Abstract
Progesterone (P), the most biologically active progestin of ovarian origin, modulates numerous cellular functions in the central nervous system to coordinate physiology and reproduction. The neurobiological activity of P is mediated not by a single form of the progestin receptor (PR), but by two neural isoforms of PRs, PR-A and PR-B. Classical model of P action assumes that these neural effects are primarily mediated via their intracellular PRs, acting as transcriptional regulators, in steroid-sensitive neurons, modulating genes and genomic networks. Evidence has emerged, however, that activation of neural PRs is much more diverse; four distinct classes of molecules, neurotransmitters, peptide growth factors, cyclic nucleotides, and neurosteroids have been shown to activate the PRs via cross-talk and pathway convergence. In addition, rapid signaling events associated with membrane receptors and/or subpopulations of cytoplasmic PRs, via activation of protein kinase cascades, regulate PR gene expression in the cytoplasm independent of PR nuclear action. The increasing in vitro and in vivo evidence of differential transcriptional activities and coregulator interactions between PR-A and PR-B predict that these isoforms could have distinct roles in mediating additional and/or alternate signaling pathways within steroid-sensitive neurons. In this minireview, we evaluate the available data and discuss the possible roles of the isoforms in the regulation of neurobiological processes.
IT HAS BEEN KNOWN since the early 19th century that progesterone (P), the most biologically active progestin of ovarian origin, plays a major role in the reproduction of vertebrate species. In the decades after this discovery, progestin receptors (PRs) have been characterized, their role as ligand (hormone)-dependent transcription factors established, and the molecular basis of activation extensively studied (1,2). We have also recognized that the PRs exist as multiple isoforms. The recent era is rife with the discoveries of the coregulators for PRs, leading to a better understanding of the function of these multicomponent protein complexes as both targets and propagators of posttranslational modifications upon P stimulation (3,4). In addition to the classical PR-mediated regulation, rapid signaling effects of P mediated by cytoplasmic protein kinase cascades (5,6,7,8), novel transmembrane G protein-coupled receptors (9), and the proline-rich (PXXXPXR) motif located in the N-terminal domain of the PRs (10) have also been identified. Adding another layer of complexity to the field of P action are the findings that in the absence of P, factors like neurotransmitters and growth factors can activate PRs via cross-talk with alternate intracellular second messenger signaling pathways (6,7,8,11). Although most in vitro studies have examined the involvement of PR isoforms in these responses, the individual contributions of PR isoforms to their normal in vivo physiological regulation has only recently begun to be elucidated.
This minireview will focus on our current knowledge on the PR isoforms and summarize our understanding of the selective contribution of the neural PR isoforms to the actions of P in the brain and behavior. Some of the major challenges to the field of neuroendocrinology in the context of an expanding repertoire of P actions in the brain and behavior will be discussed.
Neuronal Progestin Receptors: Integrators of Physiology and Behavior
As in other reproductive tissues, P modulates cellular functions in the central nervous system to coordinate physiology and reproductive functions in various species (12,13,14). Accumulating evidence over the past few years suggests that in addition to reproduction, P affects a wide repertoire of biological functions including aggression, maternal behavior, learning and memory, mood, and sexual differentiation (15,16,17,18,19,20,21,22,23). Although the mechanisms of P action in these biological processes are currently being investigated, a wide body of literature exists on the molecular basis of P action in reproductive behavior in female rodents. This behavior, regulated by the sequential release of estradiol (E2) and P, has remained one of the best-studied models for investigations into the P action in the brain.
Although diverse cellular mechanisms have been ascribed to the P action in the brain, the primary mechanism involves its interaction with E2-induced, intracellular PRs that function as transcriptional factors, regulating the expression of genes and genomic neural networks to initiate and/or sustain physiological response (12,13,14). Spatial, temporal, and functional correlations of neural PRs established using progestin binding, autoradiography, immunohistochemistry, and in situ hybridization techniques (12,13) identified the presence of two distinct classes of the neural PRs. Those that were dramatically increased with E2 priming, the E2-induced PRs, were localized to the ventromedial and arcuate nuclei in the hypothalamus; medial, periventricular, and suprachiasmatic preoptic areas; the midbrain central gray and tegmental areas; amygdala; the bed nucleus of stria terminalis; hypophysis; and pituitary gonadotropes in rats, hamsters, and guinea pigs (12,13,14). The PRs not induced by E2 were identified in the cerebellum and the cortex (24,25).
Physiological functions of the neural PRs as integrators of preovulatory LH surge and female reproductive behavior have been extensively studied in female rodents. It is well established that E2-induced neural PRs in the ventromedial nucleus of the hypothalamus and the preoptic area (POA) mediate P facilitation of proceptive and receptive (measured as lordosis response) behavioral components of the female rodent reproductive behavior, when mounted by a conspecific male (12,13,14). Studies using PR antagonists, protein and RNA synthesis inhibitors, antisense oligonucleotides to PR, and mutant mice with targeted deletion of PR gene provide irrevocable proof that the P facilitation of lordosis response involves a classical ligand (hormone)-dependent PR-mediated mechanism in female rodents (26,27,28,29,30,31,32,33).
Over the past few years, studies from our laboratory have demonstrated that in addition to P, neurotransmitter dopamine (DA) can activate neural PRs in a ligand (hormone)-independent manner to facilitate reproductive behavior (33,34). Using PR antagonists, antisense oligonucleotides and null mutants for PRs, we established a critical requirement of neural PRs as transcriptional mediators in the cross-talk between P- and DA-initiated pathways in the facilitation of female sexual receptive behavior. Studies from our laboratory also demonstrated that the DA-initiated second messenger signaling cascade involves the activation of protein kinase A (PKA) and neuronal phosphoprotein DA- and cAMP-regulated phosphoprotein-32 (DARPP-32), leading to the alterations in the phosphorylation dynamics and activation of PRs and/or its coregulators in the hypothalamus (35,36).
Cross-talk between signal transduction pathways is not exclusive to reproductive behavior and has been observed in other similar physiological situations, i.e. GnRH self-priming (37,38) and the production of GnRH surges (39,40,41). A behaviorally relevant stimulus, such as vaginal cervical stimulation, has also been shown to activate the neural PRs in the absence of P (42,43,44). Thus, afferent influences on steroid-sensitive neurons within the brain could have profound effects on physiology and behavior. The notion that a multitude of second and third messenger signaling molecules can substitute for P and facilitate reproductive behaviors in not novel to the field of neuroendocrinology and has existed since the early 1980s (45,46). However, the findings that many of these molecules can activate rapid signaling pathways using protein kinases as mediators within the neurons to converge with the PR-mediated genomic pathways provide a mechanistic interpretation of the integratory mechanisms that are being employed in the brain.
Multiple Forms of Progestin Receptors: A Primer
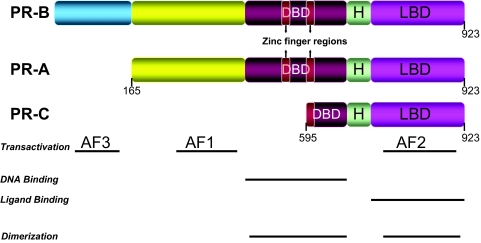
Similar to the other members of the steroid receptor superfamily, PRs have a modular protein structure consisting of distinct functional domains capable of binding the ligand at the carboxyl-terminal end [ligand-binding domain (LBD)] and the centrally located, highly conserved DNA-binding domain (DBD) required for the transcriptional activity (Fig. 1). The amino-terminal region contains a transactivation function (AF1) that modulates the level and promoter specificity of target gene activation by interacting with components of core transcriptional complex and coactivators. A second activation function (AF2) is present in the LBD, which in addition to P binding, contains sequences for dimerization, heat-shock protein association, intermolecular silencing, and intramolecular repression. A unique third activation function (AF3) is present in the B-upstream segment at the far end of the N terminus of human PR (B-isoform), which can function autonomously or synergize with downstream activation functions (AF1 and AF2) to enhance their activity (47).
Figure 1.
Structural organization of PR isoforms. The numbers in the N-terminal region denote the amino acid position in each isoform. AF1, AF2, and AF3 are the activation function domains; H is the hinge region. The sequences important for dimerization and the first and second zinc finger regions of DBD are represented.
Multiple PR isoforms are produced from a single gene on chromosome 11 at q22–23 by transcription from alternative promoters (48,49) and alternate translational sites (50). Historically, two isoforms, PR-A and PR-B, which are structurally related but functionally distinct, have been identified and investigated in most of the vertebrate species, including the chicken, rodents, primates, and the human (50,51,52,53). The only exception is the lagomorph, rabbit, which contains only one form of the PR (54,55). The two isoforms differ at the amino terminus, with the PR-A arising as an N-terminally truncated form of the larger PR-B, initiating from methionine 165, relative to PR-B. They have similar steroid hormone- and DNA-binding activities but exhibit divergent transactivational properties both in vitro (56,57) and in vivo (58,59,60). More importantly, PR-A and PR-B proteins can dimerize and bind the DNA as three distinct species: A:A and B:B homodimers and A:B heterodimers. The differential transactivational function of these complexes is contributed by the presence of a PR-B-specific AF3, which allows binding of a subset of coactivators to PR-B that is not efficiently recruited by P-bound PR-A (56). Interestingly, a third isoform, PR-C, has also been identified in the human (61,62). This protein of 60 kDa is also an N-terminally truncated isoform that lacks AF3 and AF1 and the first zinc finger of DBD and initiates at methionine 595 relative to PR-A and PR-B. PR-C isoform is thought to modulate the transcriptional activity of PR-A and PR-B (61). Thus, the differential structure of the PR isoforms confers distinct tissue-specific responses to P, through posttranslational modifications, dimerization, and recruitment of cofactor proteins contributing to the differential transactivation properties of each isoform and leading to the regulation of distinct subsets of P-dependent target genes.
PR Isoforms in the Brain: Here, There, and Everywhere
With the identification of multiple PRs in various P-responsive tissues, studies on neural PRs focused on differential expression patterns of the PR isoforms and their regulation in the brain. Although PR-A and PR-B isoforms have been identified in the brain, there are no reports of the existence of a PR-C isoform to date. Initial studies using RT-PCR analyses revealed the expression of both PR-A and PR-B mRNA transcripts in all the regions of the brain where the neural PRs are known to be present. The analyses revealed that the ratio of the isoforms varies in different brain regions during development of the female rat (63), with a region-specific ontogenic expression of PR-B mRNA from birth to postnatal d 7 in hypothalamus and POA, yielding to the PR-A mRNA expression from postnatal d 8–12. The low PR-B expression in the cerebellum and cortex, however, remained unchanged throughout this period (63,64). Interestingly, the developmental expression of murine PR isoforms in the midbrain follows a similar trend, with PR-B expression detectable until postnatal d 7, followed by a decrease on postnatal d 8 (65).
In the adult female rats, E2 and P differentially regulate the isoforms in distinct regions in the brain (66). Although mRNA transcripts for both PR-A and PR-B isoforms are induced by E2 and down-regulated by P in the hypothalamus, only PR-B mRNA expression was hormonally modulated in the POA. In the hippocampus, E2 induced only PR-A expression, whereas P had no significant effects on either isoform (66). Neither E2 nor P had any effect on PR isoform mRNA expression in both cerebellum and frontal cortex (67), confirming the binding studies that the PRs in these regions are not E2 induced. In contrast, other studies in the adult rat, report no region-specific alterations in the ratio of PR-A to PR-B transcripts in the brain (41). PR isoform transcripts also vary with estrous cycle in a region-specific manner, with PR-B being predominantly expressed on proestrus in the hypothalamus and on metestrus in the POA (68). Interestingly, the authors report no effects of the estrous cycle on the isoforms in the hippocampus, contradicting their earlier observations of E2 induction of PR-A (66). Studies in E2-treated rhesus macaques indicate a region-specific regulation of the PR isoforms, with PR-B expression being predominant in the hypothalamus and PR-A in the pituitary (69).
Sex differences are also evident in PR-A and PR-B mRNA expression patterns. In the male rat hypothalamus and hippocampus, PR-A expression, although not significant, is greater than PR-B expression, whereas the pattern is reversed in the POA (70). In contrast to the RT-PCR and binding studies in the female, E2 up-regulates PR-A isoform expression only in the cerebellum but not in the other regions in the male (67). Conflicting with these findings are the reports by Scott et al. (72) demonstrating induction of PR-B by E2 in the hypothalamus and pituitary of both male and female rats using RNase protection analyses, with minimal changes in PR-A. The levels of PR-B in the females were higher than in the males, suggesting greater responsiveness of the female brain to E2 and P.
Differential expression and altered regulation of PR-A and PR-B are also observed in rat and mouse pituitary cells (38). E2 treatment increases the transcription of both isoforms, despite the predominance of PR-A in both the species. However, species differences in pituitary response to E2 are evident in the robust effects on PR-B transcript in the rat resulting in a decreased PR-A to PR-B ratio, whereas the ratio remains unchanged in the mouse. The levels of PR-A and PR-B mRNA vary with estrous cycle, being the highest on the morning of proestrus (coinciding with high E2 levels) and decreasing by the evening in female rats (73). Although most of the studies examined mRNA expression of PR-A and PR-B, only a handful of studies have examined the PR-A and PR-B protein regulation in the brain (65,74). Interestingly, in both these studies the protein expression was incongruous with the expression of their mRNA transcripts, complicating the interpretation in terms of function.
The fact that the isoforms are differentially expressed in a region-specific manner and the ratios of the individual isoforms vary as a consequence of developmental and hormonal status raises the possibility that the isoforms could constitute a major regulatory mechanism for P regulation of diverse sets of target genes.
Beyond Localization: Defining Physiological Functions
Many of the studies detailed above provide clues to the spatiotemporal expression of PR-A and PR-B isoforms and how they subserve region-specific neuronal responses to E2 and P in the brain. They do not, however, address the functional implications of the differential hormone regulation or the contribution of the individual isoform and its relationship to a particular behavior or physiological endpoint. The generation of mutant mice in which the expression of PR-A (PRAKO−/−) and PR-B (PRBKO −/−) isoforms has been selectively ablated, has facilitated the direct analyses of the individual contributions of the PR isoforms in mediating neuronal responses to P (58,59,60). Consistent with the distinct tissue- and promoter-specific activities of PR-A and PR-B in vitro, each individual isoform has been found to modulate a distinct, but partially overlapping, subset of reproductive functions by regulation of a diverse subset of target genes as seen in their phenotypic response in the uterus, the ovary, and the mammary gland (75,76).
Recent studies have begun to address the individual contributions of the neural PR-A and PR-B isoforms in mediating physiological responses to P and the alternative ligand-independent intracellular signaling pathways using mutant mice in which the expression of PR-A (PRAKO−/−) and PR-B (PRBKO−/−) isoforms has selectively been ablated (77). Using reproductive behavior as the functional readout, these studies established a key role for neural PR-A isoform in mediating both P-dependent and -independent sexual receptive behavior in the female mice (58,60). Ablation of PR-A significantly diminished P-facilitated reproductive behavior in PRAKO−/− mice, whereas the ablation of PR-B resulted in a reduced response to P compared with their wild-type littermates. Thus, although PR-A is critical in mediating P-facilitated response, it is not adequate to mediate the full magnitude of the behavioral response. The functional participation of PR-B, most likely through heterodimerization, appears to be more effective for the display of the full complement of P-dependent receptive behavior in mice. In contrast to P, ligand-independent activation by DA appears to involve both the isoforms, PR-A and PR-B, because PRAKO−/− and PRBKO−/− mice display reduced, but not diminished, behavioral response. Surprisingly, the effects of cyclic nucleotide cAMP were primarily mediated by PR-A, because PRAKO−/− mice failed to display lordosis, compared with reduced levels in PRBKO−/− mice, suggesting that DA and cAMP are perhaps using different isoforms and distinct intracellular signaling pathways to activate PRs. It is tempting to propose that DA and cAMP activation of murine PRs could involve altered phosphorylation of distinct coactivators, several coactivators, or other phosphorylation sites on the PRs and/or coactivators in the multicomponent steroid receptor complexes that serve as a sensor for multiple modulatory signals.
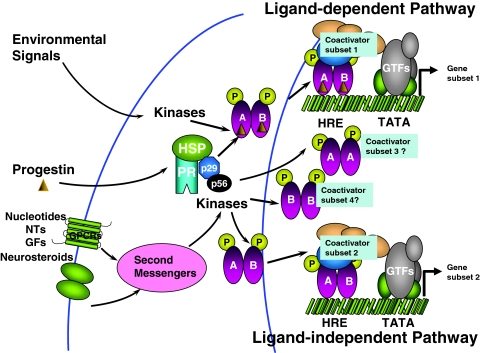
Our in vivo findings that reveal distinct functional roles for PR-A and PR-B in the mouse are in general agreement with the in vitro observations in humans and the chicken (78,79,80). However, a dominant P-dependent transcriptional role for the PR-B isoform was not evident in the mouse as seen in the human and the chicken (81,82,83,84). In the murine brain, however, it appears that PR-A has a dominant role in reproductive behavior, suggesting that perhaps bigger is not always better. This could be a reflection of tissue-selective variation because PR-B is the dominant form in the mouse mammary gland. From a mechanistic viewpoint, the differences in physiological response to P and DA, or DA and cAMP, provides an illustration of the critical role played by the N-terminal AF-3 in PR to alter physiological responses mediated by neuronal PRs. Given that the neurons respond to myriad intra- and interneuronal signals, transmitted via afferent and efferent influences, it is not surprising that the PR isoforms, via altered phosphorylation and/or coactivator recruitment, could integrate the converging responses to control and coordinate subsets of genes (Fig. 2).
Figure 2.
Schematic representation of integrated PR activation. Unliganded PR is present as an inactive complex associated with heat-shock proteins (HSP) and chaperone proteins (p29, p56). In the classical ligand-dependent pathway, P and other progestins bind to the PR, resulting in conformational change, dissociation of HSP and chaperone proteins, dimerization of the receptor, and binding to the hormone response element (HRE) in the target DNA. The P-induced conformational change facilitates the recruitment of cofactors and other general transcription factors (GTFs) to the promoter, producing a transcriptionally active complex that can direct gene transcription. Compounds such as cyclic nucleotides, neurotransmitters (NTs), growth factors (GFs), neurosteroids, and other environmental signals can activate second messengers and protein kinase pathways to activate PR and/or coactivators in a ligand-independent manner. Depending upon the activating stimulus, PRs can dimerize as homodimers (A:A, B:B) or heterodimers (A:B) and upon binding to HRE can recruit distinct sets of coactivators, leading to transcriptional activation of distinct subsets of genes.
Conclusions
The past decade has witnessed an exceptional progress in our understanding of the cellular and molecular mechanisms by which neural PRs integrate diverse physiological responses. The identification of the neural PR-A and PR-B and the availability of knockout models have facilitated the direct examination of isoform-selective responses to P and DA in the brain. From the limited behavioral studies carried to date, we have begun to appreciate that the existence of neural PR-A and PR-B isoforms is not redundant but a requirement in neuroendocrine regulation. Our studies have dealt with adult animals and the examination of one PR-dependent behavior. We have yet to understand how these isoforms could contribute to the other neurobiological processes mediated by the neural PRs. Furthermore, as detailed in the accompanying minireviews, recent data demonstrating that P synthesis is not exclusive to the ovaries, emphasizes a sophisticated regulatory scheme for P action in the central nervous system both during development and in adulthood. It remains to be seen what role the isoforms may play in the organization or sexual differentiation of the brain or how they may respond to various growth factors during development. Emerging evidence that P synthesis occurs in the Purkinje cells of the cerebellum (71,85,86,87) suggests that the isoforms could have a role in synapse formation and function. Very little is known about their compartmentalization and/or role in astrocytes and/or glial cell interactions within the brain. The spatiotemporal expression of PR-A relative to PR-B, although informative, is insufficient for inferring the biological response because the latter is a function of the regulation of the subset of target genes that are activated by the individual isoforms. Future studies on the cellular and molecular mechanisms by which the isoforms modulate a diverse repertoire of transcriptional and posttranslational modifications to integrate hormonal, humoral, and environmental factors within the brain will yield significant insights into the complexities of P action in the brain.
Acknowledgments
I thank Drs. Bhuvana Balasubramanian and Wendy Portillo for helpful discussions and comments on the manuscript.
Footnotes
This work was supported by the U.S. Public Health Service Grants MH 57442 and MH 63954 from the National Institutes of Health.
Disclosure Statement: The author has nothing to declare.
First Published Online February 28, 2008
Abbreviations: DA, Dopamine; DBD, DNA-binding domain; E2, estradiol; LBD, ligand-binding domain; P, progesterone; POA, preoptic area; PR, progestin receptor.
References
- O'Malley BW, Means AR 1974 Female steroid hormones and target cell nuclei. Science 183:610–620 [DOI] [PubMed] [Google Scholar]
- O'Malley BW, Tsai MJ 1992 Molecular pathways of steroid receptor action. Biol Reprod 46:163–167 [DOI] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O'Malley BW 1999 Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 20:321–339 [DOI] [PubMed] [Google Scholar]
- Lonard DM, O' Malley BW 2007 Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell 27:691–700 [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Edwards DP 2007 Receptor mechanisms mediating non-genomic actions of sex steroids. Semin Reprod Med 25:139–153 [DOI] [PubMed] [Google Scholar]
- Lange CA, Gioeli D, Hammes SR, Marker PC 2007 Integration of rapid signaling events with steroid hormone receptor action in breast and prostate cancer. Annu Rev Physiol 69:171–199 [DOI] [PubMed] [Google Scholar]
- Lange CA 2004 Making sense of cross talk between steroid hormone receptors and intracellular signaling pathways: who will have the last word? Mol Endocrinol 18:269–278 [DOI] [PubMed] [Google Scholar]
- Mani SK 2006 Signaling mechanisms in progesterone-neurotransmitter interactions. Neuroscience 138:773–781 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P 2003 Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA 100:2231–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP 2001 Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell 8:269–280 [DOI] [PubMed] [Google Scholar]
- Blaustein JD 2004 Neural steroid hormone receptors: they’re not just for hormones anymore. Endocrinology 145:1075–1081 [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Erskine MS 2002 Feminine sexual behavior: Cellular integration of hormonal and afferent information in the rodent brain. In: Pfaff DW, Arnold AP, Etgen, AM, Fahrbach, SE, Rubin RT, eds. Hormones, brain and behavior. Vol 1. San Diego: Academic Press; 139–214 [Google Scholar]
- Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow L 1994 Cellular and molecular mechanisms of female reproductive behavior. In: Knobil E, Neill JD, eds. Physiology of reproduction. New York: Raven Press; 107–220 [Google Scholar]
- Blaustein JD, Mani SK 2007 Feminine sexual behavior from the neuroendocrine and molecular neurobiological perspectives In: Blaustein JD, ed; Lajtha A, Series ed. Handbook of neurochemistry and molecular neurobiology: behavioral neurochemistry and neuroendocrinology. Vol 21. Berlin: Springer-Verlag; 95–150 [Google Scholar]
- Fraile IG, McEwen BS, Pfaff DW 1987 Progesterone inhibition of aggressive behaviors in hamsters. Physiol Behav 39:225–229 [DOI] [PubMed] [Google Scholar]
- Meisel RL, Fraile IG, Pfaff DW 1990 Hypothalamic sites of progestin action on aggression and sexual behavior in female Syrian hamsters. Physiol Behav 47:219–223 [DOI] [PubMed] [Google Scholar]
- Numan M, Roach JK, Del Cerro MCR, Guillamon A, Segovia S, Sheehan TP, Numan MJ 1999 Expression of intracellular progesterone receptors in the rat brain during different reproductive states, and in involvement in maternal behavior. Brain Res 830:358–371 [DOI] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Darnaudery M, Corpechot C, Young J, Koehl M, Le Moal M, Baulieu EE, Robel P, Simon H 1997 Neurosteroids: deficient cognitive performance in aged rats depends on low pregnene sulfate levels in the hippocampus. Proc Natl Acad Sci USA 94:14865–14870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood JF, Morley JE, Roberts E 1992 Memory-enhancing effects in male mice of pregnenolone and steroids metabolically derived from it. Proc Natl Acad Sci USA 89:1567–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR 2000 Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 157:924–930 [DOI] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF 2007 Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci USA 104:2465–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CK, Nakayama AY, De Vries GJ 1998 Potential role of maternal progesterone in the sexual differentiation of the brain. Endocrinology 139:3658–3661 [DOI] [PubMed] [Google Scholar]
- Wagner CK 2006 The many faces of progesterone: a role in adult and developing male brain. Front Neuroendocrinol 27:340–359 [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, McEwen BS 1978 Oestrogen modulates progestin receptor concentrations in some rat brain regions but not in others. Nature 274:276–278 [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, McEwen BS 1980 Progestin receptors in rat brain: distribution and properties of cytoplasmic progestin-binding sites. Endocrinology 106:192–202 [DOI] [PubMed] [Google Scholar]
- Glaser JH, Etgen AM, Barfield RJ 1985 Intrahypothalamic effects of progestin agonists on estrous behavior and progestin receptor binding. Physiol Behav 34:871–878 [DOI] [PubMed] [Google Scholar]
- Brown TJ, Blaustein JD 1984 Inhibition of sexual behavior in female guinea pigs by a progestin receptor antagonist. Brain Res 301:343–349 [DOI] [PubMed] [Google Scholar]
- Meisel RL, Pfaff DW 1984 RNA and protein synthesis inhibitors: effects of sexual behavior in female rats. Brain Res Bull 12:187–193 [DOI] [PubMed] [Google Scholar]
- Meisel RL, Pfaff DW 1985 Specificity and neural sites of action of anisomycin in the reduction or facilitation of female sexual behavior in rats. Horm Behav 19:237–251 [DOI] [PubMed] [Google Scholar]
- Pollio G, Xue P, Zanisi A, Nicolin A, Maggi A 1993 Antisense oligonucleotide blocks progesterone-induced lordosis behavior in ovariectomized rats. Mol Brain Res 19:135–139 [DOI] [PubMed] [Google Scholar]
- Ogawa S, Olazabal S, Pfaff DW 1994 Effects of intrahypothalamic administration of antisense DNA for progesterone receptor mRNA on reproductive behavior and progesterone receptor immunoreactivity. J Neurosci 14:1766–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SK, Blaustein JD, Allen JMC, Law SW, O'Malley BW, Clark JH 1994 Inhibition of rat sexual behavior by antisense oligonucleotides to the progesterone receptor. Endocrinology 135:1409–1414 [DOI] [PubMed] [Google Scholar]
- Mani SK, Allen JMC, Lydon, JP, Mulac-Jericevic B, Blaustein JD, DeMayo FJ, Conneely OM, O'Malley BW 1996 Dopamine requires the unoccupied progesterone receptor to induce sexual behavior in mice. Mol Endocrinol 10:1728–1737 [DOI] [PubMed] [Google Scholar]
- Mani SK, Allen JMC, Clark JH, Blaustein JD, O' Malley BW 1994 Convergent pathways for steroid hormone- and neurotransmitter-induced rat sexual behavior. Science 265:1246–1249 [DOI] [PubMed] [Google Scholar]
- Mani SK, Fienberg AF, O' Callaghan JP, Snyder GL, Allen PB, Dash PK, Moore AN, Mitchell AJ, Bibb J, Greengard P, O'Malley BW 2000 Requirement of DARPP-32 in progesterone-facilitated sexual receptivity in female rats. Science 287:1053–1056 [DOI] [PubMed] [Google Scholar]
- Mani SK, O'Malley BW 2002 Molecular mechanisms of Progesterone receptor action. In: Pfaff DW, Etgen AM, Fahrbach SE, Rubin RT, eds. Hormones, brain and behavior. Vol 3. San Diego: Academic Press; 643–682 [Google Scholar]
- Turgeon JL, Waring DW 1994 Activation of the progesterone receptor by the gonadotropin-releasing hormone self-priming signaling pathway. Mol Endocrinol 8:860–869 [DOI] [PubMed] [Google Scholar]
- Turgeon JL, Waring DW 2006 Differential expression and regulation of progesterone receptor isoforms in rat and mouse pituitary cells and LβT2 gonadotropes. J Endocrinol 190:837–846 [DOI] [PubMed] [Google Scholar]
- Chappell PE, Lee J, Levine JE 2000 Stimulation of gonadotropin-releasing hormone surges by estrogen. II. Role of cyclic adenosine 3′5′-monophosphate. Endocrinology 141:1486–1492 [DOI] [PubMed] [Google Scholar]
- Chappell PE, Levine JE 2000 Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinology 141:1477–1485 [DOI] [PubMed] [Google Scholar]
- Levine JE, Chappell PE, Schneider JS, Sleiter NC, Szabo M 2001 Progesterone receptors as neuroendocrine integrators. Front Neuroendocrinol 22:69–106 [DOI] [PubMed] [Google Scholar]
- Auger AP, Blaustein JD 1997 Progesterone treatment increases Fos-immunoreactivity within some progestin receptor-containing neurons in localized regions of female rat forebrain. Brain Res 746:164–170 [DOI] [PubMed] [Google Scholar]
- Auger AP, Moffatt CA, Blaustein JD 1997 Progesterone-independent activation of rat brain progestin receptors by reproductive stimuli. Endocrinology 138:511–514 [DOI] [PubMed] [Google Scholar]
- Auger AP, LaRiccia LM, Moffatt CA, Blaustein JD 2000 Progesterone, but not progesterone-independent activation of progestin receptors by a mating stimulus, rapidly decreases progestin receptor immunoreactivity in female rat brain. Horm Behav 37:135–144 [DOI] [PubMed] [Google Scholar]
- Whalen RE, Lauber AH 1986 Progesterone substitutes: cGMP mediation. Neurosci Biobehav Rev 10:47–53 [DOI] [PubMed] [Google Scholar]
- Beyer C, Gonzalez-Mariscal G 1986 Elevation in hypothalamic cyclic AMP as a common factor in the facilitation of lordosis in rodents: a working hypothesis. Ann NY Acad Sci 474:270–281 [DOI] [PubMed] [Google Scholar]
- Sartorius CA, Melville MY, Hovland AR, Tung L, Takimoto GS, Horwitz KB 1994 A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B-isoform. Mol Endocrinol 8:1347–1360 [DOI] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P 1990 Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor isoforms A and B. EMBO J 9:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus WL, Montano MM, Katzenellenbogen BS 1993 Cloning of the rat progesterone receptor gene 5′-region and identification of two functionally distinct promoters. Mol Endocrinol 7:1603–1616 [DOI] [PubMed] [Google Scholar]
- Conneely OM, Kettleberger DM, Tsai MJ, Schrader WT, O'Malley BW 1989 The chicken progesterone receptor A and B isoforms are products of an alternate translation initiation event. J Biol Chem 264:14062–14064 [PubMed] [Google Scholar]
- Schneider W, Ramachandran C, Satyaswaroop PG, Shyamala G 1991 Murine progesterone receptor exists predominantly as the 83-kilodalton ‘A’ form. J Steroid Biochem Mol Biol 38:285–291 [DOI] [PubMed] [Google Scholar]
- Lessey BA, Alexander PS, Horwitz KB 1983 The subunit structure of human breast cancer progesterone receptors: characterization by chromatography and photoaffinity labeling. Endocrinology 112:1267–1274 [DOI] [PubMed] [Google Scholar]
- Duffy DM, Wells TR, Haluska GJ, Stouffer RL 1997 The ratio of progesterone receptor isoforms changes in the monkey corpus luteum during the luteal phase of the menstrual cycle. Biol Reprod 57:693–699 [DOI] [PubMed] [Google Scholar]
- Loosfelt H, Logeat F, Vu Hai MT, Milgrom E 1994 The rabbit progesterone receptor. Evidence for a single steroid-binding subunit and characterization of receptor mRNA. J Biol Chem 259:14196–14202 [PubMed] [Google Scholar]
- Savouret JF, Bailly A, Misrahi M, Rauch C, Redeuilh G, Chauchereau A, Milgrom E 1991 Characterization of the hormone responsive element involved in the regulation of the progesterone receptor gene. EMBO J 10:1875–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP 2000 The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol 20:3102–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB 2002 Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem 277:5209–5218 [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM 2000 Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 289:1751–1754 [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O'Malley BW 2002 Reproductive functions of progesterone receptors. Recent Prog Horm Res 57:339–355 [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo F, Conneely OM 2003 Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA 100:9744–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LL, Gonzalez-Aller C, Wood WM, Miller LA, Horwitz KB 1990 5′-Heterogeneity in human progesterone receptor transcripts predicts a new amino-terminal truncated “C”-receptor and unique A-receptor messages. Mol Endocrinol 4:1833–1840 [DOI] [PubMed] [Google Scholar]
- Wei LL, Hawkins P, Baker C, Norris B, Sheridan PL, Quinn PG 1996 An amino-terminal truncated progesterone receptor isoform, PRc, enhances progestin-induced transcriptional activity. Mol Endocrinol 10:1379–1387 [DOI] [PubMed] [Google Scholar]
- Kato J, Hirata S, Nozawa A, Yamada-Mouri N 1994 Gene expression of progesterone receptor isoforms in the rat brain. Horm Behav 28:454–463 [DOI] [PubMed] [Google Scholar]
- Kato J, Hirata S, Nozawa A, Mouri N 1993 The ontogeny of gene expression of progestin receptors in the female rat brain. J Steroid Biochem Mol Biol 47:173–182 [DOI] [PubMed] [Google Scholar]
- Beyer C, Damm N, Brito V, Küppers E 2002 Developmental expression of progesterone receptor isoforms in the mouse midbrain. Neuroreport 13:877–880 [DOI] [PubMed] [Google Scholar]
- Camacho-Arroyo I, Guerra-Araiza C, Cerbon MA 1998 Progesterone receptor isoforms are differentially regulated by sex steroids in the rat forebrain. Neuroreport 9:3993–3996 [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Coyoy-Salgado A, Camacho-Arroyo I 2002 Sex differences in the regulation of progesterone receptor isoforms expression in the rat brain. Brain Res Bull 59:105–109 [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Cerbon MA, Morimoto S, Camacho-Arroyo I 2000 Progesterone receptor isoforms expression pattern in the rat brain during the estrous cycle. Life Sci 66:1743–1752 [DOI] [PubMed] [Google Scholar]
- Bethea CL, Widmann AA 1998 Differential expression of progestin receptor isoforms in the hypothalamus, pituitary, and endometrium of rhesus macaques. Endocrinology 139:677–687 [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Reyna-Neyra A, Salazar AM, Cerbon MA, Morimoto S, Camacho-Arroyo I 2001 Progesterone receptor isoforms expression in the prepuberal and adult male rat brain. Brain Res Bull 54:13–17 [DOI] [PubMed] [Google Scholar]
- Sasahara K, Shikimi H, Haraguchi S, Sakamoto H, Honda S, Harada N, Tsutsui K 2007 Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing Purkinje cell. J Neurosci 27:7408–7417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RE, Wu-Peng XS, Pfaff DW 2002 Regulation and expression of progesterone receptor mRNA isoforms A and B in the male and female rat hypothalamus and pituitary following oestrogen treatment. J Neuroendocrinol 14:175–183 [DOI] [PubMed] [Google Scholar]
- Szabo M, Kilen SM, Nho SJ, Schwartz NB 2000 Progesterone receptor A and B messenger ribonucleic acid levels in the anterior pituitary of rats are regulated by estrogen. Biol Reprod 62:95–102 [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Villamar-Cruz O, González-Arenas A, Chavira R, Camacho-Arroyo I 2003 Changes in progesterone receptor isoforms content in the rat brain during the oestrous cycle and after oestradiol and progesterone treatments. J Neuroendocrinol 15:984–990 [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, Lydon JP 2003 Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids 68:771–778 [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Conneely OM 2004 Reproductive tissue selective actions of progesterone receptors. Reproduction 128:139–146 [DOI] [PubMed] [Google Scholar]
- Mani SK, Reyna AM, Chen JZ, Mulac-Jericevic B, Conneely OM 2006 Differential response of progesterone receptor isoforms in hormone-dependent and -independent facilitation of female sexual receptivity. Mol Endocrinol 20:1322–1332 [DOI] [PubMed] [Google Scholar]
- Tora L, Gronemeyer H, Turcotte B, Gaub MP, Chambon P 1988 The N-terminal region of the chicken progesterone receptor specifies target gene activation. Nature 333:185–188 [DOI] [PubMed] [Google Scholar]
- Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP 1993 Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol 7:1244–1255 [DOI] [PubMed] [Google Scholar]
- Weigel NL, Bai W, Zhang Y, Beck CA, Edwards DP, Poletti A 1995 Phosphorylation and progesterone receptor function. J Steroid Biochem Mol Biol 53:509–514 [DOI] [PubMed] [Google Scholar]
- Giangrande PH, McDonnell DP 1999 The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Prog Horm Res 54:291–313 [PubMed] [Google Scholar]
- Kazmi SM, Visconti V, Plante RK, Ishaque A, Lau C 1993 Differential regulation of human progesterone receptor A and B form-mediated trans-activation by phosphorylation. Endocrinology 133:1230–1238 [DOI] [PubMed] [Google Scholar]
- Jacobsen BM, Richer JK, Schittone SA, Horwitz KB 2002 New human breast cancer cells to study progesterone receptor isoform ratio effects and ligand-independent gene regulation. J Biol Chem 277:27793–27800 [DOI] [PubMed] [Google Scholar]
- Mohamed MK, Tung L, Takimoto GS, Horwitz KB 1994. The leucine zippers of c-fos and c-jun for progesterone receptor dimerization: A-dominance in the A/B heterodimer. J Steroid Biochem Mol Biol. 51:241–250 [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Ukena K, Tsutsui K 2001 Effects of progesterone synthesized de novo in the developing Purkinje cell on its dendritic growth and synaptogenesis. J Neurosci 21:6221–6232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena K, Usui M, Kohchi C, Tsutsui K 1998 Cytochrome P450 side-chain cleavage enzyme in the cerebellar Purkinje neuron and its neonatal change in rats. Endocrinology 139:137–147 [DOI] [PubMed] [Google Scholar]
- Ukena K, Kohchi C, Tsutsui K 1999 Expression and activity of 3β-hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase in the rat Purkinje neuron during neonatal life. Endocrinology 140:805–813 [DOI] [PubMed] [Google Scholar]