Abstract
A surge of GnRH release signals the LH surge that triggers ovulation. The GnRH surge is dependent on a switch in estradiol feedback from negative to positive and, in rodents, a daily neural signal, likely from the suprachiasmatic nuclei. Vasoactive intestinal polypeptide (VIP) may be involved in suprachiasmatic nuclei-GnRH neuron communication. Here we assessed the effects of acute VIP (5 min treatment) on GnRH neuron function using targeted extracellular recordings of firing activity of GnRH neurons in brain slices. We examined the effect of VIP on firing rate at different times of day using an established ovariectomized, estradiol-treated (OVX+E) mouse model that exhibits daily LH surges timed to the late afternoon. Cells from OVX animals (no estradiol) did not respond to VIP, regardless of time of day. With estradiol, the effect of VIP on GnRH neurons was dependent on the time of recording. During negative feedback, OVX+E cells did not respond. VIP increased firing in cells recorded during surge onset, but this excitatory response was reduced at surge peak. Acute treatment of OVX+E cells during surge peak with a VIP receptor antagonist decreased GnRH neuron firing. This suggests endogenous VIP may both increase GnRH neuron firing during the surge and occlude response to exogenous VIP. These data provide functional evidence for VIP effects on GnRH neurons and indicate that both estradiol and time of day gate the GnRH neuron response to this peptide. VIP may provide an excitatory signal from the circadian clock that helps time the GnRH surge.
GNRH NEURONS FORM the final common neural pathway regulating reproduction. At the end of the follicular phase of the female reproductive cycle (proestrus in rodents), the balance of feedback effects of the steroid hormone estradiol on the GnRH neuronal system switches from negative to positive, causing a large surge in GnRH release (1,2,3), which appears to be driven by increased GnRH neuron firing activity (4). The GnRH surge causes a surge of LH release by the gonadotropes of the anterior pituitary, triggering ovulation. In addition to estradiol, a circadian timing system also appears critical for the surge in some species. In rodents, the surge is dependent on not only estradiol feedback but also a daily neuronal signal that times the surge to an appropriate time of day (i.e. late afternoon in nocturnal species) (4,5,6,7). The circadian influence may extend to humans as the LH surge tends to begin in the early morning in women (8).
The most likely source of a diurnal or circadian signal is the central mammalian circadian pacemaker in the suprachiasmatic nuclei (SCN). The LH surge is abolished by lesions of the SCN (9), and SCN cells express the neuronal activation marker c-fos during the LH surge (10). One candidate neuromodulator that may mediate SCN-GnRH signals is vasoactive intestinal polypeptide (VIP). VIP neurons in the SCN provide a direct SCN-GnRH neuron connection (11,12). Furthermore, approximately 40% of GnRH neurons in the rat express VIP2 receptor protein on proestrus (13). VIP may also participate in an indirect SCN-GnRH connection via the anteroventral periventricular area (AVPV), which expresses VIP receptors (14) and projects directly to GnRH neurons (15,16).
The effects of VIP on the GnRH/LH surge are unclear. VIP infused into the third ventricle inhibits the LH surge in ovariectomized, estradiol-treated (OVX+E) rats treated with progesterone (17). Conversely, blocking VIP action by intracerebroventricular administration of a VIP antiserum or direct SCN injection of VIP antisense oligonucleotides causes a significant delay in the time course and a strong reduction of the magnitude of the LH surge in OVX+E rats (18,19). There is a greater degree of expression of c-fos, a marker of neuronal activity, in GnRH neurons innervated by VIPergic fibers than in noninnervated GnRH neurons during the LH surge (20), suggesting VIP has a permissive effect on GnRH neuron activation. A controversy thus exists with regard to the effects of VIP on the surge. Here we examined the effects of VIP on the firing activity of GnRH neurons using an established mouse model that exhibits daily LH surges after ovariectomy and constant estradiol replacement (4). To begin to examine the roles synaptic afferents may play in mediating the response to VIP, we performed recordings in both coronal and sagittal slices. Our results suggest that VIP can excite GnRH neurons and that this effect is dependent on both estradiol treatment and time of day.
Materials and Methods
Animals
Adult female mice (2–4 months old) in which green fluorescent protein is expressed under the control of the GnRH promoter (21) were used for all experiments. Animals were housed on a 14-h light, 10-h dark cycle with lights off at 1630 h Eastern Standard Time and had ad libitum access to food (Harlan 2916 chow; Harlan, Indianapolis, IN) and water. Mice were bilaterally ovariectomized under isoflurane anesthesia (Burns Veterinary Supply, Westbury, NY) and treated with a sc SILASTIC brand capsule (Dow-Corning Co., Midland, MI) containing 0.625 μg estradiol suspended in sesame oil (OVX+E, n = 16) or not treated further (OVX, n = 4). This estradiol treatment induces daily LH surges that peak around the time of lights off as previously described; OVX animals show no diurnal change in LH levels (4). The long-acting local anesthetic bupivacaine (0.25%, 7 μl/site; Abbott Laboratories, North Chicago, IL) was used for postoperative analgesia. Estradiol treatment was solely in vivo, and estradiol was not present in any recording solutions. All procedures were approved by the University of Virginia Animal Care and Use Committee and conducted in accordance with the guidelines of the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Brain slice preparation
Reagents were purchased from Sigma (St. Louis, MO) except as noted. Two to four days after surgery, coronal or sagittal brain slices (300 μm) were prepared with slight modifications (4,22) of previous descriptions (23,24). Briefly, mice were euthanized at three different times that correspond to negative feedback (0900–1030 h), surge onset (1230–1300 h), and surge peak (1430–1500 h) in OVX+E animals. The brain was rapidly removed and placed in ice-cold high-sucrose saline solution containing (in mm) 250 sucrose, 3.5 KCl, 26 NaHCO3, 10 d-glucose, 1.3 Na2HPO4, 1.2 MgSO4, and 3.8 MgCl2. Coronal or sagittal (300 μm) slices were cut with a Vibratome 3000 (Ted Pella, Inc., Redding, CA). Slices were incubated for 30 min at 30–32 C in 50% high-sucrose saline and 50% normal saline (NS) solution, with NS containing (in mm) 135 NaCl, 3.5 KCl, 26 NaHCO3, 10 d-glucose, 1.25 Na2HPO4, 1.2 MgSO4, 2.5 CaCl2 (pH 7.4). Slices were then transferred to 100% NS solution at room temperature (∼21–23 C) for 0.5–2.5 h.
Recordings
Targeted single-unit extracellular recordings (loose patch) were used in this study because this configuration allows recording with minimal impact on the behavior of the recorded cell (23,24). Recordings were performed during negative feedback (1100–1400 h), surge onset (1400–1600), and surge peak (1600–1900). Recording pipettes (1–3 mΩ) were filled with normal HEPES-buffered solution (23,24), and low-resistance (<50 mΩ) seals were formed between the pipette and the recorded GnRH neuron. Recordings were made in voltage-clamp mode with the pipette holding potential at 0 mV and signals were filtered at 10 kHz. Experiments were performed using an EPC 8 amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany) with the Pulse Control XOP (Instrutech, Port Washington, NY) running in Igor Pro software (WaveMetrics, Lake Oswego, OR) on a G4 Macintosh computer. Signals were digitized at 16-bit resolution through an ITC-18 acquisition interface (Instrutech). The location of each GnRH neuron studied was mapped on figures of sections obtained from a mouse brain atlas (25). Cells targeted for recording were largely within the midventral preoptic area, and no effect of cell location on response was observed.
In vitro treatments of VIP (100 nm) or a VIP receptor antagonist (VIP 6–28; Bachem, King of Prussia, PA; 100 nm) were applied via the bath recording solution. Up to three cells per animal and only one cell per slice were recorded. Cells were recorded for 20–25 min: a 5- to 10-min baseline period in control NS, followed by a 5-min drug application and a 10-min washout. The last 3 min of each recording condition were used in data analyses to allow time for exchange of bath solutions. If no firing was observed during recording, 15 mm KCl was added to the bath solution to depolarize cells and induce firing for confirmation of recording integrity and cell health. If no firing occurred with KCl treatment, the data were discarded.
Data analysis
For simplicity we used the terms, firing rate or firing activity, to refer to currents recorded extracellularly. Action currents (events), the membrane currents associated with action potential firing, were detected using Event Tracker software in Igor Pro (WaveMetrics). For each event, the time of the event ± 5 msec centered on the event was recorded and stored to a digital file. Events were detected off-line using custom programs in Igor Pro (23) and binned at 30-sec intervals. Binned data were transferred to Excel (Microsoft) and InStat (GraphPad, San Diego, VA) for further analysis. Mean firing rate (Hertz) was calculated by dividing the total number of events over the duration of recording. Due to the high degree of variability in baseline GnRH neuron firing rate, the response of each cell compared with its own baseline was used in analyses. A cell was considered to respond if it showed at least a 40% change in firing with drug treatment, compared with baseline. The frequency of events before and during VIP or antagonist application was compared using paired t tests. Basal firing rate of responder cells vs. nonresponder cells was compared using Mann-Whitney tests. Statistical significance was set at P < 0.05.
Results
Response of GnRH neurons from OVX+E mice to VIP is dependent on time of day
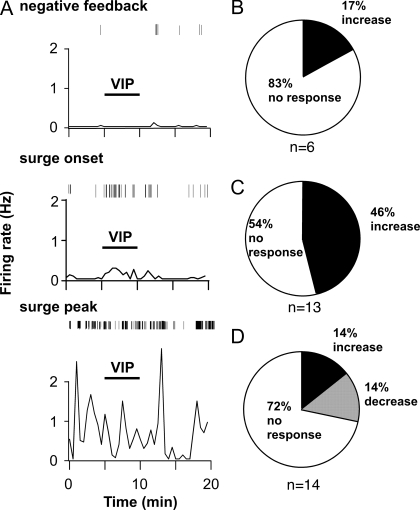
If VIP is a component of a circadian signal, the effects of VIP on GnRH neurons may be regulated by time of day. To test this, the firing of GnRH neurons in coronal slices from OVX+E mice (which show daily LH surges) was recorded before, during, and after application of VIP (100 nm). Recordings were performed during negative feedback (1100–1400 h), surge onset (1400–1600 h), and surge peak (1600–1900 h). During negative feedback, five of six cells (83%) did not respond (Fig. 1, A and B). In contrast, VIP increased firing in nearly half of OVX+E cells recorded at surge onset (46%, n = 6 of 13) (Figs. 1, A and C, and 2); the remaining cells did not respond. In responding cells, the percentage increase in firing was 673 ± 318% (P < 0.05 vs. pre-VIP levels) (Fig. 2). Baseline mean firing rate during surge onset was not significantly different from mean firing rate during negative feedback, largely because seven of the 13 cells showed very low levels of firing (0–0.01 Hz), likely due to variability in the time of transition from negative feedback to surge onset. Basal firing rate, however, did not affect the rate of response to VIP during surge onset because it was not significantly different between responders and nonresponders (responders, 0.18 ± 0.1 Hz vs. nonresponders, 0.07 ± 0.04 Hz, P > 0.25). Of note, this rate of response corresponds well with the percentage of GnRH neurons in the rat that express receptors for VIP (13). During the surge peak, the rate of response to VIP was reduced because only four of 14 cells (27%) showed a change in firing; furthermore, the direction of response was not consistent with two cells increasing and two cells decreasing firing rate (Figs. 1, A and D, and 2). VIP can thus excite GnRH neurons from OVX+E mice, but this effect appears limited to specific times of day.
Figure 1.
VIP excitation of GnRH neurons is time of day dependent. A, Representative examples of firing patterns are shown for GnRH neurons from OVX+E mice recorded during negative feedback (top panel), surge onset (middle panel), and surge peak (bottom panel). Firing rate is displayed at 30-sec intervals (bottom graph in each panel). Vertical lines at the top of each panel illustrate timing of individual action currents detected. B–D, Rate of response for OVX+E cells recorded during negative feedback (n = 6 cells) (B), surge onset (n = 13) (C), or surge peak (n = 14) (D).
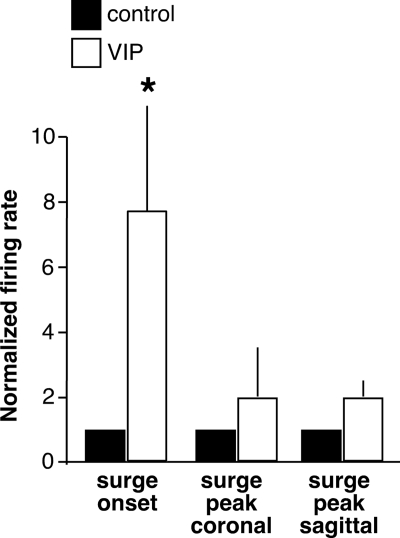
Figure 2.
Summary of VIP effects on GnRH neurons from OVX+E mice during surge onset and surge peak. Negative feedback was not included because only one cell responded. Mean ± sem firing rate changes in GnRH neurons during surge onset (n = 6) and surge peak (coronal n = 4, sagittal n = 7) that responded to VIP. *, P < 0.05 vs. control.
The orientation of brain slice preparation may affect the response to drug application because different populations of synaptic afferents are maintained in different orientations. For example, γ-aminobutyric acid (GABA) transmission to GnRH neurons during surge peak is affected by slice orientation (26), and other forms of neurotransmission may be altered as well. To examine whether the response to VIP during surge peak is dependent on maintenance of specific afferent connections, the effect of VIP on firing of GnRH neurons in sagittal slices from OVX+E mice was tested. Sagittal slices are of interest because they can contain both the SCN and GnRH neurons and thus may preserve endogenous SCN-GnRH pathways. They also can contain the AVPV, a known target of VIP that subsequently projects to GnRH neurons and is thus poised to convey transsynaptic signals. Similar to the data in coronal slices during surge peak, eight of 15 cells (53%) did not respond to VIP. Of the remaining cells, five (33%) increased firing and two (13%) decreased firing. Although this indicates no net response across the population of cells tested (Fig. 2), the change in firing in those cells that showed an increase was 159 ± 44%, indicating a substantial subpopulation of GnRH neurons in sagittal slices can respond to VIP with an increase in firing rate during surge peak.
Estradiol is required for a response to VIP
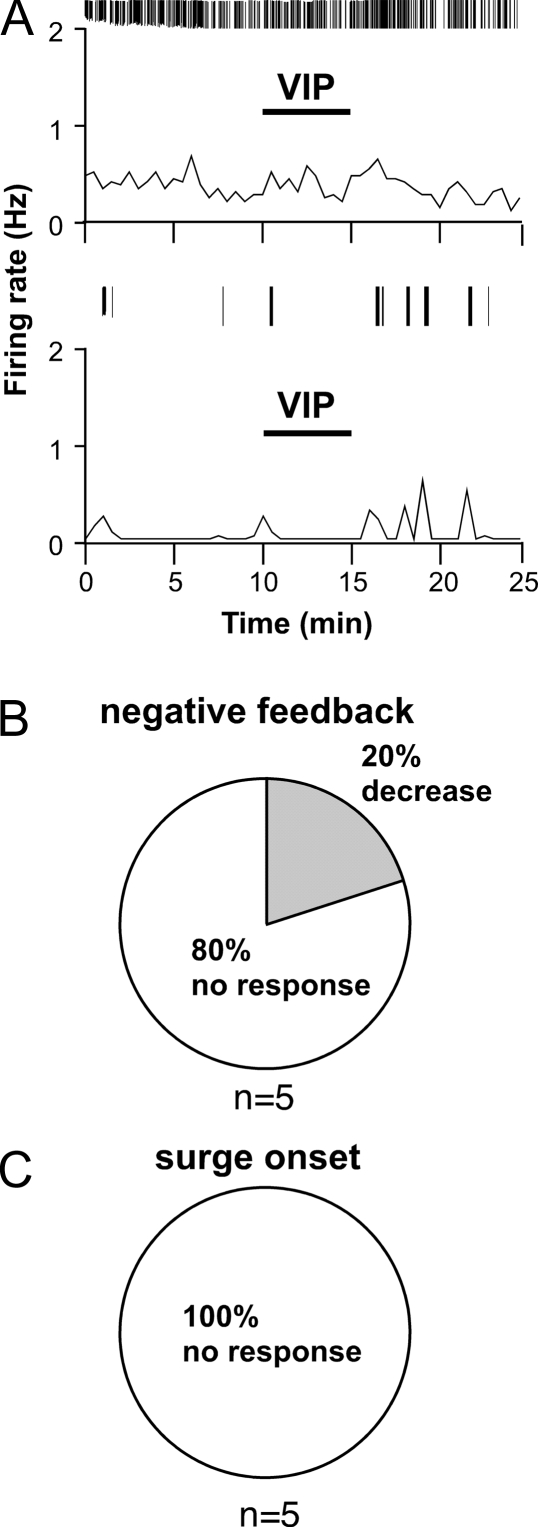
Estradiol is required for surge generation (4,5,6). We thus hypothesized that estradiol is required for a response to VIP. To test this, the effects of VIP on cells from OVX mice that were not treated with estradiol were examined. The vast majority of OVX cells (n = 9 of 10) did not respond to VIP application either during times corresponding to negative feedback or surge onset in OVX+E-treated animals (negative feedback, control 0.04 ± 0.02 Hz vs. VIP 0.01 ± 0.01 Hz, n = 5 cells, P > 0.25; surge onset, control 0.06 ± 0.05 Hz vs. VIP 0.06 ± 0.06 Hz, n = 5 cells, P > 0.72) (Fig. 3). Thus, estradiol is required for a GnRH neuron response to VIP, just as it is for the expression of diurnal changes in GnRH neuron firing activity (4).
Figure 3.
VIP does not excite GnRH neurons in OVX mice. A, Representative examples of firing patterns are shown for GnRH neurons from OVX mice. Examples from both an active cell (top panel) and an inactive cell (bottom panel) are shown to illustrate response does not depend on recent prior firing activity of the recorded cell. Firing rate is displayed at 30-sec intervals (bottom graph in each panel). Vertical lines at the top of each graph illustrate timing of individual action currents detected. B and C, Rate of response for OVX cells recorded during negative feedback (B) or surge onset (C) (n = 5 cells/group).
Endogenous VIP can excite GnRH neurons during the GnRH/LH surge
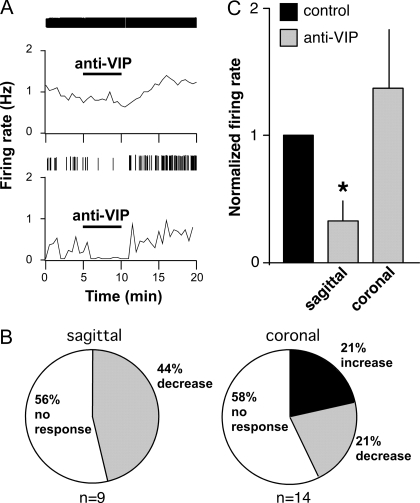
The reduced rate of response to VIP during surge peak (when OVX+E cells show an elevated level of firing activity) could be at least partially due the presence of VIP in the slice from an endogenous VIP input, in which case exogenously applied VIP would likely have no effect. To examine whether GnRH neurons receive an excitatory endogenous VIP input during the surge peak, the effect of a VIP receptor antagonist (100 nm) on GnRH neuron firing rate was tested in OVX+E cells in sagittal slices. VIP antagonist application reversibly decreased firing in four of nine cells (44%) (Fig. 4). This percentage is similar to that which was excited by VIP application around the time of surge onset, and the percentage of cells expressing VIP receptors (13). In the cells that responded, the average decrease was 67 ± 15% (P < 0.05). Basal firing rate did not affect the rate of response to VIP receptor antagonism as firing rate was not significantly different between responders and nonresponders (responders, 0.99 ± 0.31 Hz vs. nonresponders, 0.88 ± 0.22 Hz, P > 0.73). In coronal slices, which do not contain SCN cell bodies, the response to VIP antagonist was weak. Although a similar percentage of cells did not respond to antagonist as in sagittal slices (58%, eight of 14), there was not a consistent direction of change in cells that did change firing at the time of drug application. Specifically, three increased firing and three decreased firing, averaging out to no net response across the population of cells tested (P > 0.5; Fig. 4). Thus, the response to VIP antagonism appears dependent on the maintenance of connections from the SCN within the brain slice preparation. These data suggest endogenous VIP may play a role in increasing GnRH neuron firing during the surge in a substantial subpopulation of GnRH neurons.
Figure 4.
VIP receptor antagonist decreases firing in about half of cells from OVX+E mice in sagittal slices at surge peak but does not affect firing in coronal slices. A, Representative examples of firing patterns are shown for GnRH neurons that did not respond to antagonist (top panel) and cells that decreased firing with antagonist treatment (bottom panel). Firing rate is displayed at 30-sec intervals (bottom graph in each panel). Vertical lines at the top of each graph illustrate timing of individual action currents detected. B, Rate of response for all cells recorded in sagittal slices (left panel, n = 9) and coronal slices (right panel, n = 14). C, Mean ± sem firing rate changes in GnRH neurons that responded to VIP antagonist (sagittal n = 4, coronal n = 6). *, P < 0.05 vs. control.
Discussion
In some species, input from the circadian timing system appears to be critical in the regulation of reproduction, particularly in the neural control of the ovulatory process. The precise nature of this input is poorly understood. Previous studies examining the effect of VIP or VIP antagonism in rats have been inconclusive, with some indicating an inhibitory effect and others indicating an excitatory effect (17,18,19). Because measurement of GnRH levels in mice are problematic because of low peptide levels, we used electrophysiological techniques to assess the effects of VIP on the activity of GnRH neurons in brain slices. Here we present evidence supporting a role for VIP in driving increased firing of a substantial subpopulation of GnRH neurons during the GnRH surge. The effects of VIP on GnRH neurons are dependent on both estradiol feedback state and time of day, two elements that are essential in surge regulation, at least in rodent species (4,5,6). Thus, VIP is a candidate as a component of the daily neuronal signal that is required for surge generation.
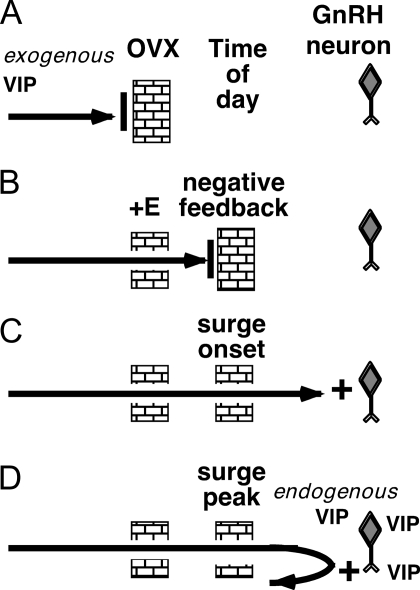
Based on these results, we postulate that both estradiol- and time-of-day-dependent gates must be open to allow for VIP excitation of GnRH neurons (Fig. 5). Without estradiol, VIP is blocked from exerting an effect on the activity of GnRH neurons (Fig. 5A). Estradiol opens this gate, but a second, time-of-day-dependent gate is closed during negative feedback (Fig. 5B), preventing VIP action on GnRH neurons. The time-of-day-dependent gate opens near the time of surge onset, allowing for effects of exogenously applied VIP (Fig. 5C). This gate remains open during the surge peak, but at this time, GnRH neurons are already receiving an endogenous VIP input, so the response to exogenously applied VIP is at least partially occluded (Fig. 5D).
Figure 5.
Working model of VIP effects on GnRH neuron firing activity, in which estradiol and time of day both gate the GnRH neuron response to VIP. A, An estradiol-dependent gate blocks exogenous VIP from exerting an excitatory effect on OVX cells, regardless of time of day. B, OVX+E treatment opens the estradiol-dependent gate, but a time-dependent gate is closed during negative feedback. C, Near surge onset; both the estradiol gate and the time-dependent gate are open, and VIP is able to exert an excitatory effect on GnRH neurons. D, Both gates remain open during surge peak, but GnRH neurons are already receiving excitatory input from endogenous VIP, so the response to exogenously applied VIP is occluded.
The SCN are a strong candidate source of a daily signal required for surge generation. Lesions of the SCN eliminate both the LH surge and ovulation (9). SCN grafts capable of restoring locomotor rhythmicity do not restore LH surges in OVX+E hamsters (27), indicating that a neural, rather than humoral, SCN output may be required for LH surge generation. Furthermore, anatomical studies have indicated the presence of ipsilateral, direct SCN-GnRH neuron connections (10,11,28,29). VIP is synthesized in the ventrolateral portion of the SCN, which receives retinal information about external light conditions both directly (30,31) and indirectly (31), and VIP fibers are found in the vicinity of GnRH neurons (11,12,32,33,34). VIP is therefore a prime candidate for transmitting information about the external light-dark cycle to GnRH and other neurons.
Generation of the daily signal (or signals) may be independent of steroid hormones (35), but estradiol is required to couple circadian input to the GnRH system (4,5,6); integration of estradiol feedback and circadian signals is thus crucial for proper surge regulation. The α-isoform of the estradiol receptor (ER) is required for surge generation (36,37). Although ERα expression has been found in the human SCN, with higher expression in females (38), it has not been detected in the rodent SCN (39). The SCN receives projections from cells expressing ERα (40), however, providing a pathway for indirect actions of estradiol on the SCN. The AVPV appears to be a site of convergence of circadian and estradiol signals because these cells express ERα, receive inputs from the SCN, and project directly to GnRH neurons (15,16,41,42). The increased percentage of GnRH neurons excited by VIP during surge peak in sagittal slices, compared with coronal slices, in the present study may indicate VIP acts through afferent pathways that are maintained in a sagittal, but not a coronal, slice preparation, such as connections from the AVPV and SCN. VIP receptor expression in the preoptic area appears to be largely confined to GnRH neurons and the AVPV (13,14), suggesting that VIP action within the preoptic area is primarily involved in the control of reproduction. The requirement for both estradiol treatment and a particular time of day for a GnRH neuron response to VIP is consistent with a possible role for this neuropeptide in transmitting successful pre- or post-SCN convergence of estradiol feedback and circadian signals to GnRH neurons.
Alterations in GnRH neuron responsiveness to VIP provide a potential point of integration of circadian and estradiol signals directly at the level of the GnRH neuron. The percentage of GnRH neurons in the rat immunoreactive for VIP2 receptor protein does not appear to change during the day of proestrus (13). Other changes, such as alterations in receptor expression or binding and activation properties, may alter the responsiveness of GnRH neurons to VIP. Future work is needed to examine whether these mechanisms play a role and display an estradiol and/or circadian dependence. Estradiol increases GnRH neuron electrical excitability (43,44), which may increase the response of GnRH neurons to depolarizing input such as VIP, providing a possible level of integration of these signals directly at the GnRH neuron.
The data presented here add to the growing body of evidence for widespread effects of VIP on neural function through changes in both neurotransmission and intrinsic properties of target cells. VIP can activate a hyperpolarization-activated cation current in thalamocortical neurons (45), reduce the amplitude of the Ca2+-dependent K+ current mediating the slow afterhyperpolarization current in hippocampal pyramidal neurons (46), and inhibit Ca2+ currents in sympathetic neurons of the rat pelvic ganglia (47). In cultures of hippocampal neurons, VIP increases GABA transmission (48). VIP also stimulates brain-derived neurotrophic factor mRNA transcription and c-fos expression in primary cultures of mouse cortical neurons (49,50). With regard to GnRH neurons, VIP may act directly on these cells through the VIP2 receptor (13) and/or indirectly through other neurons (14) or glial elements (51). VIP may also act within the SCN to modulate GnRH-targeting outputs, such as through changes in intra-SCN GABA transmission (52). In the present studies, synaptic transmission within the brain slice network was kept intact to minimize disruption to the input pathways through which VIP may act on GnRH neurons; thus, both intrinsic and synaptic effects may contribute to the observed action of VIP.
The function of GnRH neurons can be regulated both by neuromodulators and fast synaptic transmission, which in GnRH neurons is mediated by GABA and glutamate acting through ionotropic receptors (22,53). GABAA receptor activation can excite adult GnRH neurons (54,55,56), although inhibitory actions have also been suggested (57,58). Recent work has indicated a role for GABAergic mediation of estradiol feedback in surge regulation. Specifically, estradiol decreases GABA transmission to GnRH neurons during negative feedback but increases it during positive feedback (26). Further, the SCN appears to be a potential source of increased GABA transmission because disrupting inputs from the SCN to GnRH neurons in a slice preparation lowers the frequency of GABA transmission during positive feedback (26). VIP colocalizes with GABA in the SCN, and it is possible that increased GABA transmission during the surge may reflect increased co-release of GABA and VIP. Preliminary data suggest similar diurnal changes in glutamate transmission to GnRH neurons in OVX+E mice, with low glutamate transmission during negative feedback and increased transmission during positive feedback (59).
The importance of fast synaptic transmission in surge generation is demonstrated by the disruptive effects of blocking ionotropic GABA and glutamate receptors on estradiol negative and positive feedback effects on GnRH neurons. This treatment reverses some, but not all, diurnal changes in GnRH neuron firing activity (60). If the GnRH surge were dependent solely on fast synaptic transmission, the reversal should be complete. This suggests that in addition to fast synaptic transmission, neuromodulators [such as VIP, vasopressin (61,62,63), or kisspeptin (64)] likely also play a significant role in regulating GnRH neurons in different estradiol feedback states. Given the importance of the surge for ovulation and reproductive success, multiple signals may regulate diurnal changes in GnRH neuron firing activity. Only a portion of the GnRH surge itself is required to generate an LH surge, providing a further safety net. This redundancy likely accounts for fertility in VIP and VIP receptor knockout animals (65,66) as well as the maintenance of increased GnRH neuron firing activity during surge peak in coronal slices (4), which do not contain SCN cell bodies and, as shown in the present data, lack an endogenous VIPergic input.
In summary, VIP can excite GnRH neurons, and this effect is dependent on both estradiol feedback and time of day. Alterations in VIP release, along with other neuropeptides and fast synaptic transmission, are thus poised to play a critical role in regulating the diurnal and estradiol-dependent changes in GnRH neuron firing that appear to underlie surge generation.
Acknowledgments
We thank Debra Fisher and Xu-Zhi Xu for expert technical assistance and Justyna Pielecka-Fortuna, Alison Roland, Pei-San Tsai, and Heidi Walsh for helpful editorial comments.
Footnotes
This work was supported by National Institute of Child Health and Human Development/National Institutes of Health Grant R01 HD41469 and National Institute of Neurological Disorders and Stroke National Research Service Award F31 NS53253 (to C.A.C.).
Author Statement: C.A.C. and S.M.M. have nothing to disclose.
First Published Online March 6, 2008
Abbreviations: AVPV, Anteroventral periventricular area; ER, estradiol receptor; GABA, γ-aminobutyric acid; NS, normal saline; OVX+E, OVX and estradiol-treated; SCN, suprachiasmatic nuclei; VIP, vasoactive intestinal polypeptide.
References
- Sarkar DK, Chiappa SA, Fink G, Sherwood NM 1976 Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature 264:461–463 [DOI] [PubMed] [Google Scholar]
- Pau KY, Berria M, Hess DL, Spies HG 1993 Preovulatory gonadotropin-releasing hormone surge in ovarian-intact rhesus macaques. Endocrinology 133:1650–1656 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Locatelli A, Karsch FJ 1991 Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology 129:1175–1182 [DOI] [PubMed] [Google Scholar]
- Christian CA, Mobley JL, Moenter SM 2005 Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA 102:15682–15687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RL, Blake CA, Sawyer CH 1973 Estrogen-dependent 24-hour periodicity in pituitary LH release in the female hamster. Endocrinology 93:965–970 [DOI] [PubMed] [Google Scholar]
- Legan SJ, Karsch FJ 1975 A daily signal for the LH surge in the rat. Endocrinology 96:57–62 [DOI] [PubMed] [Google Scholar]
- Bronson FH, Vom Saal FS 1979 Control of the preovulatory release of luteinizing hormone by steroids in the mouse. Endocrinology 104:1247–1255 [DOI] [PubMed] [Google Scholar]
- Kerdelhue B, Brown S, Lenoir V, Queenan JT, Jones GS, Scholler R, Jones HW 2002 Timing of initiation of the preovulatory luteinizing hormone surge and its relationship with the circadian cortisol rhythm in the human. Neuroendocrinology 75:158–163 [DOI] [PubMed] [Google Scholar]
- Brown-Grant K, Raisman G 1977 Abnormalities in reproductive function associated with the destruction of the suprachiasmatic nuclei in female rats. Proc R Soc Lond B Biol Sci 198:279–296 [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Meyer J, Schwartz WJ 2003 Lateralization of circadian pacemaker output: activation of left- and right-sided luteinizing hormone-releasing hormone neurons involves a neural rather than a humoral pathway. J Neurosci 23:7412–7414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Beek EM, Horvath TL, Wiegant VM, Van den Hurk R, Buijs RM 1997 Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol 384:569–579 [DOI] [PubMed] [Google Scholar]
- Horvath TL, Cela V, van der Beek EM 1998 Gender-specific apposition between vasoactive intestinal peptide-containing axons and gonadotrophin-releasing hormone-producing neurons in the rat. Brain Res 795:277–281 [DOI] [PubMed] [Google Scholar]
- Smith MJ, Jennes L, Wise PM 2000 Localization of the VIP2 receptor protein on GnRH neurons in the female rat. Endocrinology 141:4317–4320 [DOI] [PubMed] [Google Scholar]
- Kallo I, Kalamatianos T, Wiltshire N, Shen S, Sheward WJ, Harmar AJ, Coen CW 2004 Transgenic approach reveals expression of the VPAC2 receptor in phenotypically defined neurons in the mouse suprachiasmatic nucleus and in its efferent target sites. Eur J Neurosci 19:2201–2211 [DOI] [PubMed] [Google Scholar]
- Watson RE, Langub MC, Engle MG, Maley BE 1995 Estrogen-receptive neurons in the anteroventral periventricular nucleus are synaptic targets of the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. Brain Res 689:254–264 [DOI] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen SL 2004 Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci 24:8097–8105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weick RF, Stobie KM 1992 Vasoactive intestinal peptide inhibits the steroid-induced LH surge in the ovariectomized rat. J Endocrinol 133:433–437 [DOI] [PubMed] [Google Scholar]
- Harney JP, Scarbrough K, Rosewell KL, Wise PM 1996 In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology 137:3696–3701 [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Swarts HJM, Wiegant VM 1999 Central administration of antiserum to vasoactive intestinal peptide delays and reduces luteinizing hormone and prolactin surges in ovariectomized, estrogen-treated rats. Neuroendocrinology 69:227–237 [DOI] [PubMed] [Google Scholar]
- van der Beek EM, van Oudheusden HJ, Buijs RM, van der Donk HA, van den Hurk R, Wiegant VM 1994 Preferential induction of c-fos immunoreactivity in vasoactive intestinal polypeptide-innervated gonadotropin-release hormone neurons during a steroid-induced luteinizing hormone surge in the female rat. Endocrinology 134:2636–2644 [DOI] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM 2000 Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141:412–419 [DOI] [PubMed] [Google Scholar]
- Chu Z, Moenter SM 2005 Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: a possible local feedback circuit. J Neurosci 25:5740–5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Moenter SM 2002 Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology 143:2284–2292 [DOI] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Moenter SM 2003 A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biol Proced Online 5:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ 2001 The mouse brain in stereotaxic coordinates. 2nd ed. San Diego: Academic Press [Google Scholar]
- Christian CA, Moenter SM 2007 Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci 27:1913–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL 1999 Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology 140:207–218 [DOI] [PubMed] [Google Scholar]
- Boehm U, Zou Z, Buck LB 2005 Feedback loops link odor and pheromone signaling with reproduction. Cell 123:683–695 [DOI] [PubMed] [Google Scholar]
- Yoon H, Enquist LW, Dulac C 2005 Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell 123:669–682 [DOI] [PubMed] [Google Scholar]
- Stephan FK, Berkley KJ, Moss RL 1981 Efferent connections of the rat suprachiasmatic nucleus. Neuroscience 6:2625–2641 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Ichitani Y, Okamura H, Tanaka Y, Ibata Y 1993 The direct retinal projection to VIP neuronal elements in the rat SCN. Brain Res Bull 31:637–640 [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Silver R, Gore AC, Crews D 2002 Vasoactive intestinal polypeptide contacts on gonadotropin-releasing hormone neurons increase following puberty in female rats. J Neuroendocrinol 14:685–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney MM, Smale L 2005 Arginine vasopressin and vasoactive intestinal polypeptide fibers make appositions with gonadotropin-releasing hormone and estrogen receptor cells in the diurnal rodent Arvicanthis niloticus. Brain Res 1049:156–164 [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Wiegant VM, van der Donk HA, Van den Hurk R, Buijs RM 1993 Lesions of the suprachiasmatic nucleus indicate the presence of a direct vasoactive intestinal polypeptide-containing projection to gonadotrophin-releasing hormone neurons in the female rat. J Neuroendocrinol 5:137–144 [DOI] [PubMed] [Google Scholar]
- Chappell PE, Lee J, Levine JE 2000 Stimulation of gonadotropin-releasing hormone surges by estrogen. II. Role of cyclic adenosine 3′5′-monophosphate. Endocrinology 141:1486–1492 [DOI] [PubMed] [Google Scholar]
- Herbison AE 1998 Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 19:302–330 [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone H-J, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE 2006 Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijver FP, Swaab DF 2002 Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology 75:296–305 [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW 1990 Distribution of androgen and oestrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol 294:76–95 [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Blaustein JD, Bittman EL 1999 Oestrogen receptor-α-immunoreactive neurones project to the suprachiasmatic nucleus of the female Syrian hamster. J Neuroendocrinol 11:481–490 [DOI] [PubMed] [Google Scholar]
- Simerly RB, Carr AM, Zee MC, Lorang D 1996 Ovarian steroid regulation of estrogen and progesterone receptor messenger ribonucleic acid in the anteroventral periventricular nucleus of the rat. J Neuroendocrinol 8:45–56 [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Blaustein JD, Bittman EL 1995 The suprachiasmatic area in the female hamster projects to neurons containing estrogen receptors and GnRH. Neuroreport 6:1715–1722 [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Moenter SM 2002 Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol Endocrinol 16:2255–2265 [DOI] [PubMed] [Google Scholar]
- Chu Z, Moenter SM 2006 Physiologic regulation of a tetrodotoxin-sensitive sodium influx that mediates a slow afterdepolarization potential in gonadotropin-releasing hormone neurons: possible implications for the central regulation of fertility. J Neurosci 26:11961–11973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QQ, Prince DA, Huguenard JR 2003 Vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide activate hyperpolarization-activated cationic current and depolarize thalamocortical neurons in vitro. J Neurosci 23:2751–2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug T, Storm JF 2000 Protein kinase A mediates the modulation of the slow Ca2+-dependent K+ current, IsAHP, by the neuropeptides CRF, VIP, and CGRP in hippocampal pyramidal neurons. J Neurophysiol 83:2071–2079 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yakel JL 1997 Modulation of Ca2+ currents by various G protein-coupled receptors in sympathetic neurons of male rat pelvic ganglia. J Neurophysiol 78:780–789 [DOI] [PubMed] [Google Scholar]
- Wang HL, Li A, Wu T 1997 Vasoactive intestinal polypeptide enhances the GABAergic synaptic transmission in cultured hippocampal neurons. Brain Res 746:294–300 [DOI] [PubMed] [Google Scholar]
- Pellegri G, Magistretti PJ, Martin JL 1998 VIP and PACAP potentiate the action of glutamate on BDNF expression in mouse cortical neurones. Eur J Neurosci 10:272–280 [DOI] [PubMed] [Google Scholar]
- Martin JL, Gasser D, Magistretti PJ 1995 Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide potentiate c-fos expression induced by glutamate in cultured cortical neurons. J Neurochem 65:1–9 [DOI] [PubMed] [Google Scholar]
- Gerhold LM, Wise PM 2006 Vasoactive intestinal polypeptide regulates dynamic changes in astrocyte morphometry: impact on gonadotropin-releasing hormone neurons. Endocrinology 147:2197–2202 [DOI] [PubMed] [Google Scholar]
- Itri J, Colwell CS 2003 Regulation of inhibitory synaptic transmission by vasoactive intestinal peptide (VIP) in the mouse suprachiasmatic nucleus. J Neurophysiol 90:1589–1597 [DOI] [PubMed] [Google Scholar]
- Sullivan SD, DeFazio RA, Moenter SM 2003 Metabolic regulation of fertility through presynaptic and postsynaptic signaling to gonadotropin-releasing hormone neurons. J Neurosci 23:8578–8585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Heger S, Ojeda SR, Moenter SM 2002 Activation of A-type γ-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol 16:2872–2891 [DOI] [PubMed] [Google Scholar]
- Moenter SM, DeFazio RA 2005 Endogenous γ-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology 146:5374–5379 [DOI] [PubMed] [Google Scholar]
- Yin C, Tanaka N, Kato M, Sakuma Y 2006 GABA depolarizes GnRH neurons isolated from adult GnRH-EGFP transgenic rats. Neurosci Res 55S:S159 [Google Scholar]
- Han SK, Abraham IM, Herbison AE 2002 Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology 143:1459–1466 [DOI] [PubMed] [Google Scholar]
- Han SK, Todman MG, Herbison AE 2004 Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology 145:495–499 [DOI] [PubMed] [Google Scholar]
- Christian CA, Moenter SM Diurnal changes in NMDA and AMPA/KA receptor-mediated glutamate transmission to gonadotropin-releasing hormone (GnRH) neurons are associated with the GnRH/LH surge. Proc 37th Annual Meeting of the Society for Neuroscience, San Diego, CA, 2007 (Abstract 518.4) [Google Scholar]
- Christian CA, Moenter SM Differential synaptic mediation of estradiol negative and positive feedback effects on GnRH neuron firing activity. Proc 40th Annual Meeting of the Society for the Study of Reproduction, San Antonio, TX, 2007, p 184 (Abstract 431) [Google Scholar]
- Palm IF, van der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A 1999 Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol-treated rats with lesions of the suprachiasmatic nucleus. Neuroscience 93:659–666 [DOI] [PubMed] [Google Scholar]
- Palm IF, van der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A 2001 The stimulatory effect of vasopressin on the luteinizing hormone surge in ovariectomized, estradiol-treated rats is time-dependent. Brain Res 901:109–116 [DOI] [PubMed] [Google Scholar]
- Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS 2006 Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and Clock mutant mice. Biol Reprod 75:778–784 [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA 2006 Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi J-C, Kelly JS, Maywood ES, Hastings MH 2002 The VPAC2 receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell 109:497–508 [DOI] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED 2005 Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci 8:476–483 [DOI] [PMC free article] [PubMed] [Google Scholar]