Abstract
An automated system for hydride generation - cryotrapping- gas chromatography - atomic absorption spectrometry with the multiatomizer is described. Arsines are preconcentrated and separated in a Chromosorb filled U-tube. An automated cryotrapping unit, employing nitrogen gas formed upon heating in the detection phase for the displacement of the cooling liquid nitrogen, has been developed. The conditions for separation of arsines in a Chromosorb filled U-tube have been optimized. A complete separation of signals from arsine, methylarsine, dimethylarsine, and trimethylarsine has been achieved within a 60 s reading window. The limits of detection for methylated arsenicals tested were 4 ng l−1. Selective hydride generation is applied for the oxidation state specific speciation analysis of inorganic and methylated arsenicals. The arsines are generated either exclusively from trivalent or from both tri- and pentavalent inorganic and methylated arsenicals depending on the presence of L-cysteine as a prereductant and/or reaction modifier. A TRIS buffer reaction medium is proposed to overcome narrow optimum concentration range observed for the L-cysteine modified reaction in HCl medium. The system provides uniform peak area sensitivity for all As species. Consequently, the calibration with a single form of As is possible. This method permits a high-throughput speciation analysis of metabolites of inorganic arsenic in relatively complex biological matrices such as cell culture systems without sample pretreatment, thus preserving the distribution of tri- and pentavalent species.
Keywords: Speciation analysis, Arsenic, Hydride generation atomic absorption spectrometry, Cryotrapping, Multiatomizer
1. Introduction
Inorganic As (iAs) is the prevalent form of As in the environment. Human metabolism of iAs involves the reduction of As(V) to As(III) and the oxidative methylation of As(III)-species that yields methylated arsenicals containing either As(III) or As(V)[1–3]. Toxicities of tri- and pentavalent iAs and methylated arsenicals differ significantly [4–6]. Therefore, developing methods for the oxidation state specific speciation analysis of As in biological matrices has become a key issue for As toxicology and analytical chemistry. Although the iAs(III)/iAs(V) analysis is very common, the reports on the oxidation state specific speciation analysis of methylated species -methylarsonite (MAs(III)), dimethylarsinite (DMAs(III)), methylarsonate (MAs(V)) dimethylarsinate (DMAs(V)) and trimethylarsine oxide (TMAs(V)O)- remain very scarce [1,7,8]. One of the possible analytical approaches is an oxidation state selective hydride generation followed by a cryotrapping and gas chromatography (HG-CT), i.e., trapping of volatile arsines under liquid nitrogen and their subsequent volatilization by gradual heating and separation according to boiling points and chromatographic properties of the trap [1], with the detection by methods of analytical atomic spectroscopy. The HG-CT can be used for analysis of arsenicals which produce volatile arsines upon reaction with sodium borohydride, including all known metabolites of iAs found in mammalian species [1,9]. Another advantage of HG-CT as compared to a chromatography separation of As species prior to detection approach usually by inductively coupled plasma- mass spectrometry (ICP MS) or hydride generation - atomic fluorescence [7] is that the analysis in important complex biological matrices such as urine or cell lysates can be performed directly with minimum sample pretreatment. This is especially important when dealing with unstable MAs(III) and DMAs(III) species which have been shown to quickly oxidize to MAs(V) and DMAs(V), respectively, even at temperatures below 0°C [10,11]. Notably, combined with atomic absorption spectrometry (AAS), the HG-CT approach is capable of a direct oxidation state specific speciation analysis of As in complex protein-containing matrices, including cell lysates [1]). This type of matrices would require extraction of protein-bound As-species if analyzed by HPLC-coupled techniques. Importantly, simplicity and a relatively low cost may make this method attractive for research teams working in the arseniasis endemic areas in developing countries.
The identification of tri- and pentavalent iAs and methylated arsenicals using the HG-CT approach requires in fact a two-dimensional analysis. The first dimension, the cryotrapping and separation of arsines generated from iAs, MAs, DMAs and TMAs(V)O species, regardless of the oxidation state of As, has been well developed and documented [1,9,12,13] and reviewed [14]. The main drawback that has prevented a widespread application of the HG-CT based methods is the labor intensiveness associated with the switching from cooling to heating of the cryogenic trap, which is often controlled manually. The systems controlling the cooling of the cryogenic trap by liquid N2 via vacuum pump [15], placing the cooling bath on mechanical [16] or pneumatic [14,17] stand, or the use of precooled nitrogen gas as cooling medium [18] solve to some extent this problem, but did not find wide application.
The second dimension, distinguishing between tri- and pentavalent arsenicals, is provided by the selective HG. It is usually based on the pH specific efficiency of generation of arsines from respective valencies. This approach takes advantage of the fact that generation of arsines is greatly enhanced from protonated substrate species [19] and that the pK values for iAs(III)- and iAs(V) [15,20] and supposedly also for tri- and pentavalent MAs and DMAs species significantly differ. In practice, at low pH (around 1) the reaction with borohydride generates arsines from both tri- and pentavalent arsenicals, whereas at high pH (above 6) pentavalent arsenicals are ionized and arsines can be formed only from trivalent arsenicals. It shall be noted that the change of reaction mixture pH after mixing with reductant solution must be taken into account. The amounts of As(III)- and As(V)-species can then be calculated from differences between signals obtained at low and high pH [1,15]. This approach has widely been used for the analysis of iAs(III) and iAs(V) [21,22]. Only recent work has shown that the pH-specific HG can also been applied for the analysis of methylated tri- and pentavalent arsenicals [1,9,10].
Alternatively, selective HG in the presence or absence of L-cysteine has successfully been used for speciation analysis of iAs(III) and iAs(V) [16,23,24], but not yet for tri- and pentavalent methylated species. The enhancing effect of L-cysteine on HG of As compounds is well established [13,25–29]. L-cysteine reduces pentavalent arsenicals to trivalency and reacts with trivalent arsenicals, forming arsinothiol derivatives [26,27,30]. It has also been suggested that L-cysteine is enhancing the HG performance by forming borane complexes with borohydride [19,30,31]. Importantly, uniform signals are obtained from iAs, MAs(V) and DMAs(V) species after L-cysteine treatment. [13,26,27]. However, the optimal conditions for the HG reactions in the presence and absence of L-cysteine significantly differ. The HG with L-cysteine treatment, up to now almost exclusively performed from HCl medium, suffers from a very narrow optimum of acid concentration (0.01–0.1 M) [13,16,26,27,29,30,32] for efficient generation, which is causing inconvenience and risk of error in analysis of practical samples.
A HG method, based on the continuous arrangement of the chemical reaction and on the batch arrangement of the gas-liquid separation suits best HG-CT [16] and also all kinds of inatomizer trapping [33]. Even though the concentration of acid and individual intermediates of tetrahydroborate hydrolysis change along the length of the reaction coil they do not change with time in any given section of the apparatus, allowing better control of the chemical reaction conditions. This is an advantage compared to the batch methods of HG. At the same time, no hydride is lost as the waste liquid remains in the gas-liquid separator (GLS) for the whole hydride generation and cryotrapping phase, allowing for very long reaction and hydride release times. This is an advantage compared to the conventional continuous flow methods of HG which employ the continuous arrangement of the gas-liquid separation.
Flame-in-tube atomizers are usually employed for atomization of hydrides evolved from a cryogenic trap [34,35]. The reason is that the more sensitive atomizer - conventional externally heated quartz tube is unsuitable for this task because of a demand of hydrogen and oxygen in the atomizer [22] for analyte atomization. The demand is usually covered in the case of direct transfer of generated hydride to the atomizer [22] but generally not in the case of the cryogenic trap collection. The use of the conventional externally heated quartz tube for atomization of arsines released from the cryogenic trap [12,15] is rather an exception. Indeed, the sensitivity estimated from published chromatograms [12], substantially lower than reported in the literature [36] for the same atomizer, indicates unsatisfactory performance of the atomizer for this task. The best suited atomizer for this task appears to be the multiple microflame quartz tube atomizer (multiatomizer) [37,38]. Besides the inherent advantages, i.e. prevention of the calibration curvature and substantially better resistance to atomization interferences, the multiatomizer incorporates its own introduction of oxygen and it provides the same sensitivity as the conventional externally heated quartz tube.
The aim of the paper is to develop a method for the oxidation state specific analysis of methylated As species in biological samples. The use of buffered medium for L-cysteine treatment is proposed and investigated. L-cysteine based selective HG is compared to traditional pH specific HG and the possibility of calibration on single As form is explored. An automated HG-CT system was developed for the task, featuring HG in flow arrangement with batch gas-liquid separation and the multiatomizer for atomization of arsines.
2. Experimental
2.1. Instrumentation
AAnalyst 800 AAS spectrometer (Perkin-Elmer, Norwalk, Mass, U.S.A.) equipped with FIAS 400 flow injection accessory (FIAS) was employed, except for analyses in biological matrix when identical HG-CT system on Model 5100 AAS spectrometer (Perkin-Elmer, Norwalk, Mass, U.S.A.) equipped with FIAS 200 flow injection accessory was used. Arsenic electrodeless discharge lamps System II (Perkin-Elmer) at 390 mA was used as the radiation source on both spectrometers. The slit width was 0.7 nm. If explicitly stated, deuterium background correction was used. Signals were exported as ASCII files into Origin Pro 7.5 (Origin Lab Corp.) software for further processing. For the direct transfer method with hydrides generated in the conventional continuous flow arrangement, a Perkin–Elmer 503 AAS spectrometer was utilized with Arsenic electrodeless discharge lamps System I (Perkin-Elmer) operated at 8 W. Signals from Perkin–Elmer 503 were recorded by a strip-chart recorder.
2.2. Multiatomizer
The multiatomizer was identical to that described previously (model MM5 in Ref. [38]). The inner tube of the optical bar of the atomizer used in this study was 120 mm long with 7 mm i.d and fourteen orifices having diameter between 0.1 a 1 mm [38]. The atomizer was heated to 900°C by the FIAS heating device. 35 ml min−1 of air as outer gas for the atomizer was used.
2.3. Automated system for hydride generation and cryotrapping
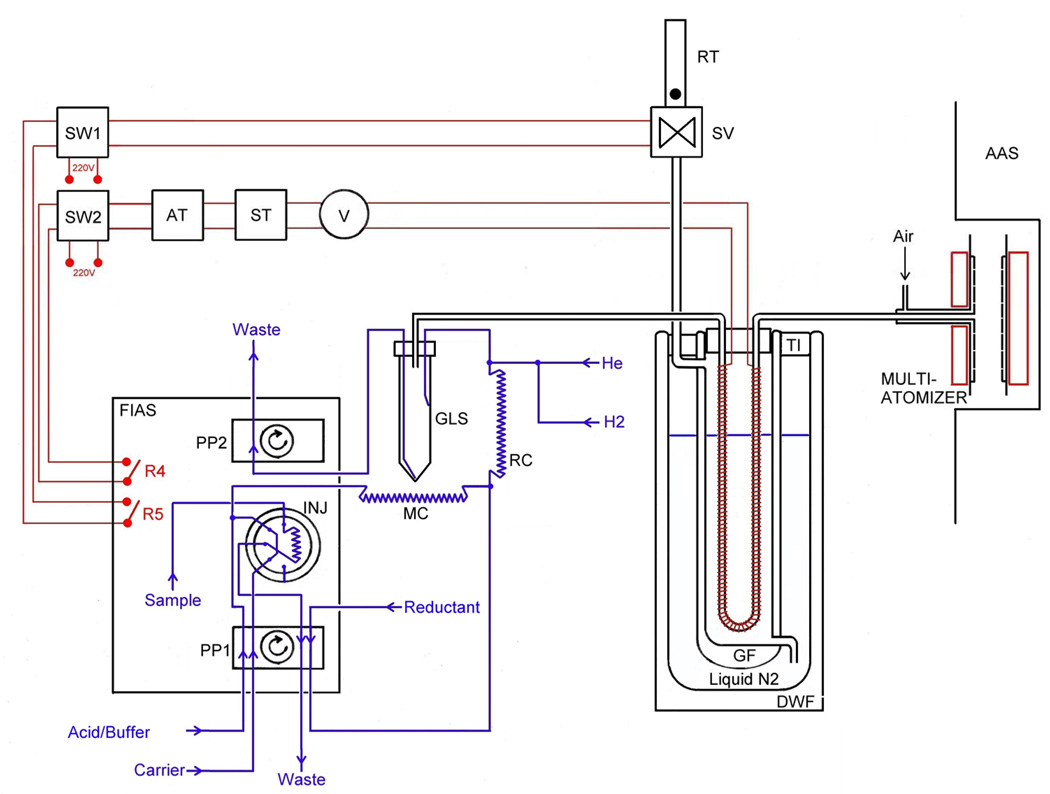
A scheme of the system for HG-CT-AAS, built around the Perkin-Elmer FIAS 400 unit, is shown in Fig. 1. All gas flows were controlled by mass flow controllers (FMA-2400 or 2600 Series, Omega Engineering, Stamford, CT, USA). The sampling loop of 500 µl was used if not specified otherwise. The manifold was built using PEEK T-pieces (0.75 mm bore) and 1/4-28 threaded connectors. The PTFE tubing was used as follows: 1/16" o.d./ 0.75 mm i.d. for the liquid leads, 1/16" o.d./ 0.5 mm i.d for the He/H2 inlet; 1/16" o.d./ 0.75 mm and 1 mm i.d for the mixing coil (280 mm-120 µl) and reaction coil (150 mm-120 µl), respectively; 1/8"o.d./ 1/16" i.d. for GLS to U-tube and U-tube to atomizer connections (150 mm each).
Fig. 1.
Experimental setup of automated HG-CT-AAS. PP1, PP2: FIAS peristaltic pump. Legend: INJ: Injection vent (500 µl loop); MC: Mixing coil; RC: Reaction coil; GLS: Phase separator; DWF: Dewar flask; GF: Glass flask; TI: Thermal insulation; R4, R5: FIAS remotes; SW1, SW2: Relay switches; AT: Autotransformer; ST:Separating transformer; V: Voltmetrer; SV: Solenoid valve; RT: Rotameter tube for gas outlet monitoring.
GLS with forced outlet [22] was used as it can handle overpressure caused by the resistance of the U tube (typically 0.2–0.3 bar). The plastic GLS shown in Fig. 1 was a polypropylene 50 ml screw-cup with a custom made acrylic lid with two 1/16" and one 1/8" inlets. The PTFE tubing was secured by 1/4-28 Rheodyne RheFlex flat bottom flangeless fittings. The mixture from reaction coil is led into half of the vial’s height by a tube through the lid, so that it enters near tangentially close to the wall and does not strike the wall to minimize aerosol production. The waste outlet starts at the cone-shaped bottom. Gas outlet tube opening is located in the free volume at approximately 3/4 of vial’s height. The GLS can accommodate up to 20 ml of liquid, which translates into 5 ml of sample volume in a single generation cycle (with equal flows of sample/carrier, buffer and reducing solution).
Two 305 mm long glass U-tubes having i.d./o.d. of 4 mm/6 mm (broad U-tube) and 2.5 mm/4.5 mm (narrow U-tube), respectively, were tested in the course of study. They were loosely filled with 2.8 g and 0.8 g of Chromosorb WAW-DCMS 45/60 (15% OV-3), respectively. After packing, the U-tubes were treated with 50 µl Rejuv-8 silylating reagent (Sigma) and flushed with helium for several hours. Both U-tubes were evenly wrapped with a Ni80/Cr20 wire (0.51 mm diameter/5.275 Ω m−1, Omega Engineering, Stamford, CT, U.S.A.) providing a total resistance of 15 Ω and 20 Ω, respectively, for broad and narrow U-tube, covering the entire Chromosorb column. Heating voltage from 30 to 60 V was used.
The U-tube was inserted into a sealed glass flask (GF in Fig. 1) with evacuated double-wall (40 mm i.d, 300 mm deep) having an inlet at the bottom and an outlet near the top. Both ends of the U-tube and leads for the heating wire were glued into a cork stopper closing gas-tight the flask. A FIAS-controlled solenoid valve (SV in Fig. 1) was connected to the flask outlet. The glass flask with evacuated double-wall was immersed into 4.3-liter Dewar flask (135 mm i.d.; DWF in Fig. 1), filled with liquid nitrogen, and covered with a polyethylene foam lid. Approximately 0.5 liter of liquid nitrogen was added into Dewar flask every 10 cycles (once an hour) to keep the nitrogen level within a 50 mm range around 3/4 of the U-tube height.
2.4. Procedure
When using L-cysteine for non-selective generation of arsines from both tri- and pentavalent arsenicals, solid L-cysteine hydrochloride to final concentration of 2% m/v was added to samples and/or standards at least one hour prior to analysis. Resulting HCl concentration in sample solutions thus was 0.11 M.
2.4.1. Direct transfer mode
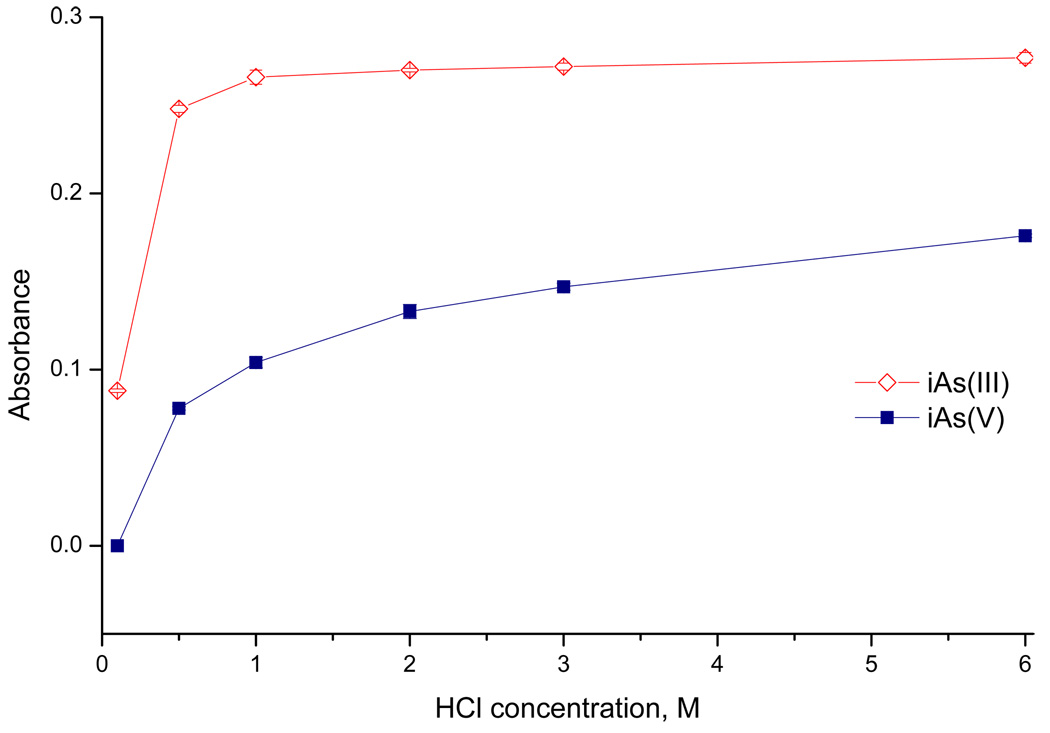
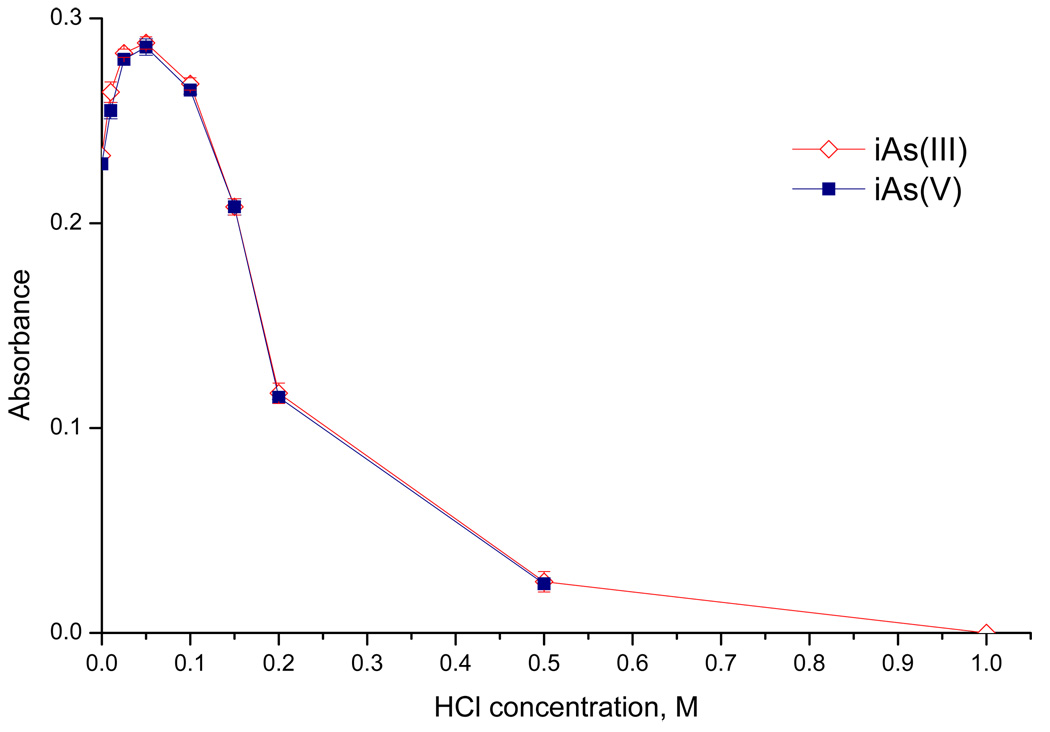
A simplified direct transfer version of the setup was employed in some experiments to supply hydride from generator directly to the atomizer aligned in the optical path of the Perkin–Elmer 503 spectrometer. Hydride generation was thus performed in the conventional continuous flow arrangement. Two continuously running peristaltic pumps (Reglo Digital 4/12, Ismatec, Switzerland) replaced the FIAS unit; the injection vent and cryotrapping unit were omitted in this case. The flow rate in individual channels was 1 ml min−1, waste pump rate was balanced so that there was a pool of liquid inside the GLS. Longer reaction coil (1000 mm- 785 µl) was used. The flow rate of carrier Ar of 75 ml min−1 was maintained throughout the analysis, with 25 ml min−1 originating from NaBH4 decomposition. The outlet from the GLS was directly connected to the atomizer by 150 mm long PTFE tubing of 1/8" o.d./ 1/16" i.d. Steady-state signals were recorded. Averages of at least 3 replicate measurements with the standard deviations as error bars are shown in Fig. 3. and Fig. 4.
Fig. 3.
The influence of HCl concentration in Acid/Buffer channel on signal of iAs(III) and iAs(V) (direct transfer mode, 20 ng ml−1 As)
Fig. 4.
The influence of HCl concentration in Acid/Buffer channel on iAs(III)/ iAs(V) signal with L-cysteine treatment (direct transfer mode, 20 ng ml−1 As)
2.4.2. Cryotrapping mode
The automated system is controlled by the FIAS unit. The FIAS program is shown in Table 1. The flow rate of carrier He (75 ml min−1 if not stated otherwise) and H2 (15 ml min−1) was maintained throughout.
Table 1.
FIAS program for automated HG-CT- AAS.
| Step | Time s | Pump1 rpm | Pump2 rpm | Valve position | Read | Solenoid valve | U-tube heating | Comment |
|---|---|---|---|---|---|---|---|---|
| P* | 30 | 55 | 100 | Fill | Open | Off | Sample loop filling | |
| 1 | 60 | 55 | 0 | Inject | Open | Off | Generation of arsines | |
| 2 | 90 | 0 | 0 | Inject | Open | Off | Reaction time | |
| 3 | 20 | 0 | 0 | Fill | Closed | On | U-tube preheat | |
| 4 | 30 | 0 | 0 | Inject | 57s | Closed | On | U-tube heating/ Read |
| 5 | 7 | 0 | 0 | Inject | Closed | Off | Heating break | |
| 6 | 58 | 0 | 0 | Inject | Closed | On | U-tube heating/cleaning | |
| 7 | 40 | 0 | 100 | Fill | Open | Off | U-tube cooling | |
| 8 | 3 |
0 | 0 | Fill | Open | Off | Ready for next run |
|
| 338 | Total time |
Prefill
Cryotrapping stage
In the Prefill step (P in Table 1), the sampling loop is loaded by the sample solution at the rate of 2.5 ml min−1. Simultaneously, there are three reagent flows pumped at the same flow rate of 1.0 ml min−1: water to the Carrier channel, the TRIS buffer solution to the Acid/Buffer channel and the reducing solution to the Reductant channel. In step 1 (see Table 1), the sample is injected to the flow of carrier water. Pump 2 is switched off so that there is no flow of the reacted solution from the GLS. Step 2 allows 90 s of waiting time to ensure complete generation of arsines and their transport into the U- tube; reaction mixture remains in the GLS. In the course of all these steps, U-tube heating is off and solenoid valve is open. The level of liquid nitrogen inside the glass cylinder containing the U-tube is the same as in the outside Dewar flask and the U-tube has the temperature of liquid nitrogen.
Volatilization stage
In step 3 (Table 1), the solenoid valve closes and the heating starts. The liquid nitrogen is displaced in few seconds from the U-tube compartment of the glass flask GF by the nitrogen gas evolved upon heating. After 20 s, in step 4, a 58 s long reading period (the longest possible allowed by the spectrometer software) is initiated. As the U-tube warms up, hydrides (arsines) evaporate according to their boiling points and separate on the chromatographic packing and enter the atomizer. In the middle of the reading period, the U-tube heating is shortly interrupted (step 5) to obtain better resolution of dimethyl- and trimethyl-arsine. In step 6 afterwards, the U-tube is further heated up to approximately 150°C to remove all the moisture; the use of membrane dryer device (Perma Pure, Toms River, N.J., U.S.A.) was found unnecessary provided that U tube was heated to sufficient temperature. In step 7, U-tube heating switches off and solenoid valve opens to allow the liquid nitrogen to re-enter the U-tube compartment. At the same time, the spent reaction mixture is pumped out of the GLS. At the end of the cycle, in step 8, the pump 2 is switched off to reset the FIAS for next run.
The total time of the procedure is 338 s. The liquid nitrogen consumption was approximately 50 ml per cycle.
2.5. Reagents
Deionized water (<0.2 µS cm−1,ULTRAPURE, Watrex) was used for all solutions . The reducing solution containing 1% NaBH4 (Fluka, Buchs, Switzerland) in 0.1% (m/v) NaOH(p.a., Lachema, Brno, Czech Rep.) was prepared daily. For analysis of samples containing cell culture medium and cell lysates containing Triton X 100 surfactant, Antifoam B emulsion (2 ml of 1% (v/v) solution per 100 ml) was added to the reducing solution to prevent foaming. A 0.75 M Tris(hydroxymethyl)aminomethane (TRIS) - HCl buffer (pH 6) was prepared from a reagent grade Trizma® hydrochloride (Sigma) and pH adjusted to 6 by NaOH. A 0.1 M citrate buffer (pH 6) was prepared by mixing of 95 ml and 415 ml of 0.1 M solutions of citric acid ( Lachema) and sodium citrate (Lachema), respectively. A phosphate buffer (pH 5.8) was prepared by dissolving 43.3g of Na2HPO4.12 H2O (Lachema) and 8.3g of citric acid in 1l of water. Other reagents included HCl (p.a., Merck, Darmstadt, Germany ) and a biochemistry grade L-cysteine hydrochloride monohydrate (Merck).
2.6. Standards
A 1000 µg l−1 As AAS standard solution (Merck, Darmstadt, Germany) was used as iAs(V) stock standard solution. A stock solution of 1000 µg As l−1 was prepared for each of other arsenic species in water using following compounds: As2O3 , Lachema, Czech republic (iAs(III)); Na2CH3AsO3.6H2O, Chem. Service, West Chester, PA, USA (MAs(V)); H (CH3)2AsO2, Strem Chemicals, Inc., Newburyport, MA, USA (DMAs(V)). (CH3AsO)4 (MAs(III)), (CH3)2As.(GS)- glutathione complex [39] and (CH3)2As.I (DMAs(III)) and (CH3)3AsO (TMAs(V)O)[40] were obtained courtesy of Dr. William Cullen (University of British Columbia, Vancouver, Canada). Working standards were prepared for individual species by serial dilution of the stock solutions in water. Mixed standards were used only in the last dilution, i.e. at the ng ml−1 level. For trivalent species, water for standard preparation was deaerated by purging with nitrogen for at least one hour. Stored at 4°C, standard solutions were stable for several weeks except for MAs(III) and DMAs(III) which had to be prepared fresh for experiments.
It was found that the As content in methylated species can differ from expected value by as much as 20% and standardization of the solutions was found necessary. The total As content was determined in approximately 100 µg l−1 solutions of individual species by liquid sampling graphite furnace- AAS, using a Perkin-Elmer Analyst 800 instrument with graphite furnace atomizer. End-capped transversely heated tubes permanently modified by 4 µg of Ir were used at the program and conditions recommended by the manufacturer. Assuming that the sensitivity for individual As forms are identical in the graphite furnace- AAS, the values found was taken as the true content. It shall be stressed that the results were consistent over several preparations of standard solutions, and therefore not due to errors in the preparation of standards, with the exception of the DMAs(III) (iodide complex) form where high variability was experienced.
2.7. Biological matrices
Human hepatocellular carcinoma (HepG2) cells were cultured in 6-well culture plates in DMEM medium (Sigma) containing 5% fetal bovine serum (Sigma). Cells were cultured to confluence for 24 h at 37°C in a cell culture incubator. After incubation, medium was collected and cells were lysed in 250 µL of 0.5% Triton X100 in deionised water. Collected media and cell lysates were kept at −20°C until analyzed. Prior analysis, cell lysates and media were diluted with deionised water.
3. Results and Discussion
3.1. System optimization and performance
The cryotrapping parameters were optimized with respect to chromatogram resolution and with the necessity to cover all the analyte peaks within the AAS instrument reading window of 58 s. The relevant parameters are the flow rate of the carrier gas, the U-tube heating rate and, finally, the U-tube internal diameter.
Carrier He flow rate was tested between 30 and 200 ml min−1 while the carrier H2 flow rate of 15 ml min−1 (to ensure H2 presence in the atomizer) was kept constant. The peak area sensitivity at He flow rate of 75 ml min−1 increased 2.2 times compared to that at 200 ml min−1. Although the full width at half maximum (FWHM) of all peaks was not significantly affected by the flow rate, the peak tailing is slightly more pronounced at the lower flow rate and became intolerable at flow rates below 75 ml min−1 which was the lowest flow rate without significantly worse separation of the arsenicals.
U-tube heating rate is the critical parameter controlling retention times and peak shapes. With increased heating rate, peaks are getting narrower and appearing at shorter relative retention times, however, their resolution is slightly worse. Due to the limitation of the reading window duration, heating rate can be utilized for optimization of retention times within the reading window, but not of the resolution.
The narrower U-tube improves the resolution due to reduced temperature gradients over the U-tube cross section in the heating stage. The resolution of the peaks was significantly improved with the narrow U-tube (2.5 mm i.d.). Because the separation of di- and trimethylarsine was difficult even in the narrow U-tube, a 7 s long U-tube heating interruption (step 5 in Table 1) at the time of dimethylarsine appearance was employed to delay the trimethylarsine retention time and to allow complete separation. Careful timing of this break was necessary not to cause the dimethylarsine peak broadening.
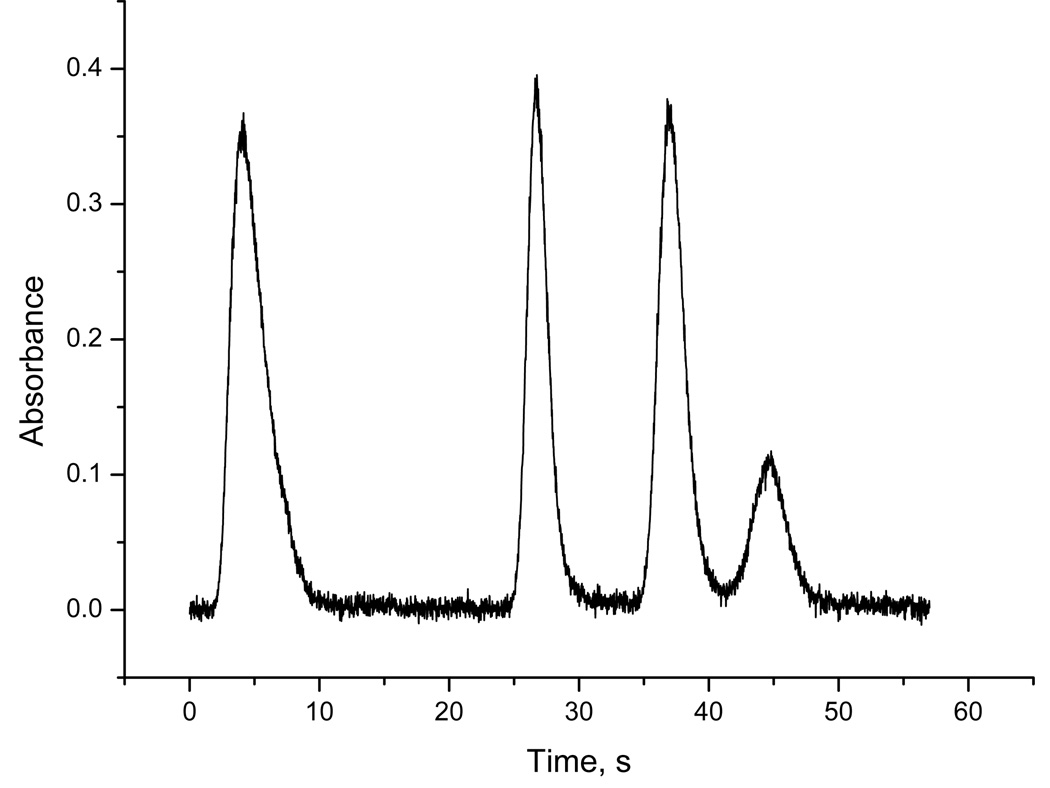
The typical separation in a form of chromatogram of mixed standard of pentavalent forms obtained with L-cysteine prereduction at optimized conditions is illustrated in Fig. 2. Typically, standard deviation of retention times was better than 0.2, 0.35, 0.45 and 0.5 s, respectively, for arsine, methylarsine, dimethylarsine and trimethylarsine peaks. Regarding the other characteristics of individual peaks, the best repeatability yielded peak area: relative standard deviation (RSD) better than 2, 3.5, 6 and 7 %, respectively, for arsine, methylarsine, dimethylarsine and trimethylarsine peaks. RSD of peak heights and of FWHM was approximately two times higher. After re-packing or re-silylation of the U-tube and between different U-tubes or when re-wrapping the resistance wire, the peak shapes, retention times and FWHM may vary, although the resolution quality remains comparable to the illustration in Fig. 2. The retention times then varied from those of the typical chromatogram (Fig. 2) within 2 s. Overall, FWHM of arsine and methylarsine peaks were between 1.5 and 3.0 s. Dimethylarsine and trimethylarsine peaks were generally slightly broader with FWHM between 2.0 and 3.5 s. The exact timing of the heating break (step 5 in Table 1) has to be carefully re-optimized for each U-tube setup, otherwise the width of dimethylarsine and/or trimethylarsine peaks can be substantially increased.
Fig. 2.
The signals for 2.5 mm U-tube. Peaks from left to right: 1 ng iAs(V), 0.9 ng MAs(V), 1 ng DMAs(V), 1 ng TMAs(V)O; with L-cysteine treatment.
Deuterium background correction was used to correct for non-specific absorption signal (see Section 3.3). Limit of detection (LOD) was about 0.06 ng for methylated forms. LOD of 0.18 ng was achieved for total iAs, due to blank contamination. Since background correction is not required to determine methylated forms, their LOD was estimated from measurements performed in the absence of background correction. The LOD’s achieved (methylated forms) were thus 0.02 ng (40 ng l−1). It should be highlighted that, assuming the same total masses of individual forms, there was no significant difference between chromatograms obtained from 0.5 ml and 5 ml sample volumes. Consequently, the concentration LODs of methylated forms were improved to 4 ng l−1 when increasing sample volume to 5 ml.
The reported LODs make a considerable improvement compared to 0.22 - 0.4 ng (peak heigth LOD’s) and 0.2–0.6 ng reported in Refs. [1] and [15], respectively, for HG-CT-AAS. The improvement is mainly due to better performance of the multiatomizer in terms of sensitivity as well as of lower baseline noise. These values also compare well with the relative LOD’s published for HPLC-ICP MS technique, e.g. [41,42], where high sensitivity of the detector is hindered by very small sample volumes permitted by a separation step.
3.2. Selective HG to distinguish between tri- and pentavalent arsenicals
In order to find optimal conditions for the generation of individual arsines, hydrides were generated in the direct transfer version of the setup (see Experimental). Then the performance of the completely automated HG-CT-AAS system was examined using aqueous As standards and biological matrices.
Since it is impossible in reality to generate arsine selectively from pentavalent forms in the presence of trivalent forms, the selective HG procedure is based on the following scheme: determination of trivalent forms in one sample aliquot (Aliquot A) and of the sum of the both forms in the second aliquot (Aliquot B). Then the content of the pentavalent arsenicals is calculated by difference. The trivalent forms are determined by selecting conditions of HG for complete conversion of the trivalent form to hydride and, simultaneously, preventing HG from the higher valency. The sum of the both forms may be determined either by selecting conditions of HG for complete reduction of both forms to hydride or after pre-reduction of the form with higher valency. It should be highlighted that it is ideal if sensitivities of the both forms in aliquot B are equal each to other and also equal to sensitivity of the trivalent form in aliquot A. In that case, the single species standardization can be used, i.e. for the estimate of both forms it is sufficient to determine sensitivity of the pentavalent form in aliquot B or of the trivalent form in either of the aliquots.
To approach the ideal situation, two sets of experimental conditions were sought for aliquots A and B, respectively: (A) conditions for selective generation of arsines exclusively from trivalent arsenicals, and (B) conditions for non-selective generation of arsines from both tri- and pentavalent arsenicals.
3.2.1. pH specific hydride generation
The selectivity of arsine generation from iAs(III) and iAs(V) has traditionally been achieved by the pH specific HG [22]. Devesa et al. [1] described a successful speciation analysis of trivalent and pentavalent forms of iAs, and also of MAs and DMAs. They used the batch arrangements of HG with TRIS buffer (pH 6) or 1M HCl as reaction medium for selective or non-selective HG, respectively. However, in the conventional continuous flow arrangement iAs(V) yields markedly lower sensitivity than iAs(III) with 1M HCl (i.e. 2M HCl in Acid/Buffer channel) as reaction medium. In fact, as shown in Fig. 3 iAs(V) provides lower sensitivity than iAs(III) at all concentrations of HCl. This is compatible with the earlier reports on the slow conversion of iAs(V) to hydride [22]( probably due to worse accessibility of anayte atom to reduction by hydridic hydrogen of borohydride [19]) - the reaction time in the reaction coil is obviously too short for the slow reaction. With 1M HCl as reaction medium, larger reaction coils were therefore tested in order to prolong the reaction time. Increasing the reaction coil length from 1 m (volume 0.8 ml, residence time approximately 0.8 s) to 5 m (volume 4 ml, residence time 4 s) did not improve the situation markedly but the coil 3 m long, 2.4 mm i.d. (volume 15 ml, residence time 15 s) provided similar sensitivities for both forms. However, the use of such a long reaction coil is not convenient because of the increased risk of liquid phase interferences [22].
When using the flow method with batch gas-liquid separation for HG (with cryotrapping) instead of the conventional continuous flow arrangement, the difference in sensitivities of the both forms with 1M HCl as reaction medium was lower than shown in Fig. 3, however, a difference in sensitivities of trivalent and pentavalent forms in aliquot B persisted (See also Table 3). In summary, the pH specific hydride generation with the continuous flow arrangement of HG cannot meet the condition for the single species standardization - it is necessary to determine all the individual sensitivities: trivalent form in aliquot A, pentavalent form in aliquot B and trivalent form in aliquot B, for each species- iAs, MAs and DMAs.
Table 3.
Relative sensitivitiesa (% ± combined standard deviation) of As species in water standards and biological matrices spiked at 2 µg l−1 level by As species measured in cryotrapping mode
| Matrix | Aliquot | iAs(III) | iAs(V) | MAs(III) | MAs(V) | DM As(III) | DMAs(V) |
|---|---|---|---|---|---|---|---|
| A: TRIS | 93 ± 4.8 | < 2 | 89 ± 3.5 | < 2 | 74 ± 4.2 | < 2 | |
| Water standards | B: TRIS+ L-cysteine | 100 | 98 ± 3.7 | 93 ± 4.3 | 93 ± 3.8 | 95 ± 4.3 | 95 ± 4.3 |
| (B): HCl 1M | 105 ± 3.5 | 63 ± 7.4 | 95 ± 4.4 | 86 ± 4.5 | 76 ± 3.5 | 75 ± 5.6 | |
| A: TRIS | 87 ± 4.8 | < 2 | 86 ± 5.1 | < 2 | 75 ± 4.1 | < 2 | |
| Cell culture medium, 1:10 dilution | B: TRIS+ L-cysteine | 105 ± 5.8 | 96 ± 5.0 | 92 ± 4.0 | 90 ± 6.6 | 93 ± 3.8 | 90 ± 7.9 |
| (B): HCl 1M | 93 ± 6.0 | 56 ± 4.5 | 83 ± 3.8 | 85 ± 3.6 | 88 ± 4.0 | 80 ± 3.5 | |
| A: TRIS | 92 ± 8.3 | < 2 | 81 ± 16 | < 2 | 59 ± 17.0 | < 2 | |
| Cell lysate, 1:5 dilution | B: TRIS+ L-cysteine | 101 ± 4.0 | 95 ± 3.5 | 95 ± 3.6 | 95 ± 3.6 | 100 ± 3.8 | 98 ± 3.5 |
| (B): HCl 1M | 102 ± 3.5 | 57 ± 7.6 | 44 ± 9.5 | 76 ± 4.5 | 67 ± 3.9 | 72 ± 7.7 |
Related to iAs(III) water standard sensitivity in Aliquot B.
3.2.2. Selective HG by L-cysteine treatment in buffered media
The alternative to the problematic pH specific approach to the selective HG is the use of L-cysteine for non-selective generation of arsines from both tri- and pentavalent arsenicals from aliquot B. The selective generation of arsines from trivalent arsenicals (aliquot A) is performed in the same way as in the previous case: by maintaining the reaction acidity at pH high enough to prevent arsine formation from pentavalent arsenicals.
Fig. 4 illustrates the advantage of this approach, the identical sensitivity of both inorganic forms converted to arsine in the presence of L-cysteine. However, it also demonstrates the narrow optimum acidity range already mentioned in the Introduction. When a "final" pH of the reaction mixture was measured after the reaction, without the addition of HCl in the Acid/Buffer channel, the pH was 9.2. Supposedly, the acidity of the reaction mixture should be maintained at lower pH to achieve the maximum efficiency of the conversion of analyte to arsine. The addition of low concentration of HCl (0.05M in our case, along with 0.11 M HCl present as a result of 2% L-cysteine hydrochloride treatment of the sample) serves to maintain the optimum acidity of the reaction mixture. In the real samples, the optimum concentration of acid may vary depending on sample matrix, and since the optimum is very narrow, this may lead to errors.
A more convenient way to maintain the optimum acidity is to use a buffer. For the sake of simplicity, the same buffer should be used for both aliquots A or B; a TRIS buffer appears to be particularly suitable as its maximum buffering capacity is in the slightly basic region. The acidity at individual HG phases using the 0.75 M TRIS buffer (pH 6), as used for aliquot A, was therefore measured for water standards and biological sample matrices (see Experimental). Regardless if the sample was treated with L-cysteine (intial pH 1.5, increasing to 1.9 after addition of TRIS buffer ) or not (initial pH 6–8, changing approx. to 6.5 after addition of TRIS buffer), the final pH of the reaction mixture was between 7.4–7.8.
In the ideal case, the buffer should provide the same sensitivity of tri- and pentavalent As forms in aliquot B and trivalent form in aliquot A. Further, the pentavalent form in aliquot A should not yield any signal. Besides TRIS, sensitivities for citrate and phosphate buffers were also tested. The sensitivity found with in the direct transfer version of the experimental set-up (see Experimental) for a given buffer introduced to the Acid/Buffer channel of the hydride generator was related to that for tri- and pentavalent inorganic forms with L-cysteine treatment at the optimum acidity, i.e. with 0.05 M HCl in the Acid/Buffer channel (Fig. 4). 0.2 M phosphate buffer (pH 5.8) and 0.75 M TRIS buffer (pH 6) performed optimally: no significant signal of pentavalent forms and sensitivities close to 100% for trivalent forms in aliquot A as well as for both forms in aliquot B. 0.1 M citrate buffer (pH 6) was found unacceptable since it yielded only 30% sensitivity of iAs(III) in aliquot A. The TRIS buffer was employed further because of higher iAs(V) blank levels in the phosphate buffer.
Table 2 shows relative sensitivities of individual forms in both aliquots with TRIS buffer. The repeatability of the measurements was 5% or better. Taking into account the uncertainty of As content in the standards (see Experimental section), following conclusions can be made: (i) All As forms with the exception of TMAs(V)O in aliquot B and the trivalent forms and TMA(V)O in aliquot A are converted to corresponding hydrides with the same efficiency and (ii) all the hydrides, i.e. arsine, methylarsine, dimethylarsine and trimethylarsine, are atomized in the multiatomizer with the same efficiency. Therefore, the procedure of selective HG based on L-cysteine treatment and TRIS buffer meets the condition for the single species standardization. Once the individual As hydrides can be separated, it is sufficient to determine sensitivity either of any As form (with the exception of TMAs(V)O) in aliquot B or of any trivalent form (or TMAs(V)O) in aliquot A. All forms can be quantified then, based on this value.
Table 2.
Relative sensitivitiesa (% ± combined standard deviation) of As forms in aliquot A and B. Direct transfer mode, single As species standards at 20 µg l−1 level
| Aliquot | iAs(III) | iAs(V) | MAs(III) | MAs(V) | DMAs(III) | DMAs(V) | TMAs(V)O |
|---|---|---|---|---|---|---|---|
| A: TRIS | 94 ± 3.6 | <1 | 103 ± 2.3 | <1 | 107 ± 2.6 | 5 ± 0.6 | 108 ± 2.5 |
| B: TRIS+L cysteine | 100 | 101 ± 3.3 | n.t.b | 96 ± 5.7 | n.t.b | 101 ± 2.9 | 35 ± 10 |
Related to iAs(III) sensitivity in aliquot B.
n.t.- not tested.
In contrast to other pentavalent forms, TMAs(V)O yields full sensitivity in the absence of L-cysteine in aliquot A. The reason is that TMAs(V)O does not have to be protonated, as it is present in the solution as a neutral species active enough for HG reaction [19]. The presence of L-cysteine decreased its sensitivity to approximately one third (Tab. 2), in agreement with earlier report [29]. Therefore, quantitative determination of TMAs(V)O should be performed without L-cysteine treatment although its qualitative presence is revealed also in the L-cysteine modified samples.
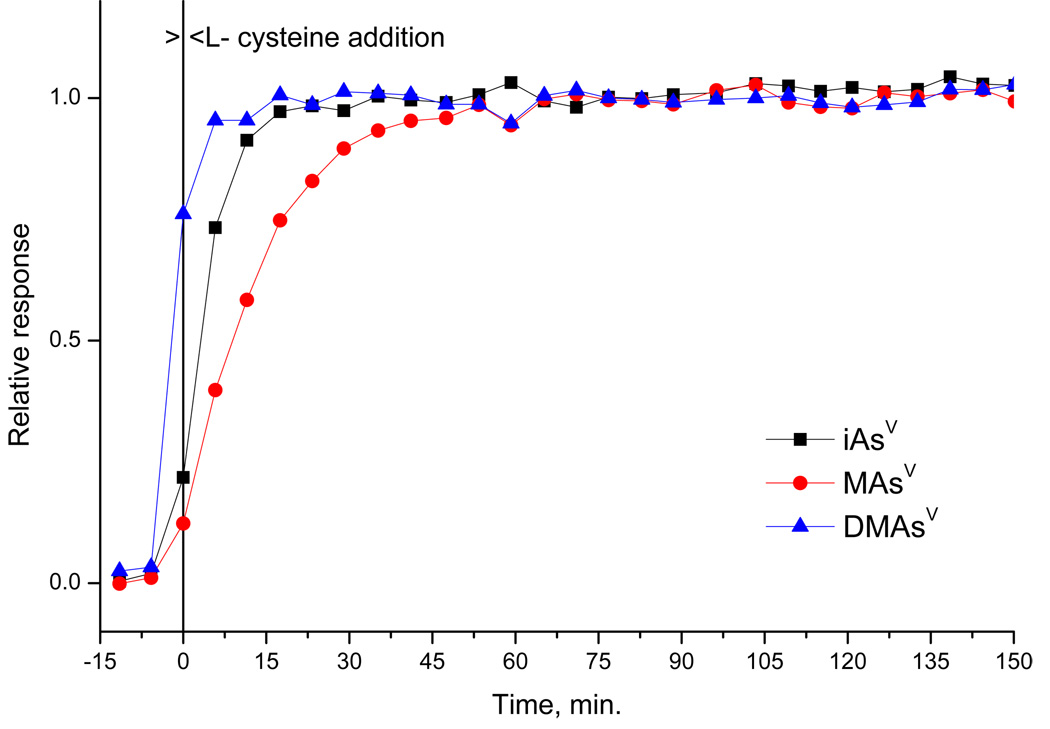
On-line L-cysteine addition would significantly enhance performance of the selective HG procedure. To test feasibility of the on-line addition in the buffered media, the influence of the time elapsed between addition of L-cysteine to the sample solution on the response of iAs(V), MAs(V) and DMAs(V) was investigated with the cryotrapping mode procedure. As shown in Fig. 5 (the time interval between data points is given by the duration of the measurement cycle), the complete L-cysteine derivatization at room temperature takes 60 minutes for MAs(V), whereas only 20 and 5 minutes is necessary for iAs(V) and DMAs(V), respectively. This corresponds to the data published previously for generation from HCl media [28,29]. Once the derivatization was complete, all species yielded constant signal till the end of investigated time interval, i.e. 150 minutes (Fig. 5). In conclusion, the on-line L-cysteine addition at room temperature is not feasible since the time required for the pentavalent arsenicals to prereduce and/or form complexes is too long.
Fig. 5.
L-cysteine treatment of pentavalent forms as function of time. HG-CT-AAS, TRIS buffer, iAs(V)- MAs(V)-DMAs(V), 1 ng each form. Responses are related to the steady state value (65–95 min.).
3.3. Non-specific signal
In aliquot B, a non-specific absorption signal was experienced on the chromatograms, manifesting itself as a peak with retention time approximately 3 s longer than for iAs and partially coincided with the iAs peak. The height of the non-specific peak was approximately 0.01, corresponding to apparent As mass roughly 0.06 ng. The source must be a chemical species generated in the hydride generator from L-cysteine (no signal in otherwise identical aliquot A) having probably the boiling point close to AsH3. Deuterium background correction was capable of solving the problem proving that the unidentified species did not contain As.
Also the baseline drifts, typically approximately to absorbance 0.005 - 0.01 at the end of the measurement interval were eliminated by the deuterium background correction.
3.4. Single species standardization, sensitivity and limits of detection
As discussed above for the direct transfer arrangement, the selective HG based on L-cysteine treatment and TRIS buffer meets the condition for the single species standardization. The feasibility of the single species standardization was therefore verified for the cryotrapping mode procedure.
The relative peak area sensitivities of all forms in both aliquots are shown in Table 3. The sensitivities of individual forms show good uniformity with L-cysteine treatment (Aliquot B). In aliquot A, slightly lower sensitivities were observed for methylated trivalent forms. We believe that the difference is due to partial oxidation of the trivalent standards to pentavalent forms, as the lowest sensitivity of 74% was achieved for DMAs(III) (as iodide complex) which is most prone to oxidation [11]. The sensitivity of all forms observed in the course of several months was in the range of 0.9–1.3 s per ng As (except TMAs(V)O with L-cysteine treatment- see Sec.3.2.2).
Uniform sensitivity of all forms proves superb performance of the whole system. Hydrides of all forms are generated with equal (presumably close to complete) efficiency in presented buffer- L-cysteine scheme. The transport, trapping and desorption efficiency is equal for arsine, mono-, di-, and trimethylarsine, as is the atomization efficiency in the multiatomizer.
The sensitivity of all individual forms was also tested in real matrices: in cell culture medium diluted 1:10 and in cell lysate diluted 1:5, spiked by As species. Similar sensitivities were found as in aqueous standards as illustrated in Table 3. The only exception were the trivalent forms in cell lysate matrix in aliquot A, where gradual oxidation of trivalent to pentavalent forms was observed (DMAs(III) >> MAs(III) > iAs(III)). Gradual decrease of signals for several replicates in the course of the measurements was experienced in this case, reflected in high relative standard deviations (see Table 3).
A traditional way of Aliquot B, i.e. the measurements at low pH (in 1 M HCl) was also tested with results clearly inferior to L-cysteine treatment (see Table 3). Pentavalent species and DMAs(III) yielded 14–37% lower sensitivity compared to iAs(III) in water, and even higher differences were observed in the cell culture medium and cell lysate matrices. Moreover, differences in sensitivity were found between water standards and these matrices .
The observations indicate that the single-species standardization might be applied for the cryotrapping mode procedure employing the L-cysteine treatment- to quantify iAs(III) iAs(V), MAs(III) , MAs(V), DMAs(III), DMAs(V), it is sufficient to determine sensitivity in a standard solution of the most stable and the most easily available As forms, i.e. of iAsIII or iAs(V), in the presence of L-cysteine, i.e. in aliquot B. This is very convenient since the availability of trivalent methylated As compounds is very problematic and the solutions of those forms are not stable so that it is hardly feasible to prepare those standards for daily analyses. In fact a standardization by one reliable single-species standard brings more accurate results than use multiple-species standards of unreliable oxidation state or even As content, as long as the conditions for the single species standardization are fulfilled.
3.5. Method selectivity and stability of standard solutions
The selectivity of presented method is limited by its principle- multiple compounds may produce identical volatile product- (methyl substituted) arsine. In the case of tri- and pentavalent compounds, those are distinguished by different reaction conditions. As those compounds readily change valency, sample storage and any pretreatment is of paramount importance. The lowest stability exhibited the DMAs(III) (iodide complex) standards, where oxidation of working standards was observed within one hour and even the stock standards could not be stored frozen overnight. MAs(III) working standards had to be prepared fresh daily, standards of other species were found stable for several weeks at 100 ng ml−1 level (stored at 4°C). We did not observe any changes in the methylation of individual forms in stock standard solutions upon storage at 4°C for several months, except for MAs(III), which partially disproportionates to iAs and DMAs (28% iAs(III), 14% iAs(V), 14% MAs(III), 38% MAs(V), 6% DMAs(V) found, similarly as described by Gong et al.[11]).
The presence of thiols such as cysteine or glutathione in the biological matrix may result in reduction of pentavalent arsenicals and formation of complexes [43], possibly unstable [44], which manifests itself as presence of trivalent arsenicals (which, of course, is technically true). This must be taken into account in metabolic studies.
The reports of trivalent methylated arsenicals are often challenged [7,45]. One possible source of error might originate from thioderivates of oxo-species, such as dimethylthioarsinic acid (DMTA), found in the sheep urine [46]. The DMTA and analogously obtained monomethylated specie were reported HG active to some extent even at pH 6 [47][48], producing mainly dimethylarsine and monomethylarsine, respectively, with rather low efficiency, and thus interfering with DMAs(III) determination. Considering that the DMTA was reported to change into DMAs inaqueous solutions [45], it is possible that signals observed from DMTA can be in fact those of DMAs, and further investigation of HG properties of DMTA and its stability is necessary to clarify this issue.
Another group of compounds possibly interfering in HG based techniques are arsenosugars, which may be also HG active. 5–20% of signal compared to iAs at pH1[47,49] was reported, producing mainly dimethylarsine [47].
4. Conclusions
It can be concluded that the described automated HG-CT system improves the throughput of the method while retaining the quality of the separation of individual peaks, making it user friendly. This makes the automated system more attractive for a routine speciation analysis, possibly in connection with even more sensitive detectors, atomic fluorescence or ICP-MS.
The selective HG procedure based on L-cysteine treatment is capable of resolution of valency of inorganic as well as methylated arsenic species. Uniform sensitivity obtained for all forms using the described procedure permits standardization by a single form of As. This is especially important as the not commercially available MAs(III) and DMAs(III) species are prone to very quick oxidation in standard solutions.
Proposed generation from buffered medium does not require to keep sample acidity in narrow range, overcoming the main disadvantage of the L-cysteine treatment. This approach, allowing more control over the pH of the reaction mixture, may be beneficiary also for chemical vapour generation of other elements, especially cadmium.
The selective HG procedure proved capable of determination of tri- and pentavalent methylated species in biological matrix. Excellent limits of detection are obtained with simple instrumentation. Ref. [48] presents further application of described system for speciation analysis of all major human metabolites of iAs in complex biological matrices. The application of the method for As speciation analysis in urines is imminent. Further developments may include the improvement of separation, especially dimethylarsine and trimethylarsine. On-line reaction modification of selective hydride generation would be desired, replacing L-cysteine by other agents reacting possibly faster with arsenicals.
More research is necessary concerning other arsenic species, especially thioderivates and arsenosugars, and their HG activity at different pH conditions and in presence of L-cysteine, as the data published on HG properties of these potentially interfering compounds and their presence in the matrices of interest do not permit a comprehensive critical discussion of toxicological importance of this issue yet. Another subject of extreme caution is the sample treatment and storage to preserve the oxidation state of methylated arsenicals.
Acknowledgments
This project was supported by NIH-FIRCA project No. 1 R03 TW007057-01 (PI M. Stýblo) and by GAASCR grant No. A400310507. The authors thank Professor William R. Cullen, Department of Chemistry, University of British Columbia, who provided methylated trivalent arsenicals and TMAs(V)O for this study. This manuscript has been reviewed in accordance with the policy of the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Devesa V, Del Razo LM, Adair B, Drobná Z, Waters SB, Hughes MF, Stýblo M, Thomas DJ. Comprehensive analysis of arsenic metabolites by pH-specific hydride generation atomic absorption spectrometry. J. Anal. At. Spectrom. 2004;19:1460–1467. [Google Scholar]
- 2.Thomas DJ, Stýblo M, Waters SB. Elucidating the pathway for arsenic methylation. Toxicol. Appl. Pharmacol. 2004;198:319–326. doi: 10.1016/j.taap.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Waters SB, Drobná Z, Devesa V, Stýblo M, Thomas DJ. Arsenic (+3 oxidation state) methyltransferase and the inorganic arsenic methylation phenotype. Toxicol. Appl. Pharmacol. 2005;204:164–169. doi: 10.1016/j.taap.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Stýblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in human cells. Arch. Toxicol. 2000;74:289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- 5.Petrick JS, Jagadish B, Mash EA, Aposhian HV. Monomethylarsonous acid (MMA(III)) and arsenite: LD(50) in hamsters and in vitro inhibition of pyruvate dehydrogenase. Chem. Res. Toxicol. 2001;14:651–656. doi: 10.1021/tx000264z. [DOI] [PubMed] [Google Scholar]
- 6.Stýblo M, Drobná Z, Jaspers I, Lin S, Thomas DJ. The role of biomethylation in toxicity and carcinogenicity of arsenic. A research update. Environ. Health Perspect. 2002;110 Suppl.5:767–771. doi: 10.1289/ehp.110-1241242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francesconi KA, Kuehnelt D. Determination of arsenic species: A critical review of methods and applications, 2000–2003. Analyst. 2004;129:373–395. doi: 10.1039/b401321m. [DOI] [PubMed] [Google Scholar]
- 8.Gong ZL, Lu XF, Ma MS, Watt C, Le XC. Arsenic speciation analysis. Talanta. 2002;58:77–96. doi: 10.1016/s0039-9140(02)00258-8. [DOI] [PubMed] [Google Scholar]
- 9.Waters SB, Devesa V, Fricke MW, Creed JT, Stýblo M, Thomas DJ. Glutathione modulates recombinant rat arsenic (+3 oxidation state) methyltransferase-catalyzed formation of trimethylarsine oxide and trimethylarsine. Chem. Res. Toxicol. 2004;17:1621–1629. doi: 10.1021/tx0497853. [DOI] [PubMed] [Google Scholar]
- 10.Del Razo LM, Stýblo M, Cullen WR, Thomas DJ. Determination of trivalent methylated arsenicals in biological matrices. Toxicol. Appl. Pharmacol. 2001;174:282–293. doi: 10.1006/taap.2001.9226. [DOI] [PubMed] [Google Scholar]
- 11.Gong ZL, Lu XF, Cullen WR, Le XC. Unstable trivalent arsenic metabolites, monomethylarsonous acid and dimethylarsinous acid. J. Anal. At. Spectrom. 2001;16:1409–1413. [Google Scholar]
- 12.Cabon JY, Cabon N. Speciation of major arsenic species in seawater by flow injection hydride generation atomic absorption spectrometry. Fresenius J. Anal. Chem. 2000;368:484–489. doi: 10.1007/s002160000526. [DOI] [PubMed] [Google Scholar]
- 13.Howard AG, Salou C. Cysteine enhancement of the cryogenic trap hydride AAS determination of dissolved arsenic species. Anal. Chim. Acta. 1996;333:89–96. [Google Scholar]
- 14.de Diego A, Pecheyran C, Tseng CM, Donard OF. Cryofocusing for on-line metal and metalloid speciation in the environment, Chap. 12. In: Sanz-Medel A, editor. Flow analysis with atomic spectrometric detection. Amsterdam: Elsevier; 1999. pp. 375–406. [Google Scholar]
- 15.Burguera JL, Burguera M, Rivas C, Carrero P. On-line cryogenic trapping with microwave heating for the determination and speciation of arsenic by flow injection hydride generation atomic absorption spectrometry. Talanta. 1998;45:531–542. doi: 10.1016/s0039-9140(97)00189-6. [DOI] [PubMed] [Google Scholar]
- 16.Ellwood MJ, Maher WA. An automated hydride generation-cryogenic trapping-ICP-MS system for measuring inorganic and methylated Ge, Sb and As species in marine and fresh waters. J. Anal. At. Spectrom. 2002;17:197–203. [Google Scholar]
- 17.Tseng CM, Amouroux D, Brindle ID, Donard OFX. J. Environ. Monitor. 2000;2:603–612. doi: 10.1039/b007499n. [DOI] [PubMed] [Google Scholar]
- 18.Wasik A, Lobinski R, Namiesnik J. An automated speciation analyser of organometallic compound content. Instrum. Sci. Technol. 2001;29:393–405. [Google Scholar]
- 19.D'Ulivo A. Chemical vapor generation by tetrahydroborate(III) and other borane complexes in aqueous media - a critical discussion of fundamental processes and mechanisms involved in reagent decomposition and hydride formation. Spectrochim. Acta B. 2004;59:793–825. [Google Scholar]
- 20.Anderson RK, Thompson M, Culbard E. Selective reduction of arsenic species by continuous hydride generation Part I. Reaction media. Analyst. 1986;111:1143–1151. [Google Scholar]
- 21.Cullen WR, Reimer KJ. Arsenic speciation in the environment. Chem Rev. 1989;89:713–764. [Google Scholar]
- 22.Dědina J, Tsalev DL. Hydride generation atomic absorption spectrometry. Chichester: Wiley & Sons, Inc.; 1995. [Google Scholar]
- 23.Shraim A, Chiswell B, Olszowy H. Speciation of arsenic by hydride generation-atomic absorption spectrometry(HG-AAS) in hydrochloric acid reaction medium. Talanta. 1999;50:1109–1127. doi: 10.1016/s0039-9140(99)00221-0. [DOI] [PubMed] [Google Scholar]
- 24.Cordos EA, Frentiu T, Ponta M, Abraham B, Mariginean J. Optimisation of analytical parameters in inorganic arsenic (III and V) speciation by hydride generation using L-cysteine as prereducing agent in diluted HCl medium. Chem. Spec. Bioavailab. 2006;18:1–9. [Google Scholar]
- 25.Chen HW, Brindle ID, Le XC. Prereduction of arsenic(V) to arsenic(III), enhancement of the signal, and reduction of interferences by L-cysteine in determination of arsenic by hydride generation. Anal. Chem. 1992;64:667–672. [Google Scholar]
- 26.Le XC, Cullen WR, Reimer KJ. Effect of cysteine on the speciation of arsenic by using hydride generation atomic absorption spectrometry. Anal. Chim. Acta. 1994;285:277–285. [Google Scholar]
- 27.Carrero P, Malave A, Burguera JL, Burguera M, Rondon C. Determination of various arsenic species by flow injection hydride generation atomic absorption spectrometry: Investigation of the effects of the acid concentration of different reaction media on the generation of arsines. Anal. Chim. Acta. 2001;438:195–204. [Google Scholar]
- 28.Howard AG, Salou C. Arsenic speciation by cryogenic trap hydride generation atomic absorption spectroscopy: Performance enhancement by pre-derivatization. J. Anal. At. Spectrom. 1998;13:683–686. [Google Scholar]
- 29.Tsalev DL, Sperling M, Welz B. Flow-injection hydride generation atomic absorption spectrometric study of the automated on-line pre-reduction of arsenate, methylarsonate and dimethylarsinate and high-performance liquid chromatographic separation of their L-cysteine complexes. Talanta. 2000;51:1059–1068. doi: 10.1016/s0039-9140(00)00297-6. [DOI] [PubMed] [Google Scholar]
- 30.Pitzalis E, Ajala D, Onor M, Zamboni R, D'Ulivo A. Chemical vapour generation of arsane in the presence of L-cysteine. Mechanistic studies and their analytical feedback. Anal. Chem. 2007;79:6324–6333. doi: 10.1021/ac070513p. [DOI] [PubMed] [Google Scholar]
- 31.Brindle ID, Le XC. Reduction of interferences in the determination of germanium by hydride generation and atomic emission- spectrometry. Anal. Chim. 1990;229:239–247. [Google Scholar]
- 32.D'Ulivo A, Bramanti E, Lampugnani L, Zamboni R. Improving the analytical performance of hydride generation non-dispersive atomic fluorescence spectrometry. Combined effect of additives and optical filters. Spectrochim. Acta B. 2001;56:1893–1907. [Google Scholar]
- 33.Kratzer J, Dědina J. Arsine and selenium hydride trapping in a novel quartz device for atomic-absorption spectrometry. Anal. Bioanal. Chem. 2007;388:793–800. doi: 10.1007/s00216-006-1048-3. [DOI] [PubMed] [Google Scholar]
- 34.Sarradin PM, Leguille F, Astruc A, Pinel R, Astruc M. Optimization of atomization parameters in the speciation of organotin compounds by hydride generation gas-chromatography electrothermal atomic-absorption spectrometry. Analyst. 1995;120:79–83. [Google Scholar]
- 35.Lespes G, Seby F, Sarradin PM, Potingautier M. Application of experimental-designs in optimization of a hydride generation quartz furnace atomic-absorption spectrometry method for selenium determination. J. Anal. At. Spectrom. 1994;9:1433–1439. [Google Scholar]
- 36.Dědina J. Atomization of volatile compounds for atomic absorption and atomic fluorescence spectrometry: on the way towards the ideal atomizer. Spectrochim. Acta Part B. 2007;62:846–872. [Google Scholar]
- 37.Dědina J, Matoušek T. Multiple microflame - a new approach to hydride atomization for atomic absorption spectrometry. J. Anal. At. Spectrom. 2000;15:301–304. [Google Scholar]
- 38.Matoušek T, Dědina J, Selecká A. Multiple microflame quartz tube atomizer - further development towards the ideal hydride atomizer for atomic absorption spectrometry. Spectrochim. Acta Part B. 2002;57:451–462. [Google Scholar]
- 39.Cullen WR, McBride WC, Reglinski J. The reaction of methylarsenicals with thiols: Some biological implications. J. Inorg. Biochem. 1984;21:179–194. [Google Scholar]
- 40.Cullen WR, McBride WC, Reglinski J. The reduction of trimethylarsine oxide to trimethylarsine by thiols: A mechanistic model for the biological reduction of arsenicals. J. Inorg. Biochem. 1984;21:45–60. [Google Scholar]
- 41.Nakazato T, Taniguchi T, Tao H, Tominaga M, Miyazaki A. Ion-exclusion chromatography combined with ICP-MS and hydride generation-ICP-MS for the determination of arsenic species in biological matrices. J. Anal. At. Spectrom. 2000;15:1546–1552. [Google Scholar]
- 42.Wangkarn S, Pergantis SA. High-speed separation of arsenic compounds using narrow-bore high-performance liquid chromatography on-line with inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2000;15:627–633. [Google Scholar]
- 43.Delmondedieu M, Basti MM, Otvos JD, Thomas DJ. Reduction and binding of arsenate and dimethylarsinate by glutathione: a magnetic resonance study. Chem. Bio. Interact. 1994;90:139–155. doi: 10.1016/0009-2797(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 44.Raab A, Meharg AA, Jaspars M, Genney DR, Feldmann J. Arsenic-glutathione complexes- their stability in solution and during separation by diferent HPLC modes. J. Anal. At. Spectrom. 2004;19:183–190. [Google Scholar]
- 45.Hansen HR, Raab A, Jaspars M, Milne BF, Feldmann J. Sulfur- containing arsenical mistaken for dimethylarsinous acid [DMA(III)] and identified as a natural metabolite in urine: Major implications for studies on arsenic metabolism and toxicity. Chem. Res. Toxicol. 2004;17:1086–1091. doi: 10.1021/tx049978q. [DOI] [PubMed] [Google Scholar]
- 46.Martin SJ, Newcombe C, Raab A, Feldmann J. Arsenosugar metabolism not unique to the sheep of North Ronaldsay. Environ. Chem. 2005;2:190–197. [Google Scholar]
- 47.Regmi R, Milne BF, Feldmann J. Hydride generation activity of arsenosugars and thioarsenicals. Anal. Bioanal. Chem. 2007;388:775–782. doi: 10.1007/s00216-006-1076-z. [DOI] [PubMed] [Google Scholar]
- 48.Hernandez-Zavala A, Matoušek T, Drobná Z, Paul DS, Walton FS, Adair BM, Dědina J, Thomas DJ, Stýblo M. Speciation analysis of arsenic in biological matrices by automated hydride generation-cryotrapping-atomic absorption spectrometry with multiple microflame quartz tube atomizer (multiatomizer) J. Anal. At. Spectrom. 2007 doi: 10.1039/b706144g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmeisser E, Goessler W, Kienzl N, Francesconi KA. Volatile analytes formed from arsenosugars: determination by HPLC-HG-ICPMS and implications for arsenic speciation analyses. Anal. Chem. 2004;76:418–423. doi: 10.1021/ac034878v. [DOI] [PubMed] [Google Scholar]