does not affect the uptake of the Francisella8. PI3K activation has been shown to be dispensable for the phagocytosis of other intra-cellular pathogens such as Salmonella32,33, Shigella33 and Type II Helicobacter pylori34. This reiterates the observation that intracellular signaling events that modulate the functional responses of host cells are dependent upon the cellular context and the nature of the stimulus.
Since the Syk/Erk axis is important for the phagocytosis of Francisella, how Syk activates Erk deserves attention. Syk is able to activate Erk through at least two independent pathways. First, Syk phosphorylates the Ras adapter Shc which can then associate with the Grb2/SoS complex, leading to the activation of Ras, an upstream activator of Erk35. Second, autophosphorylation of Syk can result in the activation of protein kinase C (PKC), ultimately leading to the phosphorylation of Erk. Specifically, using constitutively active and dominant-negative mutants of PKCδ, a key component in the phagocytic pathway of neutrophils, Ueda et al have shown that PKCδ mediates phorbol ester-induced activation of Erk in a Raf and MEK dependent but Ras-independent manner36. We are currently investigating the precise mechanism by which Erk is activated during Francisella infection.
In this study we have demonstrated that Syk is required for the activation of PI3K/Akt pathway. We previously reported an indispensable role for PI3K/Akt pathway in modulating Francisella-induced inflammatory response8. Also, in other model systems, Syk is critical for the induction of iNOS, which is important for innate immune responses against invading pathogens37. Thus, Syk may be important in regulating additional aspects of the innate immune response to Francisella. We are currently testing these possibilities.
In summary, we have identified that Syk regulates the activation of Erk and PI3K/Akt, and we further demonstrate that Erk axis but not PI3K is critical for the phagocytosis of Francisella. To our knowledge, this is the first study that reports the identity of molecular signals coordinating the phagocytosis of Francisella.
Acknowledgments
This work was sponsored by the NIH/NIAID Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (RCE) Program. The authors wish to acknowledge membership within and support from the Region V ‘Great Lakes’ RCE (NIH award 1-U54-AI-057153). This work was also supported by NIH grants R01 AI059406 and P01 CA095426 to ST. JPB is supported by T32CA090223. We acknowledge Drs. Larry Schlesinger, John Gunn, Mark Coggeshall and Axel Ullrich for kindly providing some reagents.
Abbreviations used in this paper
- CFU
colony forming unit
- MAPK
mitogen activated protein kinase
- MOI
multiplicity of infection
- PI3K
Phosphatidylinositol 3 kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Oyston PC, Sjostedt A, Titball RW. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat Rev Microbiol. 2004;2:967–978. doi: 10.1038/nrmicro1045. [DOI] [PubMed] [Google Scholar]
- 2.Santic M, Molmeret M, Abu KY. Modulation of biogenesis of the Francisella tularensis subsp novicida-containing phagosome in quiescent human macrophages and its maturation into a phagolysosome upon activation by IFN-gamma. Cell Microbiol. 2005;7:957–967. doi: 10.1111/j.1462-5822.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 3.Golovliov I, Baranov V, Krocova Z, Kovarova H, Sjostedt A. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect Immun. 2003;71:5940–5950. doi: 10.1128/IAI.71.10.5940-5950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clemens DL, Lee BY, Horwitz MA. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect Immun. 2004;72:3204–3217. doi: 10.1128/IAI.72.6.3204-3217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golovliov I, Kuoppa K, Sjostedt A, Tarnvik A, Sandstrom G. Cytokine expression in the liver of mice infected with a highly virulent strain of Francisella tularensis. FEMS Immunol Med Microbiol. 1996;13:239–244. doi: 10.1111/j.1574-695X.1996.tb00244.x. [DOI] [PubMed] [Google Scholar]
- 6.Stenmark S, Sunnemark D, Bucht A, Sjostedt A. Rapid local expression of interleukin-12, tumor necrosis factor alpha, and gamma interferon after cutaneous Francisella tularensis infection in tularemia-immune mice. Infect Immun. 1999;67:1789–1797. doi: 10.1128/iai.67.4.1789-1797.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butchar JP, Rajaram MV, Ganesan LP, Parsa KV, Clay CD, Schlesinger LS, Tridandapani S. Francisella tularensis induces IL-23 production in human monocytes. J Immunol. 2007;178:4445–4454. doi: 10.4049/jimmunol.178.7.4445. [DOI] [PubMed] [Google Scholar]
- 8.Parsa KV, Ganesan LP, Rajaram MV, Gavrilin MA, Balagopal A, Mohapatra NP, Wewers MD, Schlesinger LS, Gunn JS, Tridandapani S. Macrophage pro-inflammatory response to Francisella novicida infection is regulated by SHIP. PLoS Pathog. 2006;2:e71. doi: 10.1371/journal.ppat.0020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajaram MV, Ganesan LP, Parsa KV, Butchar JP, Gunn JS, Tridandapani S. Akt/Protein kinase B modulates macrophage inflammatory response to Francisella infection and confers a survival advantage in mice. J Immunol. 2006;177:6317–6324. doi: 10.4049/jimmunol.177.9.6317. [DOI] [PubMed] [Google Scholar]
- 10.Gavrilin MA, Bouakl IJ, Knatz NL, Duncan MD, Hall MW, Gunn JS, Wewers MD. Internalization and phagosome escape required for Francisella to induce human monocyte IL-1{beta} processing and release. Proc Natl Acad Sci U S A. 2006;103(1):141–146. doi: 10.1073/pnas.0504271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben Nasr A, Haithcoat J, Masterson JE, Gunn JS, Eaves-Pyles T, Klimpel GR. Critical role for serum opsonins and complement receptors CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in phagocytosis of Francisella tularensis by human dendritic cells (DC): uptake of Francisella leads to activation of immature DC and intracellular survival of the bacteria. J Leukoc Biol. 2006;80(4):774–86. doi: 10.1189/jlb.1205755. [DOI] [PubMed] [Google Scholar]
- 13.Clemens DL, Lee BY, Horwitz MA. Francisella tularensis enters macrophages via a novel process involving pseudopod loops. Infect Immun. 2005;73:5892–5902. doi: 10.1128/IAI.73.9.5892-5902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balagopal A, MacFarlane AS, Mohapatra N, Soni S, Gunn JS, Schlesinger LS. Characterization of the receptor-ligand pathways important for entry and survival of Francisella tularensis in human macrophages. Infect Immun. 2006;74:5114–5125. doi: 10.1128/IAI.00795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulert GS, Allen LA. Differential infection of mononuclear phagocytes by Francisella tularensis: role of the macrophage mannose receptor. J Leukoc Biol. 2006;80(3):563–71. doi: 10.1189/jlb.0306219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pierini LM. Uptake of serum-opsonized Francisella tularensis by macrophages can be mediated by class A scavenger receptors. Cell Microbiol. 2006;8:1361–1370. doi: 10.1111/j.1462-5822.2006.00719.x. [DOI] [PubMed] [Google Scholar]
- 17.Katz J, Zhang P, Martin M, Vogel SN, Michalek SM. Toll-like receptor 2 is required for inflammatory responses to Francisella tularensis LVS. Infect Immun. 2006;74:2809–2816. doi: 10.1128/IAI.74.5.2809-2816.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malik M, Bakshi CS, Sahay B, Shah A, Lotz SA, Sellati TJ. Toll-like receptor 2 is required for control of pulmonary infection with Francisella tularensis. Infect Immun. 2006;74:3657–3662. doi: 10.1128/IAI.02030-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y, Tohyama Y, Kadono T, He J, Shahjahan Miah SM, Hazama R, Tanaka C, Tohyama K, Yamamura H. Protein-tyrosine kinase Syk is required for pathogen engulfment in complement-mediated phagocytosis. Blood. 2006;107:4554–4562. doi: 10.1182/blood-2005-09-3616. [DOI] [PubMed] [Google Scholar]
- 20.Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, Tybuleewicz VLJ, DeFranco AL. A critical role for Syk signal transduction and phagocytosis mediated by Fcy recptors on macrophages. J Exp Med. 1997;186:1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kugler S, Schuller S, Goebel W. Involvement of MAP-kinases and - phosphatases in uptake and intracellular replication of Listeria monocytogenes in J774 macrophage cells. FEMS Microbiol Lett. 1997;157:131–136. doi: 10.1111/j.1574-6968.1997.tb12763.x. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Garcia E, Rosales R, Rosales C. Phosphatidylinositol 3-kinase and extracellular signal-regulated kinase are recruited for Fc receptor-mediated phagocytosis during monocyte-to-macrophage differentiation. J Leukoc Biol. 2002;72:107–114. [PubMed] [Google Scholar]
- 23.de Winter P, Rayne RC, Coast GM. The effects of intracellular signalling pathway inhibitors on phagocytosis by haemocytes of Manduca sexta. J Insect Physiol. 2007 doi: 10.1016/j.jinsphys.2007.04.001. 2007 Apr 24; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Garcia E, Sanchez-Mejorada G, Rosales C. Phosphatidylinositol 3-kinase and ERK are required for NF-kappaB activation but not for phagocytosis. J Leukoc Biol. 2001;70:649–658. [PubMed] [Google Scholar]
- 25.Song X, Tanaka S, Cox D, Lee SC. Fcgamma receptor signaling in primary human microglia: differential roles of PI-3K and Ras/ERK MAPK pathways in phagocytosis and chemokine induction. J Leukoc Biol. 2004;75:1147–1155. doi: 10.1189/jlb.0403128. [DOI] [PubMed] [Google Scholar]
- 26.Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Allan DS, Krzewski K, Ge B, Kopcow H, Strominger JL. CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proc Natl Acad Sci U S A. 2006;103:10346–10351. doi: 10.1073/pnas.0604236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison RE, Sikorski BA, Jongstra J. Leukocyte-specific protein 1 targets the ERK/MAP kinase scaffold protein KSR and MEK1 and ERK2 to the actin cytoskeleton. J Cell Sci. 2004;117:2151–2157. doi: 10.1242/jcs.00955. [DOI] [PubMed] [Google Scholar]
- 29.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Bio. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox D, Dale BM, Kishiwada M, Helgason CD, Greenberg S. A regulatory role for Src homology 2 domain-containing inositol 5′-phosphatase (SHIP) in phagocytosis mediated by Fc gamma receptors and complement receptor 3 (alpha(M)beta(2); CD11b/CD18. J Exp Med. 2001;193:61–71. doi: 10.1084/jem.193.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vachon E, Martin R, Plumb J, Kwok V, Vandivier RW, Glogauer M, Kapus A, Wang X, Chow CW, Grinstein S, Downey GP. CD44 is a phagocytic receptor. Blood. 2006;107:4149–4158. doi: 10.1182/blood-2005-09-3808. [DOI] [PubMed] [Google Scholar]
- 32.Drecktrah D, Knodler LA, Steele-Mortimer O. Modulation and utilization of host cell phosphoinositides by Salmonella spp. Infect Immun. 2004;72:4331–4335. doi: 10.1128/IAI.72.8.4331-4335.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brumell JH, Grinstein S. Role of lipid-mediated signal transduction in bacterial internalization. Cell Microbiol. 2003;5:287–297. doi: 10.1046/j.1462-5822.2003.00273.x. [DOI] [PubMed] [Google Scholar]
- 34.Allen LA, Allgood JA, Han X, Wittine LM. Phosphoinositide3-kinase regulates actin polymerization during delayed phagocytosis of Helicobacter pylori. J Leukoc Biol. 2005;78:220–230. doi: 10.1189/jlb.0205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jabril-Cuenod B, Zhang C, Scharenberg AM, Paolini R, Numerof R, Beaven MA, Kinet JP. Syk-dependent phosphorylation of Shc. A potential link between FcepsilonRI and the Ras/mitogen-activated protein kinase signaling pathway through SOS and Grb2. J Biol Chem. 1996;271:16268–16272. doi: 10.1074/jbc.271.27.16268. [DOI] [PubMed] [Google Scholar]
- 36.Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 37.Ulanova M, Marcet-Palacios M, Munoz S, Asfaha S, Kim MK, Schreiber AD, Befus AD. Involvement of Syk kinase in TNF-induced nitric oxide production by airway epithelial cells. Biochem Biophys Res Commun. 2006;351:431–437. doi: 10.1016/j.bbrc.2006.10.073. [DOI] [PubMed] [Google Scholar]


