Abstract
Epigallocatechin-3-gallate (EGCG), a main catechin of green tea, has been suggested to inhibit hepatic gluconeogenesis. However, the exact role and related mechanism have not been established. In this study, we examined the role of EGCG in hepatic gluconeogenesis at concentrations that are reachable by ingestion of pure EGCG or green tea, and are not toxic to hepatocytes. Our results show in isolated hepatocytes that EGCG at relatively low concentrations (≤ 1 μM) inhibited glucose production via gluconeogenesis and expression of key gluconeogenic genes. EGCG was not toxic at these concentrations while demonstrating significant cytotoxicity at 10 μM and higher concentrations. EGCG at 1 μM or lower concentrations effective in suppressing hepatic gluconeogenesis did not activate the insulin signaling pathway, but activated 5′-AMP-activated protein kinase (AMPK). The EGCG suppression of hepatic gluconeogenesis was prevented by blockade of AMPK activity. In defining the mechanism by which EGCG activates AMPK, we found that the EGCG activation of AMPK was mediated by the Ca2+/calmodulin-dependent protein kinase kinase (CaMKK). Furthermore, our results show that the EGCG activation of AMPK and EGCG suppression of hepatic gluconeogenesis were both dependent on production of reactive oxygen species (ROS), which was a known activator of CaMKK. Together, our results demonstrate an inhibitory role for EGCG in hepatic gluconeogenesis and shed new light on the mechanism by which EGCG suppresses gluconeogenesis.
Introduction
EGCG is the most abundant catechin contained in green tea (1). Catechins including epicatechin (EC), epicatechin-3-gallate (ECG), and EGCG account for 30–40% of the dry weight of green tea (reviewed in (1,2)). EGCG has been shown to be involved in regulation of a variety of metabolic processes, and has been used as an anti-obesity reagent in animal models and in humans (3–6). Although its effectiveness in the treatment of human diabetes has not been established, EGCG has been shown in rodents to be effective in preventing the development of Type I diabetes and treatment of Type II diabetes (7,8). The mechanism of EGCG regulation of metabolism remains to be established although a variety of different roles for EGCG have been suggested.
EGCG has been shown to reduce food intake, plasma levels of glucose, and body weight (9,10). EGCG inhibits proliferation and adipose differentiation of the 3T3-L1 preadipocyte cell line, and induces apoptosis in 3T3-L1 adipocytes (11–13). Pure EGCG and green tea extracts have both been shown in humans to stimulate brown fat thermogenesis (14,15). EGCG can modulate insulin secretion and insulin sensitivity (16,17). EGCG has also been shown in skeletal muscle to promote fatty acid oxidation (18). Furthermore, EGCG has been suggested to reduce blood pressure through enhancing vascular endothelial function and insulin sensitivity (19).
Of our interest, EGCG has previously been shown to inhibit hepatic gluconeogenesis by mimicking insulin function (20). However, concentrations used in previous studies were at high levels (>10 μM) that were capable of killing various kinds of tumor cells (1,2,20). Furthermore, catechins such as EGCG, once ingested by humans, are rapidly metabolized through glucuronidation, sulfation, methylation, and ring fission (1), and the peak plasma concentration of EGCG after ingestion of a large amount of green tea or pure EGCG can only reach approximately 1 μM (21). Therefore, in this study we set to examine whether EGCG is effective in suppressing hepatic gluconeogenesis at or lower than 1 μM. Our results show that EGCG indeed inhibited hepatic gluconeogenesis at 1 μM or lower concentrations without cytotoxicity, but was toxic to hepatocytes at 10 μM or higher concentrations. EGCG at 1 μM and lower concentrations did not activate insulin signaling, instead activated AMPK through CaMKK and ROS.
Materials and Methods
Chemicals and Antibodies
EGCG (Cat. #: E4143), 8-(4-Chlorophenyl-thio) adenosine 3′,5′-cyclic monophosphate sodium salt (cAMP, Cat. #: C3912), dexamethasome (Cat. #: D4902), Catalase (Cat. #: C1345), PEG-Catalase (PEG-CAT, Cat. #: C4963), STO-609 acetic acid (Cat. #: C1318), NADPH, H2O2, and β-actin antisera (Cat. #: A-5441) were from Sigma. LY2294002 (Cat. #: 440202) and Compound C (Cat. #: 171260) were from Calbiochem. Antibodies against AMPK (Cat. #: 2532), phospho-AMPK (Cat. #: 2531), -LKB1 (Cat. #: 3054), -ACC (Cat. #: 3661), and -AKT/total (Cat. #: 9297 and 9191) were from Cell Signaling Technology. Phospho-IRS-1pY612 was from Abcam (Cat. #: ab4868-50). The CaMKK antibody (Cat. #: 610544), anti-phosphoserine/threonine antibody (Cat. #: 612548) were from BD Transduction Laboratories. The siRNA duplexes against AMPKα were from Santa Cruz Biotechnology (Cat. #: sc-45313). The cytotoxicity detection kit (Cat. #: 1644793) was from Roche.
Isolation of Primary Hepatocytes
Primary hepatocytes were isolated from C57BL/6 mice as previously described (22). All mice used for isolation of hepatocytes were fed with a normal chow diet at a regular schedule unless otherwise noted. Briefly, under anesthesia with pentobarbital (Intraperitoneal, 30 mg/kg body weight), livers were perfused with a Ca2+-free Hanks’ balanced solution (Invitrogen) at 5 ml/min for 8 min, followed by a continuous perfusion with the serum-free Williams’ E medium containing collagenase (Worthington, type II, 50 units/ml), HEPES (10 mM), and NaOH (0.004 N) at 5 ml/min for 12 min. Hepatocytes were harvested and purified with Percoll and centrifugation. The viability of hepatocytes was examined with Trypan blue exclusion. Only cells isolated with a viability >95% were used. Hepatocytes were inoculated into collagen-coated 6-well plates (5 × 105/well) or 24 well plates in the Williams’ E medium with 10% fetal bovine serum, and were incubated for 24 h before any experimentation.
Measurement of Glucose Production in Primary Hepatocytes
The glucose production from primary hepatocytes was measured as previously described (22,23). Briefly, cells were treated with EGCG for 3 h at concentrations as noted in the serum free Williams’ E medium. Cells were then washed with a pre-warmed glucose-free DMEM medium for 3 times, and stimulated by cAMP (100 μM)/dexamethasome(Dex, 500 nM) in the presence of EGCG at concentrations as noted for another 3 h in the glucose-free DMEM medium. Gluconeogenic substrates including 20 mM sodium lactate and 2 mM sodium pyruvate were added to some cells. Glucose in the media was quantified by using a glucose assay kit from Roche (Cat. NO: 0716251) and normalized to cellular protein concentrations. The total glucose production was derived from both glycogenolysis and gluconeogenesis. The glucose production from glycogenolysis was measured in the absence of gluconeogenic substrates. The glucose production via gluconeogenesis is defined as the difference between the total glucose production and the glucose from glycogenolysis.
Immunoblotting
Immunoblottings were performed as previously described (22,24–28). Briefly, cell lysates were prepared by the homogenization and sonication, followed by adding 2X Laemmli sample buffer. Aliquots (5 μg proteins/well) were resolved with mini Tris-glycine gradient gels (4–20%, Invitrogen) and transferred to nitrocellulose membranes. Levels of phospho-/total AKT, AMPK and phospho- IRS-1pY615, -ACC, -LKB1 were detected with a 1:1,000 dilution of each specific antiserum, followed by incubation with a 1:10,000 dilution of second immunoglobulin G conjugated with the alkaline phosphatase (RPN5783, Amersham Biosciences). Fluorescent bands were visualized with a Typhoon PhosphorImager (Molecular Dynamics).
Immunoprecipitation
Immunoprecipitations were performed as previously described (24,29–31). Target cells were dissolved with ice-cold Nonidet P-40 lysis buffer (1% Nonidet P-40, 150 mM NaCl, 10% glycerol, 2 mM EDTA, 20 mM Tris (pH 8.0), 1 mM dithiothreitol, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 2 g/ml leupeptin, and 10g/ml aprotinin) at 4° C for 10 min. CaMKK proteins were precipitated with specific antibodies and protein G-agarose at 4 °C for overnight. Phosphorylation of CaMKK was detected by immunoblotting with antibodies against phosphorylated serine/threonine.
RNA Isolation and TaqMan Real-time PCR
Total RNAs from hepatocytes were prepared by using a RNA purification kit from Qiagen. mRNAs were reversely transcribed into cDNAs, and quantified with Taqman Real time PCR and normalized to the endogenous GADPH. Probes, primers, and other related reagents were from Applied Biosystems. Reactions were performed according to manuals from the manufacturer. Catalogue numbers are: Mm00440636-m1 for PEPCK, Mm00839363-m1 for G6Pase, and Mm99999915-g1 for GAPDH.
Measurement of H2O2 production in vitro
Levels of H2O2 were measured as described (32). Briefly, increasing amounts of EGCG were incubated in PBS in the presence or absence of 100 μM NADPH or NADPH plus catalase (20 Unit/ml) at 37°C for 30 min. Levels of H2O2 in the media were measured using an Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Molecular Probes) with H2O2 solution as a standard. The absorbance at 535 nm was measured by VICTOR 3 1420 Mutilabel Counter (Perkin Elmer, Finland).
Introduction of siRNA Duplexes into Primary Hepatocytes
The siRNA duplexes were introduced into primary hepatocytes via transient transfection as previously described (22). Briefly, siRNA duplexes as indicated in each experiment were mixed with 4 μl of Lipofactamine RNAiMAX Reagents (Invitrogen, Cat. No: 13778-150) in the OPTI medium (Invitrogen) and then added to hepatocytes. Six hours later, the media were replaced with fresh Williams’ E Media containing 10 % FBS; and the cells were cultured for another 20 h.
Statistical analysis
Results were analyzed using GraphPad Prism (version 4.0), and were presented as mean ± SD of at least three independent experiments. Statistical significance was determined by Student t-tests or one way ANOVA analysis.
Results
EGCG inhibits gluconeogenesis in primary hepatocytes
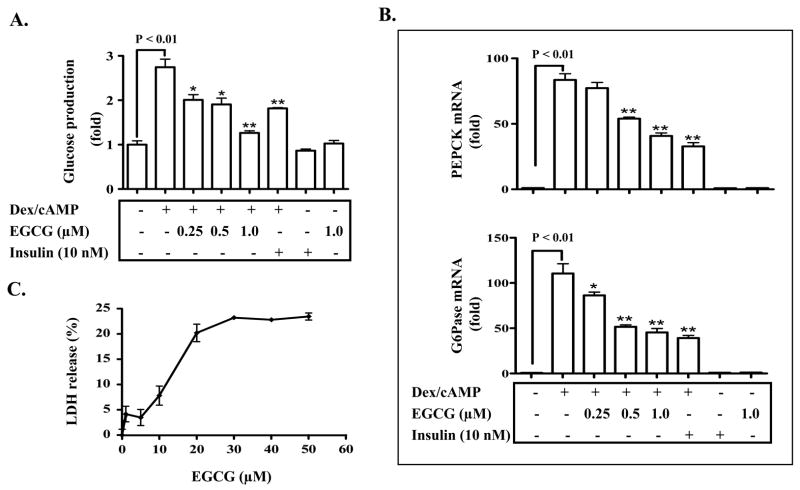
To examine the effect of EGCG on hepatic gluconeogenesis, isolated hepatocytes were stimulated by cAMP/Dex in the presence or absence of increasing amounts of EGCG, followed by measuring the glucose production via gluconeogensis and expression of key gluconeogenic genes. As shown in Fig. 1A, the glucose production via gluconeogenesis was stimulated by cAMP/Dex as expected, but the stimulation was attenuated by EGCG in a concentration-dependent manner. Similarly, the glucose production via gluconeogenesis was blocked by insulin as anticipated. Consistently, expression of PEPCK and G6Pase genes was also blocked by either EGCG or insulin (Fig. 1B). These results show that EGCG inhibits hepatic gluconeogenesis at or lower than 1 μM, and support the results from a previous study with higher concentrations that EGCG inhibits hepatic gluconeogenesis (20).
Fig. 1. EGCG inhibits gluconeogenesis in primary hepatocytes.
Mouse hepatocytes were isolated as detailed in “Materials and Methods”. (A) Cells were treated with EGCG for 3 h at concentrations as noted in the serum-free Williams’ E medium, and were washed with a pre-warmed glucose-free DMEM medium 3 times. Cells were then stimulated by cAMP/dexamethasome (Dex) in the presence of EGCG or insulin at concentrations as noted for 3 h in the glucose-free DMEM medium. Gluconeogenic substrates were added to cells as noted. The glucose production via gluconeogenesis was quantified and calculated as detailed in “Materials and Methods”. (B) Hepatocytes were similarly pretreated with EGCG, and then stimulated by cAMP/Dex for 3 h in the presence of EGCG or insulin as indicated. PEPCK and G6Pase mRNAs were subsequently measured by Taqman Real-Time PCR and normalized to GAPDH. (C) Hepatocytes were similarly treated with EGCG (1 to 50 μM) for 6 h, and levels of lactate dehydrogenase (LDH) in the medium were measured. As a positive control (100%), 1% Triton X-100 was added to some cells. *: P < 0.05 and **: P < 0.01 vs. cells treated with Dex/cAMP.
To control the possibility that EGCG suppresses hepatic gluconeogenesis through a toxic effect, LDH released from hepatocytes was measured. As shown in Fig. 1C, EGCG did not cause significant release of LDH at 1 μM, which was effective in suppressing hepatic gluconeogenesis. However, EGCG at concentrations higher than 10 μM caused significant release of LDH suggesting that EGCG is toxic to cells at concentration higher than 10 μM. It is noteworthy that EGCG caused an even stronger toxicity in H4IIE hepatoma cells (data not shown). These results are consistent with previous reports that EGCG is capable of killing cancer cells at concentrations higher than 10 μM (1,2).
EGCG inhibition of hepatic gluconeogenesis is independent of insulin signaling
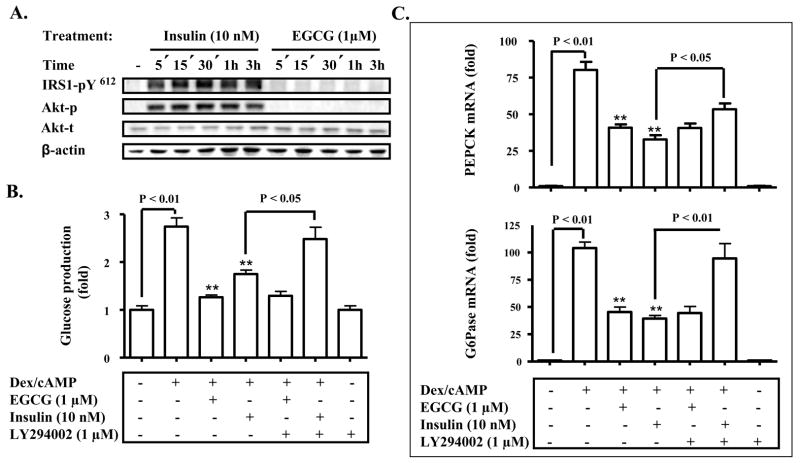
Since a previous study has shown that EGCG at concentrations higher than 10 μM represses hepatic gluconeogenesis by mimicking the insulin function (20), we examined the effect of EGCG at lower concentrations on the insulin signaling pathway. As shown in Fig. 2A, EGCG did not stimulate the tyrosine phosphorylation of IRS1 or Akt phosphorylation at 1 μM, which was effective in suppressing hepatic gluconeogenesis (Fig. 1A–B). In contrast, insulin robustly activated the tyrosine phosphorylation of IRS1 and Akt phosphorylation as expected. Furthermore, EGCG suppression of the glucose production via gluconeogenesis and expression of PEPCK and G6Pase genes were not influenced by a PI3K inhibitor, LY294002 (Fig. 2B–C). However, LY294002 completely blocked the insulin-induced suppression of the glucose production via gluconeogenesis and the expression of PEPCK and G6Pase genes. Together, these results indicate that EGCG inhibits hepatic gluconeogenesis independent of the insulin signaling pathway.
Fig. 2. EGCG does not activate the insulin signaling pathway.
(A) Mouse primary hepatocytes were treated with EGCG (1 μM) or insulin (10 nM) for 5 min to 3 h. Cellular levels of IRS1 phosphorylation at tyrosine615 and phospho-/total Akt were measured by immunoblotting. (B) and (C) Hepatocytes were pre-treated with LY294002 for 30 min as noted before the treatment with EGCG or insulin for 3 h in the serum-free Williams’ E medium. Cells were then washed with a pre-warmed glucose-free DMEM medium for 3 times, and subsequently stimulated by cAMP/dexamethasome (Dex) in the presence of EGCG or insulin for 3 h in the glucose-free DMEM medium. Gluconeogenic substrates were added to cells as noted. The glucose production via gluconeogenesis was quantified and calculated as detailed in “Materials and Methods”. Transcripts of PEPCK and G6Pase genes were quantified by the Taqman Real-time PCR and normalized to GADPH. **: P < 0.01 vs. cells treated with Dex/cAMP.
EGCG activates AMPK signaling pathway in primary hepatocytes
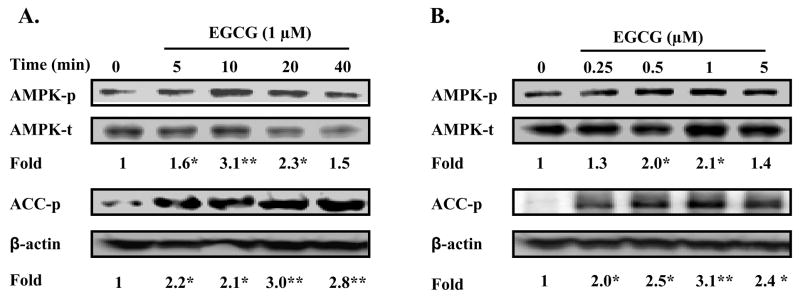
AMPK is another known suppressor of hepatic gluconeogenesis in addition to the insulin signaling, and polyphenolic compounds including resveratrol, apigenin, S17834 (a synthetic polyphenol), and EGCG have previously been shown to activate AMPK in HepG2 hepatoma cells, HT-29 colon cancer cells, or 3T3-L1 cells (33–35). It is currently unknown whether or not EGCG activates AMPK in primary hepatocytes. To address this question and the possible role of AMPK in the EGCG suppression of hepatic gluconeogenesis, we treated primary hepatocytes with EGCG and measured levels of phosphorylated AMPK and an AMPK substrate, acetyl-CoA carboxylase (ACC). As shown in Fig. 3A–B, EGCG elevated the phosphorylation of AMPK and ACC in a time- and concentration-dependent manner. These data show that EGCG activates AMPK in hepatocytes suggesting that EGCG suppresses hepatic gluconeogenesis through AMPK.
Fig. 3. EGCG activates AMPK in primary hepatocytes.
Mouse hepatocytes were isolated as described in “Materials and Methods”. Cells were treated with EGCG at 1 μM for an increasing amount of time (A) or an increasing amount of EGCG for 10 min (B). Levels of phospho-/total AMPK and acetyl Co-A carboxylase (ACC) were measured by immunoblotting, and quantified by densitometry. Levels of phospho-AMPK were normalized to the total AMPK; levels of phospho-ACC were normalized to β-actin. *: p<0.05 and **: p<0.01 vs. untreated cells.
AMPK mediates the EGCG inhibition of hepatic gluconeogenesis
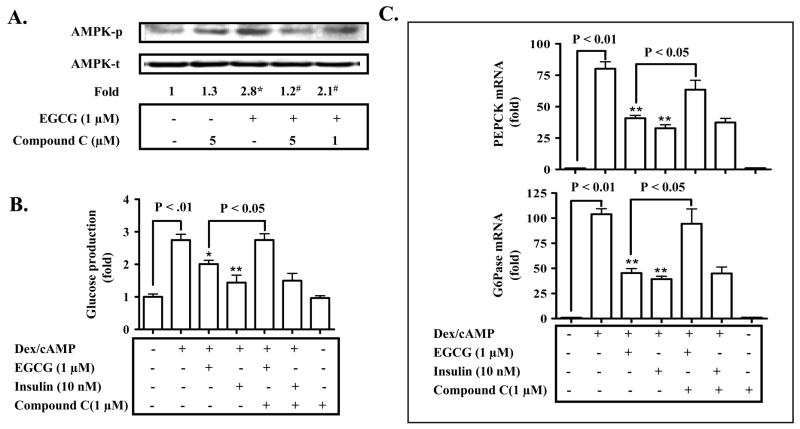
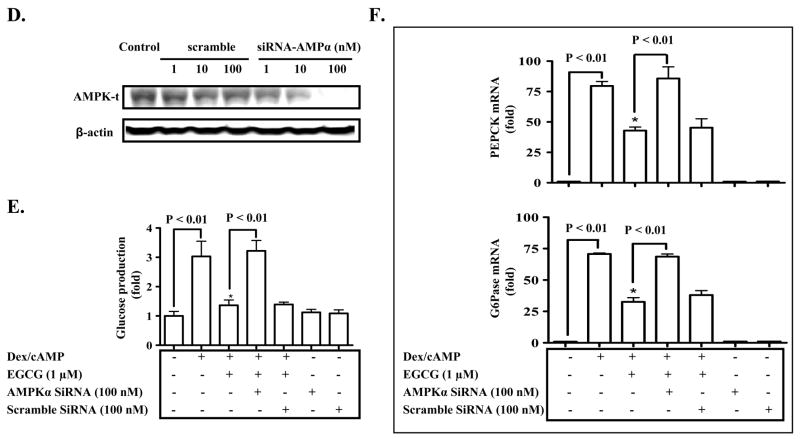
To examine the role of AMPK in EGCG inhibition of hepatic gluconeogenesis, the activity of AMPK in primary hepatocytes was blocked by either a selective chemical inhibitor, Compound C (36,37), or siRNA duplexes against the AMPKα gene prior to the treatment with EGCG, followed by the measurement of the glucose production via gluconeogenesis and expression of PEPCK and G6Pase. As showed in Fig. 4, EGCG activation of AMPK was prevented by the Compound C, and EGCG suppression of the glucose production via hepatic gluconeogenesis and expression of PEPCK and G6Pase genes were prevented by either Compound C or the specific siRNA. The scrambled siRNA had not effect. Together, these results demonstrate that AMPK is a mediator of the EGCG inhibition of hepatic gluconeogenesis.
Fig. 4. AMPK mediates the EGCG repression of hepatic gluconeogenesis.
(A) Primary hepatocytes were pre-treated with Compound C for 30 min as noted, and then treated with EGCG for 10 min. Levels of phospho-/total AMPK were measured by immunoblotting, and quantified by densitometry. Levels of phospho-AMPK were normalized to total AMPK. *: p < 0.05 compared to untreated cells; #: p < 0.05 compared to EGCG treatment. (B) Cells were sequentially treated with Compound C for 30 min, EGCG or insulin for 3 h in the serum-free Williams’ E medium, and then washed with the pre-warmed glucose-free DMEM medium 3 times. Subsequently, cells were stimulated by cAMP/dexamethasome in the presence of EGCG or insulin for 3 h in glucose-free DMEM medium. Gluconeogenic substrates were added to some cells. The glucose production via gluconeogenesis was quantified and calculated as detailed in “Materials and Methods). *: p<0.05 and **: p<0.01 compared to Dex/cAMP treatment. (C) Transcripts of PEPCK and G6Pase genes in cells similarly treated as described in (B) were quantified by the Taqman Real-time PCR and normalized to GADPH. *: p<0.05 compared to Dex/cAMP + insulin. (D) Hepatocytes were transfected with siRNA duplexes as noted. Levels of the AMPK protein were detected by immunoblotting 26 h after the transfection. (E) and (F) Cells were transfected with siRNA duplexes for 26 h as noted prior to the treatment with EGCG or insulin. Stimulation of cells with cAMP/Dex and quantification of the glucose production via gluconeogenesis were performed as described above. Transcripts of PEPCK and G6Pase genes were quantified by the Taqman Real-time PCR and normalized to GADPH. *: P < 0.05 vs. cells treated with Dex/cAMP.
The EGCG activation of AMPK and suppression of hepatic gluconeogenesis is CaMKK-dependent
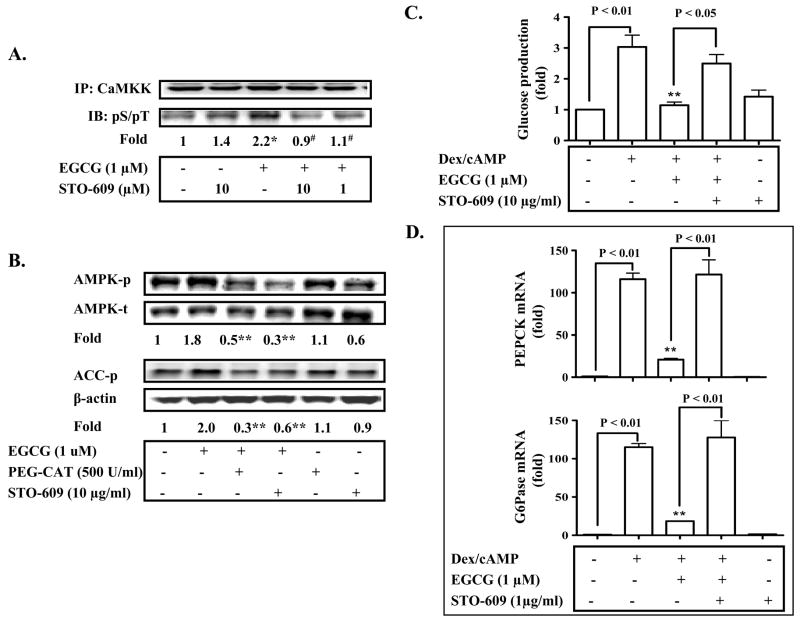
There are currently two known activators of AMPK, LKB1 and CaMKK (see (38) for review). Therefore, we examined whether or not these kinases were involved in the EGCG activation of AMPK and suppression of gluconeogenesis. Our results from primary hepatocytes indicate that EGCG did not initiate detectable phosphorylation of LKB1 (data not shown). However, EGCG stimulated phosphorylation of both CaMKK and AMPK (Fig. 5A and B). EGCG-induced phosphorylation of both CaMKK and AMPK was blocked by a CaMKK inhibitor, STO-609 (Fig. A and 5B). Furthermore, EGCG suppression of the glucose production via gluconeogenesis and expression of PEPCK and G6Pase genes were all attenuated by STO-609 (Fig. 5C–D). Together, these data indicated that hepatic CaMKK is the upstream activator of AMPK induced by EGCG.
Fig. 5. EGCG activates AMPK through Ca2+/calmodulin-dependent protein kinase kinase (CaMKK) and ROS, and consequently inhibits hepatic gluconeogenesis.
(A) Primary hepatocytes were treated with EGCG for 10 min. CaMKK molecules in the cells were then immunoprecipitated (IP) with the specific antisera against CaMKK, and presence of phosphorylated CaMKK was detected by immunoblotting (IB) with antibodies against phosphorylated serine/threonine. Levels of phospho-CaMKK were normalized to the total CAMKK. *: P < 0.05 compared to all other lanes; #: P < 0.05 compared to EGCG treatment. (B) Primary hepatocytes were pre-treated with a CaMKK inhibitor, STO-609, or a cell membrane permeable catalase (PEG-CAT) for 30 min prior to the treatment with EGCG (10 min). Levels of phospho-/total AMPK, phospho-ACC, and β-actin were measured by immunoblotting, and quantified by densitometry. **: p < 0.01 vs. EGCG alone. (C) and (D) Hepatocytes were pre-incubated with STO-609 for 30 min as noted, incubated with EGCG or insulin for 3 h in the serum-free Williams’ E medium, and were then washed with the pre-warmed glucose-free DMEM medium 3 times. Cells were subsequently treated with cAMP/dexamethasome (Dex) in the presence of EGCG or insulin for 3 h in the glucose-free DMEM medium. Gluconeogenic substrates were added to some cells. The glucose production via gluconeogenesis was quantified and calculated as detailed in “Materials and Methods”. Transcripts of PEPCK and G6Pase genes were quantified by the Taqman Real-time PCR and normalized to GADPH. **: P < 0.01 vs. cells treated with Dex/cAMP.
EGCG activation of AMPK and suppression of hepatic gluconeogenesis is ROS-dependent
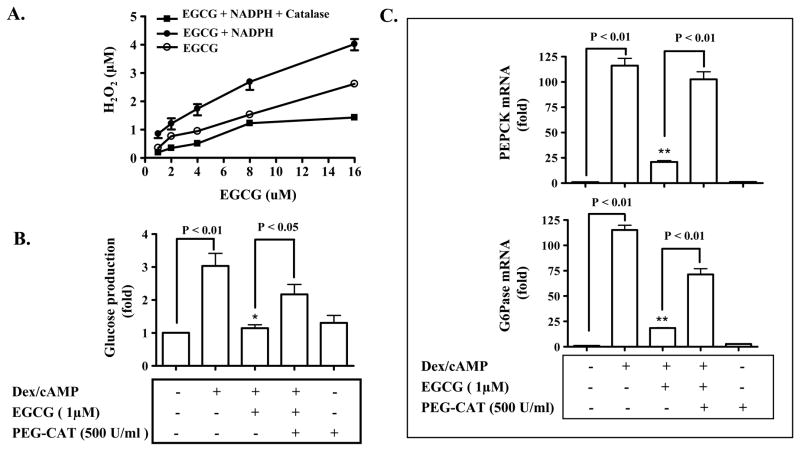
ROS is know to be able to activate AMPK through CaMKK (33,35) In particular, ROS has been shown to be involved in activation of AMPK by EGCG in HT-29 cancer cells (35). Therefore, we postulated that ROS was involved in EGCG activation of AMPK and suppression of hepatic gluconeogenesis. To test the hypothesis, we examined whether or not EGCG can induce ROS production. Our results show that in the absence of EGCG there was no detectable H2O2 in the PBS buffer (data not shown). However, EGCG alone induced production of H2O2 in a concentration-dependent manner; and the EGCG-induced H2O2 production was enhanced in the presence of NADPH as a proton donor (Fig. 6A). Furthermore, the H2O2 induced by EGCG and NADPH was neutralized by a catalase. To examine the role of ROS induced by EGCG in gluconeogenesis, primary hepatocytes as noted were treated with EGCG in the presence of a cell membrane permeable catalase (PEG-CAT), which was presumably able to eliminate H2O2. As shown in Fig. 5B, EGCG induction of AMPK phosphorylation was completely blocked by PEG-CAT. Accordingly, EGCG suppression of the glucose production via gluconeogenesis and expression of PEPCK and G6Pase genes were also prevented by PEG-CAT (Fig. 6B–C). Together, these results indicate that EGCG activates AMPK and consequently suppresses hepatic gluconeogenesis through the production of ROS.
Fig. 6. EGCG-induced production of ROS is required for EGCG suppression of hepatic gluconeogenesis.
(A) EGCG was incubated in PBS for 30 min in the presence or absence of NADPH or NADPH plus catalase (20 Unit/ml) as noted, and levels of H2O2 in the media were measured as detailed in “Materials and Methods”. (B) and (C) Primary hepatocytes were treated with a membrane permeable catalase (PEG-CAT) for 30 min before the treatment with EGCG or insulin for 3 h in the serum-free Williams’ E medium. Cells were then washed with the pre-warmed glucose-free DMEM medium 3 times, and subsequently stimulated with cAMP/dexamethasome (Dex) in the presence of EGCG or insulin for 3 h in the glucose-free DMEM medium. Gluconeogenic substrates were added to some cells. The glucose production via gluconeogenesis was quantified and calculated as detailed in “Materials and Methods”. Transcripts of PEPCK and G6Pase genes were quantified by the Taqman Real-time PCR and normalized to GADPH. *: P < 0.05 and **: P < 0.01 vs. cells treated with Dex/cAMP.
Discussion
EGCG has previously been shown to suppress hepatic gluconeogenesis both in cultured cells and in animal models; and the role of EGCG has been suggested to be mediated by Akt (20,39,40). Here we have confirmed that EGCG is indeed inhibitory to gluconeogenesis in isolated hepatocytes at non-toxic concentrations. However, our results show that EGCG at these concentrations dose not activate Akt and IRS1, which are required for insulin inhibition of hepatic gluconeogenesis, but instead activates AMPK. We further show that the blockade of AMPK activation prevents EGCG suppression of hepatic gluconeogenesis.
The role of EGCG in activation of Akt can be either stimulatory or inhibitory. The inhibitory role of EGCG is associated with its capability of counteracting the growth of tumor/cancer cells (see (1,41) for review). EGCG can not only directly down regulate Akt activation in various tumor/cancer cell, but also blocks the activation of Akt induced by either EGF or PDGF in non-tumor cells (42,43). The inhibitory effect of EGCG on Akt activation is mostly initiated by high concentrations, which are usually higher than 10 μM, typically at 50–100 μM (1). Studies have shown in pancreatic stellate cells and epidermal keratinocytes that EGCG is toxic at concentrations higher than 10 μM (43,44). Similarly, we have found that EGCG is toxic to hepatocytes at 10 μM or higher concentrations. On the other hand, EGCG has been shown in epidermal keratinocytes, vascular endothelial cells, and neuronal cells to activate Akt; and the activation of Akt is protective to these cells against various stresses such as oxidative stress and ultraviolet light irradiation (19,44–47). Although a study with epidermal keratinocytes show that EGCG activates Akt at concentrations lower than 1 μM (44), activation of Akt by EGCG in most previous studies has been elicited by concentrations higher than 10 μM. In this study, we found that EGCG did not initiate detectable phosphorylation of Akt in hepatocytes at concentrations at or lower than 1 μM, but was effective in suppressing hepatic gluconeogenesis. EGCG did not elevate tyrosine phosphorylation of IRS1, either. Therefore, EGCG appears to inhibit hepatic gluconeogenesis through a signaling pathway distinct from the insulin signaling.
AMPK is one of the two kinases that are known to be able to suppress hepatic gluconeogenesis (48–50). The other one is Akt described above and known to be activated by insulin (reviewed in (51)). Interestingly, EGCG has previously been shown to activate AMPK, and the activation of AMPK is associated with the EGCG-induced apoptosis in tumor/cancer cells and the EGCG inhibition of adipose differentiation (33,35). Other polyphenols including resveratrol (a major polyphenol in red wine), apigenin, and S17834 (a synthetic polyphenol) have been shown in HepG2 hepatoma cells and in mouse liver to activate AMPK and consequently prevent lipid accumulation (34). In this study, we show that AMPK is activated in primary hepatocytes by EGCG, and is required for EGCG suppression of hepatic gluconeogenesis.
AMPK can be activated by at least two known signaling pathways (reviewed in (38)). First, LKB1 is an activator of AMPK (see (52) for review). We examined the possible role of LKB1 in EGCG activation of AMPK, and found that EGCG did not promote detectable phosphorylation of LKB1 (data not shown). Second, CaMKK is another activator of AMPK (52). In this study, our results show that EGCG promotes phosphorylation of CaMKK, and blockade of CaMKK activity prevents EGCG activation of AMPK and mitigates the inhibitory role of EGCG in hepatic gluconeogenesis. EGCG activation of AMPK has previously been shown in colon cancer cells to be associated with ROS production (35). Our results from this study show that EGCG can directly promote ROS production; and EGCG-induced ROS is required for the activation of AMPK and the consequent inhibition of hepatic gluconeogenesis by EGCG.
In summary, results from this study demonstrate that EGCG suppresses hepatic gluconeogenesis at concentrations that are not toxic to hepatocytes and are reachable by ingestion of green tea or pure EGCG. We have also revealed a new mechanism by which EGCG suppresses hepatic gluconeogenesis through ROS, CaMKK, and AMPK. It is currently still unclear how EGCG exactly activates CaMKK through production of ROS. Future investigation on this matter and the in vivo role of CaMKK and AMPK in EGCG suppression of hepatic gluconeogenesis may provide new therapeutic approaches for the management of diabetes.
Acknowledgments
We thank Drs. Sheila Collins and Jacques Robidoux for their instrumental suggestions. This study was supported by the Development Fund from the Hamner Institutes for Health Sciences (W. C.), American Heart Association (SDG-0530244N) (W. C.), and National Institutes of Health (R01DK076039) (W.C.).
References
- 1.Lambert JD, Yang CS. J Nutr. 2003;133:3262S–3267S. doi: 10.1093/jn/133.10.3262S. [DOI] [PubMed] [Google Scholar]
- 2.Yang CS, Sang S, Lambert JD, Hou Z, Ju J, Lu G. Mol Nutr Food Res. 2006;50:170–175. doi: 10.1002/mnfr.200500105. [DOI] [PubMed] [Google Scholar]
- 3.Nelson-Dooley C, Della-Fera MA, Hamrick M, Baile CA. Curr Med Chem. 2005;12:2215–2225. doi: 10.2174/0929867054864886. [DOI] [PubMed] [Google Scholar]
- 4.Wolfram S, Raederstorff D, Wang Y, Teixeira SR, Elste V, Weber P. Ann Nutr Metab. 2005;49:54–63. doi: 10.1159/000084178. [DOI] [PubMed] [Google Scholar]
- 5.Wolfram S, Wang Y, Thielecke F. Mol Nutr Food Res. 2006;50:176–187. doi: 10.1002/mnfr.200500102. [DOI] [PubMed] [Google Scholar]
- 6.Murase T, Haramizu S, Shimotoyodome A, Tokimitsu I. Int J Obes (Lond) 2006;30:561–568. doi: 10.1038/sj.ijo.0803135. [DOI] [PubMed] [Google Scholar]
- 7.Song EK, Hur H, Han MK. Arch Pharm Res. 2003;26:559–563. doi: 10.1007/BF02976881. [DOI] [PubMed] [Google Scholar]
- 8.Wolfram S, Raederstorff D, Preller M, Wang Y, Teixeira SR, Riegger C, Weber P. J Nutr. 2006;136:2512–2518. doi: 10.1093/jn/136.10.2512. [DOI] [PubMed] [Google Scholar]
- 9.Kao YH, Hiipakka RA, Liao S. Endocrinology. 2000;141:980–987. doi: 10.1210/endo.141.3.7368. [DOI] [PubMed] [Google Scholar]
- 10.Lin JK, Lin-Shiau SY. Mol Nutr Food Res. 2006;50:211–217. doi: 10.1002/mnfr.200500138. [DOI] [PubMed] [Google Scholar]
- 11.Furuyashiki T, Nagayasu H, Aoki Y, Bessho H, Hashimoto T, Kanazawa K, Ashida H. Biosci Biotechnol Biochem. 2004;68:2353–2359. doi: 10.1271/bbb.68.2353. [DOI] [PubMed] [Google Scholar]
- 12.Hung PF, Wu BT, Chen HC, Chen YH, Chen CL, Wu MH, Liu HC, Lee MJ, Kao YH. Am J Physiol Cell Physiol. 2005;288:C1094–1108. doi: 10.1152/ajpcell.00569.2004. [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Della-Fera MA, Baile CA. Obes Res. 2005;13:982–990. doi: 10.1038/oby.2005.115. [DOI] [PubMed] [Google Scholar]
- 14.Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, Chantre P, Vandermander J. Am J Clin Nutr. 1999;70:1040–1045. doi: 10.1093/ajcn/70.6.1040. [DOI] [PubMed] [Google Scholar]
- 15.Dulloo AG, Seydoux J, Girardier L, Chantre P, Vandermander J. Int J Obes Relat Metab Disord. 2000;24:252–258. doi: 10.1038/sj.ijo.0801101. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Allen A, Kwagh J, Doliba NM, Qin W, Najafi H, Collins HW, Matschinsky FM, Stanley CA, Smith TJ. J Biol Chem. 2006;281:10214–10221. doi: 10.1074/jbc.M512792200. [DOI] [PubMed] [Google Scholar]
- 17.Anderson RA, Polansky MM. J Agric Food Chem. 2002;50:7182–7186. doi: 10.1021/jf020514c. [DOI] [PubMed] [Google Scholar]
- 18.Murase T, Haramizu S, Shimotoyodome A, Nagasawa A, Tokimitsu I. Am J Physiol Regul Integr Comp Physiol. 2005;288:R708–715. doi: 10.1152/ajpregu.00693.2004. [DOI] [PubMed] [Google Scholar]
- 19.Potenza MA, Marasciulo FL, Tarquinio M, Tiravanti E, Colantuono G, Federici A, Kim JA, Quon MJ, Montagnani M. Am J Physiol Endocrinol Metab. 2007;292:E1378–1387. doi: 10.1152/ajpendo.00698.2006. [DOI] [PubMed] [Google Scholar]
- 20.Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK. J Biol Chem. 2002;277:34933–34940. doi: 10.1074/jbc.M204672200. [DOI] [PubMed] [Google Scholar]
- 21.Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS. Cancer Epidemiol Biomarkers Prev. 2002;11:1025–1032. [PubMed] [Google Scholar]
- 22.Collins QF, Xiong Y, Lupo JEJ, Liu HY, Cao W. J Biol Chem. 2006;281:24336–24344. doi: 10.1074/jbc.M602177200. [DOI] [PubMed] [Google Scholar]
- 23.Liu HY, Collins QF, Xiong Y, Moukdar F, Lupo EG, Liu Z, Cao W. J Biol Chem. 2007;282:14205–14212. doi: 10.1074/jbc.M609701200. [DOI] [PubMed] [Google Scholar]
- 24.Cao W, Luttrell LM, Medvedev AV, Pierce KL, Daniel KW, Dixon TM, Lefkowitz RJ, Collins S. J Biol Chem. 2000;275:38131–38134. doi: 10.1074/jbc.C000592200. [DOI] [PubMed] [Google Scholar]
- 25.Cao W, Medvedev AV, Daniel KW, Collins S. J Biol Chem. 2001;276:27077–27082. doi: 10.1074/jbc.M101049200. [DOI] [PubMed] [Google Scholar]
- 26.Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BMSC. Mol Cell Biol. 2004;24:3057–3067. doi: 10.1128/MCB.24.7.3057-3067.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao WH, Collins QF, Becker TC, Robidoux J, Lupo JEG, Xiong Y, Daniel K, Floering L, Collins S. J Biol Chem. 2005;280:42731–42737. doi: 10.1074/jbc.M506223200. [DOI] [PubMed] [Google Scholar]
- 28.Xiong Y, Collins QF, Lupo JG, Liu HY, Jie A, Liu D, Cao W. J Biol Chem. 2006;282:4975–4982. doi: 10.1074/jbc.M606742200. [DOI] [PubMed] [Google Scholar]
- 29.Robidoux J, Cao WH, Quan Q, Daniel KW, Moukdar F, Bai B, Floering LMSC. Mol Cell Biol. 2005;25:5466–5479. doi: 10.1128/MCB.25.13.5466-5479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu HY, MacDonald JI, Hryciw T, Li C, Meakin SO. J Biol Chem. 2005;280:19461–19471. doi: 10.1074/jbc.M500313200. [DOI] [PubMed] [Google Scholar]
- 31.Liu HY, Meakin SO. J Biol Chem. 2002;277:26046–26056. doi: 10.1074/jbc.M111659200. [DOI] [PubMed] [Google Scholar]
- 32.Bai Y, Onuma H, Bai X, Medvedev AV, Misukonis M, Weinberg JB, Cao W, Robidoux J, Floering LM, Daniel KW, Collins S. J Biol Chem. 2005;280:19062–19069. doi: 10.1074/jbc.M500566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang JT, Park IJ, Shin JI, Lee YK, Lee SK, Baik HW, Ha J, Park OJ. Biochem Biophys Res Commun. 2005;338:694–699. doi: 10.1016/j.bbrc.2005.09.195. [DOI] [PubMed] [Google Scholar]
- 34.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 35.Hwang JT, Ha J, Park IJ, Lee SK, Baik HW, Kim YM, Park OJ. Cancer Lett. 2007;247:115–121. doi: 10.1016/j.canlet.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 36.McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. J Biol Chem. 2005;280:20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- 37.Fediuc S, Gaidhu MP, Ceddia RB. J Lipid Res. 2006;47:412–420. doi: 10.1194/jlr.M500438-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Witters LA, Kemp BE, Means AR. Trends Biochem Sci. 2006;31:13–16. doi: 10.1016/j.tibs.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Koyama Y, Abe K, Sano Y, Ishizaki Y, Njelekela M, Shoji Y, Hara Y, Isemura M. Planta Med. 2004;70:1100–1102. doi: 10.1055/s-2004-832659. [DOI] [PubMed] [Google Scholar]
- 40.Anton S, Melville L, Rena G. Cell Signal. 2006 doi: 10.1016/j.cellsig.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Zaveri NT. Life Sci. 2006;78:2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Sah JF, Balasubramanian S, Eckert RL, Rorke EA. J Biol Chem. 2004;279:12755–12762. doi: 10.1074/jbc.M312333200. [DOI] [PubMed] [Google Scholar]
- 43.Masamune A, Kikuta K, Satoh M, Suzuki N, Shimosegawa T. World J Gastroenterol. 2005;11:3368–3374. doi: 10.3748/wjg.v11.i22.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung JH, Han JH, Hwang EJ, Seo JY, Cho KH, Kim KH, Youn JI, Eun HC. Faseb J. 2003;17:1913–1915. doi: 10.1096/fj.02-0914fje. [DOI] [PubMed] [Google Scholar]
- 45.Lorenz M, Wessler S, Follmann E, Michaelis W, Dusterhoft T, Baumann G, Stangl K, Stangl V. J Biol Chem. 2004;279:6190–6195. doi: 10.1074/jbc.M309114200. [DOI] [PubMed] [Google Scholar]
- 46.Koh SH, Kim SH, Kwon H, Kim JG, Kim JH, Yang KH, Kim J, Kim SU, Yu HJ, Do BR, Kim KS, Jung HK. Neurotoxicology. 2004;25:793–802. doi: 10.1016/j.neuro.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Koh SH, Kwon H, Kim KS, Kim J, Kim MH, Yu HJ, Kim M, Lee KW, Do BR, Jung HK, Yang KW, Appel SH, Kim SH. Toxicology. 2004;202:213–225. doi: 10.1016/j.tox.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 49.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamauchi J, Nagao M, Kaziro Y, Itoh H. J Biol Chem. 1997;272:27771–27777. doi: 10.1074/jbc.272.44.27771. [DOI] [PubMed] [Google Scholar]
- 51.Whiteman EL, Cho H, Birnbaum MJ. Trends Endocrinol Metab. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 52.Towler MC, Hardie DG. Circ Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]