Abstract
Exocytosis is the major mechanism by which new membrane components are delivered to the cell surface. In most, if not all, eukaryotic cells this is also a highly spatially regulated process that is tightly coordinated with the overall polarity of a cell. The Rho/Cdc42 family of GTPases and the lethal giant larvae/Sro7 family are two highly conserved families of proteins which appear to have dual functions both in cell polarity and exocytosis. Analysis of their functions has begun to unravel the coordination between these processes and propose a model for polarized vesicle docking and fusion at the site of asymmetric cell growth.
Keywords: Rho GTPases, Lgl, exocytosis, cell polarity
The ability to deliver newly synthesized proteins and lipids to discrete sites on the cell surface is critical for the ability of eukaryotic cells to grow asymmetrically (i.e. tall and thin vs. round), to release morphogens on a discrete side of a cell layer during embryonic development, and for a variety of other processes involving both static and dynamic asymmetry of the plasma membrane. The major mechanism by which new membrane components are delivered to the cell surface is by delivery, docking, and fusion of secretory vesicles with the plasma membrane—otherwise known as exocytosis. In most, if not all, eukaryotic cells this is also a highly spatially regulated process that is tightly coordinated with the overall polarity of the cell. In epithelial cells, for example, the polarized delivery of basolateral and apical proteins to their respective sites on the plasma membrane is critical to the morphology and physiology of these cells. In neurons polarized trafficking is critical not only to the distinction between dendritic and axonal processes but also to the biogenesis and physiology of the synaptic terminal in neurotransmitter release. In all eukaryotic cells it is likely that overall cell polarity and the polarity of sites of exocytosis must be tightly coordinated.
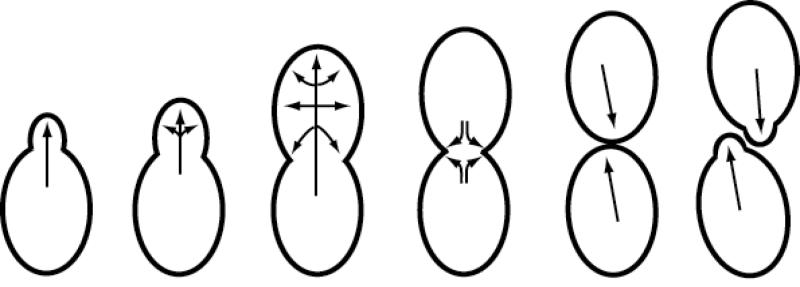
The yeast, Saccharomyces cerevisae, provides an attractive model in which to study polarized exocytosis. Yeast cells are highly polarized during most of their life cycle—this is most apparent when examining their pattern of growth which involves the development and asymmetric enlargement of the bud which following mitosis pinches off (cytokinesis) to form a new daughter cell. Growth during the cell cycle is highly polarized but dynamic (see Figure 1). The protein machinery which is involved in cell polarity and exocytosis is concentrated at bud tips in small budded cells and later relocalizes at the mother-bud neck just prior to cytokinesis. Many of the components which are involved in both cell polarization and exocytosis have been well characterized in yeast, although the mechanism by which their functions are coordinated are not at all well understood. The combination of cell biological and genetic tools available in yeast make it in an excellent system in which to unravel the coordination of these processes.
Figure 1. Changes in the sites of exocytosis during the cell division cycle in yeast.
The direction of polarized secretion and growth changes during the growth cycle of a haploid yeast cell. Growth at incipient and small buds is highly polarized at the tip, and as the bud enlarges the sites of exocytosis spreads until it becomes transiently isotropic (unpolarized) within the bud, which is followed by repolarization at the mother-bud neck just prior to cytokinesis.
Polarized exocytosis in yeast involves three distinct steps: 1) polarized delivery of vesicles along actin cables toward sites of polarized growth 2) docking of secretory vesicles with the plasma membrane and 3) polarized fusion of secretory vesicles at sites of polarized growth. Several factors have been implicated in secretory vesicle delivery most notably actin and the unconventional myosin, Myo2, (1) as well as the Sec4 exchange factor Sec2 (2). The second docking stage appears to require both the Rab GTPase, Sec4, as well as a multiprotein complex known as the Exocyst (also sometimes referred to as the Sec6/8 complex). The Exocyst complex is composed of eight proteins: Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo84, and Exo70 (3,4). Analysis of temperature-sensitive mutants in these proteins has shown that each of these is required for efficient exocytosis to the cell surface. The initial docking of secretory vesicles is thought to involve an interaction between GTP-bound Sec4, a component of the post-Golgi vesicles, and the Exocyst complex on the plasma membrane. The final step involves the actual fusion of the secretory vesicles with the plasma membrane which is thought to be dependent primarily on the action of SNARE proteins both on the vesicle, the v-SNARE Snc1 (or its close homolog Snc2), and on the plasma membrane a complex of two t-SNARE proteins, Sec9 and Sso1 (or its close homolog Sso2) (5). Genetic evidence suggests that the final two steps are likely to be tightly linked functionally and it is likely that regulation of each of these steps contributes to the overall polarity of this process. Moreover, it is clear that the polarization of the exocytic events must be coordinated with other cell polarization events such as polarization of the cytoskeleton and delivery of cell surface markers to the correct site. An example is the delivery of the Bud8 and Bud9 proteins. They are thought to serve as cell surface landmarks for bud site selection and as integral membrane proteins are carried to the cell surface by exocytosis (6). Therefore, not only must sites of exocytosis be coordinated with other polarization signals, but polarization of the exocytic process may itself play a role in the determination and maintenance of the polarized phenotype. In this review we focus on the role of two highly conserved families of proteins that appear to have dual functions both in cell polarity and exocytosis. The first is the Rho/Cdc42 family of GTPases and the second is the lethal giant larvae/Sro7 family—both of which have well ascribed roles in regulation of cell polarity in a variety of model organisms and a direct role in the regulation of exocytosis in yeast.
Rho/Cdc42 GTPases as spatial regulators of exocytosis
Rho family GTPases, including Rho, Cdc42 and Rac GTPases, are thought to have a central role in polarized growth processes (7,8). Yeast contain six Rho family members RHO1-5, and CDC42. Three of these Rho GTPases have been implicated in the polarization and function of the exocytic apparatus—all through regulation of the Exocyst complex. The role of Rho3 in exocytosis was first identified in a genetic screen for suppressors of an effector mutant in the Rab GTPase Sec4, sec4-P48(9). Rho3, in its GTP-bound state, was found to positively regulate late secretory function through physical interaction with the Exo70 component of the Exocyst complex (9,10,11). Phenotypic analysis of a collection of conditionally defective alleles of Rho3 demonstrated that Rho3 plays a direct role in exocytosis which is distinct from its role in regulation of actin polarity (9). In particular one allele, rho3-V51, which was specifically defective in its interaction with Exo70, had a severe secretory defect while actin polarity was normal (9).
Cdc42 appears to play a central role in the establishment and maintenance of polarity in yeast (8,12). Genetic analysis suggested that, like Rho3, Cdc42 participates in exocytosis in a manner that functionally overlaps with, but is not identical to, that of Rho3 (13). Like Rho3, the key to this discovery was the identification of a particular allele, cdc42-6, which had a highly specific defect in the exocytic function with little effect on the other effectors pathways regulated by Cdc42. Partial functional redundancy between Rho3 and Cdc42 was suggested by the fact that the rho3-V51 and cdc42-6 mutants are synthetically lethal in combination with each other and that each mutant is suppressed by overexpression of the wild-type form of the other. Additionally, both mutants are suppressed by a common set of dosage suppressors including SEC9, SEC4, SRO7, and SSO2--all of which are themselves components of the exocytic docking and fusion machinery. Interestingly, examination of rho3-V51 and cdc42-6 mutants by electron microscopy showed that while rho3-V51 accumulated vesicles at all stages of the cell cycle, the cdc42-6 mutant accumulated vesicles only in small budded cells (9,13). The cell cycle-specific nature of the defect in cdc42-6 was confirmed by secretion analysis of synchronized yeast cell cultures. Using these synchronized cultures, it was demonstrated that the post-Golgi secretory defect was most prominent early in the cell cycle as small buds were just emerging (13). Interestingly, both mutants displayed a polarized actin cytoskeleton as well as a robust polarization of the Exocyst components Sec8, Exo70, and Sec3, the vesicle marker Sec4, and the type V myosin, Myo2 (13,14). These observations led to the conclusion that the pathway(s) effected by the cdc42-6 and rho3-V51 mutants does not involve localization of the Exocyst complex on the plasma membrane, but rather regulation of the activity of this complex at sites of polarized growth (14).
These results are not consistent with the “Landmark” model for Exocyst function proposed by Novick and colleagues almost a decade ago (15), in which the localization of the Exocyst complex acts to mark a site (or landmark) on the plasma membrane where exocytic vesicle fusion is then targeted to. In this model factors acting upstream of the Exocyst complex (such as the Rho family GTPases) would function primarily to help localize or sequester the Exocyst complex to future sites of polarized growth. However analysis of the rho3-V51 and cdc42-6 mutants described above suggest that Rho3 and Cdc42 function in exocytosis is not directed at determining the localization of this complex (14). This led to the proposal of the “Localized Activation” model in which Cdc42 and Rho3 act as regulators of Exocyst function in a manner similar to that described for Rho/Cdc42 activation of other effectors such as Formins, WASP, and PAK/STE20 kinases (14). In this model a localized patch of Cdc42 or Rho3 in their GTP-bound state binds to the Exocyst complex through physical interaction with the Exo70 subunit. The Rho/Exo70 interaction leads to a structural change in the protein:protein interactions within the Exocyst complex. This interaction would then relieve an “autoinhibitory” interaction within the Exocyst and a basal state would be converted into an activated state (see Figure 2). This change leads to an increase in Exocyst function at sites marked by the presence of GTP-bound Rho/Cdc42 protein. The increase in docking/fusion rate at these sites would be expected to polarize any components brought to the membrane by post-Golgi trafficking including the Exocyst and Cdc42 GTPase (but not the Rho3 GTPase) leading to a positive feedback loop—and resulting in the continued polarization of these components. Several independent predictions of this model were tested by genetic and cell biological approaches and all of these examinations strongly support the notion that the Rho3 and Cdc42 GTPases act by local activation of the Exocyst rather than by sequestering the complex as a landmark for exocytosis (14). The challenge is now to o understand the mechanism by which Rho3/Cdc42 activate the function of the Exocyst and understand how this contributes to the polarization of the cell surface growth.The recent determination of the crystal structure of several of the Exocyst subunits will, no doubt, help further elucidate the molecular mechanism for the activation model (16).
Figure 2. Two Models for Regulation of Exocyst Function by Rho GTPases.
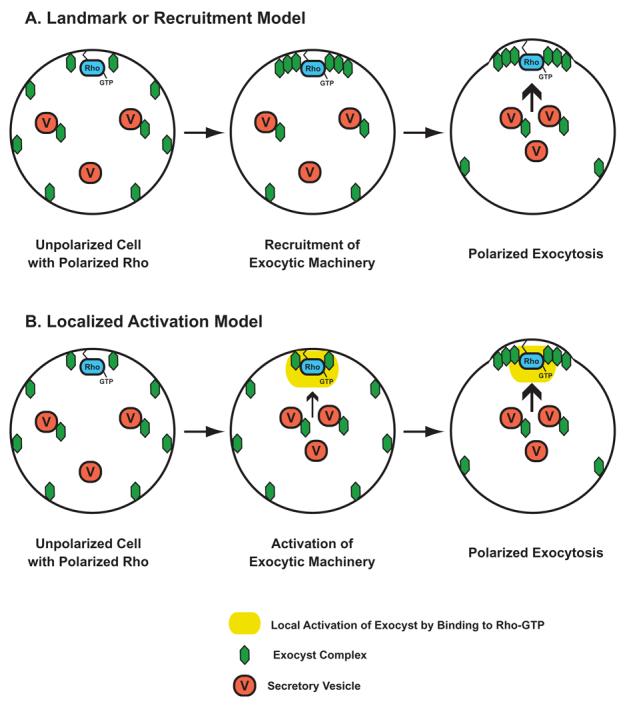
In the Landmark or Recruitment Model (A) Rho GTPases (bound to GTP) would directly recruit exocyst components to the site of polarized growth. The presence of the exocyst would then serve as a “Landmark” for the efficient docking and fusion of secretory vesicles. In the Localized Activation Model (B) the polarized Rho-GTP would locally stimulate or activate the activity of the exocyst complex function. Since exocyst components are carried to sites of polarized growth by vesicle delivery, this would ultimately result in their polarization of the exocyst as a consequence of the activation by the GTPase.
Role of the Lgl/Sro7 family in polarity and exocytosis
Discovered by Bridges in the 1930s, lethal (2) giant larvae [l(2)gl] was the first tumor suppressor identified in Drosophila melanogaster. Loss of function mutations in this gene lead to loss of polarity in at least three types of fly epithelia (17,18) and is required for cell polarity associated with asymmetric cell divisions of neuroblasts during fly development (19,20). As with the PDZ-containing proteins Scribble and Dlg, Lgl contributes to the correct targeting of apical determinants for epithelial cell polarity and mutations in l(2)gl lead to a loss of monolayer organization and the formation of epithelial derived tumors (21,18). Lgl's role as a tumor suppressor appears to be tightly linked to its role in cell polarity. However, the precise cellular role of Lgl remains controversial (22,23).
Kagami et al. (24) identified yeast homologs of Lgl in a screen for dosage suppressors of the growth defect associated with loss of the Rho3 GTPase. SRO7 and SRO77 are a redundant gene family as loss of individual genes has no detectable effect on growth but when both genes are deleted yeast cells become extremely cold-sensitive (24,25). The authors suggested a potential role for Sro7 in regulation of actin polarity downstream of Rho3 based on actin polarity defects observed in sro7Δ,sro77Δ mutants following an 18 hour shift from 37°C to 19°C. Larsson et al. (26) also isolated SOP1/SRO7 by complementation of a S. cerevisiae salt sensitive mutant and showed that sro7Δ cells were sensitive when exposed to a high sodium concentration.
Evidence that lethal giant larvae-like proteins may play a role in exocytosis was provided by the identification of a neuronal rat homolog which was found in association with the t-SNARE proteins, Syntaxin and SNAP-25 (27). This homolog, known as tomosyn, differs from its fly and yeast counterparts in that it contains a v-SNARE domain at its C-terminus (28,29). This led to the hypothesis that tomosyn might function in SNARE assembly by providing a surrogate v-SNARE-like helix during the assembly of the t-SNARE complex of Syntaxin and SNAP-25. The first functional evidence for a role of Lgl in exocytosis came from the identification of Sro7 and Sro77, in a two-hybrid screen for proteins which could bind to the t-SNARE Sec9. Consistent with the two-hybrid interaction with Sec9, Lehman et al. (25) demonstrated that sro7Δ,sro77Δ cells had a profound exocytic defect following a shift to the restrictive temperature. The Sro7 protein was localized at the cell periphery as well as in the cytosol and it was found associated with Sec9 in both pools. Consistent with the exocytic defect being due to a role in SNARE assembly, Sro7 was also found associated with ternary SNARE complexes of Sec9/Sso/Snc. In order to establish whether the primary function of Sro7/77 was in exocytosis or actin polarity the timing of the onset of secretory defects vs. actin polarity defects was determined. After a 3 hour shift to 19°C, profound secretory defects were observed in sro7Δ ,sro77Δ cells while actin polarity was virtually identical to wild-type cells (25). Only after very prolonged shifts to the non-permissive temperature of 19°C (>12 hours) did actin polarity defects become apparent. Therefore the primary function of Sro7/77 in yeast cells is in exocytosis and the effects on actin polarity are a secondary effect—perhaps due to prolonged loss of cell surface delivery. More recently Wadskog et al. have demonstrated that the salt sensitivity seen in sro7Δ mutant cells is also due to a exocytic defect as the ENA1encoded sodium pump fails to be delivered to the surface in exocytic vesicles when the mutant cells are exposed to sodium (30).
Non-neuronal homologs of Lgl have been identified in humans and mice and they appear to have properties similar to that of their Drosophila counterpart (31,32). The similarities include: 1) high levels of expression in epithelial tissues 2) presence in a high MW oligomeric complex 3) association with the lateral membrane of polarized epithelia. A mammalian homolog of Lgl, termed Mlgl, was characterized in the epithelial cell culture line, MDCK cells (33). Polyclonal antibodies raised against the mouse Mgl-1 protein recognize a single protein of the size predicted from the Mgl-1 cDNA. Interestingly, like the yeast and fly protein, this protein is present in a high MW complex that partitions between the cytosol and membrane. In MDCK cells grown in monolayer, Mlgl is found specifically associated with the lateral membrane. This is virtually identical to the staining of fly Lgl protein (34). Finally, the Mlgl protein was found to be associated with the plasma membrane SNARE proteins in MDCK cells. In particular an association of Mlgl-1with the basolateral specific t-SNARE, Syntaxin4 as well as Syntaxin4/SNAP-23 complexes were found to be present in these cells. Unfortunately attempts to demonstrate a direct function in basolateral transport have so far been unsuccessful due to the inability to perturb endogenous Mlgl proteins following treatments with siRNAs or microinjection of antibodies. This is likely due to the high abundance and stability of the endogenous Mlgl1 or due to redundancy with another closely related isoform, Mlgl2 which is also well expressed in epithelial cells—including MDCK cells (A. Muesch, personal communication). Importantly, the ability of Lgl proteins from yeast and mammalian cells to interact with SNARE proteins strongly suggests that regulation of membrane trafficking is likely to be a conserved feature of the function of the Lgl family of proteins.
While work on Lgl in yeast has now clearly demonstrated a direct role for Sro7 in polarized cell surface delivery, the relevance of this to the role of Lgl in cell polarity in other systems remains somewhat controversial (see 22 and 23 for recent reviews). However, Gangar et al., (35) found evidence that the ability to interact with t-SNAREs is a structurally conserved and functionally important feature of this family. By analyzing chimeric Lgl proteins in which large regions of yeast and mammalian Lgl proteins were exchanged, it was determined that the C-terminal domain of each protein is the primary site of SNARE interaction and that the ability to interact with SNARE proteins is likely to be critical to the ability of Lgl proteins to function in the cell (35). This analysis also strongly suggests that the overall structural organization of Lgl proteins is likely to be conserved between yeast and mammals.
A new, and we believe potentially very important piece of information in the Lgl story has come with the recent discovery that the yeast Sro7 protein may be a direct effector for the Rab GTPase, Sec4. An interaction between Sec4 and Sro7 was first suggested by a proteomic screen using recombinant GTP-locked Sec4 protein. Further work demonstrated that the interaction between Sec4 and Sro7 is direct and depends on the nucleotide state of the Rab GTPase (36). Genetic studies suggest that Sro7 has many of the properties to be a key downstream effector in transmitting Rab GTPase function onto the SNARE assembly process (25,36).
Applying Lessons from Yeast to Polarization in Animal Cells
Work over the last two decades has provided abundant evidence that the overall mechanisms involved in vesicle transport are well conserved between yeast and animal cells. However, while there are many similarities in cell polarization between yeast and animals (8,37,38), there are also likely to be key differences which animal cells have evolved in order to handle the additional complexity necessary for cells to organize into complex tissues and organ systems.
Many of the mechanistic interactions critical for the spatial regulation of trafficking in yeast are likely to be conserved in mammals (see Figure 3). For example an interaction between the Cdc42 homolog TC10 and the mammalian Exo70 homolog has been recently shown to be important for regulation of Glut4 transport to the cell surface in adipocytes (39). This pathway may be one of several redundant mechanisms by which insulin signaling is coupled to glucose transport in adipocytes. While the yeast Rab GTPase Sec4 is thought to function through interaction with the Sec15 component of the Exocyst complex, in mammalian cells Rab11 has been suggested to have an analogous regulatory interaction with the mammalian Sec15 cognate in transport from the recycling endosome to the plasma membrane (40,41). We have shown that homologs of Lgl found in mammalian polarized epithelia are found at sites of basolateral transport and are specifically associated with the basolateral t-SNARE, Syntaxin4, as well as Syntaxin4/SNAP-23 t-SNARE complexes (which are homologous to Sso1/Sec9 t-SNARE complex in yeast). This may suggest that mammalian Lgl proteins play a role in regulating t-SNARE function in basolateral vesicle docking/fusion in a manner analogous to the role of Sro7 in regulating vesicle docking in fusion in yeast. However, it is important to point out that as yet no direct functional evidence for a role for Lgl in vesicle transport has been demonstrated in animal cells. Determining whether Lgl plays a role in vesicle trafficking in animal cells, and if so what particular trafficking step it is involved in, is a key step in understanding the overall conservation of this pathway to a general model for cell polarization.
Figure 3. Conservation of Factors involved in vesicle trafficking to the cell surface between yeast and animal cells.
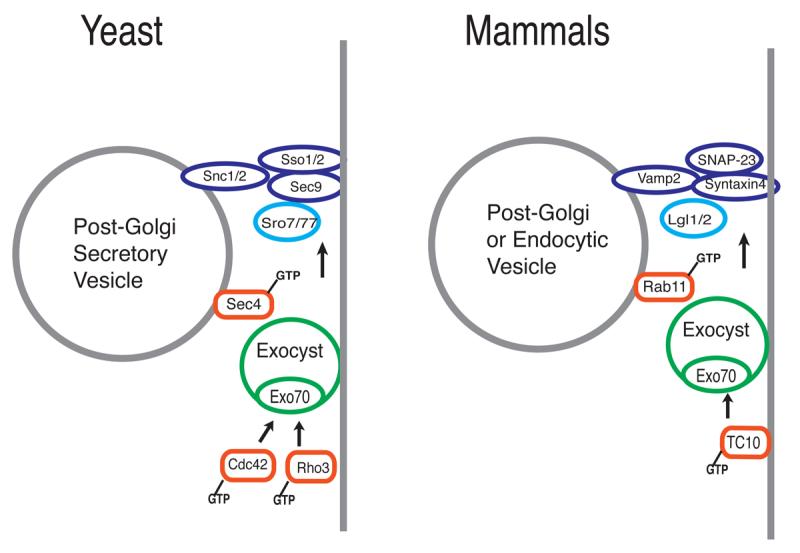
The cartoon on the left shows the names and proposed functions of factors involved in post-Golgi vesicle docking and fusion in yeast. The cartoon on the right shows the mammalian/metazoan homologs of the yeast proteins. The precise trafficking steps in which these components function are likely to vary in different cell types and in different organisms.
Final Thoughts
Previous models of polarized growth in yeast have focused on the role of the actin cytoskeleton as a primary determinant in the generation and maintenance of the polarized phenotype (42). However, more recent work has identified pathways in which key cell polarity determinants act as direct regulators of the exocytic machinery independent of the actin cytoskeleton. In particular two distinct families of polarity determinants--the Rho/Cdc42 and Lgl/Sro7 families--have each been found in yeast cells to be direct regulators of exocytic function and in each case their ability to properly function in cell polarization has been found to be linked to their ability to physically engage the exocytic apparatus. This suggests that the spatial regulation of cell surface trafficking may play a critical and distinct role in determining overall cell polarity. The fact that this regulation appears to work largely independent of the polarization of the cytoskeleton may provide the cell with an alternative mechanism to regulate polarity--which may be especially critical in situations where the cytoskeleton is being established or reorganized.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Govindan B, Bowser R, Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. J Cell Biol. 1995;128:1055–68. doi: 10.1083/jcb.128.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walch-Solimena C, Collins RN, Novick P. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J Cell Biol. 1997;137:1495–509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.TerBush DR, Maurice T, Roth D, Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–94. [PMC free article] [PubMed] [Google Scholar]
- 4.Guo W, Roth D, Walch-Solimena C, Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 1999;18:1071–80. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–53. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 6.Schenkman LR, Caruso C, Page N, Pringle JR. The role of cell cycle-regulated expression in the localization of spatial landmark proteins in yeast. J. Cell Biol. 2002;156:829–41. doi: 10.1083/jcb.200107041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridley AJ. Rho-related proteins: actin cytoskeleton and cell cycle. Curr Opin Genet Dev. 1995;5:24–30. doi: 10.1016/s0959-437x(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 8.Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- 9.Adamo J, Rossi G, Brennwald P. The Rho GTPase, Rho3, has a Direct Role in Exocytosis Which is Distinct From its Role in Actin Polarity. Mol. Biol. Cell. 1999;10:4121–4133. doi: 10.1091/mbc.10.12.4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson NG, Guo L, Imai J, Toh EA, Matsui Y, Tamanoi F. Rho3 of Saccharomyces cerevisiae, which regulates the actin cytoskeleton and exocytosis, is a GTPase which interacts with Myo2 and Exo70. Mol. Cell Biol. 1999;19:3580–7. doi: 10.1128/mcb.19.5.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong G, Hutagalung AH, Fu C, Novick P, Reinisch KM. The structures of exocyst subunit Exo70p and the Exo84p C-terminal domains reveal a common motif. Nat. Struct. Mol. Biol. 2005;12:1094–100. doi: 10.1038/nsmb1017. [DOI] [PubMed] [Google Scholar]
- 12.Irazoqui JE, Gladfelter AS, Lew DJ. Scaffold-mediated symmetry breaking by Cdc42p. Nat Cell Biol. 2003;5:1062–70. doi: 10.1038/ncb1068. [DOI] [PubMed] [Google Scholar]
- 13.Adamo JE, Moskow JJ, Gladfelter AS, Viterbo D, Lew DJ, Brennwald PJ. Yeast Cdc42 Functions at a Late Step in Exocytosis Specifically During Polarized Growth of the Emerging Bud. J. Cell Biol. 2001;155:581–592. doi: 10.1083/jcb.200106065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roumanie O, Wu H, Molk JN, Rossi G, Bloom K, Brennwald P. Rho GTPase Regulation of Exocytosis in Yeast is Independent of GTP Hydrolysis and Polarization of the Exocyst Complex. J. Cell Biol. 2005;170:583–594. doi: 10.1083/jcb.200504108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finger FP, Hughes TE, Novick P. Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell. 1998;92:559–71. doi: 10.1016/s0092-8674(00)80948-4. [DOI] [PubMed] [Google Scholar]
- 16.Munson M, Novick P. The exocyst defrocked, a framework of rods revealed. Nat. Struct. Mol. Biol. 2006;13:557–81. doi: 10.1038/nsmb1097. [DOI] [PubMed] [Google Scholar]
- 17.Manfruelli P, Arquier N, Hanratty WP, Semeriva M. The tumor suppressor gene, lethal(2)giant larvae (1(2)g1), is required for cell shape change of epithelial cells during Drosophila development. Development. 1996;122:2283–2294. doi: 10.1242/dev.122.7.2283. [DOI] [PubMed] [Google Scholar]
- 18.Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- 19.Ohshiro T, Yagami T, Zhang C, Matsuzaki F. Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature. 2000;408:593–596. doi: 10.1038/35046087. [DOI] [PubMed] [Google Scholar]
- 20.Peng CY, Manning L, Albertson R, Doe CQ. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature. 2000;408:596–600. doi: 10.1038/35046094. [DOI] [PubMed] [Google Scholar]
- 21.Gateff E. Malignant neoplasms of genetic origin in Drosophila melanogaster. Science. 1978;200:1448–1459. doi: 10.1126/science.96525. [DOI] [PubMed] [Google Scholar]
- 22.Wirtz-Peitz F, Knoblich JA. Lethal giant larvae take on a life of their own. Trends Cell Biol. 2006;16:234–41. doi: 10.1016/j.tcb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Vasioukhin V. Lethal giant puzzle of Lgl. Dev Neurosci. 2006;28:13–24. doi: 10.1159/000090749. [DOI] [PubMed] [Google Scholar]
- 24.Kagami M, Toh-e A, Matsui Y. Sro7p, a Saccharomyces cerevisiae counterpart of the tumor suppressor l(2)gl protein, is related to myosins in function. Genetics. 1998;149:1717–27. doi: 10.1093/genetics/149.4.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehman K, Rossi G, Adamo J, Brennwald P. Yeast homologues of tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, Sec9. J. Cell Biol. 1999;146:125–140. doi: 10.1083/jcb.146.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsson K, Bohl F, Sjostrom I, Akhtar N, Strand D, Mechler BM, Grabowski R, Alder L. The Saccharomyces cerevisiae SOP1 and SOP2 genes, which act in cation homeostasis, can be functionally substituted by the Drosophila lethal(2)giant larvae tumor suppressor gene. J. Biol. Chem. 1998;273:33610–8. doi: 10.1074/jbc.273.50.33610. [DOI] [PubMed] [Google Scholar]
- 27.Fujita Y, Shirataki H, Sakisaka T, Asakura T, Ohya T, Kotani H, Yokoyama S, Nishioka H, Matsuura Y, Mizoguchi A, Scheller RH, Takai Y. Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron. 1998;20:905–15. doi: 10.1016/s0896-6273(00)80472-9. [DOI] [PubMed] [Google Scholar]
- 28.Fasshauer D, Sutton RB, Brünger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc. Natl. Acad. Sci. U S A. 1998;95:15781–6. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masuda ES, Huang BC, Fisher JM, Luo Y, Scheller RH. Tomosyn binds t-SNARE proteins via a VAMP-like coiled coil. Neuron. 1998;21:479–480. doi: 10.1016/s0896-6273(00)80559-0. [DOI] [PubMed] [Google Scholar]
- 30.Wadskog I, Forsmark A, Rossi G, Schowe C, Öyen M, Goksör M, Ronne H, Brennwald P, Adler L. The Saccharomyces cerevisiae tumour suppressor homologue Sro7p/Sop1p is required for targeting of the sodium transporting ATPase to the cell surface. Mol. Biol. Cell. 2006;12:4988–5003. doi: 10.1091/mbc.E05-08-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strand D, Unger S, Corvi R, Hartenstein K, Schenkel H, Kalmes A, Merdes G, Neumann B, Krieg-Schneider F, Coy JF. A human homologue of the Drosophila tumour suppressor gene l(2)gl maps to 17p11.2-12 and codes for a cytoskeletal protein that associates with nonmuscle myosin II heavy chain. Oncogene. 1995;11:291–301. [PubMed] [Google Scholar]
- 32.Tomotsune D, Shoji H, Wakamatsu Y, Kondoh H, Takahashi N. A mouse homologue of the Drosophila tumour-suppressor gene l(2)gl controlled by Hox-C8 in vivo. Nature. 1993;365:69–72. doi: 10.1038/365069a0. [DOI] [PubMed] [Google Scholar]
- 33.Müsch A, Cohen D, Yeaman CA, Nelson WJ, Rodriguez-Boulan E, Brennwald PJ. A Mammalian Homologue of the Drosophila Tumor Suppressor lethal (2) giant larvae Interacts with Basolateral Exocytic Machinery in MDCK cells. Mol. Biol. Cell. 2002;13:158–168. doi: 10.1091/mbc.01-10-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strand D, Raska I, Mechler BM. The Drosophila lethal(2)giant larvae tumor suppressor protein is a component of the cytoskeleton. J Cell Biol. 1994;127:1345–1360. doi: 10.1083/jcb.127.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gangar A, Rossi G, Andreeva A, Hales R, Brennwald P. Structurally Conserved Interaction of Lgl Family with SNAREs Is Critical to Their Cellular Function. Curr. Biol. 2005;15:1136–1142. doi: 10.1016/j.cub.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 36.Grosshans BL, Andreeva A, Gangar A, Niessen S, Yates JR, Brennwald P, Novick P. The Yeast Lgl Family Member Sro7p is an Effector of the Secretory Rab GTPase Sec4. J. Cell Biol. 2006;172:55–66. doi: 10.1083/jcb.200510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kee Y, Yoo JS, Hazuka CD, Peterson KE, Hsu SC, Scheller RH. Subunit structure of the mammalian exocyst complex. Proc. Natl. Acad. Sci. U S A. 1997;94:14438–43. doi: 10.1073/pnas.94.26.14438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu SC, Hazuka CD, Roth R, Foletti DL, Heuser J, Scheller RH. Subunit composition, protein interactions, and structures of the mammalian brain sec6/8 complex and septin filaments. Neuron. 1998;20:1111–22. doi: 10.1016/s0896-6273(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 39.Inoue M, Chang L, Hwang J, Chiang SH, Saltiel AR. The Exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature. 2003;422:629–33. doi: 10.1038/nature01533. [DOI] [PubMed] [Google Scholar]
- 40.Wu S, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat. Struct. Mol Biol. 2005;12:879–85. doi: 10.1038/nsmb987. [DOI] [PubMed] [Google Scholar]
- 41.Zhang XM, Ellis S, Sriratana A, Mitchell CA, Rowe T. Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem. 2004;279:43027–34. doi: 10.1074/jbc.M402264200. [DOI] [PubMed] [Google Scholar]
- 42.Pruyne D, Bretscher A. Polarization of cell growth in yeast. J. Cell Sci. 2000;113:571–85. doi: 10.1242/jcs.113.4.571. [DOI] [PubMed] [Google Scholar]