Abstract
Metabolic conversion of inorganic arsenic into methylated products is a multistep process that yields mono-, di-, and trimethylated arsenicals. In recent years, it has become apparent that formation of methylated metabolites of inorganic arsenic is not necessarily a detoxification process. Intermediates and products formed in this pathway may be more reactive and toxic than inorganic arsenic. Like all metabolic pathways, understanding the pathway for arsenic methylation involves identification of each individual step in the process and the characterization of the molecules which participate in each step. Among several arsenic methyltransferases that have been identified, arsenic (+3 oxidation state) methyltransferase is the one best characterized at the genetic and functional levels. This review focuses on phylogenetic relationships in the deuterostomal lineage for this enzyme and on the relation between genotype for arsenic (+3 oxidation state) methyltransferase and phenotype for conversion of inorganic arsenic to methylated metabolites. Two conceptual models for function of arsenic (+3 oxidation state) methyltransferase which posit different roles for cellular reductants in the conversion of inorganic arsenic to methylated metabolites are compared. Although each model accurately represents some aspects of enzyme’s role in the pathway for arsenic methylation, neither model is a fully satisfactory representation of all the steps in this metabolic pathway. Additional information on the structure and function of the enzyme will be needed to develop a more comprehensive model for this pathway.
Keywords: arsenic, methylation, arsenic (+3 oxidation state) methyltransferase
Introduction
Elucidation of the pathway for conversion of inorganic arsenic (iAs) to methylated metabolites began in the 19th century with studies in microorganisms and led to identification of trimethylarsine (TMA) as a volatile metabolite (1). Implicit in identification of this highly methylated arsenical as a metabolite was the possibility that other methylated arsenicals might be formed as intermediates in this pathway. Furthermore, widespread recognition that humans who ingested iAs often exhibited a strong garlic-like odor on expired breath led to a common understanding that humans must also form methylated arsenicals. Following Braman and Foreback (2) who reported the presence of iAs, methyl arsenic (MA), and dimethyl arsenic (DMA) in human urine, Crecelius (3) provided the first definitive report of the presence of these arsenicals in the urine of a volunteer who ingested wine or water containing inorganic arsenite (iAsIII) or inorganic arsenate (iAsV). This result was consistent with the hypothesis that there is a metabolic pathway leading from iAs to these methylated products. Studies in humans and other species have provided a general understanding of the characteristics of the distribution, metabolism, retention, and excretion of arsenicals (4, 5). In particular, these studies show that many animal species convert iAs into MAs and DMAs, which are excreted in urine. Determining the basis for this conversion of iAs into methylated species depended on development of strategies to isolate, purify, and characterize the molecules that are involved in each step of the pathway that leads from iAs to its methylated metabolites and development of sufficiently sensitive and specific analytical methods to identify intermediates and products formed in the methylation pathway.
Although the details of the pathway for arsenic (As) methylation will be discussed in the course of this review, it is sufficient to say that it has commonly been assumed that the pathway leading from iAs to the methylated metabolites involves several distinct steps. In this process, oxidative methylation of arsenicals alternates with reductive processes, yielding a number of intermediates and products. Some steps in this pathway have been reported to be strictly chemical reactions; others are enzymatically catalyzed. Many details of this pathway and the nature of each constituent steps remain uncertain (for a review of some of these controversies, see Ref. 6). However, work to date has identified one methyltransferase that is clearly a participant in this pathway. Arsenic (+3 oxidation state) methyltransferase (AS3MT)1 catalyzes conversion of iAs to methylated products. This review examines the occurrence of AS3MT homologs in genomes of species ranging from sea urchins to humans and the association of occurrence of AS3MT and the As methylation phenotype; that is, the capacity to convert iAs to methylated products that can be detected in tissues and excreta. Finally, consideration of two conceptual models for As methylation by AS3MT suggests that elements of either model may be required to describe fully the role of cellular reductants in the catalytic function of this enzyme.
Phylogenetic Relations for AS3MT
Sequence Homologies in AS3MT
The AS3MT protein was the first As methyltransferase to be sufficiently purified to permit cloning of the corresponding gene (7, 8). Sequence information on rat AS3MT has provided a starting point to seek a broader perspective on its structure and function as an As methyltransferase, using genomic data to investigate the occurrence of homologous genes across a wide range of species. Inspection of predicted products for these AS3MT homologs identified proteins encoded in genomes of purple sea urchin (Strongylocentrotus purpuratus), sea squirt (Ciona intestinalis), rainbow trout (Oncorhynchus mykiss), chicken (Gallus gallus), rat (Rattus norvegicus), mouse (Mus musculus), cow (Bos taurus), chimpanzee (Pan troglodytes), and human (Homo sapiens). Alignments of predicted protein products of these AS3MT homologs identified several common features (Table 1). For all species except Pan, AS3MT ranged from 348 residues (Ciona) to 382 residues (Gallus). All AS3MT sequences contained multiple cysteine residues ranging from five in the truncated Pan sequence to 15 in the Gallus sequence. Among all predicted AS3MT sequences, there were five fully conserved cysteines. Based on the 375 residue sequence of Homo AS3MT, these are cysteines in positions 32, 61, 85, 156, and 206. Predicted AS3MT proteins contained three sequence motifs common to many S-adenosylmethionine (AdoMet)–dependent methyltransferases that catalyze methylation of proteins and small molecules (9). Interactions between AdoMet and these conserved amino acid motifs are critical for methyl group transfer to substrate (10). Motif I, with a consensus sequence of (V/I/L)(L/V)(D/I)LG(G/C)G(T/P)G with an invariant G in position 5 occurred as the sequence (I/V/ L)LDLGSGSG in AS3MT. This sequence in AS3MT conformed to the rule of hh (D/E)hGXGXG, where h was a hydrophobic amino acid, and X was any amino acid. Like other AdoMet-dependent methyltransferases, the predicted products of AS3MT in these species contained a conserved aspartate at 20 residues C-terminal to Motif I. This aspartate occurred in the common postmotif I in other methyltransferases (hhXh(D/E)). Motif II was found 65 residues C-terminal to Motif I in all AS3MT sequences. The Motif II consensus sequence ((P/G)(Q/T)(F/Y/A)DA(I/V/Y)(F/I)(C/ V/L) with an invariant aspartate at position 4 occurred as the sequence (E/N/T)(S/A)(Y/H/M/F)DI(V/I)(I/V)S in AS3MT. Motif III occurred 20 residues C-terminal to Motif II in the AS3MT sequences as VL(K/N)(H/Y/E/P/D)GGE(L/M/ F)YF. The consensus sequence for Motif III (LL(R/ K)PGG(R/I/L)(L/I)(L/F/I/V)(I/L)) has at least one of the two glycine residues conserved in most methyltransferases.
Table 1.
Multiple-Sequence Alignments of Human AS3MT and Eight Animal Homologues Illustrate Conserved Motifs I, II, and III, Conserved Asp at I′, and Five Conserved Cys Residues. Underlined sequence is UbiE methyltransferase domain. The sequences of seven species were from NCBI GenBank (accession numbers are given in parentheses following species names): Homo sapiens (Q9HBK9); Pan troglodytes (XP_508007); Bos taurus (NP_001030195); Rattus norvegicus (NP_543166); Mus musculus (AAH13468); Gallus gallus (XP_421735); Strongylocentrotus purpuratus (XP_784275). The partial sequences of another two species were from TIGR Gene Index Databases by querying human AS3MT cDNA with TBLASTX program: Rainbow trout (TC70487); Ciona intestinalis (TC65300). The missed potential residues in the N-termini are indicated with question marks (?)

|
Origins and Functions of AS3MT
The arsM gene has been identified as bacterial and archaeal homologs of AS3MT (11). ArsM protein of Rhodopseudomonas palustris catalyzes the formation of methylated arsenicals from iAsIII. AS3MT homologs occur widely in genomes of many deuterostomes, ranging from relatively simple species (S. purpuratus, C. intestinalis) to H. sapiens. Because AS3MT homologs have not been identified in the genomes of two protostomes, Drosophila melanogaster or Caenorhabditis elegans, it may have arisen in the deuterostomal lineage after the division of bilaterian animals into deuterostomal and protostomal superphyla about 656 to 575 million years before present (12). Notably, a recent study found no methylation of iAs in D. melanogaster larvae or adults (13), consistent with the absence of AS3MT from its genome. Identification of AS3MT homologs in the genomes of Strongylocentrotus and Ciona suggests that this gene arose before the division of chordates and nonchordates. Although about 60% of identified Ciona genes have homologs in protostomes, about 16% of Ciona genes lack homologs among protostomes (14). Alternatively, the absence of AS3MT in Drosophila and Caenorhabditis genomes could be an example of lineage-specific loss of a gene present in some ancestral genome (15).
Evidence of iAs methylation in protostomes, including the polychaetes, Nereis diversicolor and N. viriens, may provide some insight into phylogenetic relations for AS3MT. For these species, tetramethylarsinonium ion and arsenobetaine are the predominant arsenicals in Nereis tissues after exposure to arsenate; however, a small amount of the tissue arsenic is present as MAs (16). In another polychaete, Arenicola marina, most tissue arsenic is present as inorganic species with small percentages (~5%) present as arsenobetaine and DMAs (17). These patterns, especially the predominance of the tetramethylated form, are not consistent with the pattern seen in species in which AS3MT methylates arsenicals. For instance, tetramethylarsinonium ion is a minor constituent (<1%) in urine of rats treated with arsenite (18). Differences in metabolic profiles in polychaetes and in species in which AS3MT is expressed suggest that the pathway for arsenic methylation in polychaetes may involve other enzymes, not AS3MT.
At least three explanations can be offered for the persistence of AS3MT homologs in the genomes of organisms representing the deuterostomal lineage. First, capacity to methylate inorganic As could be a mechanism for the detoxification of this metalloid. However, because AS3MT-catalyzed methylation of iAs yields products that are more reactive and toxic than the parent compound (19), the persistence of AS3MT would be dependent on the balance of the advantageous and deleterious effects of methylation of arsenicals. Second, methylation of iAs by AS3MT could yield a metabolite that is essential for normal cellular function. Studies in experimental species (rat, hamster, minipig, goat, and chicken) suggest arsenic to be an essential nutrient (20). For example, consumption of diets with low concentrations (ng per g range) of iAs have been associated with lower growth rates and decreased reproductive success in these species. Additional studies have demonstrated interactions between dietary deficiencies of iAs, methionine, and pyridoxine (21). Although the molecular bases for these actions of arsenic have not been determined, the salutary effects of iAs in diet might be due to an uncharacterized function of a methylated metabolite of iAs. If this conjecture is correct, then AS3MT could be vital for the production of this methylated species. Third, persistence of AS3MT may reflect a function of the protein that is not related to its capacity to catalyze methylation of arsenicals. For example, AS3MT could catalyze the methylation of other metalloids, such as selenium (Se) or tellurium (Te), or of another class of substrates. In this situation, catalysis of the methylation of arsenicals by AS3MT would be simply fortuitous.
Genotype-Phenotype Correlations for Arsenic Methylation and AS3MT
The As methylation phenotype is defined as the capacity to convert arsenicals to methylated forms that are present in tissues and/or excreted in urine. An absolute dependence of As methylation phenotype on AS3MT genotype can be demonstrated in cultured cells. The heterologous expression of rat AS3MT in a simian virus 40 (SV40)-transformed human urothelial (UROtsa) cell line confers the capacity to methylate iAs on cells that otherwise do not express AS3MT and that have a null phenotype for iAs methylation (22). Conversely, silencing of AS3MT expression by RNA interference reduces the capacity for methylation of iAs in human hepatoma (HepG2) cells (23). Among animal species examined in this study, homologous proteins with essential characteristics of AS3MT can be deduced from genomic data. For all species, except Strongylocentrotus and Ciona (for which there are no data on As phenotype), there is an association between the AS3MT genotype and the As methylation phenotype (Table 2). These data on patterns of metabolites of iAs found in tissue and excreta of species ranging from Oncorhynchus to Homo indicate that methylated metabolites are formed in all of these species, except Pan. As previously suggested, the predicted 205 amino acid product of AS3MT in Pan may be catalytically inactive (25), and the expression of the truncated protein could account for null phenotype for As methylation reported in this species (26). As a caveat, data in Table 2 on the pattern of methylated arsenicals should be regarded as provisional and incomplete. Earlier studies in Bos and Oncorhynchus used analytical techniques that may not resolve fully mono- and dimethylated arsenicals. The absence of trimethylarsine oxide (TMAO) as a metabolite in most species may also reflect limitations of analytical methods used in earlier studies. Similarly, an early study (27) of iAs metabolism in the green sea urchin Strongylocentrotus droebachiensis, which might provide evidence of iAs methylation in echinoderms, should be interpreted with caution. A systematic survey with adequate analytical methods would be needed to ascertain the possible significance of interspecies difference in the patterns of metabolites and the relation between this phenotypic variation and genotypic differences among species.
Table 2.
Arsenic-Containing Metabolites Identified in Tissues (T) or Excreta (E) Following Administration of Inorganic Arsenic
Conceptual Models for Arsenic Methylation by AS3MT
Two conceptual models are currently available to describe the steps involved in the enzymatically catalyzed methylation of arsenicals by AS3MT. The first model grows out of the work of Frederick Challenger and his colleagues in the first half of the twentieth century. These studies were summarized in two comprehensive reviews (27, 28) that outlined a scheme in which the oxidative methylation of arsenicals containing AsIII alternated with the reduction of arsenicals containing AsV to trivalency. William Cullen et al. (29) summarized this general scheme for biological methylation of arsenicals:
| (1) |
Here, metabolites are produced in sequence as monomethylated, dimethylated, and trimethylated arsenical species. Neither the methyl donor (Me+) nor the source of reducing equivalents is identified. The stated order of reactions is oxidative methylation followed by reduction; therefore, methylated arsenicals containing AsIII should be found as intermediates in reactions. A more detailed sequence explicitly shows this reaction order. The substrate for the oxidative methylation reaction contains AsIII, and reduction of the oxidized arsenical precedes oxidative methylation.
| (2) |
Although more detailed, this model specifies neither the source of methyl groups nor of reducing equivalents used in these reactions.
The second conceptual model takes a different approach to the reactions involved in the methylation of arsenicals (30). Here, thiol-containing complexes of arsenicals containing AsIII are substrates for sequential reactions that transfer methyl groups to the arsenical. This general scheme can be summarized as follows:
| (3) |
In this model, methylation occurs by a nonoxidative mechanism and thiol-arsenical complexes are obligatory substrates for methylation reactions.
The validity and utility of the two conceptual models can be evaluated in reactions in which AS3MT is the enzymatic catalyst. Studies with recombinant rat AS3MT (31) and human recombinant AS3MT (30) have examined in some detail the steps involved in the methylation of arsenicals catalyzed by this enzyme. Figure 1 provides more detailed descriptions of the conceptual models under consideration. Model A follows the work of Cullen et al. (29) and experimental results obtained with recombinant rat AS3MT, and Model B is based on the work of Hayakawa et al. (30). In both models, AdoMet is the methyl group donor. In Model A, three linked reactions (1, 2, and 3) involve oxidative methylation followed by reduction. The source of reducing equivalents needed to reduce AsV to AsIII is not specified. The reactions are linked by common metabolites (MAsIII and DMAsIII) that are both products for a first reaction and substrates for a second reaction. In Model B, complexation of inorganic AsIII by the thiol-containing tripeptide glutathione (GSH) produces a substrate that acquires, in turn, two methyl groups. Here, reactions 1 and 2 overlap as the product of the first methylation step is the substrate for the second methylation reaction. The possibility of a third methylation reaction is not considered in Model B. The presence of MAsIII and DMAsIII in reactions is attributed to decomposition of the GSH-arsenical complexes, and the presence of MAsV and DMAsV is attributed to the oxidation of the methylated arsenicals containing AsIII.
Figure 1.
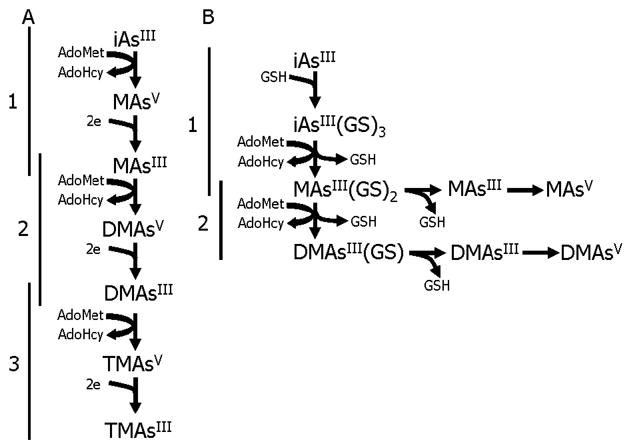
Conceptual models for methylation of inorganic arsenic catalyzed by AS3MT. In Model A, oxidative methylation of trivalent arsenicals alternates with reduction of pentavalent arsenicals. In this pathway, arsenite (iAsIII) is converted to methylarsonic acid (MAsV), which is reduced to methylarsonous acid (MAsIII). This arsenical is converted to dimethylarsinic acid (DMAsV), which is reduced to dimethylarsinous acid (DMAsIII). This dimethylated species is converted to trimethylarsine oxide (TMAsV), which is reduced to trimethylarsine (TMAsIII). Model B involves the sequential addition of methyl groups to trivalent arsenicals that are complexed to glutathione (GSH). In each model, S-adenosylmethionine (AdoMet) is the methyl group donor; S-adenosylhomocysteine (AdoHcy) is produced by removal of a methyl group from AdoMet.
Catalytic Functions for AS3MT
Activity in the Presence of Various Reductants and the Role of GSH
AS3MT was initially purified from the cytosolic fraction of the liver of adult male Fischer 344 rats, using pH-dependent fractionation, chromatofocusing, and S-adenosylhomocysteine-affinity chromatography (7). This procedure purified several thousand-fold a ~42 kDa protein that catalyzed the conversion of iAsIII to MAs and DMAs in reactions that depended on AdoMet as the methyl-group donor. Notably, the success of this scheme for purification of AS3MT from liver cytosol depended on the presence of 1 mM dithiothreitol (DTT) and 5 mM GSH in buffers used in each fractionation and chromatographic step. In the absence of either reductant, As methylating activity was quickly lost during purification. Hence, in initial studies, assays of the As methylating activity of AS3MT were conducted with both reductants in reaction mixtures.
Initial studies investigated the patterns of methylation of arsenicals by recombinant rat AS3MT in reaction mixtures in which different reductants were present (31). Because studies with nonphysiological reductants like DTT provided little information about the role of reductants in catalysis by AS3MT, primary emphasis has been placed on the role of potential physiological reductants in support of the enzyme’s function. This approach was also strongly influenced by evidence that AS3MT might function as an AsV reductase. In this assay system, formation of MAs and DMAs proceeded in the presence of AS3MT, a reductant, AdoMet, and an arsenical substrate. This suggested that reduction of pentavalent arsenicals formed in the assay mixture could be catalyzed by AS3MT. Other studies with prokaryotic and eukaryotic AsV reductases indicated that three physiological reductants (thioredoxin, Trx; glutaredoxin, Grx; dihydrolipoic acid, DHLA) could provide reducing equivalents needed to produce AsIII (32). Notably, each of these reductants contains a dithiol pair that is oxidized to a disulfide during reduction of an oxidized substrate. The regeneration of the dithiol pair in each of these reductants is accomplished by enzymatically catalyzed transfer of reducing equivalents from nicotinamide adenine dinucleotide phosphate (NADPH). For Trx and DHLA, Trx reductase (TR) catalyzes the reduction; for Grx, GSH and GSH reductase (GR) are required.
The capacity of different reductants to support catalysis of As methylation by AS3MT was determined in the absence or presence of 5 mM GSH (Fig. 2). Notably, in the absence of another reductant, addition of 5 mM GSH did not support the catalytic activity of AS3MT. Although addition of GSH had little effect on the rate of As methylation in the presence of 1 mM DTT, it sharply stimulated the methylation rate in the presence of 1 mM Tris (2-carboxylethyl) phosphine (TCEP). Compared with these nonphysiological reductants, the highest rates of methylation were found in assays containing a Trx/TR/NADPH–coupled system. Here, addition of GSH had little effect on an already high rate of As methylation. The activity of Grx as a reductant depended on addition of GSH to the reaction mixture, reflecting its role in a Grx/GSH/GR/NADPH–coupled system. DHLA also functioned as reductant when added in a DHLA/TR/NADPH–coupled system. Furthermore, the presence of methylated arsenicals containing either AsV or AsIII in reaction mixtures was consistent with the formation and conversion of these species as the reaction progressed. Several conclusions can be drawn from these studies. First, among physiological reductants, addition of Trx to reactions at concentrations approximating those found in cells (33) yielded the highest catalytic activity for AS3MT. Second, for both physiological and nonphysiological reductants, addition of GSH stimulated the overall rate for As methylation. Third, under these assay conditions, addition of GSH resulted in little, if any, conversion of iAs to methylated metabolites.
Figure 2.

Effect of glutathione (GSH) on capacity of reductants to support the methylation of inorganic arsenic by recombinant rat AS3MT. Reaction mixtures (50 μl) containing 5 μg of recombinant rat AS3MT, 1 mM AdoMet, and 1 μM [73As]-iAsIII in 100 mM Tris/100 mM Na phosphate buffer, pH 7.4, with or without reductant, were incubated at 37°C for 20 mins. Reactions were run in the presence or absence of 5 mM GSH. Reductants were 1 mM dithiothreitol (DTT), 1 mM Tris(2-carboxyethyl) phosphine (TCEP), 10 μM Escherichia coli thioredoxin (Trx) with 3 μM rat liver Trx reductase and 300 μM NADPH, 1 μM E. coli glutaredoxin 1 (Grx) with 15 nM yeast GR, 300 μM NADPH, 100 μM dihydrolipoic acid (DHLA) with 3 μM rat liver Trx reductase, and 300 μM NADPH. Methylation rate calculated as nmols of arsenite methylated per μg of protein per 20 mins. (Upper) Mean and (lower) standard deviation shown (n = 3).
In the course of identifying a trimethylated arsenical as a product of AS3MT-catalyzed reactions, the role of GSH in catalysis was further investigated (34). Although TMAO was rarely detected in reaction mixtures that contained 5 mM GSH, it accounted for nearly half of the methylated products present in reaction mixtures that contained AS3MT, AdoMet, iAsIII, and TCEP but no GSH (Fig. 3). Notably, in the absence of GSH, a physiological reductant (Trx/TR/NADPH) also supported the formation of TMAO in AS3MT-catalyzed reactions. Increasing the concentration of GSH in reaction mixtures affected the pattern and extent of As methylation, suppressing formation of TMAO but increasing overall production of methylated products. This suggested that the third methylation reaction was inhibited by GSH. When DMAsIII or dimethylthioarsenite-GSH (a 1:1 molar complex of DMAsIII and GSH) was used as substrate for AS3MT-catalyzed reactions (final concentration of 1 μM), TMAO was formed (Fig. 4). When 5 mM GSH was added to assay mixtures containing 1 μM DMAsIII, TMAO was not formed. This suggested that interactions between AS3MT and GSH that modulate the enzyme’s activity do not involve a specific role for an arsenical-GSH complex but rather reflect a direct interaction between the enzyme and GSH.
Figure 3.

Effect of the concentration of glutathione (GSH) on the pattern of metabolites produced by recombinant rat AS3MT. Reaction mixtures (50 μl) containing 5 μg of recombinant rat AS3MT, 1 mM AdoMet, 1 mM Tris(2-carboxyethyl) phosphine, and 1 μM [73As]-iAsIII in 100 mM tris/100 mM Na phosphate buffer, pH 7.4, were incubated at 37°C from 20 mins to overnight (16 hrs) in the presence of 0–10 mM of GSH. Reaction products separated by thin-layer chromatography as previously described (34). Positions for migration of inorganic arsenic (iAs), methylarsonic (MAs), dimethylarsinic (DMAs), and trimethylarsine oxide (TMA) are indicated. Arrow shows direction of chromatography.
Figure 4.

Effect of glutathione (GSH) on rat recombinant AS3MT-catalyzed methylation of dimethylarsenicals. Reaction mixtures (50 μl) contained 5 μg of rat recombinant AS3MT, 1 mM AdoMet, and 1 mM TCEP. The substrates were 1 μM dimethylarsinous acid, iodide salt (DMAsIII), 1 μM of an equimolar complex of dimethylarsinous acid with GSH (DMAsIIIGS), or 1 μM DMAsIII and 5 mM GSH. Reactions were incubated at 37°C for 120 mins and oxidation states of arsenicals in reaction mixtures were determined by pH selective hydride generation-atomic absorption spectrophotometry. Percentages of arsenic present as (White) DMAsIII, (Gray) DMAsV, or (Black) TMAO are shown.
GSH-Dependent Activity
Model B proposes that As-GSH complexes are both the substrates for, and products of, reactions catalyzed by AS3MT. Thus, although the methylation of arsenicals requires AsIII, it must be present in a GSH-containing complex. Furthermore, methylation does not oxidize AsIII but yields a complex containing a methylated arsenical and GSH. This model requires scission of an AsIII-S bond between the cysteinyl residue of GSH and AsIII. For studies of the role of GSH in AS3MT-catalyzed methylation, Hayakawa et al. (30) used reaction mixtures containing 50 μg of recombinant human AS3MT, 0.2 μM iAsIII, and 1 mM AdoMet in 25 mM phosphate buffer, pH 7. GSH concentration ranged from 0 to 5 mM and reactions were incubated for 3 hrs at 37°C. Under these conditions, methylated products were detected only in the presence of at least 2 mM GSH. The investigators found a positive relation between the percentage of concentration of iAsIII present in an iAsIII-GSH complex (at a 1:3 molar ratio of iAsIII to GSH) and the extent of AS3MT-catalyzed methylation (Fig. 5). A MAsIII-GSH complex (at a 1:2 molar ratio of MAsIII to GSH) added to reaction mixtures was rapidly oxidized to MAsV and was not converted to DMAs. However, the presence of 5 mM GSH in reaction mixtures permitted the MAsIII-GSH complex to be converted to DMAs by AS3MT. In the absence of GSH, MAsV was not a substrate for methylation by AS3MT. However, addition of 5 mM GSH to the reaction mixture permitted formation of a small amount of MAsIII-GSH complex that was converted to DMAs.
Figure 5.

Glutathione (GSH)-dependent conversion of arsenite to methylated arsenicals by recombinant human AS3MT. Relationship between the percentage of iAsIII converted to methylated (MAs) or dimethylated (DMAs) species and the percentage of iAsIII present in an triglutathione complex (As(GS)3) as GSH concentration increases from 0 to 5 mM. Reaction mixtures (100 μl) containing 50 μg of recombinant rat AS3MT, 1 mM AdoMet, and 0.2 μM iAsIII in 25 mM phosphate buffer, pH 7.0, were incubated at 37°C for 3 hrs. (Redrawn from data in fig. 5 of Ref. 30).
Recent work with recombinant human AS3MT has examined the role of reductants, including the Trx/TR/NADPH–coupled system and GSH.2 Here, reaction mixtures contained 3 to 5 μg of recombinant human AS3MT, 3 μM iAsIII, and 1 mM AdoMet in 100 mM Tris, 100 mM phosphate buffer, pH 7.4. Concentrations of Trx, TR, NADPH, and GSH varied in reaction mixtures and are indicated in figure legends. As shown in Figure 6, AS3MT catalyzed conversion of iAs to MAs and DMAs in the presence of the complete Trx/TR/NADPH–coupled system; however, omission of Trx from reaction mixtures abolished activity. In the presence of 1 mM GSH, conversion of iAs to MAs and DMAs was stimulated. Again, omission of Trx from reaction mixtures containing GSH sharply reduced activity. A small amount of MAs was detected in these reaction mixtures, suggesting a minimal effect of GSH on AS3MT’s catalytic activity. Examination of the concentration dependence of the effect of GSH on the activity of AS3MT in reaction mixtures containing the Trx/TR/ NADPH–coupled system showed that GSH stimulated the rate of conversion of iAs to MAs and DMAs (Fig. 7).
Figure 6.

Effects of glutathione (GSH) and a thioredoxin (Trx)-coupled reduction system on the activity of recombinant human AS3MT. Reaction mixtures (80 μl) containing 3 μg of recombinant human AS3MT, 1 mM AdoMet, and 3 μM [73As]-iAsIII in 100 mM Tris/100 mM Na phosphate buffer, pH 7.4, were incubated at 37°C from 60 mins in the presence or absence of 1 mM GSH. Complete reaction mixtures contained 3 μM E. coli thioredoxin (Trx) with 1 μM rat liver Trx reductase and 300 μM NADPH; Trx was omitted from some reaction mixtures (–Trx). Percentages of arsenic present as (Open) inorganic arsenic, (Black) methylarsonic, and (Gray) dimethylarsinic species are shown.
Figure 7.

Glutathione (GSH) concentration dependency of the activity of recombinant human AS3MT. Reaction mixtures (80 μl) containing 4 μg of recombinant human AS3MT, 1 mM AdoMet, and 3 μM [73As]-iAsIII, 0.26 μM E. coli thioredoxin (Trx) with 0.5 μM rat liver Trx reductase and 300 μM NADPH in 100 mM tris/100 mM Na phosphate buffer, pH 7.4, were incubated at 37°C from 60 mins in the presence of 0–10 mM GSH. Percentages of arsenic present as (Black) methylarsonic and (Gray) dimethylarsinic species are shown.
Merits and Limitations of the Conceptual Models for Arsenic Methylation by AS3MT
The first conceptual model involving alternating steps of oxidative methylation and reduction of arsenicals can serve as a framework for understanding the role of cellular reductants in catalytic activity of AS3MT. However, two general questions must be addressed in evaluating the function of these cellular reductants. First, how do these reductants act to promote the catalytic activity of the enzyme? Second, how does the function of these reductants complement the actions of GSH? The use of cellular reductants by AS3MT is reminiscent of the role of these reductants in the activity of several well-characterized AsV reductases. These enzymes use Trx/TR/NADPH– or Grx/GSH/GR/NADPH–coupled systems as the source of reducing equivalents for conversion of AsV to AsIII (32). Because AS3MT catalyzes each step in a reaction sequence that leads from iAs to TMAs, it is conceivable that this protein is multifunctional, catalyzing both oxidative catalysis and reduction of AsV. However, it should be noted that AS3MT activity is absolutely dependent on the presence of the complete Trx/ TR/NADPH–coupled system; omission of Trx abolishes activity. This suggests that Trx is not only needed to reduce methylated intermediates containing AsV (e.g., MAsV) but is also required for AS3MT to catalyze the reaction in which iAsIII is present as the substrate. This suggests that Trx could be required for the reduction of some cysteine residue in AS3MT that is critical for its function as a methyltransferase.
The second conceptual model is dependent on interactions between GSH, arsenicals, and AS3MT. Speculation about the role of GSH in As methylation was first summarized by Thompson (35), who integrated much of the evidence on the reduction of pentavalent arsenicals by GSH and the role of GSH complexes in the enzymatically catalyzed methylation of As. Although GSH can reduce AsV to AsIII in cellular environments and forms stable complexes with AsIII (36–38), good quantitative estimates are lacking the extent of reduction by this pathway or the persistence of these complexes in cellular environments. Although DMAsIII present in a GSH complex could be converted to TMAO, it is not certain if the complex was the substrate for methylation. It is entirely possible that DMAsIII could be transferred from the GSH complex to a higher affinity binding site on AS3MT. Hence, the GSH complex would serve only to maintain arsenic in a reduced state before its interaction with the enzyme. Data on the effects of GSH on iAs methylation in assay systems using crude cytosol or in tissue slices (39–41) provide little insight into the molecular processes that underlie the effects of GSH on enzymatically catalyzed methylation. It is possible that GSH functions to maintain the correct mix of reduced and of oxidized cysteine residues in AS3MT. The presence of multiple cysteines in this protein raises the distinct possibility that formation of intramolecular or intermolecular disulfide bonds affect the structure and function of the enzyme.
Conclusions and Future Directions
Similarities in the sequences of the predicted protein products of AS3MT homologs found in genomes of a variety of deuterostomes argue that this ubiquitous protein serves a function that is conserved among these organisms. If the conserved function of these AS3MT homologs is catalysis of As methylation, then there has been a persistent selective pressure for the capacity of the protein to catalyze this reaction. Further understanding of the function of AS3MT depends on physicochemical studies probing its interactions with substrates and with critical reductants. Site-directed mutagenesis offers the possibility of identifying specific amino acid residues required for its catalytic activity. Altered expression of AS3MT in cultured cells using RNA interference could be used to examine effects of altered AS3MT expression on the As methylation phenotype. AS3MT gene knockouts can be used to examine the As methylation phenotype and the toxicological consequences of altered capacity to methylate arsenicals.
Examining evidence on the catalytic activity of AS3MT in relation to the two conceptual models for As methylation supports the conclusion that its activity does not strictly depend on the presence of GSH. Methylation occurs in reaction mixtures that contain only the Trx/TR/NADPH–coupled system as a source of reducing equivalents. Because different laboratories assay As methylation rates under different experimental conditions (e.g., enzyme concentration, buffer pH, analytical methods), quantitative comparisons of data obtained in different laboratories are problematic. However, rates of conversion of iAs to methylated metabolites using recombinant human AS3MT are much higher in reaction mixtures containing Trx/TR/ NADPH than in reaction mixtures containing GSH as the sole reductant. Using data from the work of Hayakawa et al. (fig. 5 in Ref. 30), a methylation rate of 0.1 pmols of methylated substrate per μg of protein per hr was calculated for reaction mixtures containing 0.2 μM iAsIII as substrate and 5 mM GSH as the reductant. By comparison, a methylation rate of 0.8 pmols of methylated substrate per μg of protein per hr was calculated for reaction mixtures containing 0.3 μM iAsIII as substrate and 5 μM Trx, 1 μM TR, and 300 μM NADPH as the source of reducing equivalents.3 In general, it can be suggested that the presence of GSH in reaction mixtures stimulates rates of reactions catalyzed by AS3MT that are supported by other physiological reductants. The nature of the interaction between physiological reductants and AS3MT that underlie the catalytic functions of the enzyme is unknown. Do the physiological reductants serve as a source of reducing equivalents needed to reduce AsV to AsIII, or do the reductants help maintain the critical cysteine residues in the reduced state? Characterizing these interactions as well as interactions between AS3MT and GSH is an outstanding research problem that will require considerable ingenuity to solve. Studies of the catalytic function of common polymorphisms of AS3MT may provide cogent insights into the relation between enzyme structure and function (42).
A broader research topic grows out of this research on interactions between arsenicals, reductants, and AS3MT in reaction mixtures in which all elements present in the system are known and quantified. Cells have redundant systems for reduction, including GSH, Trx, Grx, and DHLA. The availability of each reductant is tightly regulated by biosynthetic control (e.g., GSH synthesis regulated by activity of glutamate-cysteine ligase; Ref 43), and there is evidence of interactions between reductants that affect their reactivity and concentration (e.g., glutathionylation of Trx; Ref. 44). Because AS3MT promiscuously uses each of these physiological reductants to support catalysis, its activity in cells must be determined at least in part by concentrations of several reductants. Availability of the reductants could contribute to the observed dose-dependency of the rate of methylation of iAs in cultured primary human hepatocytes (45). Similarly, differences in rates of methylation of iAs in cultured cells derived from different donor species or in intact organisms of different species could reflect interspecies differences in the concentrations of the reductants needed to support the catalytic activity of AS3MT. Integrating information on the regulation of AS3MT by reductants poses a considerable challenge in elucidating the control of As methylation at the cellular and systemic levels.
Acknowledgments
We thank Professor Barry P. Rosen and his colleagues in the Department of Biochemistry and Molecular Biology, School of Medicine, Wayne State University, for providing Ref. 11 before publication. This manuscript has been reviewed in accordance with the policy of the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Footnotes
This protein and its gene were initially termed cyt19 by us and other investigators, following its original identification in GenBank with a methyltransferase of unknown function (NCB accession NP065602 and NP 05133). Subsequently, we have followed the recommendations of the Human Genome Nomenclature Committee (http://www.gene.ucl.ac.uk/cgi-bin/nomenclature/searchgenes.pl) for the systematic naming of genes and proteins and refer to protein or gene as arsenic (+3 oxidation state) methyltransferase (AS3MT).
Xing W, Adair BW, Thomas DJ. Unpublished results, 2006.
W. Xing et al., unpublished results.
Former (S.B.W. and J.L.) and current (W.X.) postdoctoral fellows in the Curriculum in Toxicology, University of North Carolina at Chapel Hill, have been supported by Training Grant T901915 of the U.S. Environmental Protection Agency-University of North Carolina Toxicology Research Program. V.D. was a recipient of an MECD/ Fulbright Grant for Postdoctoral Training administered by the Ministry of Education, Culture, and Sports, Spain.
References
- 1.Cullen WR, Bentley R. The toxicity of trimethylarsine: an urban myth. J Environ Monit. 2005;7:11–15. doi: 10.1039/b413752n. [DOI] [PubMed] [Google Scholar]
- 2.Braman RS, Foreback CC. Methylated forms of arsenic in the environment. Science. 1973;182:1247–1249. doi: 10.1126/science.182.4118.1247. [DOI] [PubMed] [Google Scholar]
- 3.Crecelius EA. Changes in the chemical speciation of arsenic following ingestion by man. Environ Health Perspect. 1977;19:147–150. doi: 10.1289/ehp.7719147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Styblo M, Delnomdedieu M, Thomas DJ. Biological mechanisms and toxicological consequences of the methylation of arsenic. In: Cherian MG, Goyer RA, editors. Toxicology of Metals-Biochemical Aspects, Handbook of Experimental Pharmacology. Vol. 115. Berlin: Springer Verlag; 1995. pp. 407–433. [Google Scholar]
- 5.Vahter M. Methylation of inorganic arsenic in different mammalian species and population groups. Sci Prog. 1999;82:69–88. doi: 10.1177/003685049908200104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aposhian HV, Aposhian MM. Arsenic toxicology: five questions. Chem Res Toxicol. 2006;19:1–15. doi: 10.1021/tx050106d. [DOI] [PubMed] [Google Scholar]
- 7.Lin S, Shi Q, Nix FB, Styblo M, Beck MA, Herbin-Davis KM, Hall LL, Simeonsson JB, Thomas DJ. A novel S-adenosyl-L-methionine: arsenic(III) methyltransferase from rat liver cytosol. J Biol Chem. 2002;277:10795–10803. doi: 10.1074/jbc.M110246200. [DOI] [PubMed] [Google Scholar]
- 8.Walton FS, Waters SB, Jolley SL, LeCluyse EL, Thomas DJ, Styblo M. Selenium compounds modulate activity of recombinant rat AsIII methyltransferase and methylation of arsenite by rat and human hepatocytes. Chem Res Toxicol. 2003;16:261–265. doi: 10.1021/tx025649r. [DOI] [PubMed] [Google Scholar]
- 9.Kagan RM, Clarke S. Widespread occurrence of three sequence motifs in diverse S-adenosylmethionine-dependent methyltransferases suggests a common structure for these enzymes. Arch Biochem Biophys. 1994;310:417–427. doi: 10.1006/abbi.1994.1187. [DOI] [PubMed] [Google Scholar]
- 10.Kozbial PZ, Mushegian AR. Natural history of S-adenosylmethionine-binding proteins. [Accessed November 15, 2005];BMC Struct Biol. 2005 5:19. doi: 10.1186/1472-6807-5-19. Available at: www.biomedcentral.com/1472-6807/5/19. [DOI] [PMC free article] [PubMed]
- 11.Qin J, Rosen BP, Zhang Y, Wang G, Franke S, Rensing C. Arsenic detoxification and evolution of trimethylarsine gas by a microbial arsenite S-adenosylmethionine methyltransferase. Proc Natl Acad Sci U S A. 2006;103:2075–2080. doi: 10.1073/pnas.0506836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogura A, Ikeo K, Gojobori T. Estimation of ancestral gene set of bilaterian animals and its implication to dynamic change of gene content in bilaterian evolution. Gene. 2005;345:65–71. doi: 10.1016/j.gene.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 13.Rizki M, Kossatz E, Velazquez A, Creus A, Farina M, Fortaner S, Sabbioni E, Marcos R. Metabolism of arsenic in Drosophila melanogaster and the genotoxicity of dimethylarsinic acid in the Drosophila wing spot test. Environ Mol Mutagen. 2006;47:162–168. doi: 10.1002/em.20178. [DOI] [PubMed] [Google Scholar]
- 14.Dehal P, Satou Y, Campbell RK, Chapman J, Degnan B, De Tomaso A, Davidson B, DiGregorio A, Gelpke M, Goodstein DM, Harafuji N, Hastings KE, Ho I, Hotta K, Huang W, Kawashima T, Lemaire P, Martinez D, Meinertzhagen IA, Necula S, Nonaka M, Putnam N, Rash S, Saiga H, Satake M, Terry A, Yamada L, Wang HG, Awazu S, Azumi K, Boore J, Branno M, Chin-Bow S, DeSantis R, Doyle S, Francino P, Keys DN, Haga S, Hayashi H, Hino K, Imai KS, Inaba K, Kano S, Kobayashi K, Kobayashi M, Lee BI, Makabe KW, Manohar C, Matassi G, Medina M, Mochizuki Y, Mount S, Morishita T, Miura S, Nakayama A, Nishizaka S, Nomoto H, Ohta F, Oishi K, Rigoutsos I, Sano M, Sasaki A, Sasakura Y, Shoguchi E, Shini T, Spagnuolo A, Stainier D, Suzuki MM, Tassy O, Takatori N, Tokuoka M, Yagi K, Yoshizaki F, Wada S, Zhang C, Hyatt PD, Larimer F, Detter C, Doggett N, Glavina T, Hawkins T, Richardson P, Lucas S, Kohara Y, Levine M, Satoh N, Rokhsar DS. The draft genome of Ciona intestinalis: insights into chordate and vertebrate origins. Science. 2002;298:2157–2167. doi: 10.1126/science.1080049. [DOI] [PubMed] [Google Scholar]
- 15.Miller DJ, Ball EE, Technau U. Cnidarians and ancestral genetic complexity in the animal kingdom. Trends Genet. 2005;21:536–539. doi: 10.1016/j.tig.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Geiszinger AE, Goessler W, Francesconi KA. Biotransformation of arsenate to the tetramethylarsonium ion in the marine polychaetes Nereis diversicolor and Nereis virens. Environ Sci Technol. 2002;36:2905–2910. doi: 10.1021/es015808d. [DOI] [PubMed] [Google Scholar]
- 17.Geiszinger AE, Goessler W, Francesconi KA. The marine polychaete Arenicola marina: its unusual arsenic compound pattern and its uptake of arsenate from seawater. Mar Environ Res. 2002;53:37–50. doi: 10.1016/s0141-1136(01)00106-4. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida K, Inoue Y, Kuroda K, Chen H, Wanibuchi H, Fukushima S, Endo G. Urinary excretion of arsenic metabolites after long-term oral administration of various arsenic compounds to rats. J Toxicol Environ Health A. 1998;54:179–192. doi: 10.1080/009841098158890. [DOI] [PubMed] [Google Scholar]
- 19.Thomas DJ, Styblo M, Lin S. The cellular metabolism and systemic toxicity of arsenic. Toxicol Appl Pharmacol. 2001;176:127–144. doi: 10.1006/taap.2001.9258. [DOI] [PubMed] [Google Scholar]
- 20.Uthus EO. Evidence for arsenic essentiality. Environ Geochem Health. 1992;14:55–58. doi: 10.1007/BF01783629. [DOI] [PubMed] [Google Scholar]
- 21.Uthus E, Poellot R. Effect of dietary pyridoxine on arsenic deprivation in rats. Magnes Trace Elem. 1991–92;10:339–347. [PubMed] [Google Scholar]
- 22.Drobna Z, Waters SB, Devesa V, Harmon AW, Thomas DJ, Styblo M. Metabolism and toxicity of arsenic in human urothelial cells expressing rat arsenic (+3 oxidation state)-methyltransferase. Toxicol Appl Pharmacol. 2005;207:147–159. doi: 10.1016/j.taap.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drobna Z, Xing W, Thomas DJ, Styblo M. shRNA silencing of AS3MT expression minimizes arsenic methylation capacity of HepG2 cells. Chem Res Toxicol. 2006;19:894–898. doi: 10.1021/tx060076u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Waters SB, Drobna Z, Devesa V, Styblo M, Thomas DJ. Arsenic (+3 oxidation state) methyltransferase and the inorganic arsenic methylation phenotype. Toxicol Appl Pharmacol. 2005;204:164–169. doi: 10.1016/j.taap.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Vahter M, Couch R, Nermell B, Nilsson R. Lack of methylation of inorganic arsenic in the chimpanzee. Toxicol Appl Pharmacol. 1995;133:262–268. doi: 10.1006/taap.1995.1150. [DOI] [PubMed] [Google Scholar]
- 26.Penrose WR, Conacher HB, Black R, Meranger JC, Miles W, Cunningham HM, Squires WR. Implications of inorganic/organic interconversion on fluxes of arsenic in marine food webs. Environ Health Perspect. 1977;19:53–59. doi: 10.1289/ehp.771953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Challenger F. Biological methylation. Chem Rev. 1945;36:315–361. [Google Scholar]
- 28.Challenger F. Biological methylation. Adv Enzymol. 1951;12:429–491. doi: 10.1002/9780470122570.ch8. [DOI] [PubMed] [Google Scholar]
- 29.Cullen WR, McBride BC, Reglinski J. The reduction of trimethylarsine oxide to trimethylarsine by thiols: a mechanistic model for the biological reduction of arsenicals. J Inorg Biochem. 1984;21:45–60. [Google Scholar]
- 30.Hayakawa T, Kobayashi Y, Cui X, Hirano S. A new metabolic pathway of arsenite: arsenite-glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Arch Toxicol. 2005;79:183–191. doi: 10.1007/s00204-004-0620-x. [DOI] [PubMed] [Google Scholar]
- 31.Waters SB, Devesa V, Del Razo LM, Styblo M, Thomas DJ. Endogenous reductants support the catalytic function of recombinant rat cyt19, an arsenic methyltransferase. Chem Res Toxicol. 2004;17:404–409. doi: 10.1021/tx0342161. [DOI] [PubMed] [Google Scholar]
- 32.Mukhopadhyay R, Rosen BP. Arsenate reductases in prokaryotes and eukaryotes. Environ Health Perspect. 2002;110(Suppl 5):745–748. doi: 10.1289/ehp.02110s5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandes AP, Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 34.Waters SB, Devesa V, Fricke MW, Creed JT, Styblo M, Thomas DJ. Glutathione modulates recombinant rat arsenic (+3 oxidation state) methyltransferase-catalyzed formation of trimethylarsine oxide and trimethylarsine. Chem Res Toxicol. 2004;17:1621–1629. doi: 10.1021/tx0497853. [DOI] [PubMed] [Google Scholar]
- 35.Thompson DJ. A chemical hypothesis for arsenic methylation in mammals. Chem Biol Interact. 1993;88:89–114. doi: 10.1016/0009-2797(93)90086-e. [DOI] [PubMed] [Google Scholar]
- 36.Scott N, Hatlelid KM, MacKenzie NE, Carter DE. Reactions of arsenic(III) and arsenic(V) species with glutathione. Chem Res Toxicol. 1993;6:102–106. doi: 10.1021/tx00031a016. [DOI] [PubMed] [Google Scholar]
- 37.Delnomdedieu M, Basti MM, Otvos JD, Thomas DJ. Reduction and binding of arsenate and dimethylarsinate by glutathione: a magnetic resonance study. Chem Biol Interact. 1994;90:139–155. doi: 10.1016/0009-2797(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 38.Delnomdedieu M, Basti MM, Styblo M, Otvos JD, Thomas DJ. Complexation of arsenic species in rabbit erythrocytes. Chem Res Toxicol. 1994;7:621–627. doi: 10.1021/tx00041a006. [DOI] [PubMed] [Google Scholar]
- 39.Buchet JP, Lauwerys R. Role of thiols in the in vitro methylation of inorganic arsenic by rat liver cytosol. Biochem Pharmacol. 1988;37:3149–3153. doi: 10.1016/0006-2952(88)90313-9. [DOI] [PubMed] [Google Scholar]
- 40.Georis B, Cardenas A, Buchet JP, Lauwerys R. Inorganic arsenic methylation by rat tissue slices. Toxicology. 1990;63:73–84. doi: 10.1016/0300-483x(90)90070-w. [DOI] [PubMed] [Google Scholar]
- 41.Styblo M, Delnomdedieu M, Thomas DJ. Mono and dimethylation of arsenic in rat liver cytosol in vitro. Chem Biol Interact. 99:147–164. doi: 10.1016/0009-2797(95)03666-0. [DOI] [PubMed] [Google Scholar]
- 42.Wood TC, Salavaggione OE, Mukherjee B, Wang L, Klumpp AF, Thomae BA, Eckloff BW, Schaid DJ, Wieben ED, Weinshilboum RM. Human arsenic methyltransferase (AS3MT) pharmacogenetics: gene resequencing and functional genomics studies. J Biol Chem. 2006;281:7364–7373. doi: 10.1074/jbc.M512227200. [DOI] [PubMed] [Google Scholar]
- 43.Dalton TP, Chen Y, Schneider SN, Nebert DW, Shertzer HG. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic Biol Med. 2004;37:1511–1526. doi: 10.1016/j.freeradbiomed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 44.Casagrande S, Bonetto V, Fratelli M, Gianazza E, Eberini I, Massignan T, Salmona M, Chang G, Holmgren A, Ghezzi P. Glutathionylation of human thioredoxin: a possible crosstalk between the glutathione and thioredoxin systems. Proc Natl Acad Sci U S A. 2002;99:9745–9749. doi: 10.1073/pnas.152168599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drobna Z, Waters SB, Walton FS, LeCluyse EL, Thomas DJ, Styblo M. Interindividual variation in the metabolism of arsenic in cultured primary human hepatocytes. Toxicol Appl Pharmacol. 2004;201:166–177. doi: 10.1016/j.taap.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Le XC, Ma M. Short-column liquid chromatography with hydride generation atomic fluorescence detection for the speciation of arsenic. Anal Chem. 1998;70:1926–1933. doi: 10.1021/ac971247q. [DOI] [PubMed] [Google Scholar]
- 47.Benramdane L, Accominotti M, Fanton L, Malicier D, Valllon JJ. Arsenic speciation in human organs following fatal arsenic trioxide poisoning-a case report. Clin Chem. 1999;45:301–306. [PubMed] [Google Scholar]
- 48.Lakso JU, Peoples SA. Methylation of inorganic arsenic by mammals. J Agric Food Chem. 1975;23:674–676. doi: 10.1021/jf60200a028. [DOI] [PubMed] [Google Scholar]
- 49.Hughes MF, Kenyon EM, Edwards BC, Mitchell CT, Del Razo LM, Thomas DJ. Accumulation and metabolism of arsenic in mice after repeated oral administration of arsenate. Toxicol Appl Pharmacol. 2003;191:202–210. doi: 10.1016/s0041-008x(03)00249-7. [DOI] [PubMed] [Google Scholar]
- 50.Falnoga I, Stibilj E, Tusek-Znidaric M, Slejkovec Z, Mazej D, Jacimovic R, Scancar J. Effect of arsenic trioxide on metallothionein and its conversion to different arsenic metabolites in hen liver. Biol Trace Elem Res. 2000;78:241–254. doi: 10.1385/bter:78:1-3:241. [DOI] [PubMed] [Google Scholar]
- 51.Pizarro I, Gomez MM, Fodor P, Palacios MA, Camara C. Distribution and biotransformation of arsenic species in chicken cardiac and muscle tissues. Biol Trace Elem Res. 2004;99:129–143. doi: 10.1385/BTER:99:1-3:129. [DOI] [PubMed] [Google Scholar]
- 52.Oladimeji AA, Qadri SU, Tam GK, DeFreitas AS. Metabolism of inorganic arsenic to organoarsenicals in rainbow trout (Salmo gairdneri) Ecotoxicol Environ Saf. 1979;3:394–400. doi: 10.1016/0147-6513(79)90029-0. [DOI] [PubMed] [Google Scholar]


