Abstract
The ovarian hormones progesterone and estrogen have well-established neurotrophic and neuroprotective effects supporting both reproductive function and cognitive health. More recently, it has been recognized that these steroids also regulate metabolic functions sustaining the energetic demands of this neuronal activation. Underlying this metabolic control is an interpretation of signals from diverse environmental sources integrated by receptor-mediated responses converging upon mitochondrial function. In this study, to determine the effects of progesterone (P4) and 17β-estradiol (E2) on metabolic control via mitochondrial function, ovariectomized rats were treated with P4, E2, or E2 plus P4, and whole-brain mitochondria were isolated for functional assessment. Brain mitochondria from hormone-treated rats displayed enhanced functional efficiency and increased metabolic rates. The hormone-treated mitochondria exhibited increased respiratory function coupled to increased expression and activity of the electron transport chain complex IV (cytochrome c oxidase). This increased respiratory activity was coupled with a decreased rate of reactive oxygen leak and reduced lipid peroxidation representing a systematic enhancement of brain mitochondrial efficiency. As such, ovarian hormone replacement induces mitochondrial alterations in the central nervous system supporting efficient and balanced bioenergetics reducing oxidative stress and attenuating endogenous oxidative damage.
THE OVARIAN HORMONES estrogen and progesterone (P4) have well-established neurotrophic effects supporting both reproductive function and cognitive health (1,2,3). More recently, it has been recognized that these steroids can regulate metabolic functions sustaining the energetic demands of neuronal activation. Underlying this metabolic control is an interpretation of signals from diverse environmental sources integrated by receptor-mediated responses converging upon mitochondrial function. Mitochondria are the primary energy sources of the cell that converts nutrients into energy through cellular respiration via the electron transport chain (ETC).
Mitochondria consist of a tightly integrated functional network that regulates the energy balance of the cell. The energy balance is required not only to regulate the survival of the cell, but also, more importantly, to regulate the many specialized physiological functions of the cell. In the central nervous system (CNS), mitochondrial energy balance is critical to establish membrane excitability and to execute the complex processes of neurotransmission and plasticity. Neurons are highly specialized cells requiring large amounts of ATP to maintain the plasma membrane ionic gradients that allow for rapid propagation of neural signaling and neurotransmission. Ionic transport processes, namely Na+/K+-ATPases and Ca2+-ATPases, consume about 60–80% of ATP consumption in the CNS to maintain ionic gradients allowing for neuronal excitability (4). Thus, the CNS has an immense energetic demand critically dependent upon mitochondrial function and oxygen supply to support the generation of ATP by oxidative phosphorylation.
Many of the components of this functional bioenergetic network are vulnerable to oxidative stress that can impair cellular function or increase the chance of cell death (4,5). Compromised mitochondrial function has been linked to numerous diseases, including those of the metabolic, cardiovascular, and nervous systems. Many of these vulnerable components of mitochondrial function are ovarian controlled as demonstrated by reported sex differences or functional alterations in response to hormonal manipulation (6,7,8,9). It has been shown that the extended lifespan of female mice is due in part to a reduced oxidative load that is ovarian controlled (6). Female rat brown adipose tissue mitochondria are larger and have more cristae than males (7). In addition, there are purported effects of estrogens on mitochondria function in various tissues, including decreased mitochondrial respiratory activity as assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction in myocardium of ovariectomized rats (8). In the brain, the cytochrome c oxidase subunit II (COXII) was identified as the single 17β-estradiol (E2)-induced clone resulting from a differential screen of a cDNA library prepared from rat hippocampus (9).
For the most part, estrogen regulation of mitochondrial function has been the primary focus despite the fact that P4 has important properties that can enhance or degrade these modulatory pathways. In the current study, we investigated the role of P4 in regulating mitochondrial function in the CNS, both alone and in conjunction with E2. We propose that in addition to the well-documented protective effects, E2 and P4 enhance energy production through balanced respiration, maintaining better electron flow coupling and reduced oxidative damage. To evaluate this hypothesis, we used the ovariectomized, hormone-replaced rat model. Our data reveal that E2 and P4 potently enhance the functional efficiency of whole-brain mitochondria, as evidenced by increased electron transport and reduced oxidative leak and damage.
Materials and Methods
Chemicals
All chemicals were from MP Biomed (Irvine, CA) unless otherwise noted. E2 and P4 were obtained from Steraloids (Newport, RI). Steroids were dissolved in ethanol and diluted in sesame oil with final ethanol concentration less than 0.001%.
Animals
The use of animals for the study was approved by the Institutional Animal Care and Use Committee at the University of Southern California (Protocol No. 10256). Young adult (3–6 months old) female ovariectomized Sprague Dawley rats purchased from Harlan (Indianapolis, IN) were housed under controlled conditions of temperature (22 C), humidity, and light (14 h light, 10 h dark) with water and food available ad libitum. After at 2 wk of habituation to the facilities and surgery recovery (after ovariectomy), rats were injected sc with E2 (30 μg/kg), P4 (30 μg/kg), or sesame oil vehicle control and fasted 24 h before killing and forebrain dissection. The dose of E2 (30 μg/kg body weight) was chosen as representative of a standard systemic E2 therapy used clinically and in previous studies, which ranges from 10–100 μg/kg. To confirm administration of steroid, plasma and brain E2 levels were analyzed by commercial ELISA (IBL-Hamburg, Hamburg, Germany) after hexane-ethyl acetate extraction. The 30-μg/kg dose produced E2 levels in ovariectomized rats of 42 pg/g in brain tissue and 44 pg/ml in serum. Our previous in vitro studies indicated that P4 was most neurally effective at the same concentration as E2 (2,10). At the time of killing, uteri were removed and weighed to determine efficacy of estradiol treatment (control = 0.117 ± 0.005 g; E2 = 0.230 ± 0.012 g; P4 = 0.133 ± 0.024 g; E2+P4 = 0.216 ± 0.026 g wet weight). All experiments were approved by the Institutional Animal Care and Use Committee.
Mitochondrial isolation
Brain mitochondria were isolated from rats as previously described (11). Rats were decapitated, and the whole brain minus the cerebellum was rapidly removed, minced, and homogenized at 4 C in mitochondrial isolation buffer (MIB) (pH 7.4), containing sucrose (320 mm), EDTA (1 mm), Tris-HCl (10 mm), and Calbiochem’s Protease Inhibitor Cocktail Set I (AEBSF-HCl 500 μm, aprotonin 150 nm, E-64 1 μm, EDTA disodium 500 μm, leupeptin hemisulfate 1 μm). Single-brain homogenates were then centrifuged at 1500 × g for 5 min. The pellet was resuspended in MIB, rehomogenized, and centrifuged again at 1500 × g for 5 min. The postnuclear supernatants from both centrifugations were combined, and crude mitochondria were pelleted by centrifugation at 21,000 × g for 10 min. The resulting mitochondrial pellet was resuspended in 15% Percoll made in MIB, layered over a preformed 23%/40% Percoll discontinuous gradient, and centrifuged at 31,000 × g for 10 min. The purified mitochondria were collected at the 23%/40% interface and washed with 10 ml MIB by centrifugation at 16,700 × g for 13 min. The loose pellet was collected and transferred to a microcentrifuge tube and washed in MIB by centrifugation at 9000 × g for 8 min. The resulting mitochondrial pellet was resuspended in MIB to an approximate concentration of 1 mg/ml. The resulting mitochondrial samples were used immediately for respiratory measurements or stored at −70 C for later protein and enzymatic assays. During mitochondrial purification, aliquots were collected, and for confirmation of mitochondrial purity and integrity, Western blot analysis was performed for mitochondrial antiporin (1:500; Mitosciences, Eugene, OR), nuclear antihistone H1 (1:250; Santa Cruz Biotechnology, Santa Cruz, CA), endoplasmic reticulum anticalnexin (1:2000, SPA 865; Stressgen, now a subsidiary of Assay Designs, Ann Arbor, MI), and cytoplasmic anti-myelin basic protein (1:500, clone 2; RDI, Concord, MA) (data not shown).
Respiratory measurements
Mitochondrial oxygen consumption was measured polarographically using a Clarke-type electrode. One hundred micrograms of isolated mitochondria were placed in the respiration chamber at 37 C in respiratory buffer (130 mm KCl, 2 mm KH2PO4, 3 mm HEPES, 2 mm MgCl2, 1 mm EGTA) to yield a final concentration of 200 μg/ml. After 1 min baseline recording, mitochondria were energized by the addition of glutamate (5 mm) and malate (5 mm) as substrates. State 3 respiration was stimulated by the addition of ADP (410 μm). State 4o respiration was induced by the addition of three pulses of the adenine nucleotide translocator (ANT) inhibitor atractyloside (50 μm) to deplete ADP. The rate of oxygen consumption was calculated based on the slope of the response of isolated mitochondria to the successive administration of substrates. The respiratory control ratio (RCR) was determined by dividing the rate of oxygen consumption/min for state 3 (presence of ADP) by the rate of oxygen consumption/min for state 4o respiration (absence of ADP by addition of atractyloside).
Complex IV/cytochrome c oxidase activity
Cytochrome oxidase activity was measured spectrophotometrically by monitoring change in absorbance (550 nm) of reduced cytochrome c by permeabilized mitochondria. Mitochondria were permeabilized in 0.2 ml 75 mm potassium phosphate buffer (pH 7.5) at 25 C. The reaction was started by the addition of 0.05 ml 5% cytochrome c previously reduced with sodium hydrosulfite. Cytochrome c oxidase activity was calculated in nanomoles of oxidized cytochrome c per minute per milligram protein and reported as rate relative to the mean rate from vehicle control-treated animals.
Western blot analysis
Equal amounts of mitochondrial protein (20 μg/well) were loaded in each well of a 10% SDS-PAGE gel, electrophoresed with a Tris/glycine running buffer, and transferred to a polyvinylidene difluoride membrane. The blots were probed with anti-COXIV (1:1000; Mitosciences), anti-complex V, subunit α (1:1000; Mitosciences), anti-manganese superoxide dismutase (anti-MnSOD) (1:500; BD Biosciences) or anti-peroxiredoxin V (1:500; BD Biosciences, San Jose, CA) and a horseradish peroxidase-conjugated horse antimouse secondary antibody (Vector, Burlingame, CA). Antigen-antibody complexes were visualized with the TMB Substrate kit (Vector). Band intensities were determined using the VersaDoc System (Bio-Rad, Hercules, CA).
RNA isolation and RT-PCR
Total RNA was isolated from brain tissue with TRIzol reagent. Expression of mRNA for COXI, COXII, COXIII, and COXIV was assessed by SYBR-Green based real-time RT-PCR. cDNA was synthesized from 10 μg total RNA by RT using SuperScript II Reverse Transcription kit (Invitrogen, Carlsbad, CA). cDNA was amplified by PCR on an iCycler (Bio-Rad) using Bio-Rad iScript SYBR Green reaction buffer.
Mitochondrial biogenesis
Total DNA was isolated with TRIzol reagent and analyzed by real-time PCR. Mitochondrial biogenesis was estimated as the relative levels of β-actin to COXII DNA.
Mitochondrial peroxide production
The rate of H2O2 production by isolated mitochondria was determined by the Amplex Red peroxidase assay.
Free radical leak
Free radical leak was determined as previously described (12). H2O2 production and O2 consumption were measured in parallel in the same brain mitochondria under similar experimental conditions. This allowed for the calculation of the fraction of electrons out of sequence, which reduce O2 to reactive oxygen species (ROS) at the respiratory chain (the percent free radical leak) instead of reaching cytochrome oxidase to reduce O2 to water. Because two electrons are needed to reduce 1 mol O2 to H2O2, whereas four electrons are required to reduce 1 mol O2 to water, the percent free radical leak was calculated as the rate of H2O2 production divided by two times the rate of O2 consumption, and the result was multiplied by 100.
Lipid peroxidation
Lipid peroxides in brain mitochondria were measured using the leucomethylene blue assay, using tert-butyl hydroperoxide as a standard, by monitoring the 650-nm absorbance after 1 h incubation at RT. The aldehyde product or termination product of lipid peroxidation in brain mitochondria was determined by measuring thiobarbituric acid-reactive substances (TBARS). Brain mitochondria were mixed with 0.15 m phosphoric acid. After the addition of thiobarbituric acid, the reaction mixture was heated to 100 C for 1 h. After cooling and centrifugation, the formation of TBARS was determined by the absorbance of the chromophore (pink dye) at 531 nm using 600 nm as the reference wavelength.
Statistics
Statistically significant differences were determined by one-way ANOVA followed by Student-Neuman Keuls post hoc analysis.
Results
P4- and E2-enhanced brain mitochondrial respiratory activity
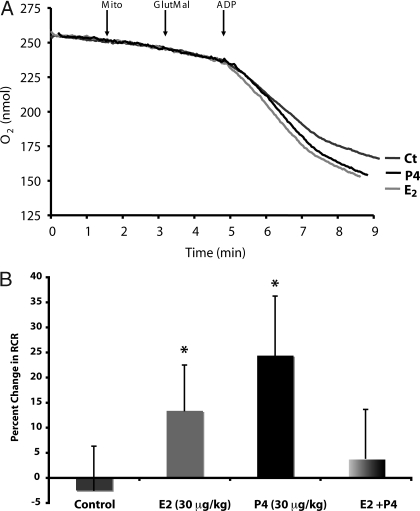
To determine P4 and E2 regulation of brain mitochondrial respiration, young adult female Sprague Dawley rats were ovariectomized to remove endogenous sources of ovarian hormones. The animals were allowed to recover from surgery for 2 wk to allow for clearance of residual ovarian hormones and to equilibrate to the state of ovarian deprivation. Ovariectomized rats were injected sc with 30 μg/kg P4, 30 μg/kg E2, and 30 μg/kg of each steroid or equivalent volume of sesame oil vehicle as a control. Rats were killed 24 h later, and whole-brain mitochondria were isolated. We first measured the respiratory rate of the isolated whole-brain mitochondria using glutamate (5 mm) and malate (5 mm) as respiratory substrates. ADP addition to the mitochondrial suspension initiated state 3 respiration. Addition of the adenine nucleotide transporter inhibitor atractyloside reduced the rate of O2 consumption to that of state 4o respiration, limited by proton permeability of the inner membrane. There was a significant increase in the RCR in the P4- and E2-treatment groups but not in the E2/P4 group (Fig. 1 and Table 1). In vivo treatment with P4 resulted in a 24.5% increase in the RCR of isolated brain mitochondria (Table 1 and Fig. 1). In vivo treatment with E2 resulted in a 13.4% increase in the RCR of isolated brain mitochondria (Fig. 1 and Table 1). In contrast to the effects of the two hormones alone, the coadministration of E2 and P4 did not result in a significant change in the RCR of isolated brain mitochondria (Fig. 1 and Table 1). There was a nonsignificant increase in rate of state 3 respiration in the P4 and E2 groups (Table 1). There was no difference in the rate of state 4o respiration in any treatment group (Table 1). These data indicate an increased efficiency of mitochondrial respiration rather than an alteration in the coupling of the ETC.
Figure 1.
P4 and E2 enhance mitochondrial respiratory activity. Oxygen electrode measurements of respiration using isolated rat brain mitochondria. A, Representative traces of mitochondrial oxygen consumption with or without in vivo E2 (30 μg/kg), P4 (30 μg/kg), E2/P4, or sesame oil vehicle control treatment (Ct) in the presence of l-malate (Mal; 5 mm), l-glutamate (Glut; 5 mm), and ADP (410 μm) to initiate state 3 respiration and atractyloside to induce state 4o respiration. The traces are representative of six to eight separate experiments. B, Percent changes in mitochondrial RCR (state 3/state 4o). The data represent mean ± sem of eight separate experiments. *, P < 0.05 compared with control; n = 8.
Table 1.
Mitochondrial respiration: oxygen electrode measurements of respiration using isolated rat brain mitochondria
| State 4o | State 3 | RCR | |
|---|---|---|---|
| Control | 4.56 ± 0.53 | 33.42 ± 2.91 | 7.85 ± 0.69 |
| E2 | 5.21 ± 0.57 | 43.94 ± 3.50 | 8.90 ± 0.71 |
| P4 | 4.54 ± 0.70 | 40.16 ± 4.18 | 9.77 ± 0.92 |
| E2 + P4 | 4.30 ± 0.51 | 32.63 ± 2.77 | 8.16 ± 0.76 |
Enhanced COX activity and expression in hormone-replaced rats
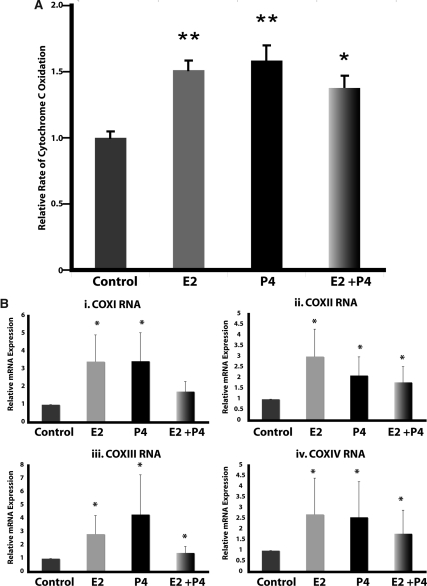
The accelerated movement of electrons down the ETC would be expected to alter the activity of the terminal complex, COX (complex IV). To test this hypothesis, we examined the enzymatic activity of COX. Brain mitochondria isolated from P4-treated rats displayed a significant 1.55-fold increase in COX activity (P < 0.01 compared with control; n = 4; Fig. 2A). E2 treatment resulted in a significant 1.5-fold increase in brain mitochondrial COX activity (P < 0.01 compared with control; n = 8; Fig. 2A). In contrast to the lack of effect of coadministration on rates of respiration, cotreatment with E2 and P4 significantly increased brain mitochondrial COX activity by 1.4-fold (P < 0.05 compared with control; n = 8).
Figure 2.
P4 and E2 enhance COX activity and expression. A, Relative rate of COX activity of isolated whole-brain mitochondria with or without in vivo E2 (30 μg/kg), P4 (30 μg/kg), E2/P4, or sesame oil vehicle control treatment. The bars represent mean ± sem from four separate experiments with two animals per group for each experiment. *, P < 0.05 compared with control; **, P < 0.01 compared with control; n = 8. B, Total RNA was isolated from brain after 24 h exposure to E2 (30 μg/kg), P4 (30 μg/kg), E2/P4, or sesame oil vehicle control. Expression of COXI, COXII, COXIII, and COXIV mRNA was assessed by real-time RT-PCR. Bars represent mean relative expression ± sem from eight animals per group. *, P < 0.05 compared with control; n = 8.
The increased COX activity could be due to modulation of enzymatic activity or to an alteration in expression levels of COX holoenzyme proteins. To determine whether there was an alteration in COX subunit expression, we assessed mRNA expression by real-time RT-PCR. Because it is unknown whether P4 or E2 regulation of mitochondrial function occurs at a nuclear or mitochondrial site of origin, we assessed the expression of both mitochondrial- and nuclear-encoded COX subunit mRNAs. The mitochondria-encoded subunits COXI, COXII, and COXIII were all significantly up-regulated by about 3-fold in the P4- and E2-treatment groups (Fig. 2B; P < 0.05 compared with control; n = 8). Likewise, the expression of the nuclear-encoded subunit COXIV was significantly increased by about 2.5-fold in the P4- and E2-treatment groups (Fig. 2B; P < 0.05 compared with control; n = 8). The coadministration of P4 and E2 resulted in a significant increase in the expression of COXII (∼2-fold), COXIII (∼1.5-fold), and COXIV (∼1.5-fold) (Fig. 2B; P < 0.05 compared with control; n = 8).
Enhanced complex V expression in hormone-replaced rats
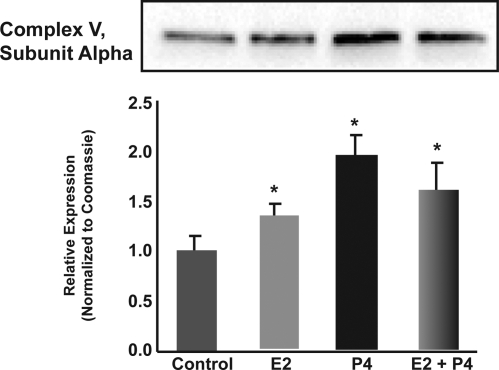
The accelerated movement of electrons down the ETC would be expected to be coupled to increased ATP production. Increased ATP in response to E2 in vitro has previously been reported (13). To determine whether there was an increase in ATP synthase/complex V to mediate the accelerated electron flow and ATP production, we assessed protein expression of complex V, subunit α by Western blot analysis. Complex V, subunit α was significantly increased by about 1.5- to 2-fold in the P4-, E2-, and E2/P4-treatment groups (Fig. 3; P < 0.05 compared with control; n = 4).
Figure 3.
P4 and E2 increase expression of complex V, subunit α protein. Total protein was isolated from brain after 24 h exposure to E2 (30 μg/kg), P4 (30 μg/kg), E2/P4, or sesame oil vehicle control. Expression of complex V, subunit α was determined by Western blot analysis. Bars represent mean relative expression ± sem from four animals per group. *, P < 0.05 compared with control; n = 4.
Mitochondrial biogenesis was unaffected by hormone replacement
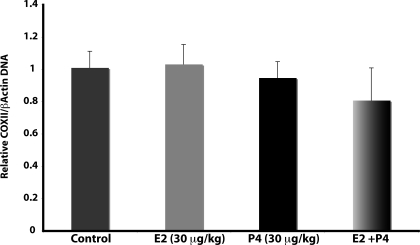
Mitochondrial respiratory efficiency can be induced by increasing the number of mitochondria (14). Thus, the increase in respiratory rate, COX activity, and expression of COX subunits may have resulted from a global increase in mitochondrial biogenesis. To test this hypothesis, we assessed the effect of E2 and P4 treatment on a marker of mitochondrial biogenesis, the ratio of mitochondrial DNA (mtDNA) to nuclear DNA. Real-time PCR for COXII (mitochondrial) and β-actin (nuclear) was performed on total brain DNA. There was no effect of P4, E2, or E2/P4 treatment on the ratio of COXII to β-actin DNA (Fig. 4). These data indicate that neither E2 nor P4 affect the rate of mitochondrial biogenesis.
Figure 4.
P4 and E2 do not alter mitochondrial biogenesis. Total DNA was isolated from brain after 24 h exposure to E2 (30 μg/kg), P4 (30 μg/kg), E2/P4, or sesame oil vehicle control. The relative copy number of COXII (mitochondrial) and β-actin (nuclear) DNA was assessed by real-time PCR, and the ratio of COXII/ β-actin was used as a marker of relative mitochondrial number. Bars represent mean ± sem from eight animals per group. *, P < 0.05 compared with control; n = 8.
Effects of P4 and E2 on ROS production by brain mitochondria
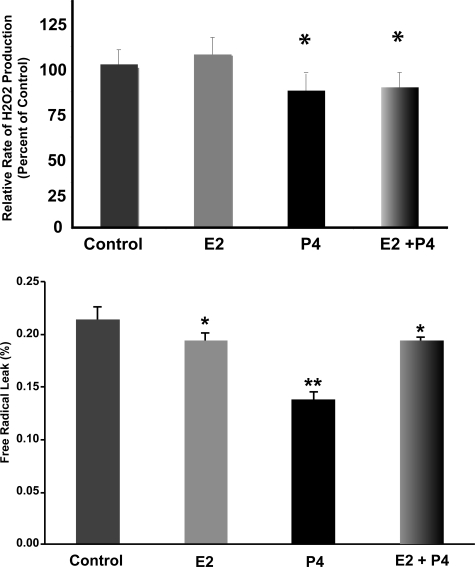
The increased number of electrons flowing through the ETC represented by the observed increased respiratory activity could result in increased free radical production due to the increased probability of electron leak. However, the increased activity of the terminal complex, COX, would be expected to deplete the electron-rich intermediaries such as ubisemiquinone, decreasing the incidental one-electron reduction of oxygen. Thus, we hypothesized that P4 and E2 would reduce mitochondrial ROS production. Fluorescent Amplex Red measurements of H2O2 were made with isolated brain mitochondria exposed to additions of malate/glutamate plus ADP (state 3). P4 treatment resulted in a significant decrease in the rate of H2O2 production by isolated brain mitochondria (Fig. 5A; P < 0.05 compared with control; n = 8). In contrast, there was no significant effect on H2O2 production by isolated brain mitochondria in the E2-treated group (Fig. 5A; n = 8). The coadministration of E2 and P4 resulted in a significant decrease in the rate of H2O2 production by state 3 mitochondria (Fig. 5A; P < 0.05 compared with control; n = 8).
Figure 5.
Ovarian hormones reduce rate of peroxide production and free radical leak in brain mitochondria. A, Fluorescent Amplex Red measurements of H2O2 production in isolated rat brain mitochondria exposed in vivo to ovarian hormones. Spectrofluorometer measurements were made of H2O2 production by isolated brain mitochondria in the presence of malate (5 mm) and glutamate (5 mm) plus ADP (410 μm) to initiate state 3 respiration. Values represent relative mean H2O2 production rates ± sem of eight separate experiments. *, P < 0.05 compared with control; n = 8. B, The percent free radical leak was calculated for isolated whole-brain mitochondria after in vivo E2 (30 μg/kg), P4 (30 μg/kg), E2/P4, or sesame oil vehicle control treatment. Free radical leak was calculated as the percentage of H2O2 production to twice the rate of O2 consumption. The bars represent mean ± sem from eight animals per group. *, P < 0.05 compared with control; **, P < 0.01 compared with control; n = 8.
The efficiency of mitochondria can also be assessed by determining the rate of free radical leak, or the percent electron flow that reduces oxygen to ROS instead of reducing O2 to water at COX. The free radical leak with malate/glutamate plus ADP (state 3) was significantly lower in the P4 (∼33%), E2 (∼11%), and E2/P4 (∼12%) groups than in the control group (Fig. 5B; P < 0.05 compared with control; n = 8).
P4 and E2 reduced lipid peroxidation of brain mitochondria
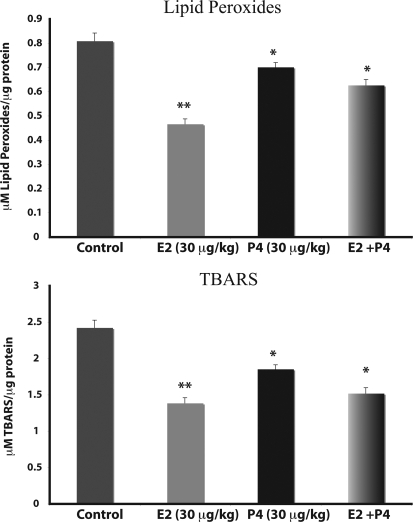
If the leakage of free radicals is reduced in mitochondria exposed to P4 and E2, we reasoned that the oxidative damage to mitochondrial membranes should also be reduced. Results of these analyses indicated that brain mitochondrial lipid peroxidation was significantly lower in ovarian hormone-replaced rats than in control vehicle-treated rats (Fig. 6). When assessed as lipid peroxides, there was a reduction in mitochondrial lipid peroxidation of 13.8, 43.7, and 22.5% in P4, E2, and E2/P4 groups, respectively (Fig. 6; n = 4; P < 0.05 compared with control). When assessed as the aldehyde product, there was a reduction in TBARS of 25, 41.7, and 37.5% in P4, E2, and E2/P4 groups, respectively (Fig. 6; n = 4; P < 0.05 compared with control).
Figure 6.
P4 and E2 reduce lipid peroxidation of brain mitochondria. Whole-brain mitochondria were isolated 24 h after in vivo exposure to E2 (30 μg/kg), P4 (30 μg/kg), E2/P4, or sesame oil vehicle control treatment. Lipid peroxides in brain mitochondria were measured using the leucomethylene blue assay (A) or by measuring TBARS (B). The bars represent mean ± sem from eight animals per group. *, P < 0.05 compared with control; **, P < 0.01 compared with control; n = 8.
P4 and E2 alter the antioxidant profile of brain mitochondria
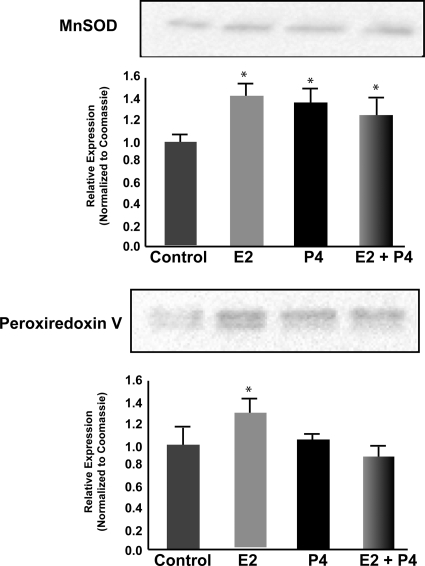
Even though we observed effects on H2O2 production in only the P4-treatment group, and not the E2-treatment group (Fig. 5), there was a pronounced reduction in lipid peroxidation in the E2-treatment group (Fig. 6). This would indicate activation of mechanisms beyond solely mitochondrial efficiency in response to E2 that may not be activated in response to P4. Thus, we determined the impact of P4 and E2 on the protein expression of the mitochondrial antioxidant proteins MnSOD and peroxiredoxin V (Fig. 7). There was a significant increase in MnSOD expression of about 1.4-fold in the P4, E2, and E2/P4 groups (Fig. 7; n = 4; P < 0.05 compared with control). In contrast, the expression of peroxiredoxin V was increased only in the E2-treatment group (Fig. 7; n = 4; P < 0.05 compared with control) but not in the P4- and E2/P4-treatment groups (Fig. 7; n = 4). These results fit with the above observations on lipid peroxidation, in that MnSOD is responsible for generating H2O2 from the superoxide anion and peroxiredoxin V is involved in clearance of H2O2 and prevention of peroxidative damage.
Figure 7.
P4 and E2 alter the antioxidant profile of brain mitochondria. Whole-brain mitochondria were isolated 24 h after in vivo exposure to E2 (30 μg/kg), P4 (30 μg/kg), E2/P4, or sesame oil vehicle control treatment. Expression of the mitochondrial antioxidant proteins MnSOD (A) and peroxiredoxin V (B) were measured using Western blot analysis. The bars represent mean ± sem from four animals per group. *, P < 0.05 compared with control; **, P < 0.01 compared with control; n = 4.
Discussion
The ovarian hormones, E2 and P4, play significant neuromodulatory and neuroprotective roles, influencing synaptogenesis, cerebral blood flow, and neuronal survival. These multiple actions during development and aging not only regulate organismal reproductive physiology but also regulate cognitive health (1). More recently, it has been recognized that E2 also regulates the metabolic functions sustaining the energetic demands of its neurotrophic effects. Underlying this metabolic control is an interpretation of signals from diverse environmental sources integrated by receptor-mediated responses converging upon mitochondrial function (15). In this study, we investigated the impact of P4 and E2 on key mitochondrial functions, oxidative respiration, and free radical generation. Results of these analyses indicate that both E2 and P4 significantly increased mitochondrial respiration 24 h after a single in vivo exposure. Consistent with an increase in oxidative respiration, P4 and E2 significantly increased COXIV enzyme activity and expression of COXIV mRNA. Remarkably, both E2 and P4 reduced free radical leak, indicating greater efficiency of electron transport. Consistent with reduced generation of free radicals, P4 and E2 induced a significant reduction in mitochondrial lipid peroxidation. The data further indicate that P4 and E2 directly regulate mitochondrial function and that the effects are not due to an increase in the number of mitochondria because neither P4 nor E2 nor their combination induced evidence for mitochondrial biogenesis. The mechanisms underlying this response are as yet unknown. Although the serum steroid levels measured at 24 h fall within the physiological range, it is known that steroid levels after a single injection are much higher at earlier time points. However, such spiking is also observed with other delivery methods including oral administration, and the systemic dosing in this study are comparable to the systemic doses observed for common oral administration of 1–2 mg/d micronized E2. Together, these data indicate that the gonadal hormones, P4 and E2, are potent regulators of mitochondrial function to increase both the magnitude and efficiency of mitochondrial respiration in brain.
Surprising to us was the efficacy of P4 and the lack of synergy when P4 and E2 were administered in combination. On all outcome measures, the combination of P4 and E2 resulted in a substantial decrement in response magnitude. Furthermore, the data indicate that the combination of P4 and E2 is not synergistic and when administered in combination leads to a lower level of response relative to either gonadal hormone alone. Although the concentration of P4 was not sufficient to inhibit E2-induced uterine proliferation, using the currently available data, it is not possible to discern the mechanism for the antagonism on mitochondrial respiration, and future studies will be required to determine whether it is an effect related to dosage or competitive mechanisms of the two compounds. Regardless of the outcome on mitochondrial respiration, there was a profound effect in the coadministration groups on free radical leak and oxidative damage that coincides with the neuroprotective ability of E2/P4 coadministration that we have previously reported (2,10). Another point of note is that the effects of the combined treatment were not consistent across all outcome measures. In some outcomes, as in mitochondrial respiration, there was no effect of the combined treatment relative to control, and in others, as in COX activity and expression, the combined treatment significantly altered the response relative to control. Although this may be due to methodological limitations, in the face of the different composite mitochondrial outcome profile between E2 and P4, it is more suggestive of alternative mechanisms of steroidal regulation of mitochondrial function. Thus, the neuroprotective abilities of E2 and P4 do not involve all aspects of mitochondrial bioenergetics, and the two steroids probably act through two different sets of overlapping sets of mechanisms. These different mechanisms may act synergistically or antagonistically resulting in the mixed profile of outcomes.
The primary function of mitochondria is to produce the ATP required for cellular bioenergetics, which in the neuron includes the maintenance of membrane potentials crucial for proper signaling and information processing. The effects of ovariectomy in Sprague Dawley rats have been characterized as progressive memory deficits, central cholinergic nerve system degeneration, and homeostatic imbalance (16). These effects are a characteristic consequence of neuronal ATP impairment, resulting from the loss of ovarian hormones. Furthermore, implicating the role of mitochondrial ATP production in the neuronal effects of E2, we have previously demonstrated that estrogen treatment of primary hippocampal neuronal cultures results in increased ATP production (13). It is unknown whether P4 exerts similar effects but would be expected based upon the increased mitochondrial functionality demonstrated in the current study.
Mitochondrial ATP production occurs through the coupling of oxidative phosphorylation with respiration. The ETC is composed of four enzyme complexes: reduced nicotinamide adenine dinucleotide dehydrogenase (complex I), succinate dehydrogenase (complex II), ubiquinol-cytochrome c reductase (complex III), and COX (complex IV). The enzymatic complexes of the ETC act in concert to couple the reduction of oxygen to water (respiration) with the generation of a chemiosmotic gradient across the mitochondrial inner membrane, the latter of which is used by ATP synthase to generate ATP. The coupled respiration occurs by the sequential transfer of electrons through the enzymatic complex, terminating at complex IV (COX) with the reduction of oxygen to water. Besides being the terminal ETC complex, regulation of COX holds special prominence due to its implication as a key regulatory point of oxidative phosphorylation (17). Although it was earlier thought that COX activity was in excess of other components of the electron transport chain, more recent experiments indicate that COX is in fact much more tightly coupled to mitochondrial respiration (17). In the current study, we demonstrated that complex IV activity is significantly increased in response to ovarian hormone replacement. These findings are consistent with reported results of other groups indicating increased COX activity with E2 treatment in various tissues (9,18,19), including brain in which E2 treatment prevented ethanol withdrawal-induced declines in COX activity (20). Our study is the first that we know of to examine the COX activity in the CNS in response to E2 and P4.
The regulation of COX is of further interest from a transcriptional vantage point due to its being encoded by a combination of mitochondrial and nuclear genes that must be coordinately expressed. The catalytic core of the multimeric COX holoenzyme comprises the two subunits COXI and COXII and is stabilized by a third subunit, COXIII, all three of which are encoded by the mtDNA. Ten additional subunits, including COXIV, are encoded by the nuclear genome and are required for full assembly and function of the COX holoenzyme (21). The increased COX activity can largely be explained by the increased expression of COX subunits. Corresponding to the responses in COX activity, we saw robust changes in COX expression in the E2 and P4 alone groups, with more modest increases in the E2/P4 coadministration group. These results are consistent with the previously reported increases in COX III mRNA expression (9). It should also be noted that there was much more variability in the COX transcript expression levels than was observed for COX holoenzyme activity. It is unknown whether this was due to relative sensitivities of the assay methodologies or whether it represented unexamined regulatory control, including allosteric modulation of COX activity or ancillary protein expression, which allowed for tighter modulation of enzymatic activity relative to gene transcription.
In addition to oxidative enzymes, other mitochondrial proteins that regulate mitochondrial respiration and ATP production may be under control of E2 and P4. Transport of substrates needed for oxidative phosphorylation, such as pyruvate, fatty acids, and phosphate may be regulated by P4 or E2, either through transcriptional control or allosteric modulation. Some authors have suggested that E2 can bind directly to and modulate the activity of ATPase. It was demonstrated that E2-BSA conjugates bind in vitro the oligomycin-sensitivity-conferring protein, which forms the stalk region between F0 and F1 subunits of F0F1-ATPase in mitochondria (22). Other in vitro studies demonstrated that estrogens can enhance or inhibit ATPase activity in a tissue- and concentration-dependent manner (23,24,25). It is unknown what effect in vivo exposure to E2 or P4 has on ATPase activity in brain or whether the alterations in respiratory activity, ATP levels, and oxidative stress are dependent upon a direct interaction of the steroids with the oligomycin-sensitivity-conferring protein.
Changes in the oxidative capacity are often due to differences in mitochondrial number, size, and density per cell as well as to changes in mitochondrial components and/or activity. The mtDNA content, which can be used as a suitable marker of mitochondrial number, was not different between any of the treatment groups; the increased mitochondrial activity observed is not explained by an increase in the number of mitochondria. Although, an effect of mitochondrial size cannot be ruled out based on the current data, the greater oxidative capacity induced by ovarian hormones could be linked to a greater machinery per mitochondrion and therefore to higher efficiency of the individual mitochondria. This lack of mitochondrial biogenesis is consistent with greater oxidative capacity in female rat liver mitochondria without a sex difference in the number of liver mitochondria (26). Furthermore, the findings of Van Itallie and Dannies demonstrated an E2-induced increase in COXII mRNA in pituitary tumor cells with no increase in COXII gene copy number (27). We observed an equivalent increase in the expression of both mitochondrial- and nuclear-encoded COX subunits. Due to the tightly coordinated regulation of mtDNA- and nuclear DNA-encoded mitochondrial genes, it is not possible to identify whether the hormones act at a nuclear site regulating COXIV that in turn signals for increased COXI, -II, and -III expression, or vice versa, or whether the hormones act at both sites. More detailed experiments are required to dissect the site of action.
Oxidative damage to mitochondria is posited to play a major role in aging and in neuronal populations may underlie cognitive declines associated with aging (28,29,30). As electrons pass through the mitochondrial ETC, some electrons leak out to molecular oxygen (O2) to form O2•−, which is dismutated by MnSOD to form H2O2. The hydrogen peroxide can in turn be reduced to water by peroxidases such as glutathione peroxidase or peroxiredoxins. A lack of sufficient peroxidase activity results in the peroxidation of lipids and proteins. Lipid peroxidation, the nonspecific oxidation of polyunsaturated fatty acids in cellular membranes, is a radical-mediated pathway and generates a number of harmful degradation products besides drastically altering the structure and function of the membrane. Our results demonstrate that ovarian hormone treatment reduced lipid peroxidation of whole-brain mitochondria. This result is consistent with previous reports indicating that E2 (31,32,33) and P4 (34) can individually reduce oxidative stress in the brain. Here, we extend these findings by demonstrating that the prevention of the lipid peroxidation is present at the level of the mitochondrial membrane and is correlated with enhanced mitochondrial functional efficiency represented by the lower values of free radical leak. The lower levels of lipid peroxidation in the E2-treatment group in the absence of a change in the rate of H2O2 production are indicative of enhanced cellular antioxidant defenses. This is consistent with reports of E2-induced increases in expression of MnSOD and interactions with the glutathione system (35,36). This E2- and P4-induced reduction in lipid peroxidation is due to a reduction the lipid peroxidation induced by the 2-wk steroid deprivation after ovariectomy in which we observed an approximately 3.3-fold induction in lipid peroxidation in the ovariectomized compared with sham ovariectomized rats (data not shown).
Respiratory control is one facet of an interlocking network regulating metabolic activity and energy production in which mitochondrial redox potential is coupled with cytosolic signaling. This tightly coupled network integrates various signals to determine cell fate based upon ATP levels, extracellular signals, and energetic demands. The response of a cell to a death signal depends upon energetic status and redox balance. Energetic status is crucial for full realization of the apoptotic cascade because many of the steps involved are ATP dependent (37). Redox balance can alter the sensitivity of the mitochondria to permeability transition (4). As such, efficient respiratory control can affect not only energy production but also response to toxic insults. In addition to the defensive effects of E2 and P4, including regulation of the Bcl-2 family proteins (38), the increased respiratory control efficiency and decreased oxidative load demonstrated in this study could establish a buffer against neuronal functional decline associated with aging and neurodegenerative diseases.
In summary, we have demonstrated that P4 and E2 increase the oxidative capacity of whole-brain mitochondria. This increased respiratory activity is correlated with decreased free radical leak and reduced lipid peroxidation. As such, ovarian hormone replacement induces mitochondrial alterations in the CNS, supporting efficient and balanced bioenergetics, reducing oxidative stress, and attenuating endogenous oxidative damage.
Footnotes
This study was supported by grants from the National Institute of Aging (5PO1AG026572; Project 1 to J.N.) and National Institutes of Mental Health (1RO1 MH67159-01 to R.D.B and J.N.), and R.W.I. is supported by National Institute of Aging Training Grant T32-AG000093-24/25 (C. E. Finch, PI).
Disclosure Statement: The authors have nothing to declare.
First Published Online February 21, 2008
Abbreviations: CNS, Central nervous system; COXII, cytochrome c oxidase subunit II; E2, 17β-estradiol; ETC, electron transport chain; MIB, mitochondrial isolation buffer; MnSOD, manganese superoxide dismutase; mtDNA, mitochondrial DNA; P4, progesterone; RCR, respiratory control ratio; ROX, reactive oxygen species; TBARS, thiobarbituric acid-reactive substances.
References
- Morrison JH, Brinton RD, Schmidt PJ, Gore AC 2006 Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci 26:10332–10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Diaz Brinton R 2002 Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology 143:205–212 [DOI] [PubMed] [Google Scholar]
- Nilsen J, Diaz Brinton R 2003 Mechanism of estrogen-mediated neuroprotection: regulation of mitochondrial calcium and Bcl-2 expression. Proc Natl Acad Sci USA 100:2842–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls DG, Budd SL 2000 Mitochondria and neuronal survival. Physiol Rev 80:315–360 [DOI] [PubMed] [Google Scholar]
- Cadenas E 2004 Mitochondrial free radical production and cell signaling. Mol Aspects Med 25:17–26 [DOI] [PubMed] [Google Scholar]
- Borras C, Sastre J, Garcia-Sala D, Lloret A, Pallardo FV, Vina J 2003 Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med 34:546–552 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cuenca S, Pujol E, Justo R, Frontera M, Oliver J, Gianotti M, Roca P 2002 Sex-dependent thermogenesis, differences in mitochondrial morphology and function, and adrenergic response in brown adipose tissue. J Biol Chem 277:42958–42963 [DOI] [PubMed] [Google Scholar]
- Zhai P, Eurell TE, Cotthaus R, Jeffery EH, Bahr JM, Gross DR 2000 Effect of estrogen on global myocardial ischemia-reperfusion injury in female rats. Am J Physiol Heart Circ Physiol 279:H2766–H2775 [DOI] [PubMed] [Google Scholar]
- Bettini E, Maggi A 1992 Estrogen induction of cytochrome c oxidase subunit III in rat hippocampus. J Neurochem 58:1923–1929 [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD 2002 Impact of progestins on estradiol potentiation of the glutamate calcium response. Neuroreport 13:825–830 [DOI] [PubMed] [Google Scholar]
- Han D, Williams E, Cadenas E 2001 Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J 353:411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz A, Caro P, Ibanez J, Gomez J, Gredilla R, Barja G 2005 Dietary restriction at old age lowers mitochondrial oxygen radical production and leak at complex I and oxidative DNA damage in rat brain. J Bioenerg Biomembr 37:83–90 [DOI] [PubMed] [Google Scholar]
- Brinton RD, Chen S, Montoya M, Hsieh D, Minaya J, Kim J, Chu HP 2000 The women’s health initiative estrogen replacement therapy is neurotrophic and neuroprotective. Neurobiol Aging 21:475–496 [DOI] [PubMed] [Google Scholar]
- Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R 2006 Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci USA 103:1768–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD 2004 Mitochondria as therapeutic targets of estrogen action in the central nervous system. Curr Drug Targets CNS Neurol Disord 3:297–313 [DOI] [PubMed] [Google Scholar]
- Sato T, Teramoto T, Tanaka K, Ohnishi Y, Irifune M, Nishikawa T 2003 Effects of ovariectomy and calcium deficiency on learning and memory of eight-arm radial maze in middle-aged female rats. Behav Brain Res 142:207–216 [DOI] [PubMed] [Google Scholar]
- Campian JL, Qian M, Gao X, Eaton JW 2004 Oxygen tolerance and coupling of mitochondrial electron transport. J Biol Chem 279:46580–46587 [DOI] [PubMed] [Google Scholar]
- Puerta M, Rocha M, Gonzalez-Covaleda S, McBennett SM, Andrews JF 1998 Changes in cytochrome oxidase activity in brown adipose tissue during oestrous cycle in the rat. Eur J Endocrinol 139:433–437 [DOI] [PubMed] [Google Scholar]
- Stirone C, Duckles SP, Krause DN, Procaccio V 2005 Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol 68:959–965 [DOI] [PubMed] [Google Scholar]
- Jung ME, Agarwal R, Simpkins JW 2007 Ethanol withdrawal posttranslationally decreases the activity of cytochrome c oxidase in an estrogen reversible manner. Neurosci Lett 416:160–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramson J, Svensson-Ek M, Byrne B, Iwata S 2001 Structure of cytochrome c oxidase: a comparison of the bacterial and mitochondrial enzymes. Biochim Biophys Acta 1544:1–9 [DOI] [PubMed] [Google Scholar]
- Zheng J, Ramirez VD 1999 Purification and identification of an estrogen binding protein from rat brain: oligomycin sensitivity-conferring protein (OSCP), a subunit of mitochondrial F0F1-ATP synthase/ATPase. J Steroid Biochem Mol Biol 68:65–75 [DOI] [PubMed] [Google Scholar]
- Keller JN, Germeyer A, Begley JG, Mattson MP 1997 17β-Estradiol attenuates oxidative impairment of synaptic Na+/K+-ATPase activity, glucose transport, and glutamate transport induced by amyloid β-peptide and iron. J Neurosci Res 50:522–530 [DOI] [PubMed] [Google Scholar]
- Massart F, Paolini S, Piscitelli E, Brandi ML, Solaini G 2002 Dose-dependent inhibition of mitochondrial ATP synthase by 17β-estradiol. Gynecol Endocrinol 16:373–377 [PubMed] [Google Scholar]
- Zheng J, Ramirez VD 1999 Rapid inhibition of rat brain mitochondrial proton F0F1-ATPase activity by estrogens: comparison with Na+, K+-ATPase of porcine cortex. Eur J Pharmacol 368:95–102 [DOI] [PubMed] [Google Scholar]
- Justo R, Boada J, Frontera M, Oliver J, Bermudez J, Gianotti M 2005 Gender dimorphism in rat liver mitochondrial oxidative metabolism and biogenesis. Am J Physiol Cell Physiol 289:C372–C378 [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Dannies PS 1988 Estrogen induces accumulation of the mitochondrial ribonucleic acid for subunit II of cytochrome oxidase in pituitary tumor cells. Mol Endocrinol 2:332–337 [DOI] [PubMed] [Google Scholar]
- Barja G 2004 Free radicals and aging. Trends Neurosci 27:595–600 [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Giuffrida Stella AM, Bates TE, Clark JB 2001 Mitochondrial involvement in brain function and dysfunction: relevance to aging, neurodegenerative disorders and longevity. Neurochem Res 26:739–764 [DOI] [PubMed] [Google Scholar]
- Reddy PH 2006 Mitochondrial oxidative damage in aging and Alzheimer’s disease: implications for mitochondrially targeted antioxidant therapeutics. J Biomed Biotechnol 2006:31372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl C, Widmann M, Trapp T, Holsboer F 1995 17-β-Estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem Biophys Res Commun 216:473–482 [DOI] [PubMed] [Google Scholar]
- Kii N, Adachi N, Liu K, Arai T 2005 Acute effects of 17β-estradiol on oxidative stress in ischemic rat striatum. J Neurosurg Anesthesiol 17:27–32 [PubMed] [Google Scholar]
- Shea TB, Ortiz D 2003 17β-Estradiol alleviates synergistic oxidative stress resulting from folate deprivation and amyloid-β treatment. J Alzheimers Dis 5:323–327 [DOI] [PubMed] [Google Scholar]
- Subramanian M, Pusphendran CK, Tarachand U, Devasagayam TP 1993 Gestation confers temporary resistance to peroxidation in the maternal rat brain. Neurosci Lett 155:151–154 [DOI] [PubMed] [Google Scholar]
- Gridley KE, Green PS, Simpkins JW 1998 A novel, synergistic interaction between 17β-estradiol and glutathione in the protection of neurons against β-amyloid 25–35-induced toxicity in vitro. Mol Pharmacol 54:874–880 [DOI] [PubMed] [Google Scholar]
- Nakamizo T, Urushitani M, Inoue R, Shinohara A, Sawada H, Honda K, Kihara T, Akaike A, Shimohama S 2000 Protection of cultured spinal motor neurons by estradiol. Neuroreport 11:3493–3497 [DOI] [PubMed] [Google Scholar]
- Nicotera P, Leist M, Fava E, Berliocchi L, Volbracht C 2000 Energy requirement for caspase activation and neuronal cell death. Brain Pathol 10:276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Chen S, Irwin RW, Iwamoto S, Brinton RD 2006 Estrogen protects neuronal cells from amyloid β-induced apoptosis via regulation of mitochondrial proteins and function. BMC Neurosci 7:74 [DOI] [PMC free article] [PubMed] [Google Scholar]