Abstract
The agouti viable yellow (Avy) spontaneous mutation generates an unusual mouse phenotype of agouti-colored coat and adult-onset obesity with metabolic syndrome. Persistent production of agouti signaling protein in Avy mice antagonizes melanocortin receptors in the hypothalamus. To determine how this disruption of neuroendocrine circuits affects leptin transport across the blood-brain barrier (BBB), we measured leptin influx in Avy and B6 control mice after the development of obesity, hyperleptinemia, and increased adiposity. After iv bolus injection, 125I-leptin crossed the BBB significantly faster in young (2 month old) B6 mice than in young Avy mice or in older (8 month old) mice of either strain. This difference was not observed by in situ brain perfusion studies, indicating the cause being circulating factors, such as elevated leptin levels or soluble receptors. Thus, Avy mice showed peripheral leptin resistance. ObRa, the main transporting receptor for leptin at the BBB, showed no change in mRNA expression in the cerebral microvessels between the age-matched (2 month old) Avy and B6 mice. Higher ObRb mRNA was seen in the Avy microvasculature with unknown significance. Immunofluorescent staining unexpectedly revealed that many of the ObR(+) cells were astrocytes and that the Avy mice showed significantly more ObR(+) astrocytes in the hypothalamus than the B6 mice. Although leptin permeation from the circulation was slower in the Avy mice, the increased ObR expression in astrocytes and increased ObRb mRNA in microvessels suggest the possibility of heightened central nervous system sensitivity to circulating leptin.
MANY FORMS OF obesity are associated with increased blood leptin levels proportionate to the adipose tissue mass. Circulating leptin can cross the blood-brain barrier (BBB) and blood-cerebrospinal fluid barrier to provide negative feedback control of feeding behavior and energy balance (1,2,3,4). In the hypothalamus, experimental evidence has led to a proposed model in which anorexigenic signals, including Janus kinases and signal transducer and activator for transcription (STAT), activated by leptin converge on melanocortin receptor (MCR)-4 neurons (5,6,7).
The agouti viable yellow (Avy) mouse provides a unique model to study the defects of MCR-4 signaling and metabolic syndromes related to adult-onset obesity. The spontaneous mutation in the Avy mice involves the insertion of a retrotransposon at the promoter region of the gene encoding the agouti signaling protein. The ectopic overexpression of agouti-related protein (AgRP) leads to antagonism of the melanocortin receptors. In the skin, dysfunction of MCR-1 signaling increases pheomelanin synthesis, resulting in subapical yellow bands of hair. In the hypothalamus, dysfunction of MCR-4 signaling results in hyperphagia and obesity. This produces the two prominent phenotypical features of Avy mice: an agouti-colored coat and obesity. Despite the discovery of Avy mice in the early 1960s (8) and the use of Avy mice for various obesity and metabolic studies in the past few decades (9,10,11,12,13,14), the role and regulation of the leptin system in these mice have not been fully addressed.
Although the BBB limits the diffusion of most peptides and proteins from blood to brain, there are transport systems for selective ingestive peptides and adipokines (4,15,16,17,18). In the past two decades, the transport system for leptin across the BBB has to play a crucial role in many forms of obesity and leptin resistance, following the classical paper by Banks et al. (1). This transport system can be regulated by many physiological or pathological factors, including diet-induced obesity (19), brain lesions (20), leptin treatment (21), triglycerides (22), α1-adrenergic agents (23), and glucose and insulin (24). The dynamic changes of leptin transport indicate that the BBB is involved even in genetic forms of obesity and leptin resistance (25,26).
There are few studies examining the permeation of ingestive peptides across the BBB in the Avy mouse. AgRP is a candidate for a reverse antagonist to MCR-4 and inhibitor of leptin-induced suppression of food intake and body weight (27). However, AgRP does not cross most parts of the BBB by a saturable transport system (28,29). By contrast, the C-terminal mahogany peptide crosses the BBB via a specific system (30). In Avy mice, the brain uptake of mahogany peptide shows age-related changes; in 1-month-old Avy mice, it is higher than in 7-month-old Avy mice, 12-month-old Avy mice, or B6 mice studied at any of these three ages (31). Because mahogany protects against obesity (32,33), the increased permeation of mahogany peptide preceding the fat surge might serve to delay the onset of obesity in the Avy mice that are susceptible to strong epigenetic influences (31).
Wolff (34) has provided a comprehensive comparison of Avy and ob/ob mice that share an obesity phenotype but have different underlying etiologies. However, the function of the leptin system in Avy mice has not been addressed. Here, we propose that leptin transport across the BBB in Avy mice not only shows adaptive changes related to the dynamics of their blood leptin concentrations, but also plays an active role in “repairing” the neuroendocrine circuitry induced by excess AgRP in the brain.
Materials and Methods
Mice
The animal protocol was approved by the Institutional Animal Care and Use Committee. Avy mice (B6.C3Fe-Avy/J) and their background control B6 mice were originally obtained from Jackson Laboratory (Bar Harbor, ME). The Avy allele occurred spontaneously in the C3H/HeJ strain, and heterozygotes have been maintained in our breeding colony for over four generations, with backcrossing to C57BL/6J mice. The mice were group housed (three to four per cage), kept on a light-dark cycle of 0700–1900 h, and fed a regular rodent chow. Male mice were used for most of the studies to reduce the variability associated with the estrous cycle. They were monitored throughout the study period, until 2–8 months of age after the development of obesity, as described in Results.
Measurement of body weight, fat composition, and serum leptin level
Body weight was measured weekly after birth for both the Avy and B6 groups. The percentage of body fat was determined with a Bruker minispec Live Mice Analyzer (model mq7.5, LF50; Bruker Optics, Inc., The Woodlands, TX), as described previously (31). At the age of 2 months, 15 wk, and 6 months, mice were weighed and placed in the cylindrical polycarbonate holding tubes that were inserted into the nuclear magnetic resonance device. In a terminal study, blood was collected from two groups of four male mice each at the age of 2 months, after anesthesia by ip urethane injection. Serum leptin concentrations were determined with an ELISA kit (Active Murine Leptin ELISA; Diagnostic Systems Laboratories, Webster, TX). The results were measured in a plate reader at an absorbance of 450 nm with a detection limit of 0.04 ng/ml.
BBB transport assay after iv injection of leptin by multiple-time regression analysis
Four groups of mice were studied (n = 6 per group for the Avy mice, and n = 7 per group for the B6 group). Recombinant full-length mouse leptin (R & D Systems, Minneapolis, MN) was radioactively labeled with 125I by the iodogen method (Pierce, Rockford, IL), and purified on Sephadex G-10 columns. The specific activity of 125I-leptin was about 60–80 Ci/g. BSA was labeled with 131I using the chloramine-T reagent and used as an internal control for BBB integrity. The iodogen method is gentler and has a better chance of preserving the biological activity of iodinated leptin. The chloramine T method is faster and less expensive; thus, it is used only to label the vascular marker that acts as an inert control substance in our study.
Multiple time-regression analysis was applied as previously detailed (28,35,36). 125I-leptin and 131I-albumin (∼30,000 cpm/μl for each mouse) were delivered in the same injectate of 100 μl lactated Ringer’s solution containing 1% (wt/vol) BSA. The injection was made into the isolated left jugular vein of anesthetized mice, each mouse representing a specific time point. We conventionally use urethane for terminal anesthesia in BBB permeability studies because of its relatively wide safety margin with minimal effects on autonomic and cardiovascular systems (37), its long-lasting steady level of surgical anesthesia, and the consistency of BBB transport assay results in contrast to the lower reproducibility with ketamine/xylazine. Urethane has dose-dependent potentiation of neuronal nicotinic acetylcholine, γ-aminobutyric acid, and glycine receptors, and inhibition of N-methyl D-aspartate and -amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (38). Because we mainly examine the transport functions and expression levels of ObR, which has not been shown to interact with ligand-gated ion channels directly, we expect similar changes in the control and experimental groups.
At time points of 1, 3, 5, 7, 10, and 15 min after iv injection, blood was collected by transection of the right carotid artery, and the mice were decapitated immediately afterwards. Based on previous HPLC studies of the degradation pattern of leptin (1,35,39), the study used a maximal circulation time of 15 min during which leptin remains intact. The amount of 125I-leptin was about 17 ng/ml, similar to the endogenous leptin concentration in the serum of a moderately obese subject.
At the end of the study, the radioactivity of 125I-leptin and 131I-albumin in brain and serum was measured in a dual-channel γ-counter (Wallac, Gaithersburg, MD). The brain to serum ratio of 125I-leptin in each gram of brain was calculated. The serum values were also used in the calculation of exposure time, a theoretical steady-state value with correction for the decrease in blood concentration of 125I-leptin after iv bolus injection (35). Because we applied iv bolus injection of 125I-leptin in the multiple-time regression analysis, the exposure time is longer than the corresponding experimental time. The influx rate and volume of distribution of 125I-leptin were determined from the linear regression correlation between the brain to serum ratio of radioactivity and exposure time. The values for 131I-albumin were determined from the linear regression correlation between the brain to serum ratio and real time because albumin showed minimal redistribution during the study interval. The unidirectional influx rate Ki is the slope of the linear regression line, and the initial volume of distribution Vi is the intercept. Differences in the regression lines between groups were compared by the least square method with the GraphPad Prism program (San Diego, CA).
BBB transport assay by in situ brain perfusion
Two groups of 2-month-old mice were studied simultaneously: the B6 mice (n = 4) and the Avy mice (n = 6), based on their age-matched availability. The mice were anesthetized by ip injection of urethane, and processed for intracardial perfusion through the left ventricle, with the right atrium severed. The descending aorta was clamped at the level of the diaphragm. The perfusion was driven by a syringe pump at a speed of 2 ml/min. The composition of the perfusate was, in g/liter: NaCl, 7.19; KCl, 0.3; CaCl2, 0.28; NaHCO3, 2.1; KH2PO4, 0.16; MgCl2 · 6H2O, 0.37; and d-glucose, 0.99, with 1% BSA added after bubbling with oxygen. The pH was adjusted to 7.4. The vascular space was cleared by 2-min pre-perfusion with perfusate only. The mice were then perfused with 125I-leptin and 131I-albumin (1000 cpm/μl each) for 5 min, followed by 1-min post-wash with perfusate only. The brain was collected after decapitation. The radioactivity of the weighed brain and 50 μl perfusate were measured with the dual-channel program in a γ-counter. Group means of the brain to perfusate ratio are shown with their ses. Any significant difference between the groups was determined by ANOVA.
RNA extraction and real-time PCR
Two groups of mice were studied: Avy and B6 males 2 months old (n = 9–11 per group). Microvessel-enriched fractions from the cerebral cortices of neonatal and adult mice were obtained by the capillary depletion method as described previously (40,41). This provides more than 40-fold enrichment of endothelial cells. The hypothalamus was dissected immediately after decapitation of the mouse. The rostral border was the optic chiasm, and the most caudal parts were the mammillary bodies. With RNAase-free forceps, the whole hypothalamus was removed from the brain, snap frozen in liquid nitrogen, and transferred to −80 C until RNA extraction within 1 wk. Total RNA was obtained with an Absolutely RNA Miniprep Kit (Stratagene, La Jolla, CA). A one-step core PCR kit (Applied Biosystems, Foster City, CA) was used for reversed transcription and mRNA amplification using TaqMan real-time PCR primers and fluorescent probes. The primers and probes for ObRa, ObRb, and the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are listed in Table 1. Standard curves proved efficient amplification of the genes. The relative amount of mRNA was calculated from the cycle threshold by the established δδ-cycle threshold method, in which ObRa and ObRb mRNA was normalized to the GAPDH mRNA. Potential differences between the Avy and B6 groups were determined by ANOVA for each fraction (microvessels or hypothalamus).
Table 1.
TaqMan primers and probes for real-time PCR
| mRNA | Primer/probe | Sequence |
|---|---|---|
| ObRa | 5′ Primer | GAAGTCTCTCATGACCACTACAGATGA |
| 3′ Primer | TTGTTTCCCTCCATCAAAATGTAA | |
| Probe | 6FAM-CCCAATCTACCAACTTCCCAACAGTCCA-TAMRA | |
| ObRb | 5′ Primer | GCATGCAGAATCAGTGATATTTGG |
| 3′ Primer | CAAGCTGTATCGACACTGATTTCTTC | |
| Probe | 6FAM-CCTCTTCTTCTGGAGCCTGAACCCATTTC-TAMRA | |
| GAPDH | 5′ Primer | TGTGTCCGTCGTGGATCTGA |
| 3′ Primer | CCTGCTTCACCACCTTCTTGA | |
| Probe | 6FAM-CCGCCTGGAGAAACCTGCCAAGTATG-TAMRA |
Immunohistochemistry of ObR and glial fibrillary acidic protein (GFAP)
Two groups of mice were studied (n = 3 per group): B6 and Avy mice 2 months old. The mice were anesthetized by ip injection of urethane and perfused transcardially with 50 ml saline, followed by 15- to 20-min 4% paraformaldehyde prepared in 0.1 m phosphate butter driven by a syringe pump. The brains were postfixed overnight in 4% paraformaldehyde, and cryoprotected with 10% (2 h), 20% (2 h), and then 30% sucrose overnight. Coronal sections of 20-μm thickness were obtained on a Microm HM 560 cryostat (Richard-Allan Scientific, Kalamazoo, MI). The free-floating sections were permeabilized with 0.3% Triton X-100, blocked with 10% normal donkey serum, incubated with primary antibodies at 4 C overnight, thoroughly washed, and incubated with Alexa dye-conjugated corresponding secondary antibodies. To identify cells expressing leptin receptors (ObR), a polyclonal antibody against the C terminus of the short form of mouse ObR was used (sc-1834, 20 μg/ml; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). To identify astrocytes, a monoclonal antibody against GFAP was used (AB5804; CHEMICON Intl. Inc., Temecula, CA). The secondary antibodies were Alexa488-conjugated donkey antigoat and Alexa594-conjugated donkey antimouse polyclonal, respectively. Two negative controls were included in the study. Some sections were incubated with secondary antibody only, with omission of the primary antibody. In others, the primary antibody against ObR was preabsorbed with a blocking peptide (sc-1834p, 5-fold m excess; Santa Cruz Biotechnology) overnight at 4 C, before incubation with the brain sections. The sections were mounted on Superfrost (Fisher Scientific, Pittsburgh, PA) glass slides with medium containing the antifading agent 1,4-diazobicyclo-[2,2,2]-octane (DAPCO) and viewed under a Nikon TE2000 epifluorescence microscope (Nikon Corp., Tokyo, Japan).
Image analyses
Epifluorescent images were acquired with a Nikon TE2000 inverted microscope with a cool-charged camera. The filters, objectives, exposure time, and magnification settings were identical during capture of the images. The thresholding and intensity measurement of immunofluorescent staining in matching regions of the brain (arcuate nucleus) from the B6 and Avy mice were performed with MetaVieu imaging analysis software (Universal Imaging, now Molecular Devices, Sunnyvale, CA). Manual cell counting and image thresholding were performed on the same surface area of matching images between the B6 and Avy groups by separate experimenters. Statistical analysis was performed for imaging threshold, intensity, and cell counts by one-way ANOVA.
Confocal microscopy
To confirm the astrocytic phenotype of ObR(+) cells, sections double stained for ObR and GFAP were imaged with a Zeiss Meta 510 confocal microscope (Carl Zeiss, Inc., Thornwood, NY) in our Cellular Imaging Core Facility. A 488-nm laser line from an argon laser was used to excite the Alexa488 fluorophore, and a 543-nm helium-neon laser line was used to excite the Alexa594 fluorophore. Band pass BP500 and long pass LP560 filters were used to collect the emission signal, respectively. Colocalization of ObR and GFAP staining was clearly seen and confirmed by Imaris image analysis (Bitplane, Zurich, Switzerland). Similarly, potential colocalization of ObR and the microglial marker CD11b (Serotec, Raleigh, NC) was analyzed by confocal microscopy after double immunolabeling.
Results
Development of adult-onset obesity phenotype in the Avy mice
The mice used in this study were produced by heterozygote mating to increase the rate of fertility. The Avy phenotype can be first identified by the early coat color at about 1 wk of age. This includes solid yellow and varying degrees of mottled yellow. Mice with a solid yellow coat are the most obese; mice with a mottled agouti but predominantly yellow coat were more obese than those with a predominantly black coat. To reduce variability, we mainly used solid yellow agouti male mice for the studies. These mice were housed together with their black-coated litter mates.
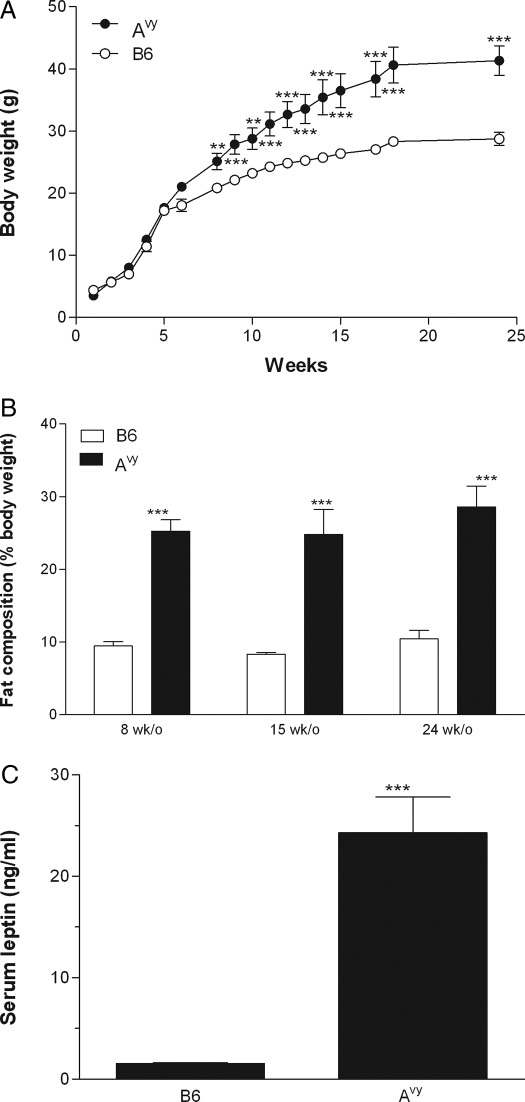
The body weights of the Avy and their black coat litter mates (controls) were monitored weekly from 1–24 wk of age. Figure 1A shows that the body weight of the Avy group was significantly higher than that of the control group (P < 0.01), determined by repeated measures ANOVA. Greater intragroup variation was seen in the Avy group, indicated by higher ses than the minimal differences in the control group. Post hoc analysis showed that a significant increase of body weight in the Avy mice was present by 8 wk of age and beyond. At age 24 wk, the Avy mice had a significantly higher percentage of fat than their black-coat littermates (P < 0.005; Fig. 1B).
Figure 1.
Body weight and metabolic indices of the B6 and Avy mice. A, Growth curve of the Avy and age-matched B6 control mice (n = 4 per group). Repeated measures ANOVA showed a significant (P < 0.01) overall increase of body weight in the Avy group. Post hoc analysis indicated that the difference was significant at 8 wk of age and persisted until the end of the study at 24 wk of age. **, P < 0.01; ***, P < 0.005. B, Body fat of the Avy and B6 mice was determined by nuclear spin resonance. The 8-wk-old (n = 3), 15-wk-old (n = 6), and 24-wk-old B6 mice had 9.47, 8.32, and 10.44% fat, respectively. The 8-wk-old (n = 6), 15-wk-old (n = 7), and 24-wk-old (n = 4) Avy mice had 25.24, 24.82, and 28.60% fat, respectively. The increase in the Avy mice was significant at all three time points. ***, P < 0.005. C, Serum leptin concentrations were measured at 8 wk of age by ELISA. The Avy mice had significantly higher concentrations of circulating leptin. ***, P < 0.005. n = 4 per group.
Blood leptin concentrations were determined in groups of Avy and B6 mice at 2 months of age. ELISA showed that the Avy group had significantly higher leptin concentrations than their nonobese littermates (P < 0.005; Fig. 1C).
BBB permeability to leptin in the Avy mice
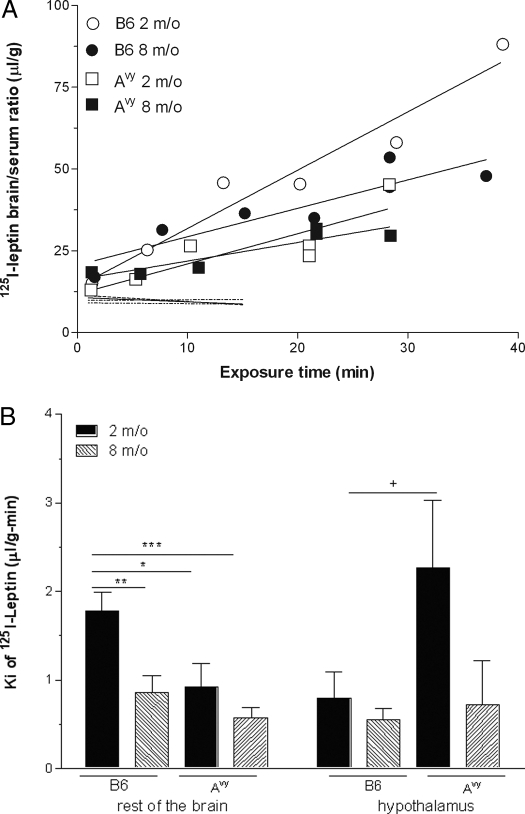
Four groups of mice were used to determine the effects of the Avy mutation and age on leptin permeation across the BBB. These mice received an iv bolus injection of 125I-leptin and the vascular permeability control 131I-albumin, and were killed at different times between 1 and 15 min after iv injection, each mouse representing one time point in the individual groups. We have shown that 125I-leptin remains intact during the study period by HPLC analysis of brain samples, and by comparison with 3H-leptin (1,35). Thus, the radioactivity measured represents the intact leptin. The influx rate, calculated from the linear regression between the brain to serum ratio of 125I-leptin and exposure time, which is longer than the respective experimental time (35), was significantly higher in the 2-month-old B6 mice than the 8-month-old B6, 2-month-old Avy, and 8-month-old Avy mice (Fig. 2A). The difference of the influx rate of 125I-leptin among the groups contrasted with the lack of significant change in the influx rate of 131I-albumin, showing that the changes in leptin influx were not caused by differences in the general permeability of the BBB.
Figure 2.
Permeation of blood-borne leptin across the BBB in the Avy mice. A, The rate of 125I-leptin crossing the BBB was determined in four groups of mice (n = 7 per group) by multiple-time regression analysis. The influx rate of leptin, shown by the slope of the regression line, was significantly higher in the 2-month-old B6 mice than in the rest of the groups (P < 0.05). The dashed, unmarked lines represent the influx of 131I-albumin. Permeation of albumin showed no difference in any of the groups, being significantly lower than that of 125I-leptin. B, In the hypothalamus, the influx rate Ki of 125I-leptin in the 2-month-old Avy mice showed a trend of increase compared with the age-matched B6 mice or the older age groups. This pattern differed from that for the rest of the brain, where a significant increase was seen in the 2-month-old B6 group compared with others. +, P < 0.1; *, P < 0.05; **, P < 0.01; ***, P < 0.005.
The hypothalamus seemed to show a different pattern of leptin influx than occurred in the rest of the brain. The 2-month-old Avy mice tended to have a higher influx rate than the other groups (P < 0.1 compared with the 2 month old B6 mice; Fig. 2B). There was no regional variation in the negligible influx of coadministered 131I-albumin.
The difference in BBB permeation was mainly related to different blood leptin concentrations in the B6 and Avy mice
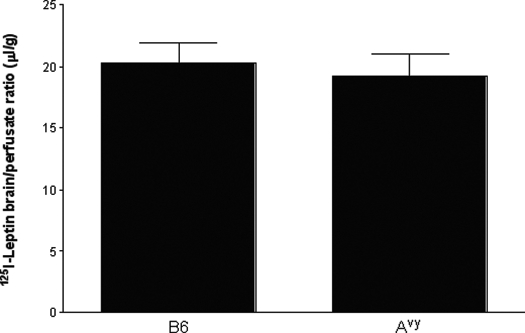
In situ brain perfusion study was used to deliver 125I-leptin for 5 min (2 ml/min) in blood-free physiological buffer into 2-month-old Avy or B6 mice. A 2-min prewash procedure was applied to remove blood from the cerebral vasculature to avoid interference from binding proteins and circulating leptin, and a post-wash step was applied to remove 125I-leptin retained in the cerebral vascular compartment. There was no significant difference in 125I-leptin uptake by the brain between the Avy and B6 groups (Fig. 3). Thus, the difference in the influx rate of 125I-leptin in the multiple-time regression analysis after iv injection appears to be mainly caused by blood-borne factors.
Figure 3.
Leptin permeation across the BBB after delivery by in situ brain perfusion for 5 min. Groups of B6 (n = 4) and Avy (n = 6) mice were studied at 2 months old. The brain uptake of 125I-leptin from the blood-free perfusate did not differ between the two groups.
The higher cerebral microvascular ObRb mRNA in the Avy mice was insufficient to increase leptin transport across the BBB
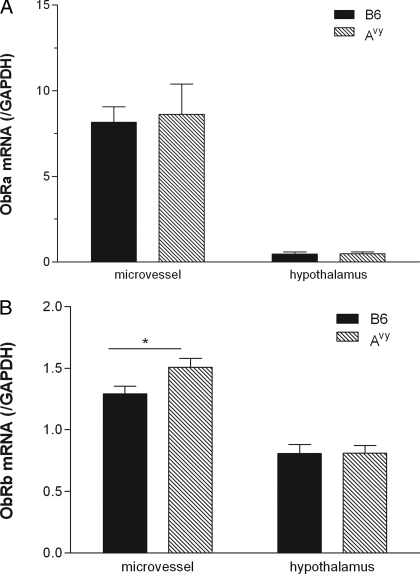
The mRNA of ObRa and ObRb in enriched microvessels from the cerebral cortex and the hypothalamus was compared in 2-month-old Avy and B6 mice. The cerebral cortex was used to avoid the leakier blood vessels of the circumventricular organs (CVOs), including those adjacent to the hypothalamus (42). In all groups, the level of ObRa was higher than ObRb in the microvessels, but the level of ObRb was higher than ObRa in the hypothalamus. This considered the potential difference in PCR amplification. For ObRa mRNA, the level of expression in the microvessels was 18 times higher than in the hypothalamus of the B6 mice. There was no difference of ObRa mRNA between the Avy and B6 mice in either region (Fig. 4A). For ObRb mRNA, the level of expression in the microvessels was about 1.6-times higher than that in the hypothalamus of the B6 mice. Avy mice showed a significant increase (P < 0.05) in microvascular ObRb mRNA, but not in hypothalamic ObRb mRNA (Fig. 4B).
Figure 4.
The level of mRNA expression of ObR isoforms in cerebral microvessels and hypothalamic homogenate. A, ObRa mRNA was unchanged between the 2-month-old B6 and Avy mice, in both the microvessels (n = 8 per group) and hypothalamus (n = 10 per group). B, ObRb mRNA in cerebral microvessels was significantly higher in the Avy mice. ObRb mRNA in the hypothalamus showed no change between the two strains at 2 months of age (n = 11 for B6 and n = 10 for Avy). *, P < 0.05.
Astrocytic ObR immunoreactivity in the Avy mice reflects a major change in the cellular phenotype with obesity
The increased mRNA of ObRb in microvessels from the Avy mice led to the question of whether there was a corresponding change in protein expression. Immunofluorescent staining was performed on the brains of 2- to 3-month-old B6 and Avy mice using an antibody recognizing all membrane-bound forms of leptin receptors (sc-1834). The specificity of immunofluorescence was shown by the absence of ObR signals in sections incubated with the primary antibody preadsorbed with ObR blocking peptide (Fig. 5A), and in sections incubated with secondary antibody only. Surprisingly, regional differences in the type of cell expression ObR were observed. Most of the ObR-positive cells in the arcuate nucleus of the hypothalamus were neurons, whereas those in the dorsomedial hypothalamus had morphology resembling astrocytes. Furthermore, Avy mice had a significantly higher number of ObR(+) astrocytes than were present in the B6 mice, in both arcuate nucleus (Fig. 5B) and dorsomedial hypothalamus (Fig. 5C). Cell counting of arcuate sections from three different mice showed that the percentage of ObR(+) astrocytes was higher in the Avy mice than those in the B6 (P < 0.005) (Table 2 and Fig. 5D). Concurrently, there was a significant reduction of the percentage of neurons that were ObR(+) in the Avy mice (P < 0.005). It appears that the mean fluorescent intensity in individual cells remained the same. Because the total number of ObR(+) cells [neurons and astrocytes because other central nervous system (CNS) cells did not show strong immunofluorescence] in the arcuate nucleus did not change significantly, this explains the lack of consistent changes of ObRa and ObRb mRNA shown previously. Overall, ObR protein expression is up-regulated in the astrocytes of Avy mice.
Figure 5.
Regional distribution and cellular phenotypes of ObR(+) cells by immunohistochemistry. A, Specificity of the ObR immunofluorescence was shown by the absence of signals with inclusion of a blocking peptide. Left panel, ObR immunofluorescence was seen in a hypothalamic section from a B6 mouse. The signal was present in the cytoplasm of neurons and occasional astrocytes (arrowheads). Right panel, A blocking peptide (sc-1834p) was included at 5-fold m excess during the overnight incubation with the ObR antibody. This preabsorption step completely abolished the immunofluorescent signal (objective, ×20; scale bar, 50 μm). B, ObR expression between the B6 (A) and Avy (B) mice differed in both neurons and astrocytes in the arcuate nucleus. The confocal microscopical images were taken with a 20× objective (scale bar, 50 μm). Seen at higher magnification (40× objective, scale bar, 30 μm), the B6 mouse (C) had fewer ObR(+) astrocytes (arrowheads) than the Avy mouse (D). C, In the dorsomedial nucleus of hypothalamus (DMH), more cells and higher staining intensity of ObR(+) astrocytes were seen in the Avy mouse (right panel) compared with the B6 control (left panel). D, The relative amount of ObR(+) astrocytes in the arcuate nucleus of the Avy mice was significantly higher than that in the B6 mice. This was inversely correlated with the relative amount of neurons. ***, P < 0.005, comparison of the same cell type between B6 and Avy; +++, P < 0.005, comparison of astrocytes and neurons of the same mouse strain (n = 3).
Table 2.
Percentage of ObR(+) astrocytes and neurons in the arcuate nucleus
| Mouse no. | Strain | No. of astrocytes (%) | Mean ± se (%) | No. of neurons (%) | Mean ± se (%) |
|---|---|---|---|---|---|
| 1 | B6 | 14 (6.1) | 215 (93.8) | ||
| 2 | B6 | 47 (11.6) | 6.87 ± 2.54 | 359 (88.4) | 93.10 ± 2.54 |
| 3 | B6 | 6 (2.9) | 201 (97.1) | ||
| 4 | Avy | 104 (27.1) | 265 (71.8) | ||
| 5 | Avy | 90 (22.1) | 24.84 ± 1.46 | 318 (77.9) | 74.79 ± 1.76 |
| 6 | Avy | 58 (25.3) | 171 (74.7) |
Double immunolabeling showed that the ObR(+) cells were astrocytes
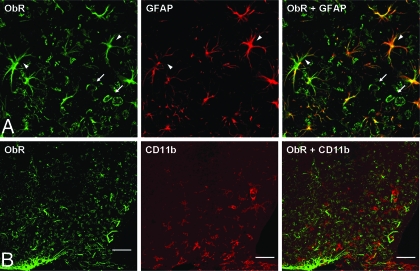
Brain sections from both Avy and B6 hypothalami were incubated with primary antibodies against ObR and GFAP and their respective secondary antibodies. Control sections were incubated with one primary antibody and its secondary antibody, or secondary antibodies only, to exclude cross-reactivity. Confocal microscopical analysis showed that all GFAP(+) cells also were ObR positive, although not all ObR-positive cells stained for GFAP. Figure 6A indicates the colocalization of ObR and GFAP in essentially all of the astrocytes in the arcuate nucleus of a B6 mouse. The colocalization in the Avy mouse was the same. By contrast, no colocalization of ObR and CD11b was seen in the Avy mouse (Fig. 6B), or its B6 controls. Thus, the microglia were not immunoreactive to ObR in the resting state.
Figure 6.
Confocal microscopical analysis of the type of cells expressing ObR. A, Colocalization of ObR (green) and GFAP (red) in the arcuate nucleus region of a B6 mouse. Essentially, all GFAP(+) astrocytes (arrowheads) coexpress ObR either at the cell body or processes. Not all ObR(+) cells are GFAP(+); the GFAP(−) cells resemble neurons (arrows). The yellow coloring indicates colocalization of ObR and GFAP in the same cells. B, Lack of colocalization of ObR (green) and CD11b (red), the marker for microglia, is shown in the Avy arcuate nucleus (scale bar, 50 μm).
Discussion
One of the advantages of the Avy model is that the extent of obesity appears to correlate well with coat color. This enables valid grouping of the mice based on their coat color at weaning. The controls are truly littermates. Growth curve measurements indicated that the Avy mice had significantly higher body weights by 8 wk of age, and the difference persisted for the rest of their lives. These obese mice show an increase of body fat composition and blood leptin concentrations, indicating the propensity to develop syndrome X (16,43).
The permeation of leptin across the BBB in two age groups of Avy and B6 mice was tested after both iv bolus delivery and in situ brain perfusion. Permeation of the iv delivered 125I-leptin was highest in the 2-month-old B6 mice, but there was no difference of the influx rate among the 2-month-old Avy, 8-month-old Avy, and 8-month-old B6 mice, all of which were lower. The difference in leptin influx was not explained by a difference in the general permeability of the BBB because the influx rate of 131I-albumin, a vascular space marker, did not change among the groups. Rather, parallel studies by in situ brain perfusion with serum-free buffer indicated that the difference was mainly caused by circulating factors and no longer persisted when leptin was delivered by perfusion.
Therefore, the higher influx rate of blood-borne 125I-leptin in the young B6 mice is probably explained by lower concentrations of circulating factors interfering with leptin transport. Endogenous leptin is certainly one of the main modulators. The Avy mice had significantly higher leptin concentrations than the B6 mice shown by ELISA. In addition, age-related changes of leptin concentrations were observed in the wild-type mice (19). The higher concentrations of leptin in the Avy and older B6 mice may exacerbate the low efficiency of leptin transport (16,21). Associated with hyperleptinemia, triglycerides also exert potent inhibition of the leptin transport system (22). In addition to the level of leptin, the soluble leptin receptors, including ObRe, also undergo developmental changes (44). We have shown that circulating soluble leptin receptors can reduce leptin transport across the BBB (45). Thus, the intrinsic capacity of the BBB to transport leptin was not altered in the Avy mice. This is similar to what has been observed in other genetic models of obesity and leptin resistance, including the ob/ob and db/db mice (25) and Kolestsky rats with deficiency of ObRa (26,46). Rather, the kinetics of leptin permeation in the Avy mice can be modulated by the high concentration of circulating leptin and the presence of leptin binding proteins, such as the soluble receptors.
We have found that the 1-month-old Avy mice have higher brain uptake of mahogany peptide than the age-matched B6 mice or older mice after the onset of obesity (31). The higher influx of leptin in the young age group was consistent with this. Both mahogany peptide and leptin are anorexigenic signals; although the saturation of BBB transport is probably a consequence, rather than a cause, of obesity, altered dynamics of permeation of ingestive peptides across the BBB conceivably exacerbates CNS pathology.
The hypothalamic region of the 2-month-old Avy mice tended to have a higher influx rate of leptin than the other study groups, as shown by multiple-time regression analysis. This differed from the higher influx rate of leptin in the 2-month-old B6 mice in the rest of the brain. Although one may speculate that the hypothalamic vasculature shows different density, surface area, volume, or length than other brain regions, these potential differences did not affect the permeation of coadministered albumin, a marker for vascular space. Leptin belongs to the class of polypeptides that penetrate the BBB slowly, and its permeation is not dependent on blood flow. Therefore, the influx rate determined by multiple-time regression analysis is equivalent to the permeability-surface area product and has considered the microvascular density. Nonetheless, the arcuate nucleus shows higher leptin uptake than many other brain regions in the classical study (1). This correlates with a high level of ObR expression in the hypothalamus shown by in situ hybridization (47,48), and by immunohistochemistry (49,50). This leads to the speculation that the Avy mice have higher ObR levels in the hypothalamus.
In parallel with the measurement of mRNA for ObR isoforms in the hypothalamus, we sampled cerebral microvessels. Rather than isolating microvessels from the hypothalamus, the cortex devoid of CVOs was used for capillary depletion. This obviates the concern that the median eminence, one of the vascular CVOs, has axonal projections and peptidergic communications to the adjacent arcuate nucleus of the hypothalamus (51). In general, the CVOs do not provide a route of direct communication between most of the CNS and circulation (42,52). This is mainly because of barriers of tanycytes and ependymal cells separating the median eminence from the arcuate nucleus (53,54), and from the cerebrospinal fluid of the fourth ventricle (55). Nonetheless, “contamination” by the median eminence is still highly probable when dissecting the hypothalamus from mouse brain without fixation.
PCR was performed for ObRa and ObRb, the two main receptor subtypes mediating leptin transport (44,56). Consistent with findings from others and our own laboratory (5,56,57,58), the level of expression of ObRa mRNA in microvessels was about six times higher than that of ObRb, although there could be differences in the efficiency of PCR amplification. This is an expected result for microvessels that constitute the main component of the BBB because ObRa is considered the main transporter of leptin due to its high abundance at microvessels.
Our results showed that there was no difference in microvascular ObRa mRNA between the B6 and Avy mice. This further supports the lack of intrinsic changes of BBB permeability to leptin shown in the transport assays. However, the expression of ObRb mRNA showed a small yet significant increase in the Avy mice compared with the age-matched B6 mice. ObRb is the longest isoform of all the leptin receptors; its cytoplasmic domain contains Box 3 essential for STAT3 activation (59). The functions of endothelial leptin signaling by ObRb are not clear; however, studies on adiponectin (60) and TNF (61,62) have shown relaying functions of BBB endothelial cells in signal transmission by secondary mediators.
In the hypothalamus, where most studies of leptin signaling have been conducted, the level of ObRa mRNA was extremely low, and ObRb mRNA showed no difference between the B6 and Avy groups. Thus, the trend of higher leptin influx in the hypothalamus of young Avy mice was not caused by differences in ObRa and ObRb expression.
The cellular components of the BBB include not only microvascular endothelial cells, but also pericytes and astrocytes. To identify the spatial relationship between leptin receptor protein expression and leptin transport across the BBB, we performed immunohistochemical studies on brain sections from the Avy and B6 mice. The results showed an astonishing difference in the number of ObR(+) cells, particularly in the hypothalamic region. The specificity of immunostaining was verified by the lack of signals in sections after preadsorption of the primary antibody with a blocking peptide. Furthermore, the increase of ObR expression in the Avy mice was seen mainly in astrocytes, rather than in neurons, microglia, or endothelial cells (despite endothelial cells showing higher ObRb mRNA expression). At this point we were not able to differentiate the subtypes of leptin receptors in the astrocytes by in situ hybridization because the differing sequences of ObRa, ObRc, and ObRd were too short for the design of specific probes. Despite the increase of ObRb mRNA in microvessels, we did not detect a significant increase of endothelial ObR immunoreactivity by immunostaining. This further supports the sensitivity of microvessel enrichment and real-time PCR for detection (40,41). Overall, the increase of astroglial ObR expression in the Avy mice was associated with the decrease of the percentage of ObR(+) neurons. This probably explains the lack of changes of ObR mRNA or leptin transport in the hypothalamus, though expression level of ObRc and ObRe, and rate of receptor degradation in the Avy mice might also play a role.
The functions of leptin receptors in astrocytes have not been fully elucidated. Cheunsuang and Morris showed that systemically delivered hydroxystilbamidine (FluoroGold equivalent) can be taken up by astrocytes in selective regions in the brain 2–6 h later, mainly in the median eminence and the adjacent ventral part of the arcuate nucleus (63). Many of these GFAP(+) but vimentin (−) and NeuN (−) cells also express leptin receptors and neuropeptide Y Y1 receptors (64). Leptin is trophic to neural progenitor cells and promotes glial as well as neuronal development. Young (65) has shown by immunohistochemistry that astrocytes that are positive for brain fatty acid binding protein are located in close proximity to STAT3(+) neurons. Wilkinson and colleagues (66,67) showed that the C6 astroglioma cells express mRNA for both ObRa and leptin, and that RNA interference of leptin increases cell death in these cells. These findings are consistent with the keen observation of the endocrine glia more than a decade ago (68). It is yet to be to determined what functions these astrocytic leptin receptors serve, whether they exert regulatory roles in the neuroendocrine circuitry of Avy mice, and how they affect overall neuronal survival and function.
In summary, the reduced permeation of blood-borne leptin across the BBB in the obese Avy mice was mainly caused by circulating factors. In situ brain perfusion study showed that the intrinsic transport system for leptin remained the same. Thus, the Avy mice exhibit peripheral leptin resistance. In the cerebral microvessels of Avy mice, there was an increase of ObRb but no change in ObRa mRNA. Most interestingly, there was a dramatic increase of ObR(+) astrocytes in the Avy mice, shown by immunohistochemistry with an antibody for which the specificity of staining was confirmed. Ongoing studies will determine the subtypes of ObR in these astrocytes. The up-regulated astrocytic ObRs may play crucial roles in the regulation of adult-onset obesity in the Avy mice.
Acknowledgments
We thank Ms. Loula Burton for her editorial assistance.
Footnotes
This work was supported by National Institutes of Health Grants DK54880 (to A.J.K.), NS45751, and NS46528 (to W.P.).
Disclosure Statement: The authors have nothing to declare.
First Published Online February 21, 2008
Abbreviations: AgRP, Agouti-related protein; Avy, agouti viable yellow; BBB, blood-brain barrier; CNS, central nervous system; CVO, circumventricular organ; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFAP, glial fibrillary acidic protein; MCR, melanocortin receptor; STAT, signal transducer and activator for transcription.
References
- Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM 1996 Leptin enters the brain by a saturable system independent of insulin. Peptides 17:305–311 [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Jovanovic S, Miao W, Samara S, Verma S, Farrell CL 2000 Differential regulation of leptin transport by the choroid plexus and blood-brain barrier and high affinity transport systems for entry into hypothalamus and across the blood-cerebrospinal fluid barrier. Endocrinology 141:1434–1441 [DOI] [PubMed] [Google Scholar]
- Banks WA 2003 Is obesity a disease of the blood-brain barrier? Physiological, pathological, and evolutionary considerations. Curr Pharm Des 9:801–809 [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ 2007 Adipokines and the blood-brain barrier. Peptides 28:1317–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uotani S, Bjorbaek C, Tornoe J, Flier JS 1999 Functional properties of leptin receptor isoforms: internalization and degradation of leptin and ligand-induced receptor downregulation. Diabetes 48:279–286 [DOI] [PubMed] [Google Scholar]
- Nijenhuis WA, Oosterom J, Adan RA 2001 AgRP(83–132) acts as an inverse agonist on the human-melanocortin-4 receptor. Mol Endocrinol 15:164–171 [DOI] [PubMed] [Google Scholar]
- Bouret SG, Simerly RB 2007 Development of leptin-sensitive circuits. J Neuroendocrinol 19:575–582 [DOI] [PubMed] [Google Scholar]
- Dickies MM 1962 A new viable yellow mutation in the house mouse. J Hered 53:84–86 [DOI] [PubMed] [Google Scholar]
- Wolff GL 1965 Body composition and coat color correlation in different phenotypes of “viable yellow” mice. Science 147:1145–1147 [DOI] [PubMed] [Google Scholar]
- Herberg L, Coleman DL 1977 Laboratory animals exhibiting obesity and diabetes syndromes. Metabolism 26:59–99 [DOI] [PubMed] [Google Scholar]
- Wolff GL, Roberts DW, Galbraith DB 1986 Prenatal determination of obesity, tumor susceptibility, and coat color pattern in viable yellow (Avy/a) mice. The yellow mouse syndrome. J Hered 77:151–158 [DOI] [PubMed] [Google Scholar]
- Shimizu H, Shargill NS, Bray GA, Yen TT, Gesellchen PD 1989 Effects of MSH on food intake, body weight and coat color of the yellow obese mouse. Life Sci 45:543–552 [DOI] [PubMed] [Google Scholar]
- Wolff GL, Whittaker P 2005 Dose-response effects of ectopic agouti protein on iron overload and age-associated aspects of the Avy/a obese mouse phenome. Peptides 26:1697–1711 [DOI] [PubMed] [Google Scholar]
- Duhl DM, Vrieling H, Miller KA, Wolff GL, Barsh GS 1994 Neomorphic agouti mutations in obese yellow mice. Nat Genet 8:59–65 [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ 1996 Passage of peptides across the blood-brain barrier: pathophysiological perspectives. Life Sci 59:1923–1943 [DOI] [PubMed] [Google Scholar]
- Banks W 2004 The many lives of leptin. Peptides 25:331–338 [DOI] [PubMed] [Google Scholar]
- Banks WA 2006 Denial versus dualism: the blood-brain barrier as an interface of the gut-brain axis. Endocrinology 147:2609–2610 [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Pan W 2000 Dynamic regulation of leptin entry into brain by the blood-brain barrier. Regul Pept 92:37–43 [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Banks WA 2004 Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol 286:R143–R150 [DOI] [PubMed] [Google Scholar]
- Banks WA, King BM, Rossiter KN, Olson RD, Olson GA, Kastin AJ 2001 Obesity-inducing lesion of the central nervous system alter leptin uptake by the blood-brain barrier. Life Sci 69:2765–2773 [DOI] [PubMed] [Google Scholar]
- Banks WA, Farrell CL 2003 Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible. Am J Physiol Endocrinol Metab 285:E10–E15 [DOI] [PubMed] [Google Scholar]
- Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE 2004 Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes 53:1253–1260 [DOI] [PubMed] [Google Scholar]
- Banks WA 2001 Enhanced leptin transport across the blood-brain barrier by alpha 1-adrenergic agents. Brain Res 899:209–217 [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V 2001 Glucose and insulin increase the transport of leptin through the blood-brain barrier in normal mice but not in streptozotocin-diabetic mice. Neuroendocrinology 73:237–242 [DOI] [PubMed] [Google Scholar]
- Maness LM, Banks WA, Kastin AJ 2000 Persistence of blood-to-brain transport of leptin in obese leptin-deficient and leptin receptor-deficient mice. Brain Res 873:165–167 [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Pan W, Maness LM, Koletsky RJ, Ernsberger P 1999 Decreased transport of leptin across the blood-brain barrier in rats lacking the short form of the leptin receptor. Peptides 20:1449–1453 [DOI] [PubMed] [Google Scholar]
- Ebihara K, Ogawa Y, Katsuura G, Numata Y, Masuzaki H, Satoh N, Tamaki M, Yoshioka T, Hayase M, Matsuoka N, Aizawa-Abe M, Yoshimasa Y, Nakao K 1999 Involvement of agouti-related protein, an endogenous antagonist of hypothalamic melanocortin receptor, in leptin action. Diabetes 48:2028–2033 [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, Hackler L 2000 Agouti-related protein(83–132) aggregates and crosses the blood-brain barrier slowly. Metabolism 49:1444–1448 [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ, Yu Y, Cain CM, Fairburn T, Stütz AM, Argyropoulos G 2005 Selective tissue uptake of AgRP (82–131) and its modulation by fasting. Endocrinology 146:5533–5539 [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V 2000 Mahogany (1377–1428) enters brain by a saturable transport system. J Pharmacol Exp Ther 294:633–636 [PubMed] [Google Scholar]
- Pan W, Kastin AJ 2007 Mahogany, blood-brain barrier, and fat mass surge in AVY mice. Int J Obes (Lond) 31:1030–1032 [DOI] [PubMed] [Google Scholar]
- Dinulescu DM, Fan W, Boston BA, McCall K, Lamoreux ML, Moore KJ, Montagno J, Cone RD 1998 Mahogany (mg) stimulates feeding and increases basal metabolic rate independent of its suppression of agouti. Proc Natl Acad Sci USA 95:12707–12712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle DL, McGrail SH, Vitale J, Woolf EA, Dussault Jr BJ, DiRocco L, Holmgren L, Montagno J, Bork P, Huszar D, Fairchild-Huntress V, Ge P, Keilty J, Ebeling C, Baldini L, Gilchrist J, Burn P, Carlson GA, Moore KJ 1999 The mahogany protein is a receptor involved in suppression of obesity. Nature 398:148–152 [DOI] [PubMed] [Google Scholar]
- Wolff GL 1997 Obesity as a pleiotropic effect of gene action. J Nutr 127:1897S–1901S [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, Pan W 2001 Validity of multiple-time regression analysis in measurement of tritiated and iodinated leptin crossing the blood-brain barrier: meaningful controls. Peptides 22:2127–2136 [DOI] [PubMed] [Google Scholar]
- Pan W, Tu H, Hsuchou H, Daniel J, Kastin AJ 2007 Unexpected amplification of leptin-induced Stat3 signaling by urocortin: implications for obesity. J Mol Neurosci 33:232–238 [DOI] [PubMed] [Google Scholar]
- Soma LR 1983 Anesthetic and analgesic considerations in the experimental animal. Ann NY Acad Sci 406:32–47 [DOI] [PubMed] [Google Scholar]
- Hara K, Harris RA 2002 The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg 94:313–318 [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Pan W 1999 Uptake and degradation of blood-borne insulin by the olfactory bulb. Peptides 20:373–378 [DOI] [PubMed] [Google Scholar]
- Pan W, Ding Y, Yu Y, Ohtaki H, Nakamachi T, Kastin AJ 2006 Stroke upregulates TNFalpha transport across the blood-brain barrier. Exp Neurol 198:222–233 [DOI] [PubMed] [Google Scholar]
- Yu C, Kastin AJ, Ding Y, Pan W 2007 Gamma glutamyl transpeptidase is a dynamic indicator of endothelial response to stroke. Exp Neurol 203:116–122 [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Pan W 2006 Intranasal leptin: blood-brain barrier bypass (BBBB) for obesity? Endocrinology 147:2086–2087 [DOI] [PubMed] [Google Scholar]
- Banks WA, Altmann J, Sapolsky RM, Phllips-Conroy JE, Morley JE 2003 Serum leptin levels as a marker for a syndrome X-like condition in wild baboons. J Clin Endocrinol Metab 88:1234–1240 [DOI] [PubMed] [Google Scholar]
- Pan W, Hsuchou H, Tu H, Kastin AJ 2008 Developmental changes of leptin receptors in cerebral microvessels: unexpected relation to leptin transport. Endocrinology 149:877–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Kastin AJ, Hsuchou H, Pan W 2007 Soluble receptor ObRe inhibits leptin transport. J Cell Physiol 214:301–305 [DOI] [PubMed] [Google Scholar]
- Banks WA, Niehoff ML, Martin D, Farrell CL 2002 Leptin transport across the blood-brain barrier of the Koletsky rat is not mediated by a product of the leptin receptor gene. Brain Res 950:130–136 [DOI] [PubMed] [Google Scholar]
- Guan XM, Hess JF, Yu H, Hey PJ, Van der Ploeg LH 1997 Differential expression of mRNA for leptin receptor isoforms in the rat brain. Mol Cell Endocrinol 133:1–7 [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB 1998 Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395:535–547 [PubMed] [Google Scholar]
- De Matteis R, Cinti S 1998 Ultrastructural immunolocalization of leptin receptor in mouse brain. Neuroendocrinology 68:412–419 [DOI] [PubMed] [Google Scholar]
- Yarnell DO, Knight DS, Hamilton K, Tulp O, Tso P 1998 Localization of leptin receptor immunoreactivity in the lean and obese Zucker rat brain. Brain Res 785:80–90 [DOI] [PubMed] [Google Scholar]
- Lantos TA, Gorcs TJ, Palkovits M 1995 Immunohistochemical mapping of neuropeptides in the premamillary region of the hypothalamus in rats. Brain Res Brain Res Rev 20:209–249 [DOI] [PubMed] [Google Scholar]
- Banks WA 2006 Blood-brain barrier and energy balance. Obesity (Silver Spring) 14(Suppl 5):234S–237S [DOI] [PubMed] [Google Scholar]
- Rethelyi M 1984 Diffusional barrier around the hypothalamic arcuate nucleus in the rat. Brain Res 307:355–358 [DOI] [PubMed] [Google Scholar]
- Rethelyi M 1997 Two decades around the hypothalamic median eminence. Neurobiology (Bp) 5:431–440 [PubMed] [Google Scholar]
- Peruzzo B, Pastor FE, Blazquez JL, Schobitz K, Pelaez B, Amat P, Rodriguez EM 2000 A second look at the barriers of the medial basal hypothalamus. Exp Brain Res 132:10–26 [DOI] [PubMed] [Google Scholar]
- Tu H, Pan W, Feucht L, Kastin AJ 2007 Convergent trafficking pattern of leptin after endocytosis mediated by ObRa-ObRd. J Cell Physiol 212:215–222 [DOI] [PubMed] [Google Scholar]
- Hileman SM, Tornøe J, Flier JS, Bjørbæk C 2000 Transcellular transport of leptin by the short leptin receptor isoform ObRa in Madin-Darby Canine Kidney cells. Endocrinology 141:1955–1961 [DOI] [PubMed] [Google Scholar]
- Hileman SM, Pierroz DD, Masuzaki H, Bjorbaek C, El Haschimi K, Banks WA, Flier JS 2002 Characterization of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology 143:775–783 [DOI] [PubMed] [Google Scholar]
- Bjørbæk C, Uotani S, da Silva B, Flier JS 1997 Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem 272:32686–32695 [DOI] [PubMed] [Google Scholar]
- Spranger J, Verma S, Gohring I, Bobbert T, Seifert J, Sindler A, Pfeiffer A, Hileman S, Tschop M, Banks W 2006 Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes 55:141–147 [PubMed] [Google Scholar]
- Yu C, Kastin AJ, Tu H, Pan W 2007 Opposing effects of proteasomes and lysosomes on LIFR: modulation by TNF. J Mol Neurosci 32:80–89 [DOI] [PubMed] [Google Scholar]
- Yu C, Kastin AJ, Pan W 2007 TNF reduces LIF endocytosis despite increasing NFκB-mediated gp130 expression. J Cell Physiol 213:161–166 [DOI] [PubMed] [Google Scholar]
- Cheunsuang O, Morris R2005 Astrocytes in the arcuate nucleus and median eminence that take up a fluorescent dye from the circulation express leptin receptors and neuropeptide Y Y1 receptors. Glia 52:228–233 [DOI] [PubMed] [Google Scholar]
- Udagawa J, Hashimoto R, Suzuki H, Hatta T, Sotomaru Y, Hioki K, Kagohashi Y, Nomura T, Minami Y, Otani H 2006 The role of leptin in the development of the cerebral cortex in mouse embryos. Endocrinology 147:647–658 [DOI] [PubMed] [Google Scholar]
- Young JK 2002 Anatomical relationship between specialized astrocytes and leptin-sensitive neurones. J Anat 201:85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morash B, Johnstone J, Leopold C, Li A, Murphy P, Ur E, Wilkinson M 2000 The regulation of leptin gene expression in the C6 glioblastoma cell line. Mol Cell Endocrinol 165:97–105 [DOI] [PubMed] [Google Scholar]
- Brown R, Morash B, Ur E, Wilkinson M 2005 RNAi-mediated silencing of leptin gene expression increases cell death in C6 glioblastoma cells. Brain Res Mol Brain Res 139:357–360 [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Chowen JA, Naftolin F 1996 Endocrine glia: roles of glial cells in the brain actions of steroid and thyroid hormones and in the regulation of hormone secretion. Front Neuroendocrinol 17:180–211 [DOI] [PubMed] [Google Scholar]