Abstract
Progesterone receptor (PR) expression is highly dependent on estradiol in the medial preoptic nucleus (MPN) and the ventromedial nucleus (VMN) of the adult rat brain. During development, males express high levels of PR in the MPN, whereas females have virtually no PR, a sex difference resulting entirely from differential exposure to estradiol. Because PR is also estradiol dependent in the adult VMN, the present study examined the regulation of PR immunoreactivity (PRir) in the developing VMN. Surprisingly, PRir was present at high levels in the VMN of both neonatal males and females. In the neonatal VMN, PR expression was dependent on gonadal hormones in males but not females. When females were ovariectomized and exposed to estradiol at various ages from neonatal to adulthood, estradiol reliably induced PRir in the MPN at postnatal d 7 but failed to induce PRir in the VMN of the same animals. Only later in development, around postnatal d 14, did estradiol increase PRir in the female VMN. There appears to be a developmental switch in the VMN when PR expression changes from estradiol independent to estradiol dependent. Furthermore, this switch is anatomically specific and does not exist in the MPN. The present results indicate that the regulation of PR expression by estradiol is dependent on age, sex, and brain region, suggesting that PR may play a critical but specific role in the normal development of these reproductively important brain areas. In addition, the neonatal female VMN may provide a unique model in which to examine the mechanisms underlying the specificity of steroid-induced gene expression.
THE SENSITIVITY OF the brain to steroid hormones is determined by the expression of nuclear steroid receptors within specific populations of neurons. As transcription factors, steroid hormone receptors regulate the transcription of specific, hormone-sensitive genes and can thereby exert powerful effects on both adult and developing brain. The factors that regulate the expression of steroid receptors in the brain have not been fully elucidated, and the mechanisms that control the regional specificity of this process are not understood.
A profound example of steroid receptor regulation of gene expression is the induction of progesterone receptor (PR) expression by estradiol, acting primarily through estrogen receptor (ER)-α (1,2,3). In the adult rodent brain, PR is highly regulated by estradiol within the medial preoptic nucleus (MPN) and the ventromedial nucleus of the hypothalamus (VMN) (4,5,6,7,8). For example, in the adult female rat, PR expression within the VMN is highly dependent on circulating estradiol levels, such that PR expression varies dramatically over the course of the estrous cycle (9) with the natural rise and fall of estradiol secretion from the ovary. Estradiol increases the levels of PR mRNA (8,10,11) and PR protein (12,13,14) in both regions. This is consistent with the evidence that the promoter region of the rat PR gene contains at least four consensus sequences for weak and imperfect, but functional, estrogen response elements (15).
Within the MPN of the developing brain, PR expression is highly regulated by estradiol in both males and females. Castration of males on the day of birth (16) or treatment of fetal males with the aromatase inhibitor, ATD (1,4,6-androstatrien-3-17-dione) (17), abolishes PR expression in the MPN, indicating that estradiol metabolized from testicular testosterone, can induce PR expression in the male MPN. Although PR expression can be induced in the perinatal female MPN by exogenous estrogen treatment (17), the ovary is steroidogenically quiescent until the second postnatal week (18,19,20,21). Therefore, PR is virtually absent in the neonatal female MPN (16,22,23). The differential secretion of steroids in developing males and females results in a dramatic sex difference in PR expression within the MPN (24). However, by postnatal day (P) 14, after the initiation of estradiol secretion by the ovary, PR expression is increased in the female MPN (16) and the sex difference is attenuated.
If PR expression were regulated by estradiol in the neonatal VMN, as it is in the adult VMN and perinatal MPN, one would predict that the male VMN would express higher levels of PR than the female VMN. Experiment 1 examined PR expression in neonatal males and females. In contrast to the MPN, the results demonstrated that the levels of PR expression are higher in females than males, suggesting that sex differences in PR expression in the neonatal VMN may be independent of gonadal hormones. Therefore, experiment 2 assessed the effects of neonatal gonadectomy on PR expression in the VMN. Results suggested that the sex difference in PR expression is independent of gonadal hormones during early development. Additionally, PR expression in the neonatal female VMN is not regulated by ovarian hormones as it is in the adult. Therefore, experiment 3 examined the ability of estradiol to induce PR expression in the female VMN over the life span. Results from the present study indicate the regulation of PR expression by estradiol is sexually dimorphic, anatomically specific, and developmentally dependent.
Materials and Methods
Animals
Subjects were the offspring of adult virgin female Sprague Dawley rats, aged 60–80 d, that were mated with males of the same strain. The day copulatory plugs were found was designated as d 1 of gestation. Pregnant and lactating females were housed singly in plastic tubs and maintained in a temperature- and light-controlled room (14 light, 10 dark; lights on at 0600 h) with food and water available ad libitum. Pregnant females were left undisturbed for the duration of their pregnancy. The day of birth was designated as P1. All animal procedures used in this study were approved by the Institutional Animal Care and Use Committee at the University of Massachusetts, Amherst, or at the University at Albany-State University of New York.
Ovariectomy
Neonatal females (P1 or P7) were anesthetized by placing them on ice. With the aid of a dissecting microscope, bilateral incisions were made through the skin and muscle wall in the flank region. Both ovaries were removed with forceps. The muscle wall, as well as the skin, was sutured with silk and the wound sealed with Nexaband (Closure Medical Corp., Raleigh, NC). Pups were allowed to recover on a warming pad before being returned to their mothers. Sham ovariectomies were performed similarly but the ovaries were left intact.
Older animals (>P7) were anesthetized with 90 mg/kg ketamine (ip, Ketaset; Henry Schein, Inc., Melville, NY) and 20 mg/kg xylazine (im, Anased; Henry Schein). Bilateral incisions were made through the skin and muscle wall in the flank region. Both ovaries were ligated and removed. The muscle wall was sutured with silk and the skin was stapled shut. Sham ovariectomies were performed similarly, but the ovaries were left intact. Subjects were allowed to recover on a warming pad before being returned to their home cages.
Castration
P1 males were anesthetized by placing them on ice. With the aid of a dissection microscope, an incision, above the phallus and at the midline, was made through the skin and muscle wall. Both testes were removed with forceps. The muscle wall was sutured with silk and the skin was sealed with Nexaband. Pups were allowed to recover on a warming pad before being returned to their mothers. Sham castrations were performed similarly but the testes were left intact.
Experiment 1: PR expression in the MPN and VMN of postnatal male and females
PR expression is sexually dimorphic in the neonatal MPN, with males expressing higher levels of PR than females (24). To determine whether a similar sex difference in PR exists in the neonatal VMN, brains of males and females were collected as follows. On P7 and P14, female and male rats were anesthetized with a lethal dose of chloral-hydrate pentobarbital (0.25 m chloral hydrate, 0.08 m magnesium sulfate, 45 mm pentobarbital, 3 m ethyl alcohol, 4.5 m propylene glycol in distilled water, ip) and were intracardially perfused with 0.9% saline followed 5% acrolein in 0.1 m phosphate buffer [PB (pH 7.6)]. Brains were removed from the skull and postfixed in 5% acrolein in 0.1 m PB followed by cryoprotection in 30% sucrose in 0.1 m PB until time of sectioning. Brains were sectioned at 50-μm-thick sections in the coronal plane on a rotary microtome. Sections were stored in cryoprotectant (30% sucrose, 0.1% polyvinyl-pyrrolidone-40 in ethylene glycol and 0.1 m PB) at −20 C until processing for immunocytochemistry as described below.
Experiment 2: the effect of gonadectomy on PR expression in the MPN and VMN males and females
To assess the contribution of gonadal hormones to PR expression within the neonatal MPN and VMN of both sexes, male and female pups were gonadectomized on the day of birth, and PR expression was examined either before (P4) or after (P14) the onset of ovarian steroidogenesis. Within 18 h after birth, female and male pups were either gonadectomized (GDX) or sham GDX as described above. Brains from sham GDX and GDX animals were collected on P4 or P14 as follows. On P4, animals were anesthetized by hypothermia, and after rapid decapitation, brains were removed from the skull and immediately immersion-fixed in 5% acrolein in 0.1 m PB for 6 h and then cryoprotected in 30% sucrose in 0.1 m PB until the time of sectioning. On P14, animals were killed with a lethal dose of Nembutal (50 mg/kg, ip; pentobarbital sodium; Henry Schein). Animals were intracardially perfused with 0.9% saline followed by 5% acrolein in 0.1 m PB. Brains were removed from the skull and postfixed in 5% acrolein in 0.1 m PB followed by cryoprotection in 30% sucrose until time of sectioning. All brains were sectioned at 50 μm thickness in the coronal plane on a rotary microtome and stored in cryoprotectant at −20 C until immunocytochemical processing. P4 and P14 tissues were run in separate immunocytochemical trials and were therefore analyzed separately.
Experiment 3: estradiol induction of PR in the MPN and VMN of females across development
PR expression in the adult female VMN is highly dependent on estradiol. In contrast, PR expression in the neonatal female VMN does not appear to be dependent on ovarian hormones. To determine when during development PR expression becomes estradiol dependent in the VMN, female rats were challenged with a dose of estradiol benzoate (EB) at different ages, and PR expression was examined. On P1, female pups were randomly assigned to one of five age groups: P7, P14, P35, P49, and P67. Seven days before the animals were killed (i.e. on P1, P7, P28, P42, and P60) females were ovariectomized as described above. Two days before the animals were killed, half of the females in each age group were weighed and administered a single injection of EB (20 μg/kg in sesame oil, sc), whereas the other half received an equal volume of sesame oil alone.
On the day the animals were killed, animals were administered a lethal dose of Nembutal (50 mg/kg, ip; pentobarbital sodium; Henry Schein) followed by rapid decapitation. Brains were removed from the skull. Under a dissecting microscope, brains were sectioned at the midsagittal plane with a scalpel. Half of the brain was immediately immersed overnight in 5% acrolein in 0.1 m PB (pH 7.6), whereas the other half was frozen on powdered dry ice and stored at −80 C for use in another experiment. The acrolein-fixed half of each brain was cryoprotected in 30% sucrose in 0.1 m PB (pH 7.6). Brains were sectioned in the coronal plane on a rotary microtome at a thickness of 50 μm and stored in cryoprotectant at −20 C until immunocytochemical processing.
In addition, uterine horns from each animal were collected as a bioassay for EB treatment. Excess fat was removed and the uterine horns were weighed to the nearest 0.01 g.
Immunocytochemistry
Free-floating brain sections from each experiment were processed for immunocytochemistry in separate trials using a rabbit polyclonal antibody (Dako Inc., Glostrup, Denmark) directed against a region adjacent to the DNA binding domain of the human PR. This antibody detects both the A and B isoforms of PR (25). Details of this immunocytochemical process have been previously described in detail (17,24). Briefly, brain sections were incubated in 1% sodium borohydride in Tris-buffered saline followed by incubation in blocking serum containing 20% normal goat serum, 1% H2O2, and 1% BSA. PR antiserum was diluted to 1:1000 and incubated with brain sections for 72 h. Sections were then incubated in biotinylated goat antirabbit IgG (Vector Laboratories, Burlingame, CA) at a concentration of 2.5–5 μg/ml. Next, sections were incubated in the ABC reagent (Vectastain Elite kit, Vector Laboratories) followed by incubation in a solution containing 0.05% diaminobenzidine, 0.75 mm nickel ammonium sulfate, 0.15% -β-d-glucose, 0.04% ammonium chloride, and 0.001% glucose oxidase. Sections were then rinsed in Tris-buffered saline, mounted onto gelatin-coated slides and allowed to air dry. Sections were then dehydrated, delipidated, and coverslipped with Permount (Fisher Scientific, Hampton, NH).
Analysis
A single representative section through the caudal aspect of the MPN and VMN ventrolateral division for each rat was selected for image analysis. Each section of the MPN and VMN was anatomically matched across rats using distinguishing landmarks (26,27). Rats that did not contain an anatomical match for a region were excluded from the analysis of that region. Microscopic images of the PR immunoreactivity (PRir) in the MPN and VMN were captured with an Olympus BH-2 microscope fitted with a CCD72 camera (Dage MTI, Michigan City, MI) that was connected to a QuickCapture frame grabber board (Data Translation Inc., Marlboro, MA) in a MacIntosh IIfx computer. NIH Image software (hhtp://rsb.info.nih.gov/nih-image) was used to analyze captured image. The relative amount of PRir in the MPN and VMN was determined by measuring the area (square micrometers) covered by thresholded pixels [i.e. those pixels with a gray level higher than a defined threshold density (specific immunoreactive staining)]. Threshold was determined as a constant function of the background OD defined as the mean OD 3–5 times the sd higher than the mean background density. The mean background density was measured in a region devoid of PRir, immediately lateral to the analyzed region containing PRir.
Statistical analyses
In all experiments, relative total amount of PRir was analyzed using a two-way ANOVA followed by preplanned multiple post hoc comparisons using Student-Neuman-Keuls post hoc test (P < 0.05). In experiment 1, separate two-way ANOVAs (sex × age) was performed for the MPN and VMN. In experiment 2, separate two-way ANOVAs (GDX × sex) were performed for each region at P4 and P14. In experiment 3, separate two-way ANOVAs (EB treatment × age) were performed for the MPN and VMN. The effect of EB on uterine weights, in experiment 3, was assessed by a Student’s t test at each age. Uterine weights were not compared across ages because weights increased dramatically simply due to somatic growth.
Results
Experiment 1: PR expression in the MPN and VMN of postnatal male and females
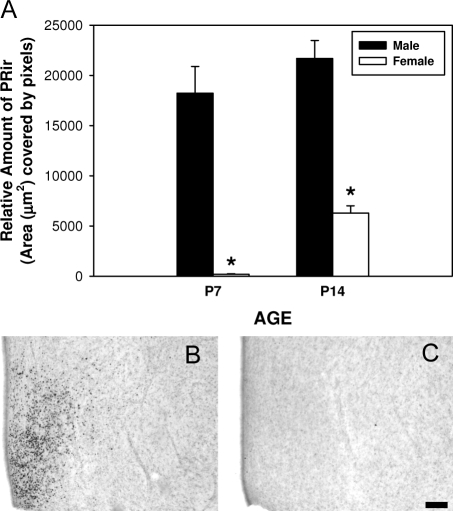
The direction of the sex difference in PRir was dependent on region. In the MPN, males had higher levels of PRir than females at P7 and P14, whereas in the VMN, females had higher levels of PRir than males at both ages.
VMN.
Two-way ANOVA (age × sex) revealed significant main effects of age [F(1,21) = 4.9, P < 0.05] and sex [F (1, 21) = 18.9, P < 0.001]. There was no significant interaction between sex and age. Post hoc analysis revealed that females had significantly higher levels of PRir in the VMN, compared with males at both P7 (P < 0.05) and P14 (P < 0.01). There was no significant effect of age in either sex (Fig. 1).
Figure 1.
PRir in the VMN of P7 and P14 is sexually dimorphic. A, The relative amount of PRir in males (black bars) and females (white bars). PRir in representative sections of P7 male (B) and female (C). *, Significantly different from the opposite sex of the same age. Bar, 100 μm.
MPN.
Two-way ANOVA (age × sex) revealed a significant main effect of age [F(1,22) = 9.8, P < 0.01] and a significant main effect of sex [F (1, 22) = 119.0, P < 0.001]. There was no significant interaction between sex and age. Post hoc analysis revealed that males had significantly higher levels of PRir in the MPN, compared with females at both P7 and P14 (P < 0.001). PRir in the MPN of females significantly increased between P7 and P14 (P < 0.01), but there was no effect of age in males (Fig. 2).
Figure 2.
PRir in the MPN of P7 and P14 is sexually dimorphic. A, The relative amount of PRir in males (black bars) and females (white bars). PRir in representative sections of P7 male (B) and female (C). *, Significantly different from the opposite sex of the same age. Bar, 100 μm.
Experiment 2: the effect of gonadectomy on PR expression in the MPN and VMN of males and females
Gonadectomy on the day of birth resulted in a complex interaction between sex, age, and region on the levels of PRir. Before ovarian steroidogenesis, gonadectomy decreased PRir levels in the MPN and VMN of P4 males but had no effect on PR expression in either region in P4 females. After ovarian steroidogenesis, gonadectomy reduced PRir in the MPN of both P14 males and females. In contrast, it decreased PRir in the VMN of P14 females but not in P14 males.
P4
VMN.
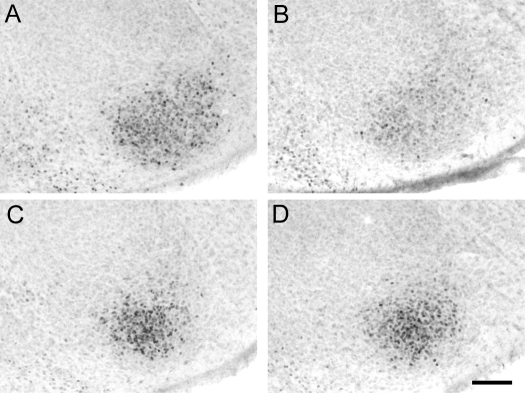
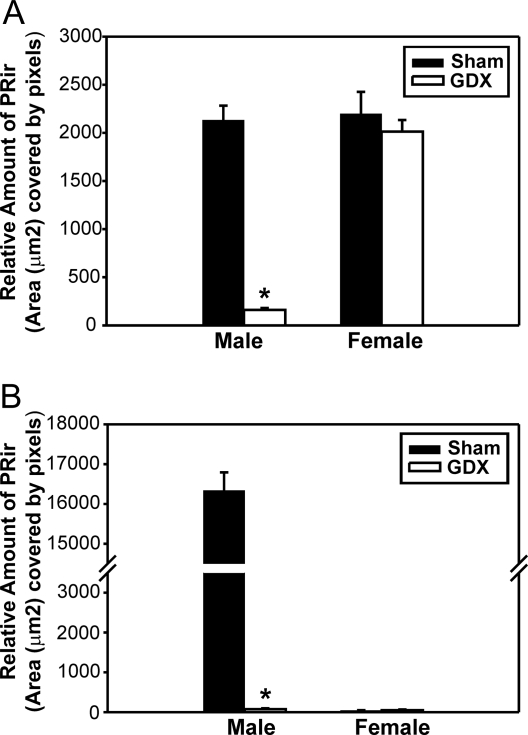
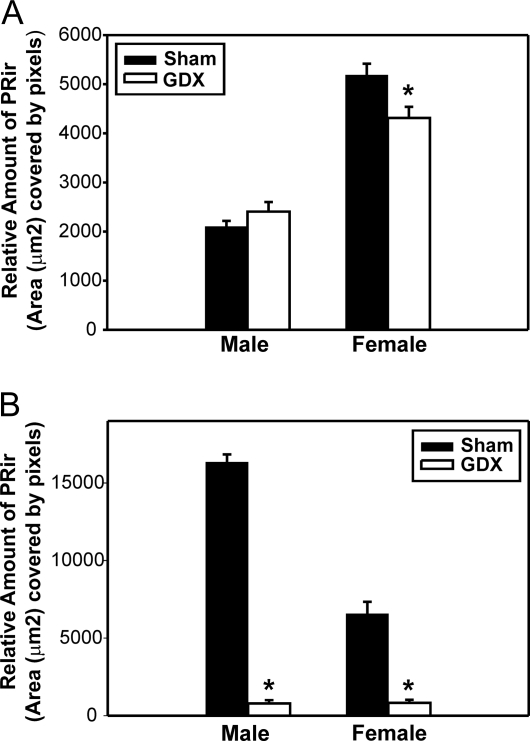
Two-way ANOVA (sex × gonadectomy) revealed that there was a significant main effect of sex [F(1,38) = 38.5, P < 0.001] and gonadectomy [F (1, 38) = 48.6, P < 0.001] and a significant interaction between sex and gonadectomy [F (1, 38) = 33.4, P < 0.001]. Post hoc analysis revealed that gonadectomy on the day of birth significantly reduced PRir in the VMN of P4 males (P < 0.001) but had no effect in females. PRir levels in the VMN did not differ between intact males and females, but gonadectomized females had significantly higher levels of PRir in the VMN, compared with GDX males (P < 0.05) (Figs. 3 and 4A).
Figure 3.
PRir in the VMN in P4 males (A and B) that were sham castrated (A) or castrated (B) on the day of birth or in P4 females (C and D) that were sham ovariectomized (C) or ovariectomized (D) on the day of birth. Bar, 100 μm.
Figure 4.
Hormonal regulation of PRir on P4. The relative amount of PRir in the VMN (A) or the MPN (B) in P4 males and females that were either GDX (white bars) or sham-GDX (Sham; black bars) on the day of birth. *, Significantly different from sham.
MPN.
Two-way ANOVA (sex × gonadectomy) revealed significant main effects of sex [F(1,39) = 1258.3, P < 0.001] and gonadectomy [F (1, 39) = 1247.1, P < 0.001] and a significant interaction between sex and gonadectomy [F (1, 39) = 1249.9, P < 0.001]. Post hoc analysis revealed that gonadectomy on the day of birth significantly reduced PRir in males, compared with sham-gonadectomized males (P < 0.001) but had no effect in females. Intact males had significantly higher levels of PRir in the MPN, compared with intact females (P < 0.001), and castration abolished this sex difference (Fig. 4B).
P14
VMN.
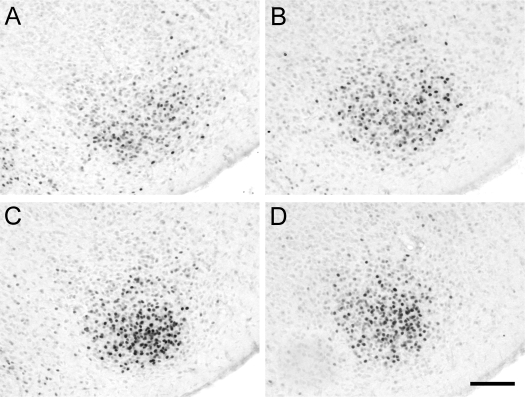
There was a significant main effect of sex [F(1,41) = 153.1, P < 0.001] but not gonadectomy. There was a significant interaction between sex and gonadectomy [F(1,41) = 8.5 (P < 0.01]. Post hoc analysis revealed that gonadectomy on the day of birth significantly reduced PRir in the VMN of females (P < 0.005) but had no effect in males. Intact females had significantly higher levels of PRir in the VMN than intact males (P < 0.001), and this sex difference persisted, even after gonadectomy (P < 0.001) (Figs. 5 and 6A).
Figure 5.
PRir in the VMN in P14 males (A and B) that were sham-castrated (A) or castrated (B) on the day of birth or in P14 females (C and D) that were sham-ovariectomized (C) or ovariectomized (D) on the day of birth. Bar, 100 μm.
Figure 6.
Hormonal regulation of PRir on P14. The relative amount of PRir in the VMN (A) or the MPN (B) in P14 males and females that were either GDX (white bars) or sham-gonadectomized (Sham; black bars) on the day of birth. *, Significantly different from sham.
MPN.
There was a significant main effect of sex [F(1,37) = 78.6, P < 0.001] and gonadectomy [F(1,37) = 376.4, P < 0.001] and a significant interaction between sex and gonadectomy [F (1, 37) = 79.9, P < 0.001]. Post hoc analysis revealed that gonadectomy on the day of birth significantly reduced PRir in the MPN of P14 males (P < 0.001) and females (P < 0.001). Intact males had significantly higher levels of PRir in the MPN than females (P < 0.001), and gonadectomy on the day of birth abolished the sex difference (Fig. 6B).
Experiment 3: estradiol induction of PR in the VMN and MPN of females across development
The effect of estradiol on PRir levels was dependent on age and region. Estradiol induced PRir in the MPN at P7 but did not alter PRir in the VMN of the same animals. However, estradiol increased PRir at all other ages in both regions.
VMN.
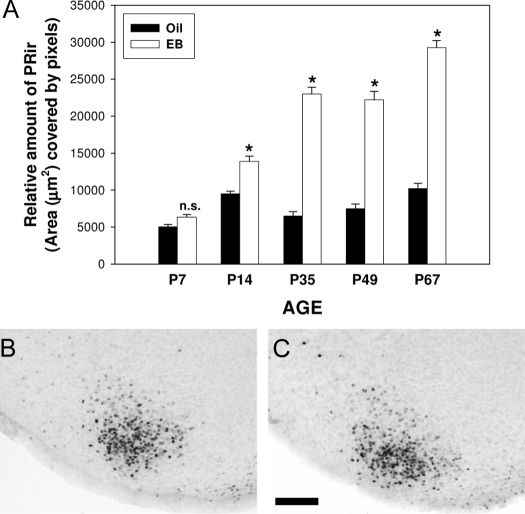
Estradiol significantly induced PRir in the VMN at all ages, except P7. Two-way ANOVA (EB treatment × age) revealed a significant main effect of age [F(4, 92) = 113.9, P < 0.001] and EB treatment [F (1, 92) = 630.479, P < 0.001] as well as a significant interaction between age and EB treatment [(F (4, 92) = 63.8, P < 0.001]. Post hoc analyses revealed that EB treatment significantly increased PRir in the VMN at P14, P35, P49, and P67 (P < 0.001). There was no significant effect of EB treatment at P7 (P = 0.163). Within oil-treated females, there was a significant increase in PRir levels between P7 and P14 (P < 0.001) and between P49 and P67 (P < 0.05). There was a significant decrease in PRir between P14 and P35 (P < 0.005) and no significant difference between P35 and P49. Within EB-treated females, PRir levels in the VMN were significantly higher at each age, compared with the previous age (P < 0.05) except at P49 (Fig. 7).
Figure 7.
Estradiol regulation of PRir is developmentally dependent in the VMN. A, The relative amount of PRir in the VMN of females that were ovariectomized 7 d before the animals were killed and administered either oil (black bars) or EB (white bars) 48 h before the animals were killed. PRir in representative sections of P7 ovariectomized females that were treated with EB (B) or the oil vehicle alone (C). n.s., Not significant. *, Significantly different from oil-treated controls. Bar, 100 μm.
MPN.
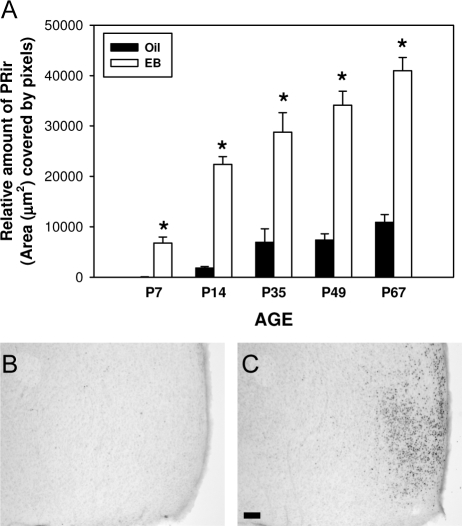
Estradiol significantly induced PRir in the MPN at all ages examined. Two-way ANOVA (EB treatment × age) revealed a significant main effect of age [F(4,96) = 38.8, P < 0.001] and EB treatment [F (1, 96) = 273.0, P < 0.001] as well as a significant interaction between EB treatment and age [F (4, 96) = 10.7, P < 0.001]. Post hoc analysis revealed that EB treatment significantly increased PRir at all ages (P7, P < 0.05; P14, P35, P49, P67, P < 0.001). Within oil-treated females, there was no significant effect of age on PRir between consecutive ages. Within EB-treated females, PRir levels in the MPN were significantly higher at each age, compared with the previous age (P < 0.05) except at P49 (Fig. 8).
Figure 8.
Estradiol consistently regulated PRir in the MPN of females at all ages. A, The relative amount of PRir in the MPN of females that were ovariectomized 7 d before the animals were killed and administered either oil (black bars) or EB (white bars) 48 h before the animals were killed. PRir in representative sections of P7 ovariectomized females that were treated with EB (B) or the oil vehicle alone (C). n.s., Not significant. *, Significantly different from oil-treated controls.
Uterine weight.
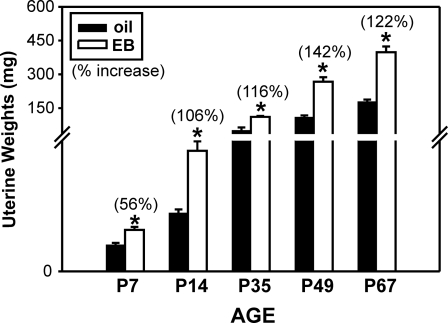
EB treatment significantly increased uterine weight, compared with oil controls at all ages examined (P < 0.001) (Fig. 9).
Figure 9.
Alterations in uterine weights after exposure to oil vehicle (oil) or EB. Mean (±sem) uterine weight (milligrams) in ovariectomized females at P7, P14, P35, P49, and P67 that were treated 48 h prior with either EB (white bars) or oil (black bars). *, Significantly different from oil-treated controls. Numbers in parentheses indicate the percent increase in mean uterine weight in EB-treated females, compared with oil-treated controls.
Discussion
The present results demonstrate that estradiol-mediated regulation of PR expression in the neonatal brain differs between males and females. Within females, estradiol regulates PR in an anatomically specific and developmentally dependent manner. Within the MPN, PR expression was highly dependent on estradiol in both sexes and throughout development. In contrast, results from the VMN indicate a more complex regulatory mechanism underlying PR expression.
Sex differences in PR
In the MPN, males expressed higher levels of PRir, compared with females, consistent with previous reports from our laboratory (16,24). This sex difference in the MPN is completely dependent on estradiol, resulting from the aromatization of testicular testosterone (17,24). In the adult female VMN, PR expression is also highly dependent on circulating estradiol levels (10,11). Therefore, it would be predicted that PR expression in the neonatal VMN would demonstrate a similar sex difference to that seen in the MPN. In stark contrast, however, PR expression was significantly higher in females, compared with males, at both P7 and P14. Moreover, PR expression in the P7 female VMN is relatively high, particularly in comparison with the virtual absence of PR expression in the MPN of the same females. Thus, the regulation of PR expression by estradiol in the neonatal female appears to be anatomically specific (i.e. highly dependent on estradiol in the MPN but seemingly independent of estradiol in the VMN). This suggests the existence of a mechanism that contributes to region-specific regulation of the PR gene.
Sex differences in the regulation of PR by gonadal hormones
The observed sex difference in PR levels in the VMN suggests that PR gene expression is regulated differentially in males and females. In males, the testes begin to secrete testosterone during gestation (19,28,29), whereas ovarian steroidogenesis does not occur until late in the second postnatal week (18,19,20,30). In the present study, castration on the day of birth virtually abolished PR expression in the MPN and VMN of P4 males, suggesting that PR expression in these two regions in the neonatal male VMN is highly estradiol dependent (present findings and Ref. 16). Interestingly, when examined at P14, PR in the MPN abolished in castrated males, but there was no significant effect of castration on PR in the male VMN. In females, at P4, before the onset of ovarian estradiol secretion, there was no significant effect of neonatal ovariectomy on PRir in either the MPN or VMN. At P14, after the onset of ovarian steroidogenesis, PRir was only slightly reduced by neonatal ovariectomy in the VMN of females. In contrast, PRir was virtually abolished in the MPN of the same animals (present findings and Ref. 16). These findings demonstrate an interaction between age and sex in the estradiol-dependent regulation of PR expression in the VMN. Furthermore, these results are consistent with the idea that within the VMN, nongonadal factor(s) may be responsible for PR expression in males (at P14) and females (at P4).
The identity of such nongonadal factors is presently unknown. However, it is possible that PR expression in the developing female VMN is independent of estradiol but still dependent on ERα activation. Previous reports clearly demonstrate that ERα, rather than ERβ, is responsible for the induction of PR gene transcription (31,32) and increasing PR protein in the brain (3,33). Numerous in vitro studies now suggest that ERα can regulate gene expression in a ligand-independent manner (35,36). For example, within MCF-7 as well as HeLa cell lines, ERα can be activated in the absence of ligand, recruit necessary transcription complexes and activate gene expression (35). The activation of ERα is dependent on its phosphorylation state. Under steroid-dependent conditions, ligand binding to the receptor results in phosphorylation. In the absence of ligand, cross talk with other intracellular signaling pathways may result in receptor phosphorylation. In the case of ERα, evidence indicates the involvement of growth factor receptor signaling pathways, in particular epidermal growth factor (37) and IGF-I (38). Whereas activation of ERα within the brain by growth factors has yet to be demonstrated, Olesen et al. (39) demonstrated the potential for ER activation via ligand-independent dopamine signaling pathways.
Regulation of PR by estradiol across development
The induction of PR expression by estradiol in the adult female VMN is well documented and represents one of the most robust examples of steroid-induced gene expression. In the adult female VMN, estradiol treatment of ovariectomized females increases PR binding (4,5,6), protein levels (12,13,14), and PR mRNA (8,10,11). In the present study, exogenous EB treatment significantly induced PR expression in the MPN at P7 but failed to alter PR levels in the VMN of the same animals. This is in stark contrast to the VMN at all other ages examined, in which EB treatment significantly increased PR beginning on, at least, P14. Furthermore, the dose of EB used in the present study was physiologically active because it significantly increased uterine weights in females of all ages, including P7. Therefore, these results reveal a developmental switch within the VMN that becomes engaged sometime between P7 and P14 and permits estradiol to induce the PR gene. The underlying factor(s) and molecular mechanisms involving changes in responsiveness to estradiol presently remain unexplored.
The inability of estradiol to induce PR in the neonatal rat VMN is a phenomenon that appears to be species specific. Estradiol-mediated regulation of PR expression in brain is a highly conserved phenomenon present in whiptail lizards (40,41), sheep (42), cows (43), and chickens (44) as well as frogs (45) among other species. Even within the neonatal mouse, PRir is highly dependent on estradiol and ERα (2), elucidating interesting and important species differences between rats and mice (46), even in what is considered to be a relatively robust endocrine phenomenon. In this light, the neonatal VMN of the female rat may be a unique and powerful model in which to study the mechanisms underlying the specificity of steroid-induced gene transcription.
The mechanisms that permit this specificity are not well understood. Differential expression of ERα cannot account for this specificity because ERα is expressed at high levels in both the neonatal MPN and VMN (47,48). However, factors that influence local estradiol levels or activity of ERα are possible. For example, it has long been accepted that the developing female brain was protected from estradiol exposure by high levels of the estrogen-binding protein α-fetoprotein (49,50,51,52,53 but see also Refs. 54 and 55), which is at high levels in circulation during perinatal life but declines over the second and third weeks after birth (56). However, circulating α-fetoprotein cannot explain the region-specific sensitivity to estradiol in the present study in which EB treatment increased PR expression almost 120-fold over controls in the MPN at P7 but less than 1.5-fold in the VMN of the same animals. The presence of α-fetoprotein within neurons (57,58), though, suggests that estradiol may be bound, and perhaps sequestered, by α-fetoprotein within individual cells of the brain and may do so in an anatomically specific manner.
Another possibility is that a factor or factors may be inhibiting ERα in the neonatal female VMN, preventing estradiol binding and/or ERα transcriptional activity. Indeed, in vitro studies demonstrate that the LIM (Lin-11, Isl-1, and Mec 3)/homeodomain protein, Islet-1, can interfere with the formation of ERα homodimers, thus preventing DNA binding and transcription of estrogen sensitive genes (59). Interestingly, preliminary evidence from our laboratory demonstrates that levels of Islet-1 expression are consistent with an inhibitory role of this protein in ERα activity, suggesting that factors such as Islet-1 may regulate anatomical and developmental specificity, as well as sex differences in steroid-induced gene expression.
Conclusions
PR appears to be uniquely regulated in the VMN of neonatal female rats and appears to be disengaged from estradiol regulation. Unlike most other regions of the hypothalamus, PR expression in the developing VMN is not predictably regulated by estradiol. Factors such as sex, age, and region appear to illuminate the conditions under which PR may be regulated by estradiol. This may represent a critical factor in the normal development of the female VMN because estradiol is known to exert masculinizing effects on the developing rat brain (for review see Ref. 60), and the VMN is critically involved in female reproductive behavior in adulthood (for review see Refs. 34 and 61). Furthermore, the present findings reveal the VMN as a unique and important in vivo model in which to examine the factors that regulate the specificity underlying steroid-induced gene transcription.
Acknowledgments
We are grateful for the technical assistance provided by Lisa McFayden-Ketchum and Erin Guiney in the completion of these experiments.
Footnotes
This work was supported by National Institutes of Health Grant HD37244 and National Science Foundation Grant IOB0447492 (to C.K.W.) and predoctoral National Research Service Award MH65874 (to P.S.Q.).
Current address for C.K.W.: Department of Psychology, Center for Neuroscience Research, Social Science 369, University at Albany, Albany, New York 12222. E-mail: cwagner@albany.edu.
Disclosure statement: P.S.Q. and C.K.W. have nothing to declare.
First Published Online February 28, 2008
Abbreviations: EB, Estradiol benzoate; ER, estrogen receptor; GDX, gonadectomized; MPN, medial preoptic nucleus; P, postnatal day; PB, phosphate buffer; PR, progesterone receptor; PRir, PR immunoreactivity; VMN, ventromedial nucleus.
References
- Moffatt CA, Rissman EF, Shupnik MA, Blaustein JD 1998 Induction of progestin receptors by estradiol in the forebrain of estrogen receptor-α gene-disrupted mice. J Neurosci 15:9556–9563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CK, Pfau JL, De Vries GJ, Merchenthaler IJ 2001 Sex difference in progesterone receptor immunoreactivity in neonatal mouse brain depend on estrogen receptor α expression. J Neurobiol 47:176–182 [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Rissman EF 2003 Double oestrogen receptor α and β knockout mice reveal differences in neural oestrogen-mediated progestin receptor induction and female sexual behaviour. J Neuroendocrinol 15:978–983 [DOI] [PubMed] [Google Scholar]
- Moguilewsky M, Raynaud JP 1979 Estrogen-sensitive progestin-binding sites in the female rat brain and pituitary. Brain Res 164:165–175 [DOI] [PubMed] [Google Scholar]
- Brown TJ, Clark AS, MacLusky NJ 1987 Regional sex differences in progestin receptor induction in the rat hypothalamus: effect of various doses of estradiol benzoate. J Neurosci 7:2529–2536 [PMC free article] [PubMed] [Google Scholar]
- Brown TJ, MacLusky NJ, Shanabrough M, Naftolin F 1990 Comparison of age- and sex-related changes in cell nuclear estrogen-binding capacity and progestin receptor induction in the rat brain. Endocrinology 126:2965–2972 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I 1997 Regulation of progesterone receptor messenger ribonucleic acid in the rat medial preoptic nucleus by estrogenic and antiestrogenic compounds: an in situ hybridization study. Endocrinology 138:5476–5484 [DOI] [PubMed] [Google Scholar]
- Funabashi T, Kleopoulos SP, Brooks PH, Kimura F, Pfaff DW, Shinohara K, Mobbs CV 2000 Changes in estrogenic regulation of estrogen receptor α mRNA and progesterone receptor mRNA in the female rate hypothalamus during aging: an in situ hybridization study. Neurosci Res 38:85–92 [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Krey LC, MacLusky NJ, McEwen BS 1981 Steroid receptor levels in intact and ovariectomized estrogen-treated rats: an examination of quantitative, temporal and endocrine factors influencing the efficacy of an estradiol stimulus. Neuroendocrinology 33:158–165 [DOI] [PubMed] [Google Scholar]
- Lauber AH, Romano GJ, Pfaff DW 1991 Sex difference in estradiol regulation of progestin receptor mRNA in rat mediobasal hypothalamus as demonstrated by in situ hybridization. Neuroendocrinology 53:608–613 [DOI] [PubMed] [Google Scholar]
- Romano GJ, Krust A, Pfaff DW 1989 Expression and estrogen regulation of progesterone receptor mRNA in neurons of the mediobasal hypothalamus: an in situ hybridization study. Mol Endocrinol 3:1295–1300 [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Turcotte J 1989 Estradiol-induced progestin receptor immunoreactivity is found only in estrogen receptor-immunoreactive cells in guinea pig brain. Neuroendocrinology 49:454–461 [DOI] [PubMed] [Google Scholar]
- Blaustein JD, King JC, Toft DO, Turcotte J 1988 Immunocytochemical localization of estrogen-induced progestin receptors in guinea pig brain. Brain Res 474:1–15 [DOI] [PubMed] [Google Scholar]
- Warembourg M Jolivet A, Milgrom E 1989 Immunohistochemical evidence of the presence of estrogen and progesterone receptors in the same neurons of the guinea pig hypothalamus and preoptic area. Brain Res 480:1–15 [DOI] [PubMed] [Google Scholar]
- Kraus WL, Montaono MM, Katzenellenbogen BS 1994 Identification of multiple, widely spaced estrogen-responsive regions in the rat progesterone receptor gene. Mol Endocrinol 8:952–969 [DOI] [PubMed] [Google Scholar]
- Quadros PS, Golstein AY, De Vries GJ, Wagner CK 2002 Regulation of sex differences in progesterone receptor expression in the medial preoptic nucleus of postnatal rats. J Neuroendocrinol 14:761–767 [DOI] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Goldstein AY, De Vries GJ, Wagner CK 2002 Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology 143:3727–3739 [DOI] [PubMed] [Google Scholar]
- Schlegel RJ, Farias E, Russo NC, Moore JR, Gardner LI 1967 Structural changes in the fetal gonads and gonaducts during maturation of an enzyme, steroid 3-β-ol-dehydrogenase, in the gonads, adrenal cortex and placenta of fetal rats. Endocrinology 81:565–572 [DOI] [PubMed] [Google Scholar]
- Smeaton TC, Arcondoulis DE, Steele PA 1975 The synthesis of testosterone and estradiol-17β by the gonads of neonatal rats in vitro. Steroids 26:181–192 [DOI] [PubMed] [Google Scholar]
- Quattropani SL, Weisz J 1973 Conversion of progesterone to estrone and estradiol in vivo by the ovary of the infantile rat in relation to the development of its interstitial tissue. Endocrinology 53:1269–1276 [DOI] [PubMed] [Google Scholar]
- Carson R, Smith J 1996 Development and steroidogenic activity of preantral follicles in the neonatal rat ovary. J Endocrinol 110:87–92 [DOI] [PubMed] [Google Scholar]
- Kato J, Onouchi T 1983 Progestin receptors in female rat brain and hypophysis in the development from fetal to postnatal stages. Endocrinology 113:29–36 [DOI] [PubMed] [Google Scholar]
- Kato J, Hirata S, Nozawa A, Mouri N 1993 The ontogeny of gene expression of progestin receptors in the female rat brain. J Steroid Biochem Mol Biol 47:173–182 [DOI] [PubMed] [Google Scholar]
- Wagner CK, Nakayama AY, De Vries GJ 1998 Potential role of maternal progesterone in the sexual differentiation of the brain. Endocrinology 139:3658–3661 [DOI] [PubMed] [Google Scholar]
- Traish AM, Wotiz HH 1990 Monoclonal and poly clonal antibodies to human progesterone receptor peptide-(533–547) recognize a specific site in unactivated (8S) and activated (4S) progesterone receptor and distinguish between intact and proteolyzed receptors. Endocrinology 127:1167–1175 [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA 1995 Atlas of prenatal rat brain development. Boca Raton, FL: CRC Press [Google Scholar]
- Paxinos G, Watson C 1998 The rat brain in stereotaxic coordinates. New York: Academic Press [Google Scholar]
- Warren DW, Haltmeyer GC, Eik-Nes KB 1973 Testosterone in the fetal rat testis. Biol Reprod 8:560–565 [DOI] [PubMed] [Google Scholar]
- Weisz J, Ward IL 1980 Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology 106:306–316 [DOI] [PubMed] [Google Scholar]
- Greco TL, Payne AH 1994 Ontogeny of expression of the genes for steroidogenic enzymes P450 side-chain cleavage, 3β-hydroxysteroid dehydrogenase, P450 17α-hydroxylase/C17–20 lyase, and P450 aromatase in fetal mouse gonads. Endocrinology 135:262–268 [DOI] [PubMed] [Google Scholar]
- Flototto T, Niederacher D, HohmannD, Heimerzheim T, Dall P, Djahansouzi S, Bender HG, Hanstein B 2004 Molecular mechanism of estrogen receptor (ER) α-specific, estradiol-dependent expression of the progesterone receptor (PR)-B isoform. J Steroid Biochem Mol Biol 88:131–142 [DOI] [PubMed] [Google Scholar]
- Rickard DJ, Waters KM, Rueskin TJ, Khosla S, Katzenellenbogen JA, Katzenellenbogen BS, Riggs BL, Spelsberg TC 2002 Estrogen receptor isoform-specific induction of progesterone receptors in human osteoblasts. J Bone Miner Res 17:580–592 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lubahn DB, Negro-Vilar A, Korach KS, Merchenthaler I 1997 Responses in the brain of estrogen receptor α-disrupted mice. Proc Natl Acad Sci USA 94:11008–11012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SK, Blaustein JD, O'Malley BW 1997 Progesterone receptor function from a behavioral perspective. Horm Behav 31:244–255 [DOI] [PubMed] [Google Scholar]
- Dutertre M, Smith CL 2003 Ligand-independent interactions of p160/steroid receptor coactivators and CREB-binding protein (CBP) with estrogen receptor-α: regulation by phosphorylation sites in the AB region depends on other receptor domains. Mol Endocrinol 17:1296–1314 [DOI] [PubMed] [Google Scholar]
- Fowler AM, Solodin N, Preisler-Mashek MT, Zhang P, Lee AV, Alarid ET 2004 Increases in estrogen receptor-α concentration in breast cancer cells promote serine 118/104/106-independent AF-1 transactivation and growth in the absence of estrogen. FASEB J 18:81–93 [DOI] [PubMed] [Google Scholar]
- Hua KT, Way TD, Lin JK 2006 Pentagalloylglucose inhibits estrogen receptor α by lysosome-dependent depletion and modulates ErbB/PI3K/Akt pathway in human breast cancer MCF-7 cells. Mol Carcinog 45:551–560 [DOI] [PubMed] [Google Scholar]
- Chen WF, Lau WS, Cheung PY, Guo DA, Wong MS 2006 Activation of insulin-like growth factor I receptor-mediated pathway by ginsenoside Rg1. Br J Pharmacol 147:542–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen KM, Jessen HM, Auger CJ, Auger AP 2005 Dopaminergic activation of estrogen receptors in the neonatal brain alters progestin receptor expression and juvenile social play behavior. Endocrinology 146:3705–3712 [DOI] [PubMed] [Google Scholar]
- Young LJ, Nag PK, Crews D 1995 Regulation of estrogen receptor and progesterone receptor messenger ribonucleic acid by estrogen in the brain of the whiptail lizard (Cnemidophorus uniparens). J Neuroendocrinol 7:119–125 [DOI] [PubMed] [Google Scholar]
- Godwin J, Crews D 1999 Hormonal regulation of progesterone receptor mRNA expression in the hypothalamus of whiptail lizards: regional and species differences. J Neurobiol 39:287–293 [PubMed] [Google Scholar]
- Scott CJ, Pereira AM, Rawson JA, Simmons DM, Rossmanith WG, Ing NH, Clarke IJ 2000 The distribution of progesterone receptor immunoreactivity and mRNA in the preoptic area and hypothalamus of the ewe: upregulation of progesterone receptor mRNA in the mediobasal hypothalamus by oestrogen. J Neuroendocrinol 12:565–575 [DOI] [PubMed] [Google Scholar]
- Dufourny L, Skinner DC 2002 Progesterone receptor, estrogen receptor α, and the type II glucocorticoid receptor are expressed in the same neurons of the ovine preoptic area and arcuate nucleus: a triple immunolabeling study. Biol Reprod 67:1605–1612 [DOI] [PubMed] [Google Scholar]
- Gasc JM, Baulieu EF 1988 Regulation by estradiol of the progesterone receptor in the hypothalamus and pituitary: an immunohistochemical study in the chicken. Endocrinology 122:1357–1365 [DOI] [PubMed] [Google Scholar]
- Roy EJ, Wilson MA, Kelley DB 1986 Estrogen-induced progestin receptors in the brain and pituitary of the South African clawed frog, Xenopus laevis. Neuroendocrinology 42:51–56 [DOI] [PubMed] [Google Scholar]
- Wagner CK 2006 The many faces of progesterone: a role in adult and developing male brain. Front Neuroendocrinol 27:340–359 [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, Handa RJ 1994 Developmental profile of estrogen receptor mRNA in the preoptic area of male and female neonatal rats. Dev Brain Res 79:283–289 [DOI] [PubMed] [Google Scholar]
- Yokosuka M, Okamura H, Hayashi S 1997 Postnatal development and sex difference in neurons containing estrogen receptor-α-immunoreactivity in the preoptic brain, the diencephalons, and the amygdala in the rat. J Comp Neurol 389:81–93. [DOI] [PubMed] [Google Scholar]
- Vannier B, Raynaud JP 1975 Effect of estrogen plasma binding on sexual differentiation of the rat fetus. Mol Cell Endocrinol 3:323–337 [DOI] [PubMed] [Google Scholar]
- Attardi B, Ruoslahti E 1976 Foetoneonatal oestradiol-binding protein in mouse brain cytosol is α foetoprotein. Nature 263:685–687 [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Lieberburg I, McEwen BS 1979 The development of estrogen receptors in the rat brain and pituitary: perinatal development. Brain Res 178:129–142 [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Chaptal C, McEwen BS 1979 The development of estrogen receptor systems in the rat brain and pituitary: postnatal development. Brain Res 178:143–160 [DOI] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C 2006 α-Fetoprotein protexts the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci 9:220–226 [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J 2002 The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J Neurosci 22:9104–9112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Baum MJ 2008 Role for estradiol in female-typical brain and behavioral sexual differentiation. Front Neuroendocrinol 29:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud JP 9173 Influence of rat estradiol binding plasma protein (EBP) on uterotrophic activity. Steroids 21:249–258 [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD 1980 Coexistence of α-fetoprotein, albumin and transferrin immunoreactivity in neurones of the developing mouse brain. Nature 286:733–735 [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD 1982 Regional differences in intraneuronal localization of α-fetoprotein in developing mouse brain. Brain Res 281:213–217 [DOI] [PubMed] [Google Scholar]
- Gay F, Anglade I, Gong Z, Salbert G 2000 The LIM/homeodomain protein islet-1 modulates estrogen receptor function. Mol Endocrinol 14:1627–1648 [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Simerly RB 2002 Anatomy, development, and function of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Moss RL, Rubin RT, eds. Hormones, brain, and behavior. Vol IV. San Diego: Academic Press; 137–191 [Google Scholar]
- Pfaff DW, Schwartz-Giblins S, McCarthy MM, Kow L-M 1994 Cellular and molecular mechanisms of female reproductive behavior. In: Knobil E, Neills JD, eds. The physiology of reproduction. 2nd ed. New York: Raven Press; 107–220 [Google Scholar]