Abstract
The effects of gonadectomy and hormone treatment on spatial learning were evaluated in adult male and female rats using a modified version of a 12-arm radial maze task. In this version, procedures were used to minimize the effectiveness of strategies less reliant on working and reference memory. Results demonstrate significant sex differences favoring male performance on the working memory component of the task. In contrast, females performed slightly better than males on the reference memory component of the task. In females, ovariectomy produced a decrease in overall accuracy (i.e. an increase in the number of arm entries necessary to obtain all food pellets) as well as declines in working and reference memory performance. Both accuracy and working memory performance, but not reference memory performance, were restored by estradiol treatment. In males, castration impaired working memory performance but did not significantly affect overall accuracy or reference memory performance. Surprisingly, all groups of males performed poorly on the reference memory component of the task, and testosterone treatment appeared to worsen, rather than improve, both accuracy and reference memory performance in males. This may reflect a male preference for certain strategies that were rendered ineffective on this task. Significant sex differences, as well as treatment effects, on arm preference patterns were also detected; however, these differences were not sufficient to account for the effects of sex and treatment on acquisition. Collectively, the data demonstrate robust effects of gonadectomy and hormone treatment on acquisition of this modified radial arm maze task in females, with lesser effects in males.
PRIOR STUDIES BOTH in humans and in animals have demonstrated significant sex differences in the performance of specific cognitive tasks. In humans, studies suggest that, on average, men perform better than women on tests of mental rotation, spatial visualization, and target-directed motor tasks, whereas women tend to perform better than men on tests of verbal memory, verbal reasoning, and perceptual fluency (1,2,3,4,5). In rodents, studies likewise show that males tend to outperform females on tests of spatial navigation and spatial working memory (6); however, reports do not always agree, most likely due to species- and strain-specific factors, differences in how specific tests are operated, as well as differences in social and rearing conditions.
The reasons for sex-specific differences in cognitive performance are not entirely known. Some studies suggest that men and women use different strategies to solve spatial tasks (7,8,9). Animal studies suggest that organizational effects of gonadal hormones on brain development are responsible for at least some of the sex differences in cognitive performance that manifest as adults (10,11,12,13,14). However, hormonal changes in adulthood also can affect cognitive performance in both animals and humans, suggesting that activational effects of gonadal hormones also play a role (reviewed in Refs. 15,16,17,18).
In adult female rodents, ovariectomy has been shown to impair performance on a variety of tasks including tasks of spatial navigation, e.g. delayed matching to position (19,20), T-maze alternation (21), various radial maze tasks (22,23,24,25), object and spatial recognition (26,27,28), and contextual fear conditioning (29,30). In many of these studies, effects were reversed by treatment with estradiol or another potent estrogen. Fewer studies have been done in males; however, there is some evidence that castration and androgen replacement can affect performance on certain spatial and inhibitory avoidance tasks in males (31,32,33,34).
To better understand the cognitive workings that are affected by gonadectomy and hormone treatment in males and females, we investigated effects on spatial learning using a modified version of a 12-arm radial maze task and compared effects directly between male and females rats. Importantly, the task was designed to limit the effectiveness of certain strategies less dependent on spatial working and reference memory. Our results demonstrate significant sex differences in working and reference memory performance on this task as well as robust sex differences in the effects of gonadectomy and hormone treatment.
Materials and Methods
Animals
A total of 75 young adult Sprague Dawley rats (36 females, 39 males) were purchased from Hilltop Laboratories (Scottdale, PA). Twenty-two of the females, and 24 of the males were gonadectomized by the supplier before delivery. All rats were housed individually upon arrival at Duquesne University, and all procedures were carried out in accordance with Public Health Service policies and with the approval of Duquesne University’s Institutional Animal Care and Use Committee.
Treatments
Two weeks after arrival, 12 of the ovariectomized (Ovx) females received a 5-mm SILASTIC brand capsule (Dow Corning Corp., Midland, MI) containing 17β-estradiol. Likewise, 10 castrated male rats received an 8-mm SILASTIC brand capsule containing testosterone propionate. Remaining rats received empty capsules as a control. All capsules were 0.058 in. inner diameter and 0.077 in. outer diameter and were implanted under the skin in the dorsal neck region. This resulted in a total of six treatment groups: intact females (n = 14), Ovx females (n = 10), Ovx females that received estradiol (Ovx+E; n = 12), intact males (n = 15), castrated males (n = 14), and castrated males that received testosterone (n = 10).
Radial arm maze (RAM) training and testing
Beginning at least 2 wk after capsule implantation, rats were handled daily and food restricted to 85% body weight before being adapted to the maze. Throughout the study, males and females were housed separately and were never run concurrently in the maze. The radial maze consisted of a clear Plexiglas hub (16 in. diameter) and 12 radiating arms (3.5 in. wide × 30 in. long), with clear Plexiglas sides and a recessed food cup at the end of each arm. Access to each arm was controlled by a clear Plexiglas door that was raised and lowered by a motor device and was operated remotely. After entering an arm, rats returned to the hub to access other arms of the maze.
Rats were first trained to traverse the maze to acquire food pellets (formula 5TUM 45 mg pellets from Test Diets, Inc., Richmond, IN) located in the food cups. The first 2 d, rats were placed in the hub with food pellets located in the hub and along each arm. On d 3–5 (longer if necessary), rats were placed in the hub with food pellets located in the arms or in the food cups at the ends of the arms. Acclimation trials continued until rats learned to traverse the maze for food located in the food cups at the end of the arms.
Radial maze training commenced with rats receiving one training trial per day. For each rat, three arms were randomly selected to serve as reference arms (never baited), with the caveat that the three arms could not be contiguous. The nine remaining arms (working memory arms) were baited with one food pellet placed in the food cup at the end of each arm. Three of these arms were randomly selected for initial entry. A rat was placed into the hub, and the doors to the three initial entry arms were opened (arm entry 1; AE1). After the rat had obtained all three food pellets and had returned to the hub, the nine remaining doors were opened (arm entry 2; AE2). The rat remained in the maze until it had consumed all six remaining food pellets or until 10 min had passed. Rats received one trial per day for a total of 43 d. Note that the purpose of the initial entry arms was to limit the effectiveness of using certain strategies to solve the task. For example, this limits the effectiveness of a response strategy (e.g. always making a specific turn, always moving to the next available arm on the right, etc.) as well as the effectiveness of proceeding to a specific location at the start and then following a set pattern of arm choices.
Hormone assays
After the completion of RAM training, rats were anesthetized with Nembutal (100 mg/kg). Trunk blood was collected, and serum was assayed for levels of estradiol (females) and testosterone (males) by RIA. Hormone assays were performed by the Assay Core of the University’s Center for Reproductive Physiology. The estradiol assay had a minimum detection limit of approximately 1.3 pg/ml serum. The testosterone assay had a minimum detection limit of approximately 43.0 pg/ml serum.
Data analysis
Performance data from d 2–43 of training were grouped into six 7-d blocks. Five performance measures were analyzed. The number of arm entries required to collect all food pellets during AE2 was used as a measure of overall accuracy (reflecting the ability to accurately identify baited arms). Working memory errors (WMEs) were defined as reentry into any arm that was initially baited. Reference memory errors (RMEs) were defined as entry into any of the three arms that were never baited. Working memory performance was evaluated by the number of WMEs made during the first six choices of AE2 and by the number of correct choices before making a WME during the first six choices of AE2. Reference memory performance likewise was evaluated by the number of RMEs made during the first six choices of AE2 and by the number of correct choices before making a RME during the first six choices of AE2. Data were first analyzed by three-way ANOVA using sex and treatment as between factors with repeated measures on block. Data for males and females were then analyzed separately by two-way ANOVA with treatment as the between factor and repeated measures on block. When a significant effect of treatment was obtained, effects of treatment on each block of training were analyzed by one-way ANOVA. When significant, this was followed by a Tukey post hoc test to compare group means. All statistical analyses were performed using JMP-IN version 5.1 for Macintosh.
In addition, the observed probability of entering an arm based on its position relative to the arm being exited was calculated for each group for each block of testing. These probabilities were then used to conduct simulations to test whether differences in the observed probabilities could account for effects of treatment on the performance measures listed above.
Results
Hormone levels
The mean serum level of estradiol in gonadally intact females was 26.8 ± 3.1 pg/ml with a range of 9.3–51.8 pg/ml. The mean level in estradiol-treated females was 28.8 ± 5.6 pg/ml with a range of 7.8–68.0 pg/ml. Estradiol levels were undetectable in Ovx, untreated females. The mean serum level of testosterone in gonadally intact males was 3.3 ± 0.4 ng/ml, with a range of 1.8–7.0 ng/ml. The mean level in testosterone-treated males was 3.6 ± 0.2 ng/ml, with a range of 2.9–4.9 ng/ml. Testosterone levels were below detection in untreated castrated males.
Effects on RAM performance
Number of arm entries required to obtain all food pellets.
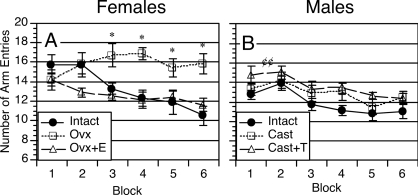
Learning curves are shown in Fig. 1. A three-way ANOVA of sex × treatment with repeated measures on block revealed a significant effect of sex [F(1,66) = 5.4; P = 0.02], a significant effect of treatment [F(2,66) = 5.2; P < 0.01], and a significant interaction between the two [F(2,66) = 5.1; P < 0.01]. Further analysis shows that at the beginning of training (block 1), intact males required significantly fewer arm entries than intact females to obtain all food pellets (12.7 ± 0.77 vs. 15.8 ± 0.79; P < 0.01); however, by block 3, the difference between these groups was no longer significant.
Figure 1.
Learning curves showing number of arm entries required by females and males to collect all food pellets on a trial. Data represent group means ± sem for each block of training. *, Ovx females differed significantly from both intact and Ovx+E groups (P < 0.05); ¢¢, castrated, testosterone-treated (Cast+T) males differed significantly from gonadally intact controls (P < 0.02 by comparison of marginal group means).
The three-way ANOVA also revealed a significant effect of block [F(5,330) =11.9; P < 0.0001], a significant interaction between treatment × block [F(10,300) =3.7; P = 0.0001], and a nearly significant three-way interaction between sex × treatment × block [F(10,330) = 1.8; P = 0.06], indicating sex and treatment-specific interactions with performance over time.
An analysis of females alone revealed a significant effect of treatment [F(2,31) = 5.4; P < 0.01], a significant effect of block [F(5,155) = 4.3; P < 0.002], and a significant interaction between treatment × block [F(10,155) = 4.2; P < 0.0001]. Post hoc analyses confirmed that Ovx females performed significantly worse that gonadally intact and estradiol-treated groups on blocks 3–6 (Fig. 1A).
The analysis of males alone revealed a significant effect of treatment [F(2,35) = 3.3; P < 0.05], a significant effect of block [F(5,175) = 8.9; P < 0.0001], but no significant interaction between treatment × block [F(10,175) = 0.48; P = 0.9]. Analysis of the marginal group means revealed that the effect of treatment was due to gonadally intact males performing significantly better than testosterone-treated males throughout training (P < 0.02).
Working memory performance.
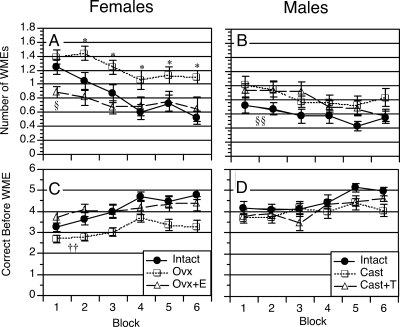
Number of WMEs, and the number of correct choices before making a WME are shown in Fig. 2. A three-way ANOVA of WMEs revealed a significant effect of sex [F(1, 66) = 11.4; P = 0.002], a significant effect of treatment [F(2, 66) =13.1; P < 0.0001], and a significant interaction of sex × treatment [F(2, 66) = 4.1; P < 0.05]. A three-way ANOVA of correct choices before making a WME produced similar results, with a significant effect of sex [F(1,66) = 7.0; P < 0.02], a significant effect of treatment [F(2,66) = 9.5; P < 0.0005], and a trend for the interaction of sex × treatment [F(2,66) = 2.7; P = 0.07]. Both analyses also revealed significant effects of block [F(5,330) = 11.2; P < 0.0001 for WMEs; F(5,330) = 13.7; P < 0.0001 for correct choices before making a WME], but no significant interactions with block.
Figure 2.
Learning curves showing working memory performance by females and males across six blocks of training. Upper panels show number of WMEs committed during the first six choices of AE2. Lower panels show number of correct choices before making a WME during the first six choices of AE2. Data represent group means ± sem. *, Ovx females differed significantly from both intact and Ovx+E groups (P < 0.05); §, Ovx+E differed significantly from both intact and Ovx groups (P < 0.05); ††, Ovx differed significantly from intact group (P < 0.002 by comparison of marginal group means); §§, intact males differ significantly from castrate group (P < 0.05 by comparison of marginal group means).
Analysis revealed that gonadally intact males outperformed gonadally intact females on measures of working memory performance, particularly at the start of training. Specifically, gonadally intact males made significantly fewer WMEs than gonadally intact females on blocks 1 (P < 0.002), 2 (P < 0.05), and 5 (P < 0.05) (Fig. 2, A and B) and made significantly more correct choices before making a WME than gonadally intact females on block 1 (P < 0.02) (Fig. 2, C and D). By the end of training, both gonadally intact males and females were averaging 0.5–0.6 WMEs and 4.6–5.0 correct choices before making a WME.
An analysis of WMEs in females alone revealed a significant effect of treatment [F(2,31) = 11.9; P < 0.0001] and a significant effect of block [F(5,155) = 7.9; P < 0.0001] but no significant interaction of treatment × block [F(10,155) = 1.1; P = 0.34]. Similar results were obtained for the analysis of correct choices before making a WME: significant effect of treatment [F(2,31) = 11.5; P < 0.0005], significant effect of block [F(5,155) =7.8, P < 0.0001], but no significant interaction of treatment × block [F(10,155) = 1.0; P = 0.44]. Post hoc comparisons revealed that Ovx rats made significantly more WMEs than intact and Ovx+E rats on blocks 2–6, and that Ovx+E rats made significantly fewer WMEs than intact and Ovx rats on block 1 (Fig. 2A). A comparison of marginal group means revealed a significant difference between Ovx rats and both intact and Ovx+E rats on the number of correct choices before making a WME (Fig. 2C).
The analysis of WMEs in males revealed a significant effect of treatment [F(2,35) = 4.8; P < 0.02], a significant effect of block [F(5,175) = 4.0; P < 0.002], but no significant interaction of treatment × block [F(10,175) = 0.49; P = 0.89]. A comparison of marginal group means revealed a significant difference between intact males and castrated males (Fig. 2B). The analysis of correct choices before making a WME in males revealed no significant effect of treatment [F(2,35) = 2.1; P = 0.14] (Fig. 3C), a significant effect of block [F(5,175) = 7.2; P < 0.0001], and no significant interaction of treatment × block [F(10,175) = 1.10; P = 0.37] (Fig. 2D).
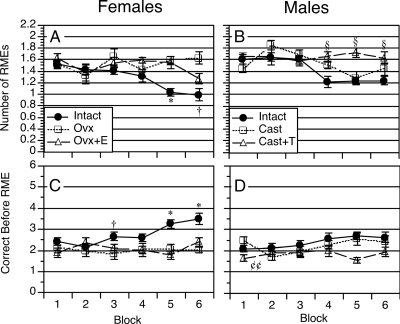
Figure 3.
Learning curves showing reference memory performance by females and males across six blocks of training. Upper panels show number of RMEs committed during the first six choices of AE2. Lower panels show number of correct choices before making a RME during the first six choices of AE2. Data represent group means ± sem. *, Intact females differed significantly from both Ovx and Ovx+E groups (P < 0.5); †, intact differed significantly from Ovx group (P < 0.05); §, castrated, testosterone-treated (Cast+T) males differed significantly from gonadally intact controls (P < 0.05); ¢¢, Cast+T males differed significantly from gonadally intact controls (P < 0.01 by comparison of marginal group means).
Reference memory performance.
Number of RMEs and the number of correct choices before making a RME are shown in Fig. 3. A three-way ANOVA of RMEs revealed a significant effect of sex [F(1,66) = 8.8; P < 0.005], a significant effect of treatment [F(2,66) = 15.1; P < 0.0001], but no significant interaction of sex × treatment [F(2,66) = 1.6; P = 0.21]. The analysis also revealed a significant effect of block [F(5,330) = 6.0; P < 0.0001], a significant interaction of sex × block [F(5,330) = 3.0; P = 0.01], a significant interaction of treatment × block [F(5,330) = 4.1; P < 0.0001], and a significant three-way interaction of sex × treatment × block [F(10,330) = 2.3; P = 0.01] (Fig. 3, A and B). In contrast, a three-way ANOVA of correct choices before making a RME revealed no significant effect of sex [F(1,66) = 2.5; P = 0.12], a significant effect of treatment [F(2,66) = 16.3; P < 0.0001], and a significant interaction of sex × treatment [F(2,66) = 3.6; P = 0.03]. There was also a significant effect of block [F(5,330) = 3.9; P < 0.005], a significant interaction of treatment × block [F(10,330) = 3.1; P < 0.001], but no other significant interactions (Fig. 3, C and D).
Notably, males did not outperform females on either measure of reference memory performance. Gonadally intact males and females made similar numbers of RMEs (1.6 ± 0.07 vs. 1.5 ± 0.07) and similar numbers of correct choices before making a RME (2.1 ± 0.17 vs. 2.4 ± 0.17) on block 1. Females improved slightly more than males, such that by block 6, females were making fewer RMEs (1.0 ± 0.09 vs. 1.2 ± 0.09) and more correct choices before making a RME (3.5 ± 0.22 vs. 2.6 ± 0.22) than gonadally intact males. The latter measure was statistically significant (P < 0.05).
An analysis of RMEs in females alone revealed a significant effect of treatment [F(2,31) = 10.6; P < 0.0005], a significant effect of block [F(5,155) = 3.1; P < 0.01], and a significant interaction of treatment × block [F(10,155) = 3.1; P = 0.001]. Post hoc comparisons confirmed that gonadally intact female rats made significantly fewer RMEs than both Ovx and estradiol-treated rats on block 5 (P < 0.05), and fewer RMEs than Ovx rats on block 6 (P < 0.05) (Fig. 3A). An analysis of correct choices before making a RME in females revealed a significant effect of treatment [F(2,31) = 16.9; P < 0.0001], a significant effect of block [F(5,155) = 2.3; P < 0.05], and a significant interaction of treatment × block [F(10,155) = 2.6; P = 0.01]. Post hoc comparisons confirmed that gonadally intact female rats made significantly more correct choices before making a RME than Ovx rats on block 3, and more correct choices than Ovx and Ovx+E rats on blocks 5 and 6 (P < 0.05 in each case) (Fig. 3C).
The analysis of RMEs in males revealed a significant effect of treatment [F(2,35) = 6.3; P < 0.005], a significant effect of block [F(5,175) = 6.1; P < 0.0001], and a significant interaction of treatment × block [F(10,175) = 3.1; P = 0.001]. Post hoc comparisons confirmed that testosterone-treated males made significantly more RMEs than gonadally intact males on blocks 4–6 (P < 0.05 in each case) (Fig. 3B). The analysis of correct choices before making a WME in males revealed a significant effect of treatment [F(2,35) = 5.4; P < 0.01], a nearly significant effect of block [F(5,175) = 2.2; P = 0.06], but no significant interaction of treatment × block [F(10,175) = 1.5; P = 0.15]. Contrasts of marginal means confirmed that testosterone-treated males differed significantly from gonadally intact males (P < 0.01) (Fig. 3D).
Analysis of arm choice patterns
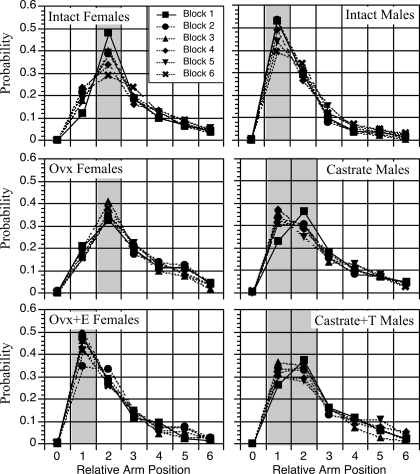
Arm choice patterns were evaluated by calculating the observed probability of entering arms located at each of the seven possible positions relative to the arm being exited, during choices 2–6 of AE2, across each block of training. Figure 4 shows the analysis for each treatment group and each block of training, collapsed across choices 2–6. A distinct sex difference in arm choice preference was observed. Gonadally intact males were more likely to enter an arm adjacent to the arm that was exited, whereas gonadally intact females were more likely to enter an arm located two positions away. These patterns were maintained throughout training, although the preferences tended to diminish in later blocks. In females, gonadectomy did not alter the pattern; however, estradiol treatment shifted the pattern to that of males. In males, castration initially produced a female pattern on block 1 of training, which reverted to a male pattern on subsequent blocks. Testosterone treatment shifted responses further toward the female pattern such that rats showed similar preferences for entering adjacent arms and arms located two positions away on blocks 2–6. A similar analysis was conducted for each treatment group and each choice, collapsed across blocks, and the same sex differences and effects of treatment were observed, regardless of which choice (i.e. choices 2–6) was being executed (data not shown).
Figure 4.
Graphs summarizing the observed probability of entering an arm based on its relative position to the arm being exited, for each block of training. Shaded areas highlight the peaks. Note that intact males show a strong preference for entering an adjacent arm, whereas intact females show a strong preference for entering an arm located two positions away. Note also that whereas ovariectomy had no effect, estradiol treatment caused females to shift to a male pattern. In contrast, both gonadectomy and testosterone treatment caused males to shift toward a female pattern.
Computer simulations were then conducted to determine whether the differences in arm choice patterns might account for the significant effects of sex and treatment on RAM performance. Simulations showed that the differences in arm choice preference produced no significant differences in the simulated performance of males vs. females on any of the five behavioral measures (data not shown).
Discussion
Sex differences in performance among gonadally intact rats
The data show significant sex differences in overall performance accuracy and in working memory performance during early stages of training between gonadally intact males and females. Specifically, males performed better than females (i.e. required fewer arm entries to collect all of the remaining food and made fewer WMEs and more correct choices before making a WME). This is consistent with a recent metaanalysis by Jonasson (6) showing robust male advantages in working memory for rats tested on RAM tasks.
In contrast, at no time did gonadally intact males outperform gonadally intact females on measures of reference memory performance. This differs markedly from Jonasson’s metaanalysis (6). In addition, rat studies have demonstrated a significant male advantage on acquisition of a Morris water maze task (6,35,36), which is a test of spatial reference memory, and Astur and co-workers (9,37) have demonstrated similar sex differences in humans using a virtual water maze task.
Several factors may account for the lack of a male advantage on reference memory in this study. One factor may be housing. The analysis by Jonasson (6) suggests that rats reared in isolation show smaller and less reliable sex differences than rats reared in groups. All of the rats in this study were individually housed. Nevertheless, we did observe significant sex differences in overall accuracy and working memory performance, suggesting that social conditions alone are not likely to account for the lack of male advantage in reference memory performance. A more likely explanation may be in the details of the task. Our protocol was designed to limit the effectiveness of certain strategies, such as visiting arms located at specific spatial intervals or selecting a specific landmark or location to begin and then proceeding in a predetermined pattern. These limitations reduce the number of strategies that can be used effectively to solve the task and may account for the lack of male advantage on the reference memory component. Notably, in human studies that have shown substantial sex differences in performance of a virtual water maze task, the differences appear to reflect strong male-female differences in strategy (9,37). These data suggest that sex differences in the performance of reference memory tasks may be related to differences in preferred strategy as opposed to differences in reference memory per se.
Effects of gonadectomy and hormone treatment
The data also show significant sex differences in the effects of gonadectomy and hormone treatment on RAM performance. In females, gonadectomy substantially impaired all measures of performance (e.g. Ovx rats showed no improvement in overall accuracy, no improvement in reference memory performance, and significantly less improvement in working memory performance relative to gonadally intact controls). Overall accuracy and working memory performance were restored by estradiol treatment. In fact, estradiol treatment improved working memory performance in Ovx rats to a level commensurate with males. In contrast, estradiol had much less effect on reference memory performance. This is similar to results reported by others showing significant effects of estradiol and/or ovariectomy on working but not reference memory in rats using an eight-arm radial maze task (22,38,39), although it should be noted that positive effects of estradiol on reference memory performance in mice have been reported (40,41).
In contrast, gonadectomy did not significantly impair overall accuracy or reference memory performance in males. Castration did, however, impair working memory performance in males but in a different way from ovariectomy in females. Specifically, Ovx females started off by making similar numbers of WMEs and slightly fewer correct choices before making a WME than gonadally intact females but improved less over time than the gonadally intact controls. In contrast, castrated males made more WMEs than gonadally intact males throughout training. This suggests that ovariectomy impairs learning (i.e. improvement) in females, whereas castration impairs performance without necessarily affecting learning in males. The distinction between learning and performance is important because factors other than cognitive ability (e.g. motivation and perception) can affect performance independent of effects on learning. Our previous studies have shown no effects of castration on acquisition of a delayed matching-to-position T-maze task (42) and no effect of ovariectomy or castration on acquisition of a configural association operant conditioning task (42,43). Because these tasks rely on many of the same motivational and perceptual abilities as the RAM task, it is unlikely that nonspecific performance factors account for the sex-specific effects of gonadectomy and hormone treatment observed here.
Notably, testosterone treatment did not significantly restore working memory performance in castrated males. Why testosterone was not effective in males is not known. Testosterone has been shown to enhance memory and spatial cognition in men (44). Kritzer et al. (31) reported that testosterone restored performance of castrated male rats on a delayed-alternation T- maze task. Other studies have shown that both testosterone and dihydrotestosterone (a nonaromatizable 5α-reduced metabolite of testosterone with potent androgenic activity) can enhance performance of male rats on inhibitory avoidance and conditioned fear tasks and that these effects are either mimicked by 3α-diol or blocked by a 3α-hydroxysteroid dehydrogenase inhibitor (33,34). Bimonte-Nelson et al. (32) also showed that testosterone enhanced working memory performance of aged male rats in a water RAM task; however, in this case, dihydrotestosterone was not effective. This suggests that nonandrogenic metabolites of testosterone (e.g. estradiol, 3α-diol) may be responsible for some effects of testosterone on working memory. Consistent with this, Edinger and Frye (45) reported that the effect of 3α-diol on inhibitory avoidance in males was reduced by antisense knockdown of ERβ in the hippocampus. Luine and Rodriguez (46) reported that estradiol, but not testosterone, was effective at enhancing working memory performance of aged male rats on a RAM task. Similarly, our own studies show that estradiol, but not testosterone, enhances acquisition of a delayed matching-to-position T-maze task by castrated male rats (42). Hence, testosterone effects on cognitive performance are complex and likely are task-specific and involve interaction with several metabolic enzymes.
Even though testosterone treatment did not restore working memory performance in males, it should be noted that castrated males consistently outperformed Ovx females with respect to both the number of WMEs committed as well as the number of correct choices before making a WME at all stages of training. This indicates that sex differences in working memory performance persist even in the absence of significant gonadal steroids. One possibility is that there may be genetic influences associated with the Y chromosome that confer male superiority on certain types of visuospatial tasks (47). Another possibility is that the levels of androgens in castrated males, although low, are still higher than those in Ovx females and may be sufficient to produce sex differences in performance. In the present study, the levels of testosterone in the castrated males were below detection; however, this does not preclude the possibility of differences in the levels of other androgenic steroids in gonadectomized males vs. females. A third possibility is that the differences in performance are due to organizational effects of gonadal steroids and other factors during critical stages of development (11). This agrees with earlier studies showing that castration and estradiol treatment during early neonatal development in rats can cause reversal of male- and female-specific patterns of RAM performance (10). Likewise, Newhouse et al. (48) recently showed that male-female differences in performance on a virtual water maze task are present before puberty, consistent with the idea that sex differences in performance are established during development and precede postpubertal differences in gonadal hormone secretions, even in humans.
With respect to reference memory performance, gonadectomy in males appeared to have no significant effect relative to gonadally intact controls. Closer examination, however, shows that gonadally intact males performed relatively poorly on the reference memory component of the task and that castrated males showed little improvement. Hence, the lack of an effect on reference memory may be related more to the poor performance of males on this component than to the absence of any effect of gonadectomy. Notably, testosterone treatment appeared to worsen, rather than improve, reference memory performance in castrated males. This was surprising given that males typically do better on spatial reference memory tasks than females. The lack of a beneficial effect of testosterone treatment in this study may again relate to details of the task. If our hypothesis is correct, i.e. that the performance of males on the reference memory component is related to the use of specific preferred strategies, then it may be that testosterone predisposes males to use strategies that were rendered ineffective in this study. This would account for the lack of an effect of castration on reference memory as well as the poorer performance of testosterone-treated males. This would also explain why, in other studies where alternate strategies could be used, castration was found to impair, and testosterone to improve, reference memory performance.
Response patterns and strategy
Studies show that rats can solve spatial tasks using a variety of strategies (49) and that estradiol treatment can increase the likelihood of using place vs. response strategies in female rats (50,51). In the present study, the effectiveness of strategies less dependent on working and reference memory was limited by forcing animals to enter three randomly selected baited arms each day before having access to all 12 arms. This does not guarantee, however, that rats were not using response strategies, nor can it be assumed that the predisposition to use such strategies is not affected by sex, gonadectomy, and hormone treatment. Indeed, our analysis demonstrated a sex difference in preferred arm choice, with males preferring to enter arms adjacent to the arm being exited and females preferring arms located two positions away. This replicates the work of Williams et al. (10), who also showed that these preferences change in response to neonatal castration and hormone manipulation. Hence, these preferences appear to arise from organizational effects of gonadal hormones during early neonatal development.
Our analysis also shows that these preferences can be altered to some degree in adults by gonadectomy and hormone treatment. It is interesting that in females, gonadectomy did not alter arm preference, whereas estradiol treatment shifted arm preference to that of males. Note that this was associated with a male-type pattern of working memory performance. The reason estradiol treatment produced a male pattern of arm preference is not known but may be due to differences in daily hormone levels between intact vs. Ovx+E rats. Ovx+E rats received continuous and sustained estradiol treatment, in contrast to the naturally cycling levels of estradiol and progesterone presumed present in the intact controls. Therefore, differences in performance may reflect specific effects of progesterone, or perhaps specific effects of cyclical vs. continuous estradiol. Nor is it known why testosterone produced a shift toward a female pattern of arm preference in castrated males. Again, this may be related to the production of nonandrogenic metabolites of testosterone.
Despite the differences in arm choice preference, computer simulations show that these differences are not sufficient in the absence of significant learning to account for the sex differences in performance detected in this study, nor can they account for the differential effects of gonadectomy and hormone treatment on acquisition of this task.
Summary and conclusions
This study demonstrates significant sex differences in the performance of a modified 12-arm RAM task as well as sex difference in the effects of gonadectomy and hormone treatment on both working and reference memory components of the task. Most notable are the robust effects of gonadectomy and hormone treatment on working memory performance in females, with much less of an effect in males. Also notable is the fact that a male advantage, which was observed on the working memory component of the task, was not observed on the reference memory component, possibly due to the inability to use certain strategies effectively on this task. Given the robust effects of gonadectomy and hormone treatment observed in females, this task should prove useful for identifying the neural circuits that underlie the effects of gonadal hormones on cognitive processes involved in spatial learning and memory as well as response patterns and strategy selection.
Acknowledgments
We thank Rhiannon Mauk, Christine Close, and Tamera Kirkland for their excellent technical assistance with this project.
Footnotes
This work was supported by National Institutes of Health Grant R01 AG021471.
Disclosure Statement: The authors have nothing to disclose.
First Published Online February 21, 2008
Abbreviations: AE1, Arm entry 1; Ovx, ovariectomized; Ovx+E, Ovx females that received estradiol; RAM, radial arm maze; RME, reference memory error; WME, working memory error.
References
- Halpern D 1992 Sex differences in cognitive abilities. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates [Google Scholar]
- Hampson E 1990 Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology 15:97–111 [DOI] [PubMed] [Google Scholar]
- Sanders G, Sjodin M, de Chastelaine M 2002 On the elusive nature of sex differences in cognition: hormonal influences contributing to within-sex variation. Arch Sex Behav 31:145–152 [DOI] [PubMed] [Google Scholar]
- Goodrich GA, Damin PB, Ascione FR, Thompson TM 1993 Gender differences in Piagetian visual-spatial representation of verticality and horizontality. J Genet Psychol 154:449–458 [DOI] [PubMed] [Google Scholar]
- Gouchie C, Kimura D 1991 The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinology 16:323–334 [DOI] [PubMed] [Google Scholar]
- Jonasson Z 2005 Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci Biobehav Rev 28:811–825 [DOI] [PubMed] [Google Scholar]
- Lawton CA 1994 Gender differences in way-finding strategies: Relationship to spatial ability and spatial anxiety. Sex Roles 30:765–779 [Google Scholar]
- Sandstrom NJ, Kaufman J, Huettel SA 1998 Males and females use different distal cues in a virtual environment navigation task. Brain Res Cogn Brain Res 6:351–360 [DOI] [PubMed] [Google Scholar]
- Astur RS, Tropp J, Sava S, Constable RT, Markus EJ 2004 Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behav Brain Res 151:103–115 [DOI] [PubMed] [Google Scholar]
- Williams CL, Barnett AM, Meck WH 1990 Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behav Neurosci 104:84–97 [DOI] [PubMed] [Google Scholar]
- Williams CL, Meck WH 1991 The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology 16:155–176 [DOI] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR 1998 Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Horm Behav 34:183–198 [DOI] [PubMed] [Google Scholar]
- Roof RL, Havens MD 1992 Testosterone improves maze performance and induces development of a male hippocampus in females. Brain Res 572:310–313 [DOI] [PubMed] [Google Scholar]
- Lund TD, Lephart ED 2001 Manipulation of prenatal hormones and dietary phytoestrogens during adulthood alter the sexually dimorphic expression of visual spatial memory. BMC Neurosci 2:21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DM, Wittert G, Burns NR 2007 Gonadal steroids and visuo-spatial abilities in adult males: implications for generalized age-related cognitive decline. Aging Male 10:17–29 [DOI] [PubMed] [Google Scholar]
- Daniel JM 2006 Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol 18:787–795 [DOI] [PubMed] [Google Scholar]
- Dohanich GP 2002 Gonadal steroids, learning and memory. In: Pfaff DW, Arnold AP, Etgen AM, Farbach SE, Rubin RT, eds. Hormones, brain and behavior. San Diego: Academic Press; 265–327 [Google Scholar]
- Sherwin BB 2003 Estrogen and cognitive functioning in women. Endocr Rev 24:133–151 [DOI] [PubMed] [Google Scholar]
- Gibbs RB 2007 Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm Behav 52:352–359 [DOI] [PubMed] [Google Scholar]
- Gibbs RB 1999 Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav 36:222–233 [DOI] [PubMed] [Google Scholar]
- Fader AJ, Hendricson AW, Dohanich GP 1998 Estrogen improves performance of reinforced T-maze alternation and prevents the amnestic effects of scopolamine administered systemically or intrahippocampally. Neurobiol Learn Mem 69:225–240 [DOI] [PubMed] [Google Scholar]
- Fader AJ, Johnson PEM, Dohanich GP 1999 Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine on a radial-arm maze. Pharmacol Biochem Behav 62:711–717 [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP 1997 Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav 32:217–225 [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH 1999 Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology 24:161–173 [DOI] [PubMed] [Google Scholar]
- Heikkinen T, Puolivali J, Liu L, Rissanen A, Tanila H 2002 Effects of ovariectomy and estrogen treatment on learning and hippocampal neurotransmitters in mice. Horm Behav 41:22–32 [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ 2003 Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology 144:2836–2844 [DOI] [PubMed] [Google Scholar]
- Vaucher E, Reymond I, Najaffe R, Kar S, Quirion R, Miller MM, Franklin KB 2002 Estrogen effects on object memory and cholinergic receptors in young and old female mice. Neurobiol Aging 23:87–95 [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA 2006 Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem 86:35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S 2001 Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1). Brain Res 888:356–365 [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, Pfaff DW 2006 Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav 49:197–205 [DOI] [PubMed] [Google Scholar]
- Kritzer MF, McLaughlin PJ, Smirlis T, Robinson JK 2001 Gonadectomy impairs T-maze acquisition in adult male rats. Horm Behav 39:167–174 [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Nelson ME, Eckman CB, Barber J, Scott TY, Granholm AC 2003 Testosterone, but not nonaromatizable dihydrotestosterone, improves working memory and alters nerve growth factor levels in aged male rats. Exp Neurol 181:301–312 [DOI] [PubMed] [Google Scholar]
- Edinger KL, Lee B, Frye CA 2004 Mnemonic effects of testosterone and its 5α-reduced metabolites in the conditioned fear and inhibitory avoidance tasks. Pharmacol Biochem Behav 78:559–568 [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger KL, Seliga AM, Wawrzycki JM 2004 5α-Reduced androgens may have actions in the hippocampus to enhance cognitive performance of male rats. Psychoneuroendocrinology 29:1019–1027 [DOI] [PubMed] [Google Scholar]
- Blokland A, Rutten K, Prickaerts J 2006 Analysis of spatial orientation strategies of male and female Wistar rats in a Morris water escape task. Behav Brain Res 171:216–224 [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Kostenuik MA, Ossenkopp KP, Kavaliers M 1996 Sex differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training. Behav Neurosci 110:1309–1320 [DOI] [PubMed] [Google Scholar]
- Astur RS, Ortiz ML, Sutherland RJ 1998 A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behav Brain Res 93:185–190 [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD 1998 Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav 34:149–162 [DOI] [PubMed] [Google Scholar]
- Wilson IA, Puolivali J, Heikkinen T, Riekkinen P 1999 Estrogen and NMDA receptor antagonism: effects upon reference and working memory. Eur J Pharmacol 381:93–99 [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC 2002 Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience 115:547–558 [DOI] [PubMed] [Google Scholar]
- Iivonen S, Heikkinen T, Puolivali J, Helisalmi S, Hiltunen M, Soininen H, Tanila H 2006 Effects of estradiol on spatial learning, hippocampal cytochrome P450 19, and estrogen α and β mRNA levels in ovariectomized female mice. Neuroscience 137:1143–1152 [DOI] [PubMed] [Google Scholar]
- Gibbs RB 2005 Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav 48:268–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R 2003 Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res 74:637–643 [DOI] [PubMed] [Google Scholar]
- Janowsky JS 2006 Thinking with your gonads: testosterone and cognition. Trends Cogn Sci 10:77–82 [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA 2006 Androgens’ effects to enhance learning may be mediated in part through actions at estrogen receptor-β in the hippocampus. Neurobiol Learn Mem 87:78–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V, Rodriguez M 1994 Effects of estradiol on radial arm maze performance of young and aged rats. Behav Neural Biol 62:230–236 [DOI] [PubMed] [Google Scholar]
- Stavnezer AJ, McDowell CS, Hyde LA, Bimonte HA, Balogh SA, Hoplight BJ, Denenberg VH 2000 Spatial ability of XY sex-reversed female mice. Behav Brain Res 112:135–143 [DOI] [PubMed] [Google Scholar]
- Newhouse P, Newhouse C, Astur RS 2007 Sex differences in visual-spatial learning using a virtual water maze in pre-pubertal children. Behav Brain Res 183:1–7 [DOI] [PubMed] [Google Scholar]
- Dudchenko PA 2001 How do animals actually solve the T maze? Behav Neurosci 115:850–860 [PubMed] [Google Scholar]
- Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ 2005 Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiol Learn Mem 84:132–137 [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL 2002 Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci 116:411–420 [DOI] [PubMed] [Google Scholar]