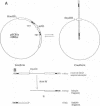
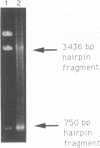
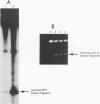
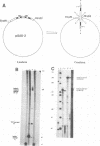
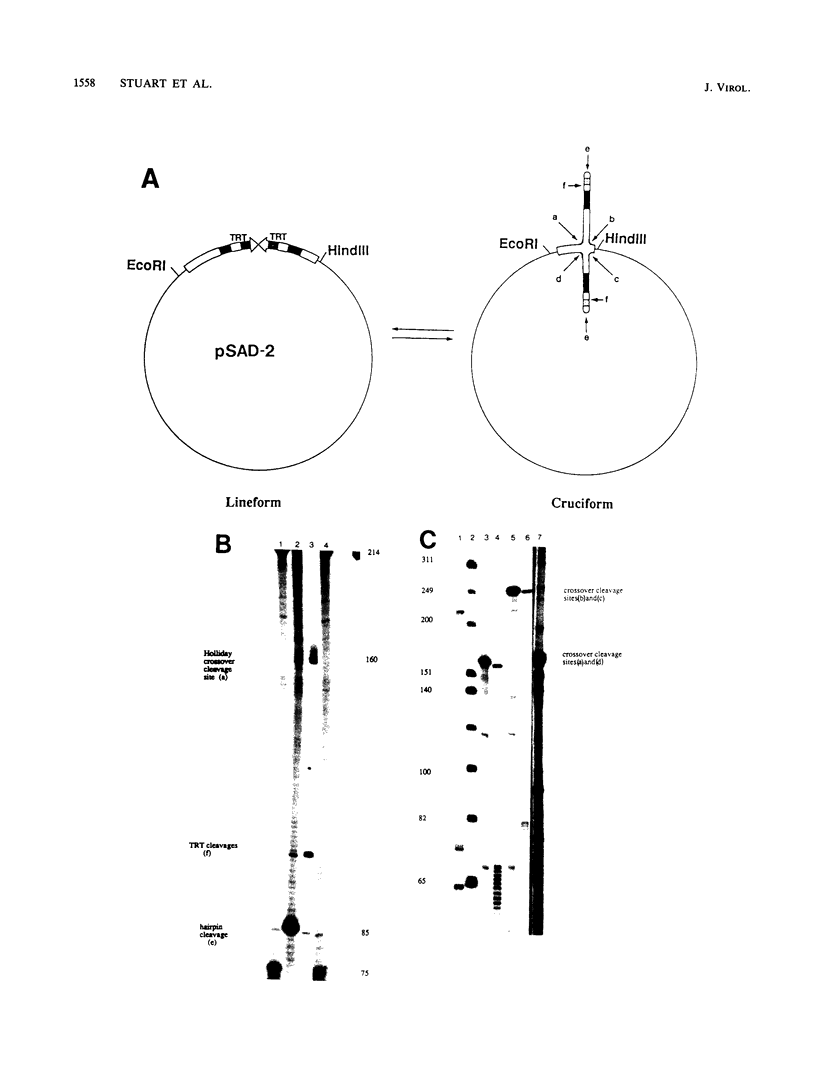
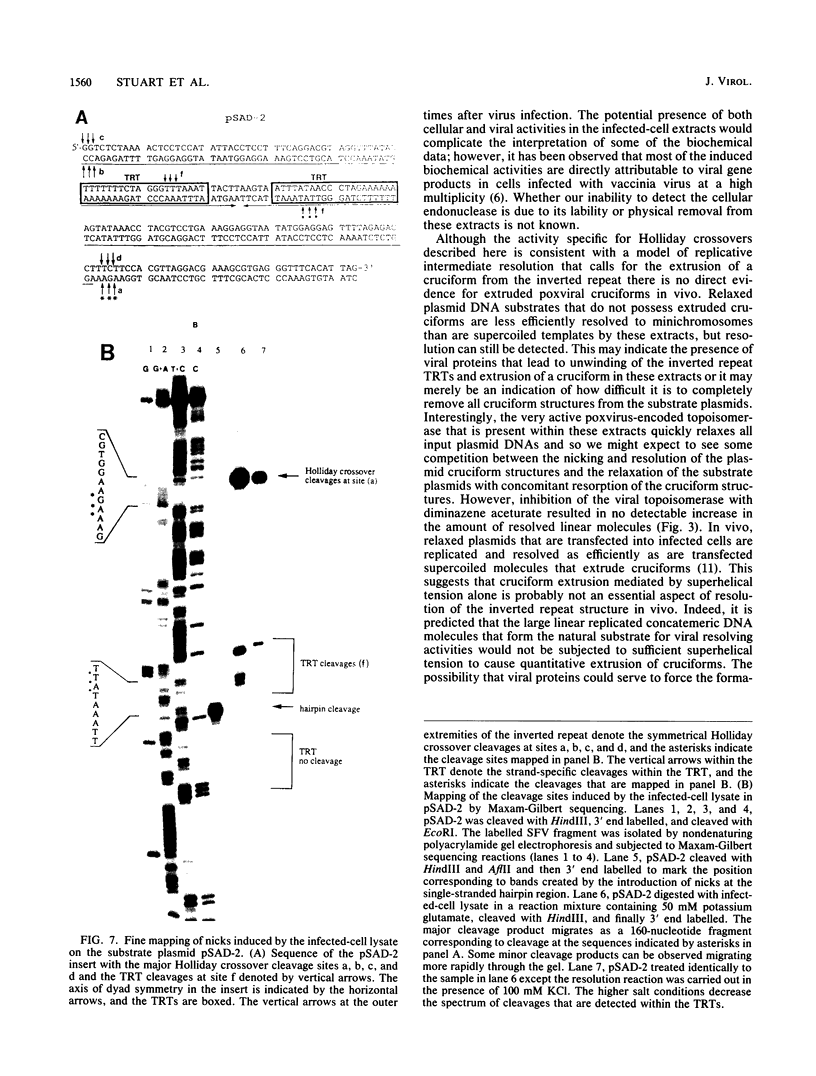
Abstract
Available evidence suggests that one or more late viral gene products are involved in processing poxvirus replicative intermediates into mature progeny hairpin-terminated genomes. Cloned versions of the Shope fibroma virus (SFV) replicated telomere in the inverted repeat configuration were used as substrates to assay lysates from poxvirus-infected cells for protein fractions that participate in the resolution of the circular substrate plasmid into a linear minichromosome with viral hairpin termini. An activity in a crude protein fraction obtained from vaccinia virus-infected cells at late times during the replicative cycle was capable of accurately resolving all poxviral inverted repeat replicative intermediates tested. The resolved linear products are identical to the products of in vivo resolution and possessed symmetrical nicks which mapped at the borders of the inverted repeat sequence. Strand-specific nicks were also identified, which mapped within the telomere resolution target sequence known to be required for telomere resolution in vivo. The resolving activity that we have identified is specific to virus-infected cells at late times during replication and cleaves cloned poxviral telomeric substrates in a fashion expected of a classic Holliday junction-resolving enzyme in addition to possessing a telomere resolution target-specific nicking activity. Although a Holliday junction-resolving activity would also be expected to play a role in the recombination induced by poxvirus infection, the appearance of the activity described here only after the commencement of viral late protein synthesis suggests that it functions strictly at late times. Other non-viral Holliday junction analogs can also be cleaved by this extract, suggesting that this component of the resolution activity may also play a role in other viral processes that require cleavage of a branched DNA structure. Thus, we have identified a poxviral activity that may be a part of a protein complex which resolves concatemeric replicative intermediates of viral DNA as well as participate in general recombination late during infection.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M. The DNA enzymology of protein machines. Cold Spring Harb Symp Quant Biol. 1984;49:1–12. doi: 10.1101/sqb.1984.049.01.003. [DOI] [PubMed] [Google Scholar]
- Ball L. A. High-frequency homologous recombination in vaccinia virus DNA. J Virol. 1987 Jun;61(6):1788–1795. doi: 10.1128/jvi.61.6.1788-1795.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles S. S., Moss B. Sedimentation of an RNA polymerase complex from vaccinia virus that specifically initiates and terminates transcription. Mol Cell Biol. 1987 Jan;7(1):7–14. doi: 10.1128/mcb.7.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M. S., DeLange A. M. A temperature-sensitive lesion in the small subunit of the vaccinia virus-encoded mRNA capping enzyme causes a defect in viral telomere resolution. J Virol. 1991 Aug;65(8):4042–4050. doi: 10.1128/jvi.65.8.4042-4050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981 Aug;113(1):224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Condit R. C., Niles E. G. Orthopoxvirus genetics. Curr Top Microbiol Immunol. 1990;163:1–39. doi: 10.1007/978-3-642-75605-4_1. [DOI] [PubMed] [Google Scholar]
- Cunningham R. P., Berger H. Mutations affecting genetic recombination in bacteriophage T4D. II. Genetic properties. Virology. 1978 Jul 1;88(1):62–70. doi: 10.1016/0042-6822(78)90110-1. [DOI] [PubMed] [Google Scholar]
- DeLange A. M. Identification of temperature-sensitive mutants of vaccinia virus that are defective in conversion of concatemeric replicative intermediates to the mature linear DNA genome. J Virol. 1989 Jun;63(6):2437–2444. doi: 10.1128/jvi.63.6.2437-2444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange A. M., McFadden G. Efficient resolution of replicated poxvirus telomeres to native hairpin structures requires two inverted symmetrical copies of a core target DNA sequence. J Virol. 1987 Jun;61(6):1957–1963. doi: 10.1128/jvi.61.6.1957-1963.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange A. M., McFadden G. The role of telomeres in poxvirus DNA replication. Curr Top Microbiol Immunol. 1990;163:71–92. doi: 10.1007/978-3-642-75605-4_3. [DOI] [PubMed] [Google Scholar]
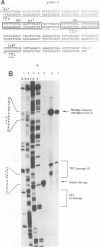
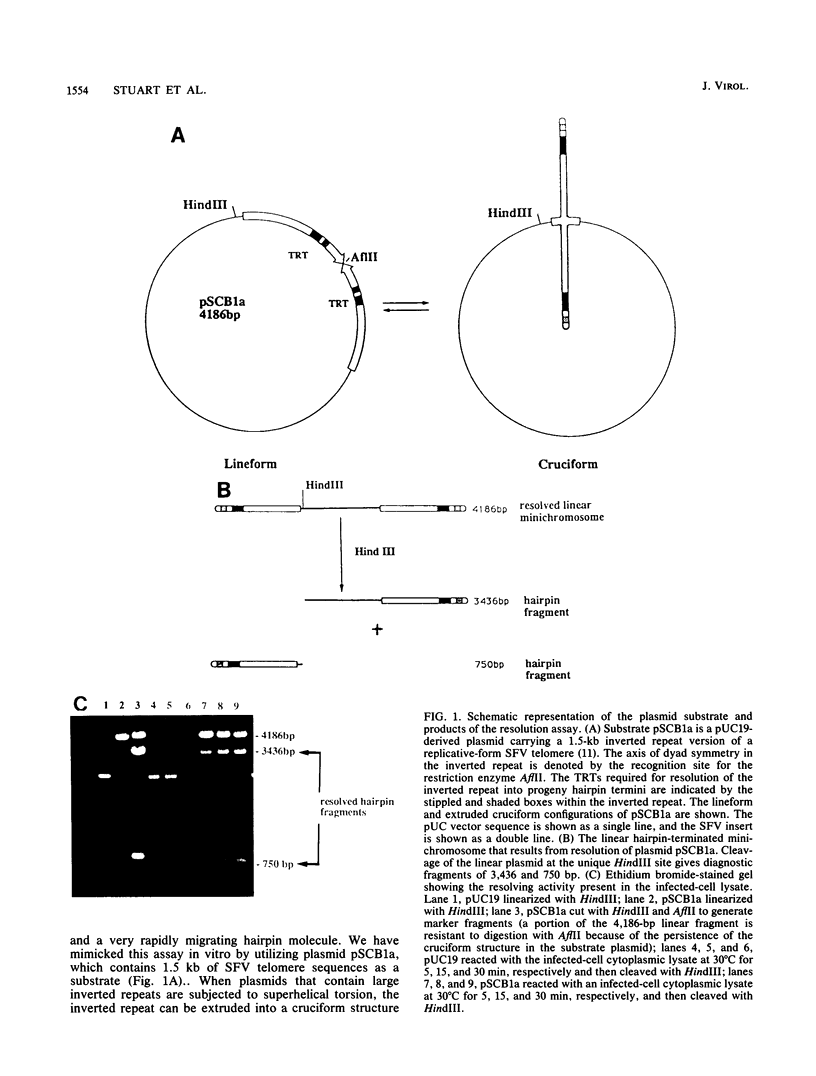
- DeLange A. M., Reddy M., Scraba D., Upton C., McFadden G. Replication and resolution of cloned poxvirus telomeres in vivo generates linear minichromosomes with intact viral hairpin termini. J Virol. 1986 Aug;59(2):249–259. doi: 10.1128/jvi.59.2.249-259.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie P., Morgan A. R., McFadden G. Cruciform extrusion in plasmids bearing the replicative intermediate configuration of a poxvirus telomere. J Mol Biol. 1987 Aug 5;196(3):541–558. doi: 10.1016/0022-2836(87)90031-3. [DOI] [PubMed] [Google Scholar]
- Doermann A H, Hill M B. Genetic Structure of Bacteriophage T4 as Described by Recombination Studies of Factors Influencing Plaque Morphology. Genetics. 1953 Jan;38(1):79–90. doi: 10.1093/genetics/38.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elborough K. M., West S. C. Resolution of synthetic Holliday junctions in DNA by an endonuclease activity from calf thymus. EMBO J. 1990 Sep;9(9):2931–2936. doi: 10.1002/j.1460-2075.1990.tb07484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. H., Kolodner R. Effect of DNA structure and nucleotide sequence on Holliday junction resolution by a Saccharomyces cerevisiae endonuclease. J Mol Biol. 1988 May 5;201(1):69–80. doi: 10.1016/0022-2836(88)90439-1. [DOI] [PubMed] [Google Scholar]
- Evans D. H., Stuart D., McFadden G. High levels of genetic recombination among cotransfected plasmid DNAs in poxvirus-infected mammalian cells. J Virol. 1988 Feb;62(2):367–375. doi: 10.1128/jvi.62.2.367-375.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENNER F., COMBEN B. M. [Genetic studies with mammalian poxviruses. I. Demonstration of recombination between two strains of vaccina virus]. Virology. 1958 Jun;5(3):530–548. doi: 10.1016/0042-6822(58)90043-6. [DOI] [PubMed] [Google Scholar]
- Frankel F. R., Batcheler M. L., Clark C. K. The role of gene 49 in DNA replication and head morphogenesis in bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):439–463. doi: 10.1016/0022-2836(71)90147-1. [DOI] [PubMed] [Google Scholar]
- GEMMELL A., CAIRNS J. Linkage in the genome of an animal virus. Virology. 1959 Jul;8(3):381–383. doi: 10.1016/0042-6822(59)90037-6. [DOI] [PubMed] [Google Scholar]
- Geshelin P., Berns K. I. Characterization and localization of the naturally occurring cross-links in vaccinia virus DNA. J Mol Biol. 1974 Oct 5;88(4):785–796. doi: 10.1016/0022-2836(74)90399-4. [DOI] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Haniford D. B., Pulleyblank D. E. Transition of a cloned d(AT)n-d(AT)n tract to a cruciform in vivo. Nucleic Acids Res. 1985 Jun 25;13(12):4343–4363. doi: 10.1093/nar/13.12.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Ball L. A. Mapping and identification of the vaccinia virus thymidine kinase gene. J Virol. 1982 Aug;43(2):403–409. doi: 10.1128/jvi.43.2.403-409.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensch F., Kemper B. Endonuclease VII resolves Y-junctions in branched DNA in vitro. EMBO J. 1986 Jan;5(1):181–189. doi: 10.1002/j.1460-2075.1986.tb04194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensch F., Kosak H., Seeman N. C., Kemper B. Cruciform cutting endonucleases from Saccharomyces cerevisiae and phage T4 show conserved reactions with branched DNAs. EMBO J. 1989 Dec 20;8(13):4325–4334. doi: 10.1002/j.1460-2075.1989.tb08619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaseelan R., Shanmugam G. Human placental endonuclease cleaves Holliday junctions. Biochem Biophys Res Commun. 1988 Oct 31;156(2):1054–1060. doi: 10.1016/s0006-291x(88)80951-3. [DOI] [PubMed] [Google Scholar]
- Jones E. V., Moss B. Mapping of the vaccinia virus DNA polymerase gene by marker rescue and cell-free translation of selected RNA. J Virol. 1984 Jan;49(1):72–77. doi: 10.1128/jvi.49.1.72-77.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B., Garabett M. Studies on T4-head maturation. 1. Purification and characterization of gene-49-controlled endonuclease. Eur J Biochem. 1981 Mar 16;115(1):123–131. [PubMed] [Google Scholar]
- Kerr C., Sadowski P. D. The involvement of genes 3,4,5 and 6 in genetic recombination in bacteriophage T7. Virology. 1975 May;65(1):281–285. doi: 10.1016/0042-6822(75)90031-8. [DOI] [PubMed] [Google Scholar]
- Lakritz N., Foglesong P. D., Reddy M., Baum S., Hurwitz J., Bauer W. R. A vaccinia virus DNase preparation which cross-links superhelical DNA. J Virol. 1985 Mar;53(3):935–943. doi: 10.1128/jvi.53.3.935-943.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee W. E., Miller O. V. Immunological evidence for the appearance of a new DNA-polymerase in cells infected with vaccinia virus. Virology. 1967 Jan;31(1):64–69. doi: 10.1016/0042-6822(67)90008-6. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Gefter M. L. Transcription of mammalian genes in vitro. Gene Amplif Anal. 1981;2:369–382. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Merchlinsky M., Garon C. F., Moss B. Molecular cloning and sequence of the concatemer junction from vaccinia virus replicative DNA. Viral nuclease cleavage sites in cruciform structures. J Mol Biol. 1988 Feb 5;199(3):399–413. doi: 10.1016/0022-2836(88)90613-4. [DOI] [PubMed] [Google Scholar]
- Merchlinsky M. Intramolecular homologous recombination in cells infected with temperature-sensitive mutants of vaccinia virus. J Virol. 1989 May;63(5):2030–2035. doi: 10.1128/jvi.63.5.2030-2035.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
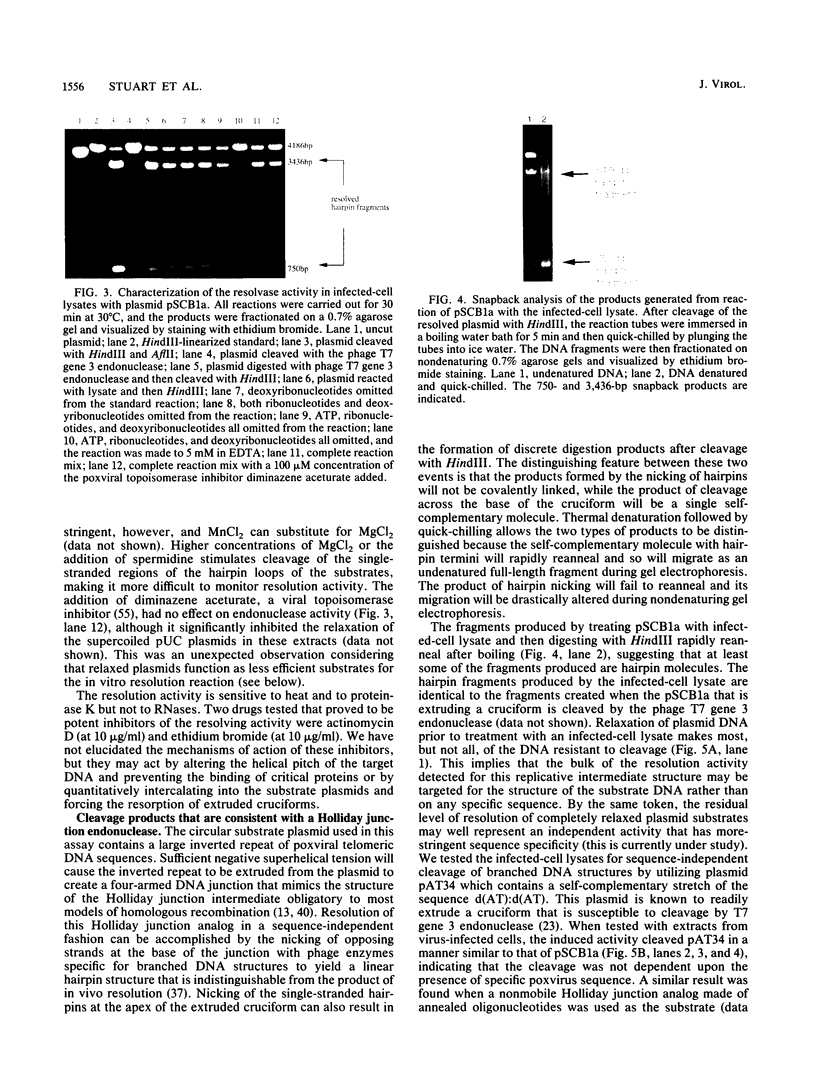
- Merchlinsky M., Moss B. Resolution of vaccinia virus DNA concatemer junctions requires late-gene expression. J Virol. 1989 Apr;63(4):1595–1603. doi: 10.1128/jvi.63.4.1595-1603.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
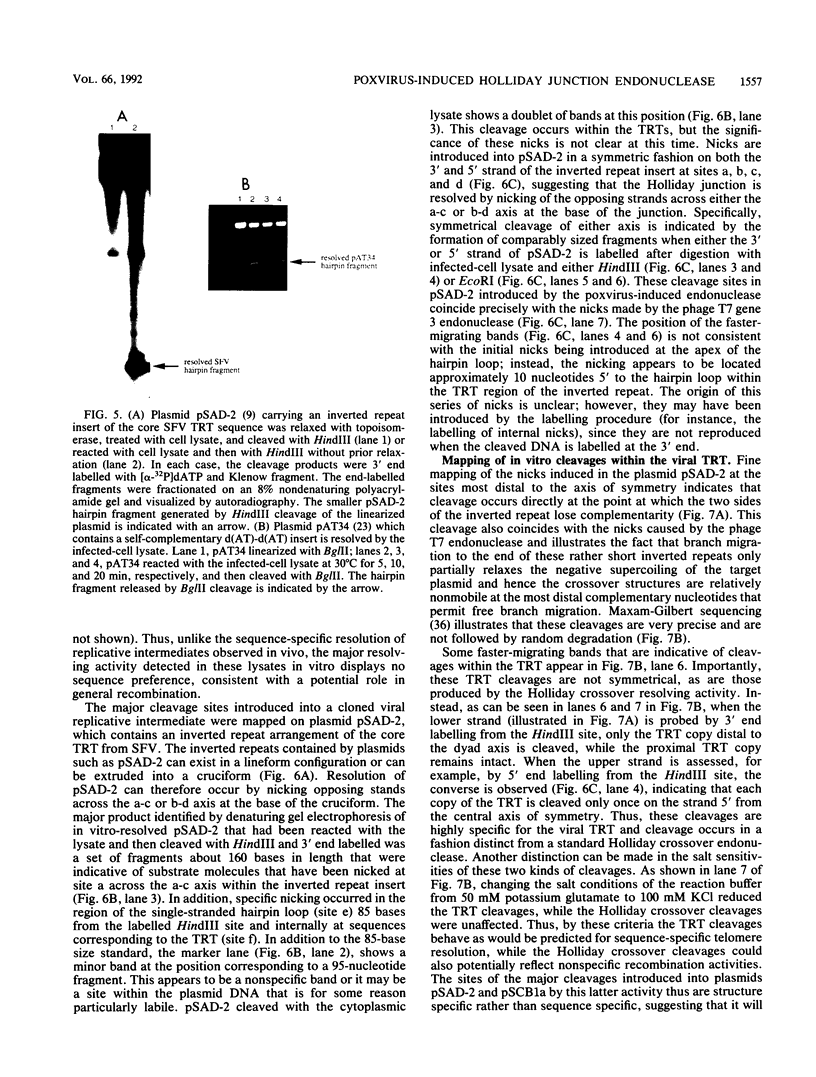
- Merchlinsky M. Resolution of poxvirus telomeres: processing of vaccinia virus concatemer junctions by conservative strand exchange. J Virol. 1990 Jul;64(7):3437–3446. doi: 10.1128/jvi.64.7.3437-3446.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi K., Kemper B., Hays J., Weisberg R. A. T4 endonuclease VII cleaves holliday structures. Cell. 1982 Jun;29(2):357–365. doi: 10.1016/0092-8674(82)90152-0. [DOI] [PubMed] [Google Scholar]
- Mosig G., Shaw M., Garcia G. M. On the role of DNA replication, endonuclease VII, and rII proteins in processing of recombinational intermediates in phage T4. Cold Spring Harb Symp Quant Biol. 1984;49:371–382. doi: 10.1101/sqb.1984.049.01.044. [DOI] [PubMed] [Google Scholar]
- Moyer R. W., Graves R. L. The mechanism of cytoplasmic orthopoxvirus DNA replication. Cell. 1981 Dec;27(2 Pt 1):391–401. doi: 10.1016/0092-8674(81)90422-0. [DOI] [PubMed] [Google Scholar]
- Moyer R. W. The role of the host cell nucleus in vaccinia virus morphogenesis. Virus Res. 1987 Sep;8(3):173–191. doi: 10.1016/0168-1702(87)90014-1. [DOI] [PubMed] [Google Scholar]
- Nakano E., Panicali D., Paoletti E. Molecular genetics of vaccinia virus: demonstration of marker rescue. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1593–1596. doi: 10.1073/pnas.79.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paetkau V., Langman L., Bradley R., Scraba D., Miller R. C., Jr Folded, concatenated genomes as replication intermediates of bacteriophage T7 DNA. J Virol. 1977 Apr;22(1):130–141. doi: 10.1128/jvi.22.1.130-141.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Two deoxyribonuclease activities within purified vaccinia virus. Proc Natl Acad Sci U S A. 1969 Jul;63(3):820–827. doi: 10.1073/pnas.63.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powling A., Knippers R. Some functions involved in bacteriophage T7 genetic recombination. Mol Gen Genet. 1974;134(2):173–180. doi: 10.1007/BF00268418. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Kates J., Kirkpatrick J. B. Replication of vaccinia virus DNA in enucleated L-cells. J Mol Biol. 1971 Aug 14;59(3):505–508. doi: 10.1016/0022-2836(71)90313-5. [DOI] [PubMed] [Google Scholar]
- Reddy M. K., Bauer W. R. Activation of the vaccinia virus nicking-joining enzyme by trypsinization. J Biol Chem. 1989 Jan 5;264(1):443–449. [PubMed] [Google Scholar]
- Rosemond-Hornbeak H., Paoletti E., Moss B. Single-stranded deoxyribonucleic acid-specific nuclease from vaccinia virus. Purification and characterization. J Biol Chem. 1974 May 25;249(10):3287–3291. [PubMed] [Google Scholar]
- Shaffer R., Traktman P. Vaccinia virus encapsidates a novel topoisomerase with the properties of a eucaryotic type I enzyme. J Biol Chem. 1987 Jul 5;262(19):9309–9315. [PubMed] [Google Scholar]
- Shuman S., Moss B. Identification of a vaccinia virus gene encoding a type I DNA topoisomerase. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7478–7482. doi: 10.1073/pnas.84.21.7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabaugh M. B., Johnson T. L., Mathews C. K. Vaccinia virus induces ribonucleotide reductase in primate cells. J Virol. 1984 Nov;52(2):507–514. doi: 10.1128/jvi.52.2.507-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabaugh M., Roseman N., Davis R., Mathews C. Vaccinia virus-encoded ribonucleotide reductase: sequence conservation of the gene for the small subunit and its amplification in hydroxyurea-resistant mutants. J Virol. 1988 Feb;62(2):519–527. doi: 10.1128/jvi.62.2.519-527.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Chan Y. S., Kerr S. M. Transcriptional mapping and nucleotide sequence of a vaccinia virus gene encoding a polypeptide with extensive homology to DNA ligases. Nucleic Acids Res. 1989 Nov 25;17(22):9051–9062. doi: 10.1093/nar/17.22.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., de Carlos A., Chan Y. S. Vaccinia virus encodes a thymidylate kinase gene: sequence and transcriptional mapping. Nucleic Acids Res. 1989 Oct 11;17(19):7581–7590. doi: 10.1093/nar/17.19.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadari S. Properties of DNA ligase from uninfected and virus-infected HeLa cells. Nucleic Acids Res. 1976 Aug;3(8):2155–2167. doi: 10.1093/nar/3.8.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyropoulos D. D., Roberts B. E., Panicali D. L., Cohen L. K. Delineation of the viral products of recombination in vaccinia virus-infected cells. J Virol. 1988 Mar;62(3):1046–1054. doi: 10.1128/jvi.62.3.1046-1054.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strätling W., Krause E., Knippers R. Fast sedimenting deoxyribonucleic acid in bacteriophage T7-infected cells. Virology. 1973 Jan;51(1):109–119. doi: 10.1016/0042-6822(73)90371-1. [DOI] [PubMed] [Google Scholar]
- Stuart D., Graham K., Schreiber M., Macaulay C., McFadden G. The target DNA sequence for resolution of poxvirus replicative intermediates is an active late promoter. J Virol. 1991 Jan;65(1):61–70. doi: 10.1128/jvi.65.1.61-70.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington L. S., Kolodner R. Partial purification of an enzyme from Saccharomyces cerevisiae that cleaves Holliday junctions. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7247–7251. doi: 10.1073/pnas.82.21.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. L., Hooda-Dhingra U., Condit R. C. Fine structure mapping of five temperature-sensitive mutants in the 22- and 147-kilodalton subunits of vaccinia virus DNA-dependent RNA polymerase. J Virol. 1989 Feb;63(2):705–713. doi: 10.1128/jvi.63.2.705-713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traktman P. The enzymology of poxvirus DNA replication. Curr Top Microbiol Immunol. 1990;163:93–123. doi: 10.1007/978-3-642-75605-4_4. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Ogawa H. Intermediates in genetic recombination of bacteriophage T7 DNA. Biological activity and the roles of gene 3 and gene 5. J Mol Biol. 1978 Nov 5;125(3):255–273. doi: 10.1016/0022-2836(78)90402-3. [DOI] [PubMed] [Google Scholar]
- Vos J. C., Sasker M., Stunnenberg H. G. Promoter melting by a stage-specific vaccinia virus transcription factor is independent of the presence of RNA polymerase. Cell. 1991 Apr 5;65(1):105–113. doi: 10.1016/0092-8674(91)90412-r. [DOI] [PubMed] [Google Scholar]
- Waldman A. S., Liskay R. M. Resolution of synthetic Holliday structures by an extract of human cells. Nucleic Acids Res. 1988 Nov 11;16(21):10249–10266. doi: 10.1093/nar/16.21.10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Bajszár G., Moss B. Mapping of the vaccinia virus thymidine kinase gene by marker rescue and by cell-free translation of selected mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1210–1214. doi: 10.1073/pnas.79.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. C., Körner A. Cleavage of cruciform DNA structures by an activity from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6445–6449. doi: 10.1073/pnas.82.19.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills A., Delange A. M., Gregson C., Macaulay C., McFadden G. Physical characterization and molecular cloning of the Shope fibroma virus DNA genome. Virology. 1983 Oct 30;130(2):403–414. doi: 10.1016/0042-6822(83)90095-8. [DOI] [PubMed] [Google Scholar]
- de Massy B., Weisberg R. A., Studier F. W. Gene 3 endonuclease of bacteriophage T7 resolves conformationally branched structures in double-stranded DNA. J Mol Biol. 1987 Jan 20;193(2):359–376. doi: 10.1016/0022-2836(87)90224-5. [DOI] [PubMed] [Google Scholar]