Abstract
Our previous studies showed that axonal outgrowth from dorsal root ganglia (DRG) transplants in the adult rat brain could be directed toward a specific target location using a preformed growth-supportive pathway. This pathway induced axon growth within the corpus callosum across the midline to the opposite hemisphere. In this study, we examined whether such pathways would also support axon growth either through or around a lesion of the corpus callosum. Pathways expressing GFP, NGF, or FGF2/NGF were set up by multiple injections of adenovirus along the corpus callosum. Each pathway included the transplantation site in the left corpus callosum, 2.8 mm away from the midline, and a target site in the right corpus callosum, 2.5 mm from the midline. At the same time, a 1 mm lesion was made through the corpus callosum at the midline in an anteroposterior direction. A group of control animals received lesions and Ad-NGF injections only at the transplant and target sites, without a bridging pathway. DRG cell suspensions from postnatal day 1 or 2 rats were injected at the transplantation site three to four days later. Two weeks after transplantation, brain sections were stained using an anti-CGRP antibody. The CGRP-positive axons were counted at 0.5 mm and 1.5 mm from the lesion site in both hemispheres. Few axons grew past the lesion in animals with control pathways, but there was robust axon growth across the lesion site in the FGF2/NGF and NGF-expressing pathways. This study indicated that preformed NGF and combination guidance pathways support more axon growth past a lesion in the adult mammalian brain.
Keywords: Axon growth, Dorsal root ganglion (DRG), Transplantation, Neurotrophic factors, Gene therapy, Brain injury
Introduction
Injuries in the adult mammalian central nervous system (CNS) often result in devastating consequences partly due to the failure of damaged axons to regenerate. The injured CNS environment is refractory to axon regeneration due to active inhibition, the lack of a supportive substrate, or a combination of the two. Many molecules, such as chondroitin sulfate proteoglycans (CSPGs), myelin-associated glycoprotein (MAG), and Nogo have been found in the glial scar that act to inhibit axon growth in the adult CNS. However, MAG and CSPGs also exist in lesioned peripheral nerves, yet do not interfere with axon growth in that setting (Chen et al., 2002; Fawcett and Asher, 1999; McKerracher et al., 1994; Zuo et al., 2002). This difference indicates that axon regeneration depends not on the presence or absence of single molecules, but on the complex interplay of growth-supportive and growth-inhibitory signals within the environment.
Various strategies have been employed to promote axon regeneration after CNS injury, including exogenous administration of neurotrophic factors, transplantation of fetal tissue (Jakeman and Reier, 1991), Schwann cells (Xu et al., 1995), olfactory ensheathing cells (Santos-Benito and Ramon-Cueto, 2003), or stem cells (McDonald et al., 1999), neutralization of inhibitory molecules from myelin (Bandtlow and Schwab, 2000; McKerracher et al., 1994; Mukhopadhyay et al., 1994; Schnell and Schwab, 1990; Wang et al., 2002) and degradation of CSPGs (Fawcett and Asher, 1999; Fitch and Silver, 1997). The exogenous application of neurotrophic factors increased neuronal survival and axon growth and sprouting. Tissue culture experiments have demonstrated that application of NGF influenced growth cone migration and enhanced neurite growth from adult dorsal root ganglia and retinal neurons (Gundersen and Barrett, 1980; Horie and Akahori, 1994). A combination of NT-3 and BDNF enticed the growth of supraspinal neurons into Schwann cell grafts placed into the lesioned rat spinal cord (Xu et al., 1995). In order to prolong the presence of neurotrophic factors, various types of delivery systems have been used in the injured CNS, such as osmotic pumps, genetically modified cells, and viral vectors. Injections of adenovirus encoding neurotrophins into the CNS induce persistent high levels of transgene expression in numerous cell types including astrocytes, microglia and oligodendrocytes (Le Gal La Salle et al., 1993, Romero et al., 2001).
Although increasing expression of neurotrophic factors in the injured CNS promotes axon growth or sprouting, very few studies show whether axons can grow long distances when supported by such molecules. Previous studies in our lab demonstrated that by using recombinant adenoviruses to express nerve growth factor (NGF) and basic fibroblast growth factor (FGF-2), we could get axons from transplanted nociceptive sensory neurons to grow along the corpus callosum, across the midline and toward an NGF-expressing target in the contralateral striatum: a distance of 7–8 millimeters including a 90-degree turn from white matter into grey matter (Ziemba et al., 2008). Furthermore, expression of semaphorin 3A slightly dorsal and lateral to the turning point sharpened the turning and increased the number of axons growing toward the striatal target. In this study, we examined whether axons from postnatal neuronal transplants could grow such distances along neurotrophin-expressing pathways even if a lesion was present at the midpoint of the pathway.
Methods
Female adult Sprague-Dawley rats (225–250g, Harlan) were used for all experiments. All procedures were carried out under the supervision of the Institutional Animal Care and Use Committee and according to the NIH Guide for the Care and Use of Laboratory Animals. Animals were maintained under conditions of controlled light and temperature, with food and water available ad libitum.
Pathway setup and brain lesion
Rats were anesthetized with a mixture of ketamine (80mg/kg, i.p.) and xylazine (10mg/kg, i.p.) and placed into a stereotactic frame after the fur over the head was shaved, and the skin was cleaned with betadine followed by 70% ethanol. Animals were randomly divided into four groups: GFP adenovirus (Ad-GFP), Ad-NGF, Ad-FGF2/NGF, and no pathway. The guidance pathways were created by injecting each adenovirus along the corpus callosum with a transplantation site in the left corpus callosum and target site in the right corpus callosum (Fig. 1). Using Bregma as a landmark, holes were drilled into the skull along the coronal plane of Bregma to allow injections at the following coordinates, depths relative to dura: transplant site, +2.8mm lateral (left side), −3.0mm deep; pathway within the corpus callosum, +/− 0.7mm lateral, 3.2 mm deep, +/− 1.5mm lateral, 2.9mm deep, + 2.5mm lateral, 2.8mm deep; target site, −2.5mm lateral, 2.8mm deep. Adenovirus concentrations for pathway and target site were 5×106 pfu/μl, except for combination Ad-FGF2/NGF injection, where the Ad-NGF concentration was reduced to 2×106 pfu/ul for a 5:2 ratio of Ad-FGF2 to Ad-NGF. The concentration of Ad-NGF for transplantation site was 5×105 pfu/ul. Injection volumes ranged between 0.2 μl (Ad-NGF at transplantation site, +2.8mm lateral; 0.4ul at +2.5mm; 0.6ul at +1.5mm; 0.8ul at +0.7mm; 1.0ul at −0.7mm; 1.2ul at −1.5mm) to 2.0 μl (Ad-NGF at target, −2.5mm lateral), and increased along the pathway to create a gradient effect. Volumes were injected at a rate of 0.4 μl/min using a 10μl Hamiltom syringe and a 30-gauge beveled needle, and the needle remained in place for 2 minutes at the end of each injection. During the same operation, a 1mm lesion was made through the corpus callosum in an anterior and posterior direction at the midline using a micro-knife.
Figure 1.
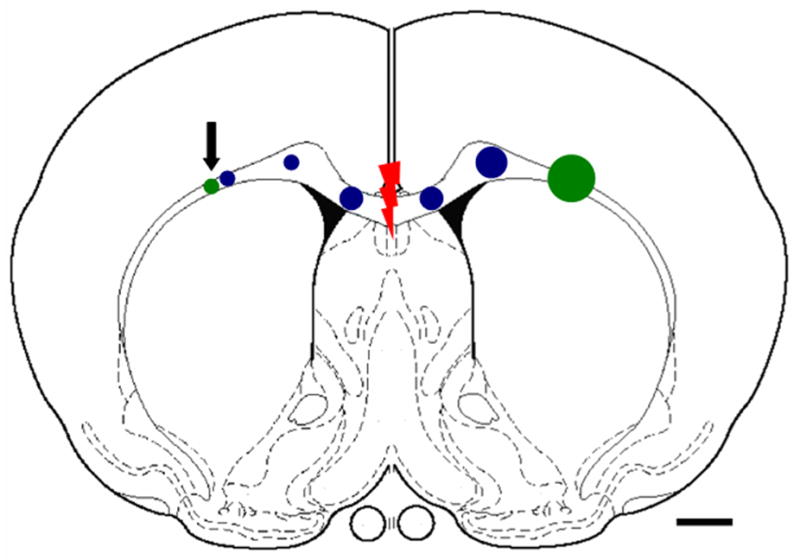
Schematic diagram showing locations for adenovirus injections. Green = Ad-NGF (transplant site at left w/arrow, target at right); Blue = a pathway along the corpus callosum with Ad-GFP, Ad-NGF or Ad-NGF/FGF. Red indicates lesion site. Larger circles indicate more virus injected. Scale bar =1mm.
DRG isolation and transplantation
Three to four days after adenovirus injection, DRG neurons were isolated from postnatal day 1 or 2 Sprague-Dawley rat pups (timed-pregnant dams from Harlan). Each pup was quickly decapitated and DRGs were removed using sterile Dumont forceps and placed into Hank’s buffer containing 1% collagenase, kept on ice until all dissections were complete. After a 20-minute incubation at 37°C, DRGs were washed then trypsinized for 10 minutes at 37°C, treated with DNase and washed twice more with 10% FBS/DMEM, then triturated to disperse the ganglia into a cell suspension. Cells were then plated out in 10%FBS/DMEM containing 50ng/ml NGF for 30–45 minutes at 37°C to allow adhesion of Schwann cells. Cells remaining in suspension were then spun down and washed in N2-supplemented media three times to eliminate the serum. The number of live neurons per microliter was determined by treatment with trypan blue and counting on a hemocytometer. The final cell suspension was supplemented with NGF (50ng/ml) and kept on ice until transplant (up to 3 hours total). Rats previously injected with adenovirus were re-anesthetized and their skulls exposed again at the transplant site (2.8mm lateral to Bregma). 4000–5000 of DRG neurons were injected with a Hamilton syringe/30-guage needle in a volume of <2μl (0.4μl/minute) into the left corpus callosum, 2.8mm below the dura. The needle was left in place for 10 minutes, withdrawn 0.4mm, left for an additional 5 minutes, then slowly retracted the rest of the way.
Immunocytochemistry (ICC)
At 2 weeks post-transplantation, rats were perfused transcardially with cold saline followed by 4% paraformaldehyde. Brains were removed and post-fixed in 4% paraformaldehyde overnight, then transferred to a 30% sucrose solution for two days before cryosectioning. Antibody to calcitonin gene-related peptide (CGRP) was used for staining to identify transplanted nociceptive sensory neurons, a subset of the DRG neuronal population. Floating sections were washed with phosphate buffered saline (PBS), incubated with 3% H2O2 for 10 min, blocked with 5% normal goat serum for 1 hr, then transferred into the following antibodies: rabbit primary antibody to CGRP (1:20,000, Sigma), mouse anti-ED-1 (1:1000, Chemicon), mouse anti-NG2 (1:1000, Zymed Laboratories) and incubated overnight at room temperature on a shaker. The following day, sections were rinsed and incubated in biotin-SP-conjugated affinipure goat anti mouse or rabbit IgG (1:1200, Jackson) for 1 hour, then incubated in Vectastain Elite ABC Reagents (1:100, Vector) for 1 hour and developed for 2–10 min in a 0.05% solution of 3,3,8-diaminobenzidine (DAB) and 0.01% H2O2. Sections were mounted on the glass slides, air dried, dehydrated and coverslipped with permount (Fisher). Some sections were also performed double immunofluorescent labeling to assess glial scar and axon growth in the lesion area. The following antibodies were used: anti-GFAP (1:100, Chemicon), anti-CGRP (1:2000, Sigma), mouse anti-CSPG (1:100, Chemicon). All primary antibodies were applied overnight at room temperature. Secondary antibodies were goat anti-mouse fluorescein (FITC), goat anti-mouse Texas Red, goat anti-rabbit FITC or goat anti-rabbit Texas Red (1:500, Jackson) and were applied for 1 hour. Sections were mounted on slides and coverslipped with 5% propyl-gallate in glycerol for fluorescence microscopy.
Quantification of axonal growth
To quantify cell survival, the total number of visible CGRP+ cell bodies at the transplant site was manually counted at 200x total magnification and summed over three sections in the 1:5 series containing the most cells. For axon growth, CGRP+ fibers were counted manually at 200x total magnification at the following points along the pathway: in the corpus callosum 0.5 and 1.5mm from midline lesion ipsilateral (+) and contralateral (−) to transplant. To correct for differences in transplant size and survival, axon counts at the more distal path points (−1.5mm, −0.5mm, +0.5mm) were divided by a count closer to the transplant (at +1.5mm) and expressed as a percentage of the axon growth. For each animal, these counts were conducted and averaged over three sections including the lesion site and three sections with axon growth but not including the lesion site. All counts were carried out by observers blinded to treatment.
Statistical Analyses
One-way ANOVA was used to test for differences in cell survival and percentage of CGRP+ axons at the different points followed by Tukey post hoc analysis. Differences were considered statistically significant if p-values were less than or equal to 0.05.
Results
Survival of transplanted neurons
In our previous studies, we found that without NGF at the transplant site, no CGRP-positive cells could be found in any animals two weeks after transplantation. However, with NGF expression at the transplant site, DRG neurons survived very well (Ziemba et al., 2008). In this study, a low concentration of Ad-NGF (5×105 pfu/ul) was injected at the transplant site in all animals at the same time as virus injections along the guidance pathway. Three to four days after virus injections, DRG neurons were isolated from P1-2 pups and injected at the transplant site. Two weeks after transplantation, immunostaining for CGRP showed that nociceptive neurons survived very well at the transplantation site in all groups (Fig. 2A–C). There was no significant difference in the number of CGRP+ neurons at the transplantation site across groups (Fig. 2D).
Figure 2.
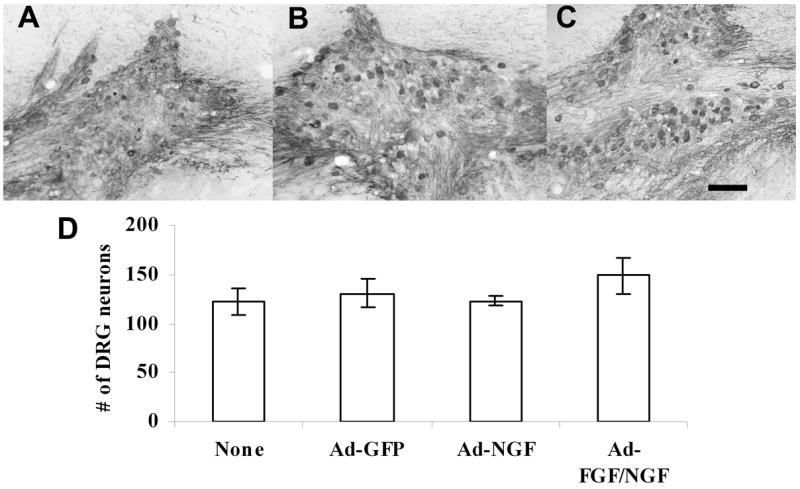
DRG neurons survived very well at the transplantation site. DRGs from postnatal day 1–2 rats survived very well with NGF support in Ad-GPF (A), Ad-NGF (B), and Ad-FGF/NGF pathways (C). There was no significant difference in the numbers of DRG neurons at the transplantation site between groups (D, p>0.05, mean ± S.E.M.). Scale bar = 100μm.
Comparison of axon growth along various pathways through a lesion site
For comparison to animals with virus-mediated expression pathways, some animals without any pathway injections were used as controls. This was done in order to determine if the injection procedure itself increased inhibition due to the development of gliosis around the needle tracts. In this group, animals received Ad-NGF injections at only the transplantation site (5×105 pfu/ul) and target site (5×106 pfu/ul), as well as a lesion at the midline of the corpus callosum. Although CGRP+ neurons survived very well at the transplantation site, axons did not grow very far from the transplant along the corpus callosum. Out of four animals, two showed a few CGRP+ axons that grew across the midline into the opposite corpus callosum. In other two animals, hardly any CGRP+ axons even reached the lesion site at the midline of the corpus callosum. About 19% of CGRP+ axons reached a point 0.5mm proximal to the lesion site. Less than 7% of CGRP+ axons reached the target area in the contralateral corpus callosum. In a second control group, six animals received pathway injections of Ad-GFP along the corpus callosum between transplant and target sites. As in the animals without an expression pathway, CGRP+ neurons survived and extended axons into the corpus callosum, but they did not grow very far (Fig. 3A). Again, very few CGRP+ axons even reached the lesion site at the midline of the corpus callosum (Fig 3B). Out of six animals, only one showed a few CGRP+ axons that grew across the midline and into the contralateral corpus callosum. Only 1% of CGRP+ axons made it to a point 0.5mm proximal to the lesion site. No CGRP+ axons reached the target area.
Figure 3.
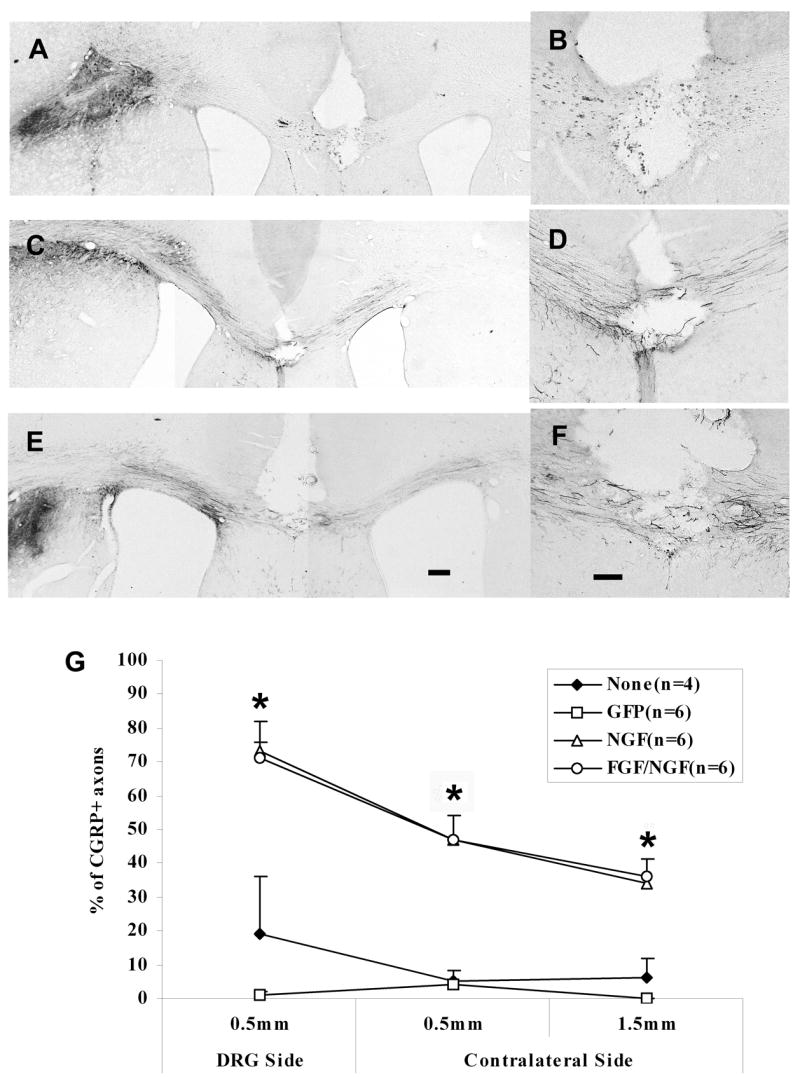
CGRP+ axons have grown across the lesion into the contralateral corpus callosum by 2 weeks post-transplant. A & B) Representative section from an animal with GFP expressed along the pathway (B=higher magnification at lesion site). C& D) Representative section from an animal with NGF expressed along the pathway (D=higher magnification at lesion site). E & F) Representative section from an animal with a combination FGF/NGF pathway (F=higher magnification at lesion site). G) Percentage of CGRP+ fibers that grew across the lesion of the corpus callosum. A higher percentage of CGRP+ axons grew across the lesion at the midline of corpus callosum into the contralateral side in both NGF and FGF/NGF pathways. *p<0.001, FGF/NGF, NGF vs GFP, p<0.01, FGF/NGF, NGF vs None (no pathway); p>0.05 FGF/NGF vs NGF; GFP vs None. Data= Mean± SEM. E: Scale bar = 200μm; F: Scale bar = 100μm.
In contrast to the control groups, many CGRP+ axons grew out of the transplants and traveled long distances along both NGF- and FGF/NGF–expressing pathways (Fig. 3C and 3E). On the transplant side, many CGRP+ fibers grew from the transplants along both pathways toward to the lesion at the midline. More than 70% of CGRP+ axons reached to the lesion area in both pathways. Some CGRP+ fibers were found across the lesion site in the contralateral corpus callosum (Fig. 3D and 3F). Although the numbers of CGRP+ fibers dropped on the contralateral side, more than 40% of the CGRP+ axons were found at 0.5 mm distal to the lesion site. Furthermore, 34% and 36% of CGRP+ axons in NGF and FGF/NGF pathways reached the target area 2.5mm distal to the lesion.
Comparing the growth of CGRP+ axons across the lesion in different pathways, there was a significant difference in FGF/NGF and NGF pathways compared to GFP group at all points (+0.5mm from lesion on the transplant side, −0.5mm, and −1.5mm from the lesion on the contralateral side, p<0.001), and no pathway group at all points (p<0.01) There were no differences between FGF/NGF and NGF at any point, or between GFP and no pathway at any point, p>0.05 (Fig. 3G).
Comparison of axon growth around the midline lesion
Since the lesion of the corpus callosum was small (1 mm in the anteroposterior direction), some axons would be more accurately described as growing around the lesion rather than through it. It is impossible to determine the exact longitudinal trajectory of individual fibers, but we counted CGRP+ axons in sections which clearly did not include the midline lesion and characterized these as axons growing around it (Fig 4). Again, we counted the number of CGRP+ axons at 0.5mm and 1.5mm from the midline on the transplant side and on the target side. About 24% and 4% of CGRP+ axons in the no pathway and GFP pathway groups (Fig. 4A) reached a point 0.5mm proximal to the midline. Very few CGRP+ axons were found at 0.5mm distal to the midline, with about 18% and 5% of CGRP+ axons in the no pathway and GFP pathway groups. Less than 10% of CGRP+ axons reached the target area in each of these groups. However, in both NGF and FGF/NGF expressing pathways (Fig. 4B and 4C), many CGRP+ axons grew from the transplants along the pathways and around the lesion into target area. More than 75% of CGRP+ axons were found near the midline on the transplant side in both NGF and FGF/NGF-expressing pathways. Although the number of CGRP+ axons decreased on the target site, more than 40% of CGRP+ axons reached the target area in both pathways.
Figure 4.
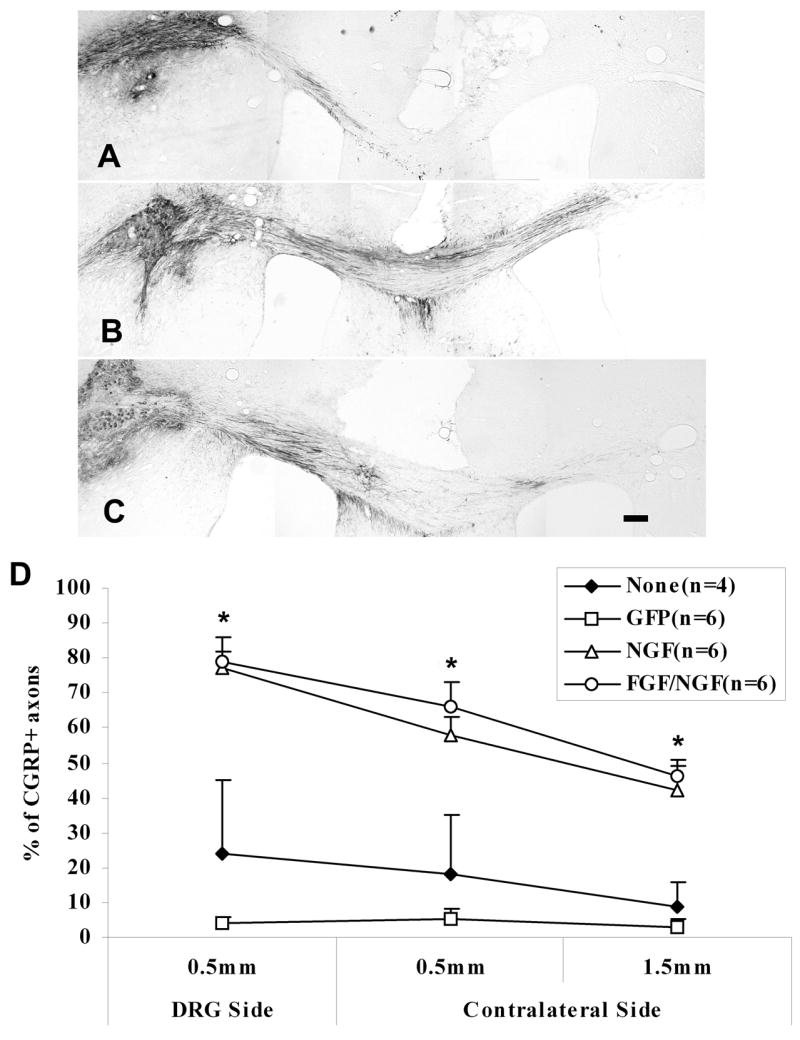
CGRP+ axons have grown around the lesion into the contralateral corpus callosum by 2 weeks post-transplant. A) Representative section from an animal with GFP expression along the pathway. Very few CGRP+ fibers grew around the lesion into the contralateral corpus callosum. B and C) Representative sections from the animals with either NGF (B) or FGF/NGF (C) expression along the pathway. More CGRP+ fibers grew around the lesion into the contralateral corpus callosum. D) Percentage of CGRP+ fibers that grew around the lesion of the corpus callosum. This shows in a similar pattern as in Figure 3, with a higher percentage of CGRP+ axons that grew around the lesion into the contralateral side in both NGF and FGF/NGF pathways. *p<0.001, FGF/NGF, NGF vs GFP, p<0.01, FGF/NGF, NGF vs None (no pathway); p>0.05, FGF/NGF vs NGF; GFP vs None (no pathway). Data = Mean ± SEM. Scale bar = 200μm.
The growth of CGRP+ axons around the lesion showed a similar pattern as across the lesion in all pathways. There was a significant difference in NGF and FGF/NGF groups at all points compared to GFP and no-pathway groups (NGF and FGF/NGF to GFP, p<0.001, at all points; NGF and FGF/NGF to none-pathway, p<0.01, at all points). There was no significant difference between NGF and FGF/NGF groups at any point, or between GFP and no pathway at any point (Fig. 4D, NGF to FGF/NGF, p>0.05; GFP to none-pathway, p>0.05).
Lesion site morphology
Lesions were created in this experiment using a micro-knife to transect the corpus callosum at the midline in an anteroposterior direction. Some sections from all groups were stained for ED-1, NG2, GFAP or CSPG. Immunostaining for the monocyte/macrophage marker ED-1 showed that macrophages were abundant in the lesion area (Fig. 5A, section from NGF pathway). Cells at the lesion site also expressed NG2, a chondroitin sulfate proteoglycan produced by glial progenitor cells (Fig. 5B, section from NGF pathway). Sections with CSPG and CGRP double staining showed that although CSPGs were up-regulated around the lesion site, CGRP+ axons still grew into that area in both NGF and FGF/NGF pathway groups (Fig. 5C–D, section from FGF/NGF pathway). Very few CGRP+ axons grew into CSPG expressing area in GFP and no pathway groups (data not shown). Similar results were found in GFAP and CGRP double staining. Sections stained with GFAP and CGRP showed that GFAP+ reactive astrocytes accumulated around the lesion site to form a scar barrier (Fig. 6A, section from NGF pathway). However, CGRP+ axons were able to grow across or around the lesion area into the contralateral corpus callosum wherever a bridge was available (Fig. 6B–D, section from NGF pathway in B, section from FGF/NGF pathway in D). There was no difference in the expression of ED-1, NG2, GFAP, or CSPG at the lesion site between experimental groups (data not shown).
Figure 5.
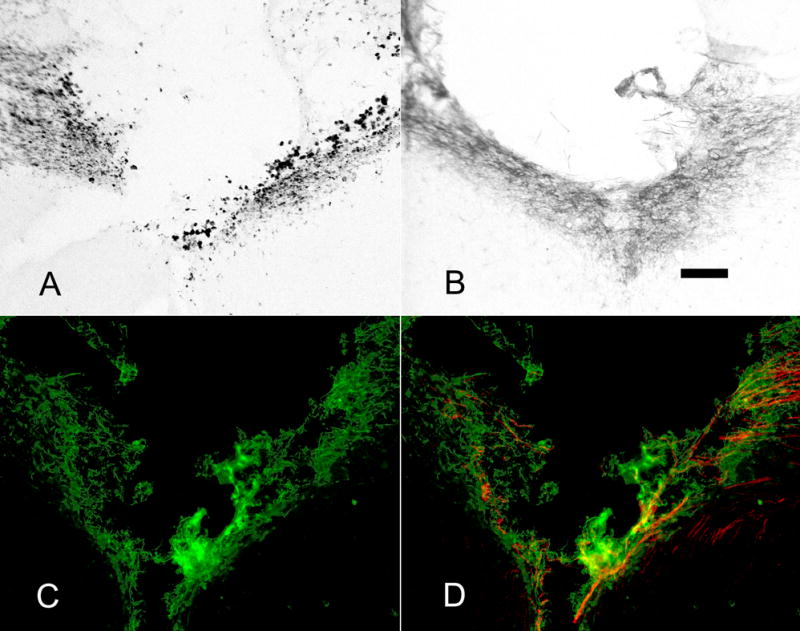
ED-1, NG2 and CSPG staining around the lesion. There was no difference in ED-1, NG2 or CSPG staining between groups – sections are representative. A) A lesion at the midline of the corpus callosum induces macrophages stained with ED-1 antibody around the lesion area (NGF pathway). B) NG2 expressing cells also presented around the lesion after injury (NGF pathway). C) CSPGs were up-regulated around the lesion area. D) In both NGF and FGF/NGF groups, CGRP+ axons (red) grew into CSPG (green) expressing lesion area. Sections shown in C and D are from the FGF/NGF pathway group. Scale bar = 100μm.
Figure 6.
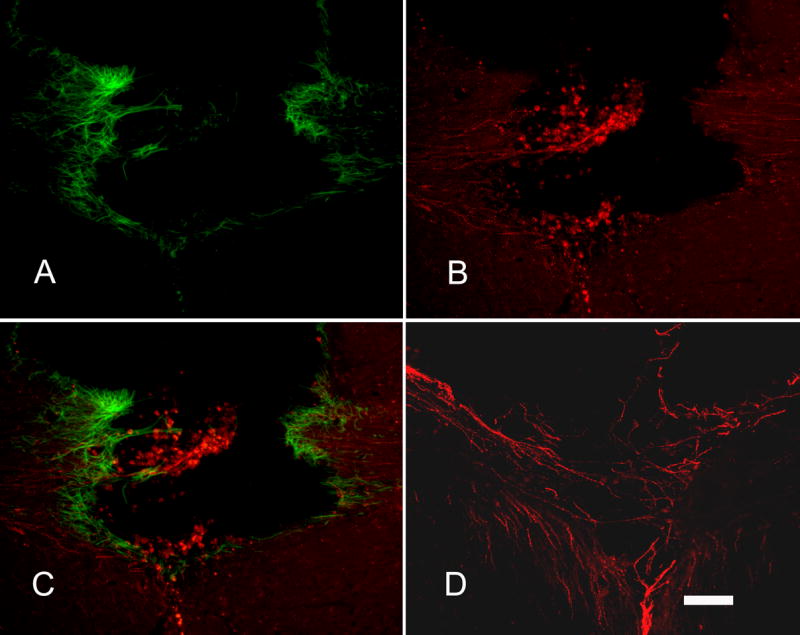
CGRP+ axons grew into the lesion area where reactive astrocytes were present around the lesion. There was no difference in GFAP staining between groups. A) Reactive astrocytes stained with GFAP (green) antibody accumulated around the lesion site. B) CGRP+ axons (red) from DRG transplants grew into the lesion area; some grew into the opposite corpus callosum. C) Merged image of A and B to demonstrate the relationship between axon growth and glial scar around the lesion site. D) Some CGRP+ axons (red) are seen bridging the lesion site to grow into the opposite corpus callosum. Sections were from NGF pathway group in A, B, C. Section was from FGF/NGF pathway group in D. Scale bar = 100μm.
Discussion
Within the adult mammalian CNS, axonal regeneration is usually abortive (Cajal, 1928). This abortive regeneration is not primarily due to an intrinsic inability for adult neurons to regenerate but to the lack of a growth-supportive environment within the CNS. Numerous studies have demonstrated that lesion sites within the CNS are particularly non-permissive to axon growth, due to the production of potent neurite growth inhibitory molecules by damaged myelin (Caroni and Schwab, 1988; McKerracher et al., 1994) and reactive astrocytes (McKeon et al., 1991; Snow et al., 1990) as well as reduced expression of neurotrophins and other growth-promoting molecules (Varon and Conner, 1994).
In spite of these well-described limitations, axons have been observed to grow within the adult CNS under certain experimental conditions. Embryonic DRG neurons, for instance, have been shown to extend axons through adult CNS white matter tracts after being transplanted there using fine-needle microinjection techniques (Davies et al., 1994; Wictorin et al., 1990). It has been proposed that neurons in the embryonic state lack receptors to neurite-inhibitory molecules (Shewan et al., 1995). As DRG neurons age, their ability to grow within these white matter tracts diminishes, so that transplanted postnatal or adult DRG neurons only extend axons if the microinjection does not cause any damage at the transplant site (Davies et al., 1997; Davies et al., 1999). These axons may be observed growing through degenerated white matter tracks, but stop abruptly upon reaching scar surround a spinal cord lesion (Davies et al., 1999). The present study was designed to determine if these limitations could be overcome by establishing a growth permissive pathway through the lesion site.
The model we used in our previous and present studies differed from the one developed by Davies and colleagues in that we made relatively large DRG transplants (4–5,000 cells in 1–2μl). This large transplant and the multiple injections used to create the pathway cause some reactive gliosis and high expression of CSPGs throughout the corpus callosum (Ziemba et al., 2008). This in combination with degenerated myelin from a lesion creates a very non-permissive growth environment which prevents the vast majority of axons from growing more than a millimeter in GFP-expressing controls. Even in these controls, the limited amount of axonal growth that does occur is most likely due to the expression of NGF at the transplantation site. In both control and experimental groups, all axons were observed to grow parallel to the orientation of endogenous axons and myelin within the corpus callosum. Such parallel growth patterns have been observed previously and are most likely due to the natural cellular architecture of myelinated tracts, which supports parallel axon growth but inhibits perpendicular growth (Davies et al., 1999; Pettigrew and Crutcher, 1999). In addition, the vast majority of DRG axons growing in white matter tracts appear to be associated with astrocytes, which also have their processes in a parallel orientation within tracts (Davies et al., 1999). In our experiments, these astrocytes are most likely the preferred substrate since, unlike oligodendrocytes, they are highly transfected by adenovirus (Romero et al., 2000).
In order to increase the number of nociceptive sensory axons extending along the corpus callosum and beyond the midline lesion, we expressed either NGF alone or a combination of NGF and FGF along the path by multiple injections of adenoviral vectors encoding those molecules. Many studies in vitro and in vivo have demonstrated that NGF can act as a soluble guidance molecule for neurons (Gundersen and Barrett, 1980; Letourneau, 1978; Levi-Montalcini, 1982). In vitro studies have shown a chemoattractive effect of NGF on neurite outgrowth of embryonic DRG neurons (Gundersen and Barrett, 1980). Previous studies in our lab demonstrated that injecting adenovirus encoding NGF in the dorsal spinal cord induced extensive sprouting of nociceptive fibers into the dorsal horn (Romero et al., 2000). Furthermore, overexpression of NGF induced lesioned sensory axons to re-grow into the dorsal horn, which would normally not happen after dorsal root injury (Romero et al., 2001; Tang et al., 2004; Tang et al., 2007). FGF-2 is also known to be a neurotrophic factor that supports neuronal survival (Morrison et al., 1986) and promotes axonal regeneration (Sapieha et al., 2003). Our previous studies showed that FGF2 induced robust regeneration of DRG neurons within the spinal cord (Romero et al., 2001), as well as long-distance axon growth from DRG transplants across the corpus callosum and into the contralateral striatum (Ziemba et al., 2008).
In both NGF and FGF2/NGF expressing pathways, many CGRP+ axons grew out of the transplants along the white matter pathway of the corpus callosum and reached the lesion at the midline of the corpus callosum. Some axons stopped near the lesion area, but some continued to grow through and/or around the lesion and into the contralateral corpus callosum toward the target which was located at 2.5mm from the midline and 5.3mm from the transplant. The number of CGRP+ axons found at each distance along the guidance pathway dropped gradually from near the transplant to contralateral target. In all groups, some CGRP+ axons were found to grow toward the target within coronal planes that did not include the injury site, without actually growing through the lesion. Even in these sections, however, pathways without NGF or FGF2/NGF expression (either with no virus or with Ad-GFP injections) did not have much axon growth while pathways expressing neurotrophins showed robust axon growth across the midline. These data suggest that expression of growth factors not only supports axon growth along the pathway, but also through and/or around a lesion, further demonstrating that expression of a growth-supportive molecules can counteract the endogenous inhibitory environment of the CNS.
In this study, we created gradient pathways in all virus-injected groups in order to promote long distance axon growth. Previous studies have shown that transplants of genetically modified cells expressing neurotrophic factors in spinal cord lesion sites provide support for axon growth into the lesion site, but not beyond it to reinnervate the distal cord (Tuszynski et al., 2002; Jin et al., 2002; Blesch et al., 2004). A recent study by Taylor and colleagues indicated that a neurotrophin gradient created by a single viral injection in the rat spinal cord promoted axonal growth just beyond a lesion filled with cellular grafts, but axon growth was not supported over long distances (Taylor et al., 2006). Based on our data, we predict that long-distance axon growth could be supported in a spinal cord injury model if the appropriate gradient pathway extended further beyond the lesion, with multiple virus injections rather than a single distal injection site.
Injuries to the CNS cause a sequence of pathological changes including hemorrhage, edema, axonal and neuronal necrosis, and demyelination followed by cyst formation and infarction (Tator, 2002). In our model, we found that a lesion at the midline of the corpus callosum caused macrophages to appear around the lesion site by two weeks post-injury. Reactive astrocytes had also accumulated near the lesion area forming a glial scar with disrupted orientation. Accumulated macrophages near the lesion site may function as host defense. Several studies have demonstrated that activated macrophages promote CNS regeneration (Ohlsson et al., 2004; Shibata et al., 2003). Reactive astrocytes occur in response to all types of CNS injury. After injury, reactive astrocytes accumulate in the lesion area and their processes entangle each other to form scar networks around the lesion which impede axonal regeneration (Fawcett and Asher, 1999; Silver and Miller, 2004; Sofroniew, 2005).
Chondroitin sulfate proteoglycan and NG2 were also expressed adjacent to the lesion in this model, most likely being produced along the surface of glia and oligodendrocyte precursor cells. Recent studies have shown a contradictory role of NG2 in response to CNS injuries. Fawcett and colleagues showed that oligodendrocyte precursor cells recognized by the NG2 antibody respond rapidly to CNS injuries, with hypertrophy and upregulation of the NG2 proteoglycan within 24 hours (Rhodes et al., 2006). These cells participate in glial scar formation, remaining around the injury site for several weeks. Other studies demonstrated that NG2 is highly inhibitory to growing axons (Chen et al., 2002; Fidler et al., 1999). However, data from several other labs are not consistent with the notion of an inhibitory effect of NG2. Using NG2-null mutant mice, de Castro and colleagues reported no difference in axonal regeneration after spinal cord transection compared to wild-type mice (de Castro et al., 2005). Yang and colleagues also demonstrated in vitro that NG2+ cells do not repel but rather promote axonal growth, regardless of the level of NG2 expression (Yang et al., 2006).
In this study, we do not know whether the increased expression of NG2 at the lesion site was attractive or repulsive to axons, but the presence of reactive astrocytes implies that there was an impediment to axon growth in that area. However, our data indicate that with NGF or FGF/NGF support, CGRP+ axons from DRG transplants can grow through or around the lesion and toward a distant target site on the contralateral side of the brain. Without appropriate molecular guidance, very few CGRP+ axons succeed in even reaching the midline. Overexpression of neurotrophic factors along the pathway may function not only to drive intrinsic determinants of growth cone advancement but also to counterbalance the inhibitory environment in the lesion area.
In summary, this study successfully demonstrated that with appropriate molecular support, axons from transplanted neurons can grow through and/or around a lesion in the adult mammalian brain. Further studies using this technique will be carried out in the context of spinal cord injury and neurodegenerative disease to examine whether axons can grow along preformed pathways through injured tissue, make functional distal connections, and lead to recovery of the damaged adult CNS.
Acknowledgments
This work was supported by National Institute of Health/National Institute for Neurological Disorders and Stroke RO1 NS38126. We thank Melody King and Jun Yang for help with axon quantification.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bandtlow CE, Schwab ME. NI-35/250/nogo-a: a neurite growth inhibitor restricting structural plasticity and regeneration of nerve fibers in the adult vertebrate CNS. Glia. 2000;29:175–181. doi: 10.1002/(sici)1098-1136(20000115)29:2<175::aid-glia11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Blesch A, Yang H, Weidner N, Hoang A, Otero D. Axonal responses to cellularly delivered NT-4/5 after spinal cord injury. Mol Cell Neurosci. 2004;27:190–201. doi: 10.1016/j.mcn.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Cajal SR. Cajal’s degeneration and regeneration of the nervous system. New York: Oxford University Press; 1928. [Google Scholar]
- Caroni P, Schwab ME. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 1988;1:85–96. doi: 10.1016/0896-6273(88)90212-7. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Ughrin Y, Levine JM. Inhibition of axon growth by oligodendrocyte precursor cells. Mol Cell Neurosci. 2002;20:125–139. doi: 10.1006/mcne.2002.1102. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Field PM, Raisman G. Long interfascicular axon growth from embryonic neurons transplanted into adult myelinated tracts. J Neurosci. 1994;14:1596–1612. doi: 10.1523/JNEUROSCI.14-03-01596.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19:5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro R, Jr, Tajrishi R, Claros J, Stallcup WB. Differential responses of spinal axons to transection: influence of the NG2 proteoglycan. Exp Neurol. 2005;192:299–309. doi: 10.1016/j.expneurol.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Fidler PS, Schuette K, Asher RA, Dobbertin A, Thornton SR, Calle-Patino Y, Muir E, Levine JM, Geller HM, Rogers JH, Faissner A, Fawcett JW. Comparing astrocytic cell lines that are inhibitory or permissive for axon growth: the major axon-inhibitory proteoglycan is NG2. J Neurosci. 1999;19:8778–8788. doi: 10.1523/JNEUROSCI.19-20-08778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Silver J. Glial cell extracellular matrix: boundaries for axon growth in development and regeneration. Cell Tissue Res. 1997;290:379–384. doi: 10.1007/s004410050944. [DOI] [PubMed] [Google Scholar]
- Gundersen RW, Barrett JN. Characterization of the turning response of dorsal root neurites toward nerve growth factor. J Cell Biol. 1980;87:546–554. doi: 10.1083/jcb.87.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie H, Akahori Y. Three-dimensional cell aggregation enhances growth-promoting activity of NGF in adult DRG. Neuroreport. 1994;6:37–40. doi: 10.1097/00001756-199412300-00011. [DOI] [PubMed] [Google Scholar]
- Jakeman LB, Reier PJ. Axonal projections between fetal spinal cord transplants and the adult rat spinal cord: a neuroanatomical tracing study of local interactions. J Comp Neurol. 1991;307:311–334. doi: 10.1002/cne.903070211. [DOI] [PubMed] [Google Scholar]
- Jin Y, Fischer I, Tessler A, Houle JD. Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury. Exp Neurol. 2002;177:265–275. doi: 10.1006/exnr.2002.7980. [DOI] [PubMed] [Google Scholar]
- Le Gal La Salle G, Robert JJ, Berrard S, Ridoux V, Stratford-Perricaudet LD, Perricaudet M, Mallet J. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993;259:988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- Letourneau PC. Chemotactic response of nerve fiber elongation to nerve growth factor. Dev Biol. 1978;66:183–196. doi: 10.1016/0012-1606(78)90283-x. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. Nerve growth factor--target cells. A model of choice in the study of neuron differentiation. Boll Ist Sieroter Milan. 1982;61:164–169. [PubMed] [Google Scholar]
- McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Morrison RS, Sharma A, de Vellis J, Bradshaw RA. Basic fibroblast growth factor supports the survival of cerebral cortical neurons in primary culture. Proc Natl Acad Sci U S A. 1986;83:7537–7541. doi: 10.1073/pnas.83.19.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Ohlsson M, Mattsson P, Wamil BD, Hellerqvist CG, Svensson M. Macrophage stimulation using a group B-streptococcus exotoxin (CM101) leads to axonal regrowth in the injured optic nerve. Restor Neurol Neurosci. 2004;22:33–41. [PubMed] [Google Scholar]
- Pettigrew DB, Crutcher KA. White matter of the CNS supports or inhibits neurite outgrowth in vitro depending on geometry. J Neurosci. 1999;19:8358–8366. doi: 10.1523/JNEUROSCI.19-19-08358.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KE, Raivich G, Fawcett JW. The injury response of oligodendrocyte precursor cells is induced by platelets, macrophages and inflammation-associated cytokines. Neuroscience. 2006;140:87–100. doi: 10.1016/j.neuroscience.2006.01.055. [DOI] [PubMed] [Google Scholar]
- Romero MI, Rangappa N, Garry MG, Smith GM. Functional regeneration of chronically injured sensory afferents into adult spinal cord after neurotrophin gene therapy. J Neurosci. 2001;21:8408–8416. doi: 10.1523/JNEUROSCI.21-21-08408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MI, Rangappa N, Li L, Lightfoot E, Garry MG, Smith GM. Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J Neurosci. 2000;20:4435–4445. doi: 10.1523/JNEUROSCI.20-12-04435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Benito FF, Ramon-Cueto A. Olfactory ensheathing glia transplantation: a therapy to promote repair in the mammalian central nervous system. Anat Rec B New Anat. 2003;271:77–85. doi: 10.1002/ar.b.10015. [DOI] [PubMed] [Google Scholar]
- Sapieha PS, Peltier M, Rendahl KG, Manning WC, Di Polo A. Fibroblast growth factor-2 gene delivery stimulates axon growth by adult retinal ganglion cells after acute optic nerve injury. Mol Cell Neurosci. 2003;24:656–672. doi: 10.1016/s1044-7431(03)00228-8. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Shewan D, Berry M, Cohen J. Extensive regeneration in vitro by early embryonic neurons on immature and adult CNS tissue. J Neurosci. 1995;15:2057–2062. doi: 10.1523/JNEUROSCI.15-03-02057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata A, Zelivyanskaya M, Limoges J, Carlson KA, Gorantla S, Branecki C, Bishu S, Xiong H, Gendelman HE. Peripheral nerve induces macrophage neurotrophic activities: regulation of neuronal process outgrowth, intracellular signaling and synaptic function. J Neuroimmunol. 2003;142:112–129. doi: 10.1016/s0165-5728(03)00253-4. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Heron P, Mashburn C, Smith GM. Targeting sensory axon regeneration in adult spinal cord. J Neurosci. 2007;27:6068–6078. doi: 10.1523/JNEUROSCI.1442-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XQ, Tanelian DL, Smith GM. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J Neurosci. 2004;24:819–827. doi: 10.1523/JNEUROSCI.1263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26:9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tator CH. Strategies for recovery and regeneration after brain and spinal cord injury. Inj Prev. 2002;8(Suppl 4):IV33–36. doi: 10.1136/ip.8.suppl_4.iv33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski MH, Grill R, Jones LL, McKay HM, Blesch A. Spontaneous and augmented growth of axons in the primate spinal cord: effects of local injury and nerve growth factor-secreting cell grafts. J Comp Neurol. 2002;449:88–101. doi: 10.1002/cne.10266. [DOI] [PubMed] [Google Scholar]
- Varon S, Conner JM. Nerve growth factor in CNS repair. J Neurotrauma. 1994;11:473–486. doi: 10.1089/neu.1994.11.473. [DOI] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Wictorin K, Clarke DJ, Bolam JP, Bjorklund A. Fetal striatal neurons grafted into the ibotenate lesioned adult striatum: efferent projections and synaptic contacts in the host globus pallidus. Neuroscience. 1990;37:301–315. doi: 10.1016/0306-4522(90)90401-o. [DOI] [PubMed] [Google Scholar]
- Xu XM, Guenard V, Kleitman N, Bunge MB. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995;351:145–160. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- Yang Z, Suzuki R, Daniels SB, Brunquell CB, Sala CJ, Nishiyama A. NG2 glial cells provide a favorable substrate for growing axons. J Neurosci. 2006;26:3829–3839. doi: 10.1523/JNEUROSCI.4247-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemba KS, Chaudhry N, Jin Y, Rabchevshy AG, Smith GM. Targeting axon growth from neuronal transplantation in the adult central nervous system along preformed guidance pathways. J Neurosci. 2008 doi: 10.1523/JNEUROSCI.3819-07.2008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Neubauer D, Graham J, Krekoski CA, Ferguson TA, Muir D. Regeneration of axons after nerve transection repair is enhanced by degradation of chondroitin sulfate proteoglycan. Exp Neurol. 2002;176:221–228. doi: 10.1006/exnr.2002.7922. [DOI] [PubMed] [Google Scholar]


