Abstract
Whereas mutations in the bmp15 gene cause infertility in ewes and women due to defects in folliculogenesis, most defects in female mice lacking bone morphogenetic protein (BMP)-15 are confined to the ovulation process, supportive of the observation that functional mouse BMP-15 is barely detected in oocytes in vivo until after the LH surge. In addition, the mouse BMP-15 proprotein is not processed into the functional mature protein in transfected cells. However, a chimeric protein consisting of the human proregion, human cleavage site, and mouse mature region (termed hhmBMP-15) is processed and the mature protein secreted. To study the role of BMP-15 in folliculogenesis, we generated transgenic mice overexpressing hhmBMP-15, exclusively in oocytes during folliculogenesis and confirmed the overexpression of mouse BMP-15 mature protein. Immature transgenic mice exhibited accelerated follicle growth with decreased primary follicles and an increase in secondary follicles. Granulosa cells of immature mice displayed an increased mitotic index and decreased FSH receptor mRNA expression. Adult mice had normal litter sizes but an increased number of atretic antral follicles. Interestingly, aging mice exhibited an early onset of acyclicity marked by increased diestrus length and early occurrence of constant diestrus. These findings indicate the role of BMP-15 in vivo in promoting follicle growth and preventing follicle maturation, resulting in an early decline in the ovarian reserve of transgenic mice. Therefore, the lack of mouse BMP-15 during early folliculogenesis in the wild-type mice may be relevant to their polyovulatory nature as well as the preservation of ovarian function as the mice age.
BONE MORPHOGENETIC PROTEIN-15 (BMP-15) is an oocyte-secreted factor that, along with its closest homolog, growth and differentiation factor (GDF)-9, plays a key role in the regulation of fertility in monoovulatory mammals (1). Both BMP-15 and GDF-9 are known to be important determinants of ovulation quota in ewes, in which heterozygous point mutations in the either the bmp15 or gdf9 gene result in increases in ovulation quota and litter size, whereas homozygous mutations lead to infertility with an arrest at the primary stage of folliculogenesis (2,3,4). BMP-15 was also shown to be involved in human fertility in sisters carrying a heterozygous mutation in the bmp15 gene, which caused an ovarian phenotype similar to that of the homozygous mutant sheep (5). GDF-9 null mice, like BMP-15 and GDF-9 mutant sheep, are infertile with a block in folliculogenesis at the primary stage (6). Mutations in the bmp15 and gdf9 genes have also been found at high incidence in patients with premature ovarian failure (7,8,9,10,11,12).
The first indication that BMP-15 may have distinct functions in monoovulatory vs. polyovulatory species came with the development of the BMP-15 null mouse. Unlike the GDF-9 null mouse, mice lacking BMP-15 exhibited normal folliculogenesis but were instead subfertile due to defects in ovulation and early embryonic development (13). Because many important functions of recombinant human BMP-15 have been elucidated using rodent granulosa cell models, it appears that rodent granulosa cells have the ability to respond to BMP-15 (14,15,16,17,18). Therefore, we hypothesized that the species-specific differences in BMP-15 function may be due to differences in the BMP-15 proteins themselves. Indeed, human and mouse BMP-15 exhibit particularly low homology, compared with other members of the TGF-β superfamily, with amino acid identities of only 60 and 77% for the proprotein and mature protein, respectively (19).
To further investigate the functional differences in mouse and human BMP-15, we used an in vitro system of transfected 293T cells to study their processing and secretion (20). Interestingly, whereas human BMP-15 proprotein was readily processed and the mature protein secreted in vitro, mouse BMP-15 proprotein was not processed to form the mature protein. To further investigate this processing defect, several chimeric constructs consisting of various combinations of the human and mouse proregions, cleavage sites, and mature regions were tested (20). Only one combination yielded processing and secretion of mouse mature protein. It consisted of the human proregion and cleavage site with the mouse mature protein encoding region.
Because defects in BMP-15 null mice are confined to the ovulation process, we next examined mouse BMP-15 expression in oocytes during a time course of pregnant mare serum gonadotropin (PMSG)/human chorionic gonadotropin (hCG) treatment (21). BMP-15 mature protein was almost undetectable in oocytes of mice treated with PMSG alone for 48 h or PMSG followed by hCG for 2 or 5 h. However, in oocytes of mice treated with PMSG followed by hCG for 9 h, production of mature BMP-15 was greatly increased. The increase in BMP-15 mature protein was not related to an increase in transcription because BMP-15 mRNA remained constant throughout the time course (21). Similar results were subsequently observed in samples from mouse ovary and cumulus-oocyte complexes (22).
Therefore, it is possible that in polyovulatory species such as mice, a posttranslational event initiated by the LH surge results in increased production of mouse BMP-15 mature protein, which then functions in the processes of cumulus expansion and ovulation. In monoovulatory sheep and humans, however, bioactive mature BMP-15 must be present beginning at the primary follicle stage for folliculogenesis to proceed normally because mutations in the bmp15 gene in ewes and women cause the arrest of primary follicle growth and development (2,3,4,14). Whereas ewes homozygous for mutations in the bmp15 gene are infertile, ewes heterozygous for mutations in bmp15 exhibit an increased ovulation quota (2,3,4). We hypothesized that the polyovulatory nature of mice might be associated with the lack (or extremely low level) of functional BMP-15 mature protein during folliculogenesis (1).
To investigate this hypothesis, we chose to overexpress mouse BMP-15 in mouse oocytes in vivo. However, given our previous findings that the mouse BMP-15 proprotein is not processed to form the mature protein in vitro (20) and that mouse BMP-15 mature protein is present in vivo in mouse oocytes only during the ovulation process (21), it was anticipated that processing of mouse BMP-15 proprotein during folliculogenesis would not occur in vivo. Therefore, to increase the chances of processing occurring throughout folliculogenesis and the ovulation process, we chose to overexpress the chimeric construct consisting of the human proregion and cleavage sequence with the human proregion, human cleavage site, and mouse mature region [hhm; (hhmBMP-15)]. We first generated a strain of mice carrying a flox-stopped hhmBMP-15 transgene (hhmBMP-15flox) in a configuration such that it is silent until recombined. Thus, using Cre recombinase, mice can be generated overexpressing hhmBMP-15 in a tissue-specific manner. Here we crossed hhmBMP-15flox female mice with male mice expressing Cre recombinase under control of the ZP3 promoter (23) to generate transgenic mice overexpressing mouse BMP-15 exclusively in the oocytes beginning at the primary stage of folliculogenesis, when the ZP3 oocyte-specific promoter is first active.
In the present study, we generated transgenic mice overexpressing mouse BMP-15 mature protein exclusively in the oocytes. Unexpectedly, overexpression of mouse BMP-15 has no effect on ovulation quota and litter size. However, it has profound effects on early folliculogenesis and ovarian function in aging mice.
Materials and Methods
The PCALNL5 vector was constructed by Dr. Izumu Saito (University of Tokyo, Tokyo, Japan) and provided by the Riken BioResource Center (Tokyo, Japan). Anti-FLAG M2 antibody conjugated to peroxidase was from Sigma Aldrich (St. Louis, MO). The antihuman BMP-15 antibody was prepared as described (14). ZP3-Cre transgenic mice were generously provided by Dr. Jamey Marth (University of California, San Diego).
Construction of transgene and generation of hhmBMP-15flox transgenic mice
All animal protocols were approved by the University of California, San Diego, Institutional Animal Care and Use Committee. Transgenic mice carrying a flox-stopped hhmBMP-15 transgene were generated using a Cre/loxP expression plasmid constructed with the vector pCALNL5 (24). The pCALNL5 vector contains a CAG promoter (cytomegalovirus LE enhancer, chicken β-actin promoter), an intron, the neo resistance gene, and Simian virus 40 early polyA sequence flanked by a loxP site, a multicloning site, and a rabbit β-globin polyadenylation signal. A chimeric transgene consisting of the human BMP-15 proregion fused to the mature region of mouse BMP-15 upstream of a Flag epitope tag (hhmBMP-15) (20) was inserted into the multicloning site. Microinjection of the linearized transgene, performed by the University of California, San Diego transgenic core, resulted in the production of 36 mice, three of which (two males and one female) were determined to be transgenic by PCR genotyping using primers C1 and H1. Genotyping of more than 100 offspring of each mouse revealed that only one of these mice transmitted the transgene through the germline, as established by PCR genotyping. This male founder was bred with a wild-type female to establish a single strain of hhmBMP-15flox transgenic mice. Genomic DNA was isolated using the Spin Doctor genomic DNA isolation kit (Gerard Biotech, Oxford, OH) according to the manufacturer’s instructions. PCR analysis for detection of both the floxed (1300 bp) and recombined (350 bp) forms of the transgene was performed using primers C1 (5′-CTGCTAACCATGTTCATGCC-3′) (25) and H1 (5′-GATTCTTCTAGCAGCTCCTC-3′). Primers for the control glyceraldehyde-3-phosphate dehydrogenase were G1 (5′-GTCTTCACCACCATGGAGAAG-3′) and G2 (5′-TCTTACTCCTTGGAGGCCATG-3′).
Generation of transgenic mice expressing hhmBMP-15 exclusively in oocytes
Female mice heterozygous for the hhmBMP-15flox transgene were crossed with male mice heterozygous for the ZP3-Cre transgene (23) to generate mice heterozygous for both the hhmBMP-15 and ZP3-Cre transgenes. Primers Z1 (5′-CTGCATTACCGGTCGATGCA-3′) and Z2 (5′-ACGTCCACCGGCATCAACGT-3′) were used for PCR genotyping. Female mice heterozygous for both the hhmBMP-15flox transgene and the ZP3-Cre transgene will be referred to as transgenic mice.
Analysis of BMP-15 and FLAG protein expression in ovaries
Ovary homogenate was prepared by homogenizing the ovaries of 8-wk-old wild-type or transgenic mice on ice in lysis buffer (tissue-PE LB containing 5 mm dithiothreitol, 5 mm EDTA, and 10 μl/ml protease arrest). Thirty micrograms of each lysate were subjected to Western immunoblotting using antihuman BMP-15 antibody as described (14) or anti-FLAG M2 antibody conjugated to peroxidase according to the manufacturer’s instructions. Western immunoblotting analysis was carried out as described (20). Blots were reprobed with anti-β-actin antibody to confirm protein loading levels. NIH Image (National Institutes of Health, Bethesda, MD) was used for quantification of BMP-15 protein levels normalized to β-actin after immunoblotting. Immunohistochemical analysis using the antihuman BMP-15 antibody was performed as described (22).
Histological analysis
Ovaries from 25-d-old and 5-month-old wild-type or transgenic mice (n = 4 per age and genotype) were fixed in Carnoy’s fluid, dehydrated in ethanol, and embedded in Paraplast. Ovaries from 5-month-old mice were collected at diestrus 1 of the estrous cycle. The ovaries were serially sectioned at a thickness of 8 μm and stained with hematoxylin and eosin. The study was blinded to prevent bias, and every fifth section was counted in both ovaries and total follicle numbers were determined. Only follicles with a visible nucleolus were counted. Follicles were categorized as follows: primordial follicles, oocyte surrounded by a single layer of squamous granulosa cells; primary/transition follicles, oocyte surrounded by a single layer of predominantly cuboidal granulosa cells with some squamous granulosa cells (transition) or a single layer of cuboidal granulosa cells (primary); secondary follicles, oocyte surrounded two to eight layers of cuboidal granulosa cells, with no visible antrum; and antral follicles, containing a visible antrum (26). Mitotic index was determined as described previously (27). Briefly, a portion (50 sections per mouse) of the sections used for classification of follicles was photographed under ×400 magnification. A 10 × 20 grid consisting of 200 small squares was used to facilitate counting, similar to the method described (28). Each small square of the grid, when placed over a ×400 image, was the size of approximately one granulosa cell; thus, approximately 200 granulosa cells were covered by the grid. The grid was randomly overlayed on each image and the number of mitotic events occurring within the area covered by the grid were counted. The mitotic index is defined as the number of mitotic events per 200 granulosa cells.
Antral follicles were classified based on the number of pyknotic nuclei (pn) as either healthy (0 pn) or atretic (1 or more pn) as described (29).
RNA extraction and real-time PCR
Preparation of total RNA from granulosa cells, reverse transcription, and real-time PCR analysis were performed as described previously (30). Primers for FSH receptor (FSHR) were F1 (5′-TCCTTCATGGGACTGAGCTT-3′) and F2 (5′-AGAGGCTCCCTGVAAAACAT-3′). Primers for L19 were described previously (21).
Serum FSH analysis
Serum FSH analysis was performed by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core. Serum from 25-d-old wild-type and transgenic mice was collected and prepared as described by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.
Fertility study
For evaluation of female fertility, 1.5- or 6-month-old wild-type and transgenic females (n = 7–13 for each age and genotype) were continuously housed with wild-type males for 4 and 5 months, respectively. Litter size was determined on the day of birth.
Evaluation of estrous cycles
The stages of the estrous cycle were determined in wild-type (n = 15) and transgenic (n = 15) mice by daily vaginal smears. Vaginal smears were obtained using a cotton swab moistened in 0.9% NaCl and stained with 0.05% methylene blue. The phases of the estrous cycle were categorized as described (31). Mice were evaluated for at least three complete cycles (the length of time between two occurrences of estrus). Mice that failed to enter estrus and exhibited smears characteristic of diestrus for at least 36 consecutive days were determined to be in constant diestrus. Evaluation of estrous cycles began at 10 months old and was continued bimonthly until the mice were 20 months old or entered constant diestrus.
Statistical analysis
Statistical significance was calculated by one-way ANOVA. The unpaired t test was used to compare results between control and treated samples in the quantitative PCR study. P ≤ 0.05 was considered to be statistically significant.
Results
Generation of hhmBMP-15flox transgenic mice and production of mice expressing hhmBMP-15 exclusively in oocytes
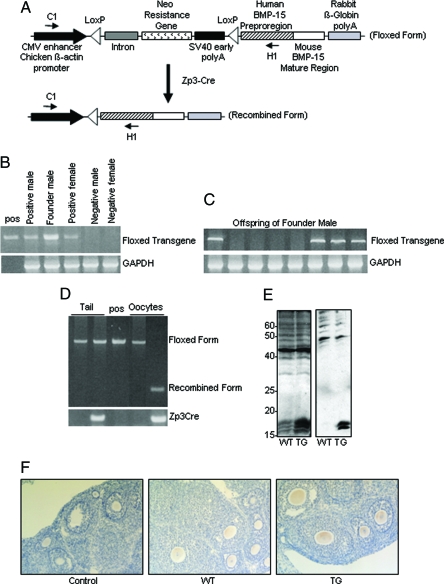
Transgenic mice carrying a flox-stopped hhmBMP-15 transgene were generated using a Cre/loxP expression plasmid constructed with the vector pCALNL5 (24). The pCALNL5 vector contains a CAG promoter (cytomegalovirus LE enhancer, chicken β-actin promoter) followed by a loxP-flanked cassette containing an intron, the neo-resistance gene and Simian virus 40 early polyA sequence. The second loxP site is followed by a multicloning site and a rabbit β-globin polyadenylation signal. A chimeric transgene consisting of the human BMP-15 proregion fused to the mature region of mouse BMP-15 upstream of a Flag epitope tag (hhmBMP-15) (20) was inserted into the multicloning site (Fig. 1A). A total of 36 mice were born from embryos microinjected with the linearized transgene, three of which (two males and one female) were determined to be transgenic by PCR genotyping using primers C1 and H1 (Fig. 1B). Genotyping of more than 100 offspring of each mouse revealed that only one of these mice transmitted the transgene through the germline, as established by PCR genotyping (Fig. 1C). This male founder was bred with a wild-type female to establish a single strain of hhmBMP-15flox transgenic mice.
Figure 1.
Oocyte-specific overexpression of hhmBMP-15F. A, The loxP-flanked Neo resistance gene is expressed under control of the cytomegalovirus (CMV) enhancer and chicken β-actin promoter. The hhmBMP-15 transgene is composed of the human BMP-15 preproregion and mouse BMP-15 mature region. Recombination after Cre recombinase expression under control of the ZP3 promoter results in conditional overexpression of hhmBMP-15 exclusively in the oocytes. Primers C1 and H1 were used for PCR genotyping. SV40, Simian virus 40. B, PCR analysis of genomic DNA from tails of mice positive or negative for the hhmBMP-15flox transgene, including the founder male. Positive control (pos) is the transgene plasmid. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase. C, PCR analysis of genomic DNA from tails of offspring of the founder male. D, PCR analysis of genomic DNA from tails or oocytes of mice carrying the ZP3-Cre and/or hhmBMP-15Fflox transgene. E, Western blot analysis of wild-type or transgenic ovaries using antihuman BMP-15 (left panel) or anti-FLAG (right panel) antibodies. F, Immunohistochemical analysis of BMP-15 in 8-wk-old wild-type (WT) or transgenic (TG) ovaries using antihuman BMP-15 antibody.
To study the effect of overexpression of hhmBMP-15 exclusively in the oocytes, females transgenic for hhmBMP-15flox were bred with males transgenic for ZP3-Cre, which express Cre recombinase under control of the ZP3 promoter. These mice have been shown to express Cre recombinase exclusively in oocytes beginning at the primary stage of folliculogenesis (23). Genomic DNA extracted from tail and oocytes of females heterozygous for either the hhmBMP-15flox transgene or both the hhmBMP-15Fflox transgene and the ZP3-Cre transgene was examined by PCR genotyping using primers C1 and H1 (Fig. 1D). Whereas the flox-stopped form of the transgene (1300 bp) was detected in tail DNA from both mice, the recombined form of the transgene (350 bp) was detected only in oocytes from the mouse carrying both transgenes. The original flox-stopped form of the transgene was absent in oocytes from mice carrying both transgenes, indicating full recombination of the transgene. We examined genomic DNA from other cells and tissues, including granulosa cells, spleen, liver, lung, heart, brain, and testes, of male and female mice heterozygous for both the hhmBMP-15flox transgene and the ZP3-Cre transgene, and the recombined form of the transgene was not detected in any of these samples (data not shown). In all studies to follow, female mice heterozygous for both the hhmBMP-15flox transgene and the ZP3-Cre transgene will be referred to as transgenic mice.
We next confirmed that hhmBMP-15 protein is produced by examining ovary homogenate from wild-type and transgenic mice using Western immunoblot analysis. Using the human BMP-15 antibody (14), three bands corresponding to the mouse BMP-15 mature proteins were detected at approximately 16–18 kDa in both wild-type and transgenic ovaries; (Fig 1E, left panel). In this regard, Gueripel et al. (22) detected a single band for the BMP-15 mature protein in mouse ovary. We are unaware of the cause of the difference at present; however, the different sizes of the mature protein bands likely correspond to different degrees of glycosylation, which may vary in samples collected from mice of different ages or in different strains of mice. Nonetheless, the level of BMP-15 mature proteins in transgenic ovaries was 4.9-fold more than that in wild-type ovaries. Using anti-FLAG antibody, two bands corresponding to the FLAG-tagged mature form of mouse BMP-15 were detected at approximately 16–18 kDa only in ovary from transgenic mice (Fig. 1E, right panel). We then confirmed that the oocytes are the source of the BMP-15 protein in the ovary by immunohistochemistry using the human BMP-15 antibody. BMP-15 was detected exclusively in the oocytes of both wild-type and transgenic mice, with increased BMP-15 detected in the oocytes of transgenic mice (Fig. 1F). As expected, based on the expression pattern of Cre recombinase in ZP3-Cre mice (23), BMP-15 was detected in the transgenic mice beginning at the primary follicle stage and throughout folliculogenesis.
Accelerated follicle development in transgenic females
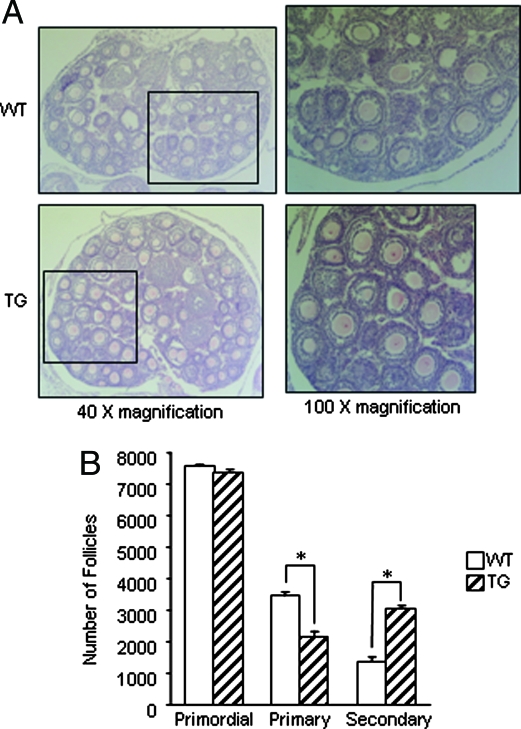
In view of the important function of BMP-15 in stimulating granulosa cell mitosis (14), we first investigated whether overexpression of hhmBMP-15 in the oocytes of transgenic mice had any effect on early folliculogenesis. Immature (25 d old) mice were chosen for this study to eliminate differences related to the estrous cycle. Primordial, primary, and secondary follicles were counted in serially sectioned ovaries. As shown in Fig. 2, we observed a difference in the number of primary and secondary follicles between wild-type and transgenic ovaries. However, there is no difference in primordial follicle numbers between wild-type and transgenic mice, which is expected given that hhmBMP-15 is overexpressed beginning at the primary follicle stage. Thus, formation of primordial follicles and the primordial to primary follicle transition occur normally in transgenic mice. However, a significant decrease in primary follicles with a concomitant increase in secondary follicles was found in transgenic mice. As expected for immature mice, few antral follicles were found in either wild-type or transgenic ovaries (data not shown).
Figure 2.
Follicle development in wild-type (WT) and transgenic (TG) ovaries. A, Histology of ovaries from 25-d-old wild-type and transgenic mice. Ovaries were photographed at ×40 (left panels) magnification. The boxed areas are shown at ×100 magnification (right panels). B, Numbers of primordial, primary, and secondary follicles were determined in wild-type and transgenic ovaries at 25 d of age. Ovaries were serially sectioned and every fifth section was counted, and total follicle numbers were determined. These data represent the mean ± sem of combined results from analysis of four mice per age and genotype (*, P ≤ 0.05).
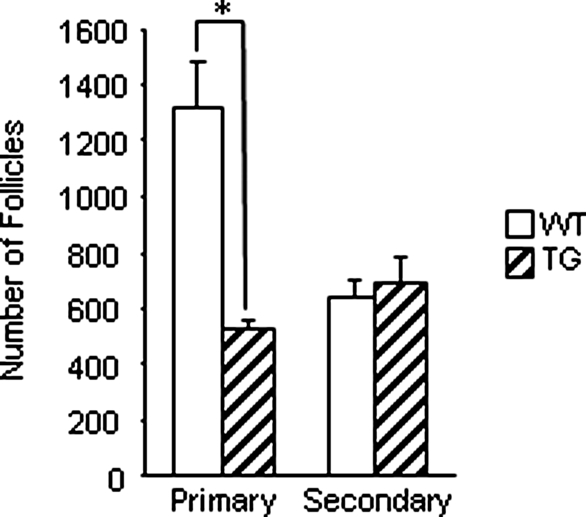
We next examined follicle development in ovaries of cycling 5-month-old mice. Like immature transgenic mice, 5-month-old transgenic mice exhibited a significant decrease in primary follicle numbers (Fig. 3). However, an increase in secondary follicles was not observed in 5-month-old transgenic mice.
Figure 3.
Follicle development in 5-month-old wild-type (WT) and transgenic (TG) ovaries. Numbers of primary and secondary follicles were determined in wild-type and transgenic ovaries at 5 months of age. Ovaries were serially sectioned and every fifth section was counted, and total follicle numbers were determined. These data represent the mean ± sem of combined results from analysis of four mice per age and genotype (*, P ≤ 0.05).
We further examined whether the increase in secondary follicles in immature transgenic mice was due to increased granulosa cell mitosis. Interestingly, the mitotic index of secondary follicles from transgenic mice was twice as high as that of wild-type mice (Fig. 4), suggesting an increased rate of proliferation of granulosa cells in transgenic mice. These results suggest that the decrease in primary follicles is not due to an increase in the primordial to primary follicle transition but is caused by an increased rate of granulosa cell mitosis in primary follicles that leads them to proceed to the secondary follicle stage at an accelerated rate.
Figure 4.
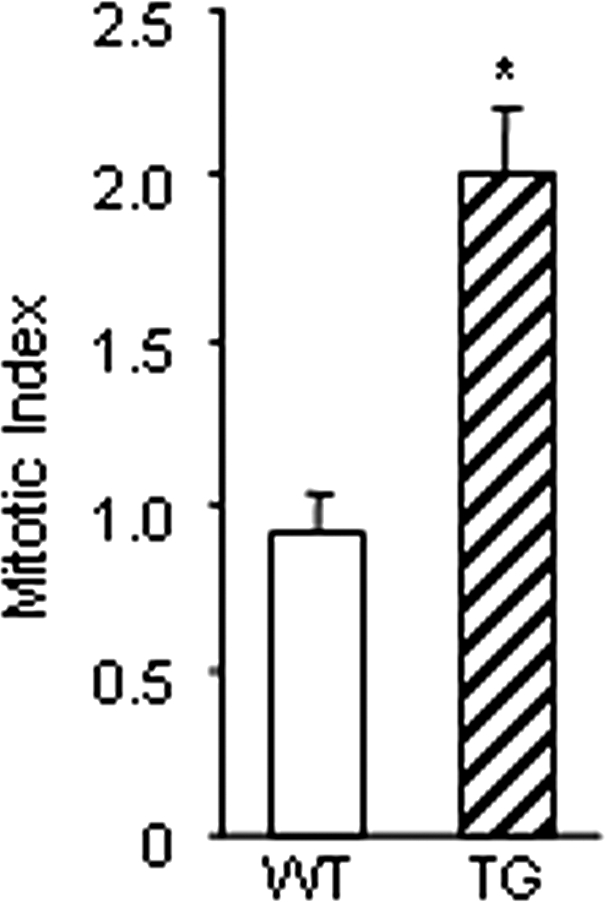
Mitotic index in secondary follicles of 25-d-old wild-type (WT) and transgenic (TG) ovaries. Ovaries were serially sectioned, and 50 sections from each mouse were photographed under ×400 magnification. A grid corresponding to 200 granulosa cells was overlayed and the number of mitotic events was counted. These data represent the mean ± sem of combined results from analysis of three mice per genotype (*, P ≤ 0.05).
BMP-15 is known to decrease FSHR mRNA expression in cultured rat granulosa cells (32). We compared the steady-state mRNA expression of FSHR in granulosa cells from 25-d-old wild-type and transgenic mice. Interestingly, FSHR mRNA expression was decreased by half in granulosa cells of transgenic mice (Fig. 5). We also compared the serum FSH levels in 25-d-old wild-type and transgenic mice and found no statistical difference in serum FSH (data not shown).
Figure 5.
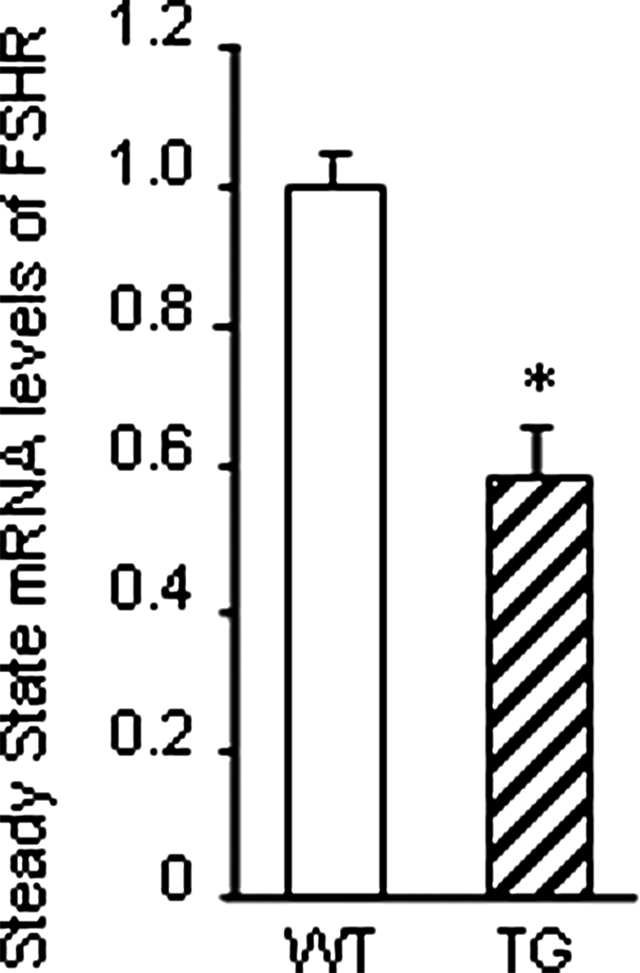
Steady-state mRNA levels of FSHR in 25-d-old wild-type (WT) and transgenic (TG) mice. Total RNA was extracted from granulosa cells and subjected to real-time PCR to determine the mRNA levels of FSHR. Data are normalized to L19 mRNA levels and shown as a relative ratio to the mRNA level of wild-type controls. These data represent the mean ± sem of combined results from the analysis of three mice per genotype (*, P ≤ 0.05).
Fertility analysis of transgenic females
Given that follicle development was accelerated in 25-d-old transgenic females, we next examined whether this had an effect on fertility and litter size. Female wild-type and transgenic mice were continuously housed with wild-type males beginning at either 1.5 months or 6 months of age and breeding was maintained for 4 or 5 months, respectively. Surprisingly, there was no significant difference (P ≤ 0.05) in the number of pups per litter or in the number of litters per month between wild-type and transgenic females (Table 1). However, the number of litters per month decreased significantly between transgenic females bred at 1.5–5 months old and those bred at 6–11 months old, indicating a possible increase in estrous cycle length, whereas this did not occur in wild-type mice.
Table 1.
Litter size and litters per month (mean ± sem) in wild-type (WT) and transgenic (TG) mice bred from 1.5 to 5 or to 11 months of age
| Genotype | Breeding period | Pups per litter |
|---|---|---|
| WT | 1.5–5 months old | 8.09 ± 0.378 |
| WT | 6–11 months old | 7.96 ± 0.224 |
| TG | 1.5–5 months old | 7.95 ± 0.221 |
| TG | 6–11 months old | 8.35 ± 0.254 |
| Genotype | Breeding period | Litters per month |
|---|---|---|
| WT | 1.5–5 months old | 0.88 ± 0.06 |
| WT | 6–11 months old | 0.82 ± 0.05 |
| TG | 1.5–5 months old | 0.98 ± 0.03a |
| TG | 6–11 months old | 0.77 ± 0.05a |
Statistically significantly different.
Increased atresia in antral follicles of transgenic females
Given that transgenic mice exhibit increased secondary follicles, compared with wild-type mice, but litter size in transgenic mice was not altered, we examined antral follicle numbers in 5-month-old mice. Interestingly, transgenic mice had more atretic antral follicles than wild-type mice (Fig. 6), suggesting that the excess secondary follicles observed in immature mice die by atresia before reaching the preovulatory stage.
Figure 6.
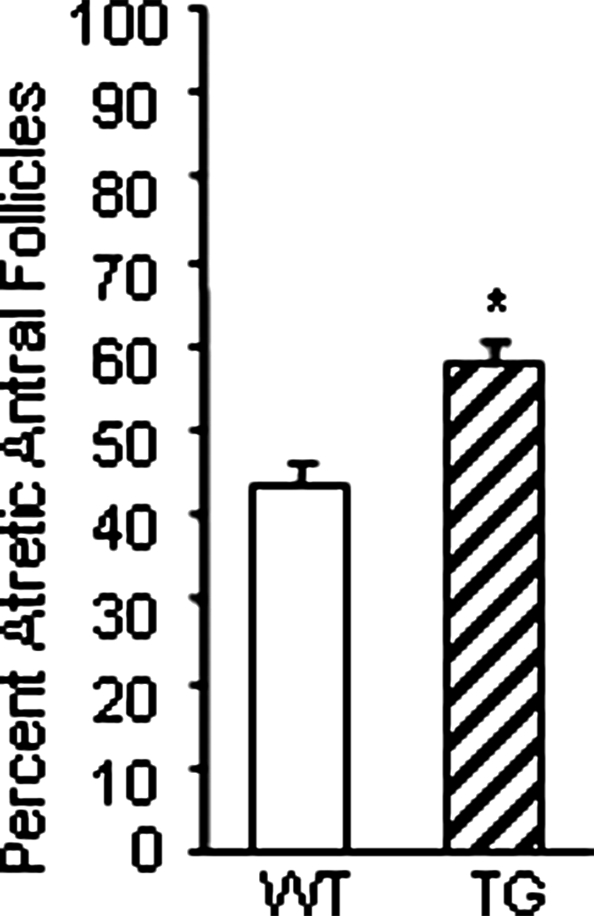
Antral follicle atresia in 5-month-old wild-type (WT) and transgenic (TG) ovaries. Ovaries were serially sectioned and total antral follicles (healthy and atretic) were counted and the percentage of atretic antral follicles was determined. These data represent the mean ± sem of combined results from analysis of three mice per genotype (*, P ≤ 0.05).
Prolonged diestrus and early onset of constant diestrus in adult transgenic females
Due to the decrease in frequency of litters between transgenic mice bred at 1.5–5 months of age and those bred at 6–11 months of age, we next examined the length of each stage of the estrous cycle in aging mice to observe the effect of overexpression of hhmBMP-15 as the mice aged. Beginning at 10 months of age, wild-type (n = 15) and transgenic (n = 15) mice were examined by daily vaginal smears for at least three complete cycles (the time between two occurrences of estrus). Examinations were repeated bimonthly until the mice reached 20 months of age or entered constant diestrus. Mice were determined to be in constant diestrus if they exhibited vaginal smears characteristic of diestrus for at least 36 consecutive days without entering estrus. Mice that were determined to be in constant diestrus were reexamined in the following months to confirm that they had not resumed cycling.
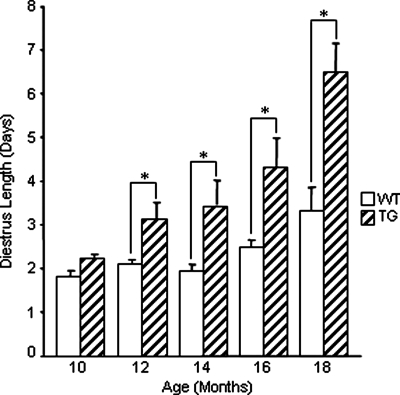
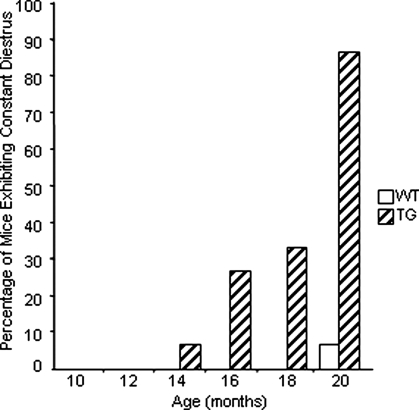
Interestingly, transgenic mice began to show increased diestrus length starting at 12 months of age, whereas this did not occur in wild-type mice until 18 months of age (Fig. 7). Surprisingly, by 20 months of age, 13 of 15 transgenic mice had entered constant diestrus, compared with only one of 15 wild-type mice (Fig. 8). These results indicate that the onset of acyclicity was shifted by approximately 6 months in transgenic mice.
Figure 7.
Diestrus length in wild-type (WT) and transgenic (TG) females. The stages of the estrous cycle were determined by daily vaginal smears. Estrous cycles were examined bimonthly for a minimum of three complete cycles in wild-type and transgenic females (n = 15 for each genotype) from 10 to 20 months of age or until the onset of constant diestrus. These data represent the mean ± sem of combined results (*, P ≤ 0.05).
Figure 8.
Percentage of mice exhibiting constant diestrus. The stages of the estrous cycle were determined by daily vaginal smears. Estrous cycles were examined bimonthly for a minimum of three complete cycles in wild-type (WT) and transgenic (TG) females (n = 15 for each genotype) from 10 to 20 months of age.
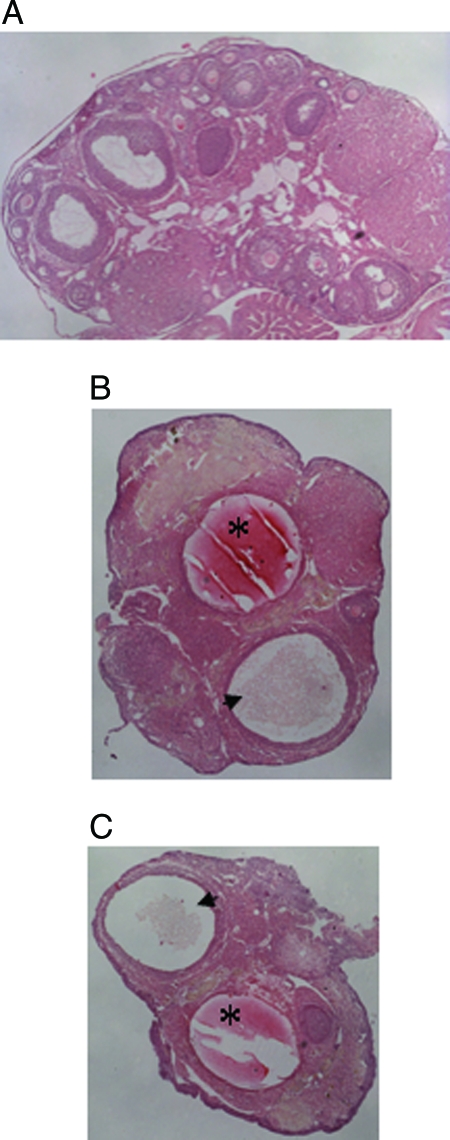
Transgenic mice exhibiting constant diestrus displayed hallmarks of ovarian failure including the presence of large fluid-filled ovarian cysts or hemorrhagic cysts (Fig. 9, B and C). Ovaries of transgenic mice that had entered constant diestrus were also characterized by a lack of developing follicles, in some cases resulting in abnormally small ovaries (Fig. 9, B and C). These defects were not observed in cycling wild-type mice examined at the same ages.
Figure 9.
Histological analysis of transgenic ovaries. Ovarian sections were stained with hematoxylin and eosin and photographed at ×40 magnification. A, Normal ovary from a cycling mouse exhibiting follicles of various developmental stages and corpora lutea. B and C, Ovaries from transgenic mice that had entered constant diestrus, exhibiting hemorrhagic (asterisk) and fluid-filled (arrowhead) cysts and a lack of developing follicles.
Discussion
In the current study, we addressed the role of BMP-15 in the mouse by overexpressing it in the oocyte during folliculogenesis, timing that mimics the expression pattern in monoovulatory animals such as human and sheep. By crossing hhmBMP-15flox female mice with male mice expressing Cre recombinase under control of the ZP3 promoter, we generated transgenic mice overexpressing mouse BMP-15 exclusively in the oocytes beginning at the primary stage of folliculogenesis.
Immature (25 d old) transgenic mice exhibit a decrease in primary follicles coupled with an increase in secondary follicles. This is unrelated to the pool of primordial follicles or the primordial/primary follicle transition, as both wild-type and transgenic mice exhibit equal numbers of primordial follicles. Examination of granulosa cells in immature ovaries revealed that the mitotic index of granulosa cells in transgenic mice was twice as high as in wild-type mice, confirming our previous finding that BMP-15 stimulates granulosa cell mitosis (14). This indicates that the increase in secondary follicles in transgenic mice is due to increased granulosa cell proliferation, leading to accelerated follicle development. BMP-15 is also known to inhibit FSHR mRNA expression in rat granulosa cells (32). Thus, we examined FSHR mRNA expression in granulosa cells from immature mice and found that FSHR mRNA was decreased by half in transgenic mice compared with wild-type mice.
Immature transgenic mice overexpressing mouse BMP-15 exhibited normal but accelerated follicle development. Therefore, we next examined their fertility and litter size. Female wild-type and transgenic mice were continuously housed with wild-type males from ages 1.5–5 or 6–11 months. Female transgenic mice were fertile, indicating that follicle development to the preovulatory stage proceeds normally despite the decrease in FSHR mRNA. Interestingly, the increase in secondary follicles in immature transgenic mice did not alter litter size in mice of either age group, indicating that the number of follicles reaching the preovulatory stage remains unchanged in transgenic mice.
Like immature transgenic mice, 5-month-old transgenic mice also exhibited a decrease in primary follicles. However, 5-month-old transgenic mice did not display a significant increase in secondary follicles. This result is likely due to the fact that many of the secondary follicles in 5-month-old transgenic mice have developed to the antral stage, making the difference less obvious. Furthermore, 5-month-old transgenic mice exhibited an increase in antral follicle atresia, suggesting that the excess secondary follicles observed in immature mice develop to the antral follicle stage and die by atresia before reaching the preovulatory stage. The decrease in FSHR in transgenic mice is likely to cause the increase in antral follicle atresia.
The phenotype observed in these transgenic mice corresponds to our hypothesis regarding the ovulation quota of BMP-15 mutant sheep. Normal sheep have an ovulation quota of 1 or 2 due to the competing actions of BMP-15 in increasing granulosa cell mitosis and decreasing FSH sensitivity. Sheep heterozygous for naturally occurring mutations in the bmp15 gene exhibit a decreased rate of granulosa cell mitosis coupled with an increase in FSH sensitivity (2,3,4). This increase in FSH sensitivity allows more follicles to proceed to the preovulatory stage, thus increasing ovulation quota (1). Mice overexpressing BMP-15 display the opposite phenotype to the heterozygous mutant sheep; they exhibit increased granulosa cell mitosis coupled with a decrease in FSH sensitivity. We hypothesized that the lack of BMP-15 during early folliculogenesis in wild-type mice may be involved in ovulation quota. However, transgenic mice exhibited normal litter size. Thus, the lack of BMP-15 during folliculogenesis does not explain the polyovulatory nature of wild-type mice. Instead, the lack of BMP-15 during early folliculogenesis in wild-type mice functions to restrain follicle development during the FSH-independent phase of folliculogenesis.
Interestingly, we noticed that the number of litters per month significantly decreased between transgenic mice bred from 1.5–5 months of age and those bred from 6–11 months of age, which did not occur in wild-type mice. This led us to compare the estrous cycles of aging transgenic mice (10–20 months old). In wild-type C57BL/6 mice, the onset of acyclicity occurs around 20 months of age and is marked by progressively increasing cycle length (33). Whereas the onset of acyclicity in rats is usually associated with prolonged estrus, many strains of mice instead exhibit a prolongation of the diestrus phase of the cycle (34). We observed a progressively lengthening diestrus phase beginning in 12-month-old transgenic mice but not until 18 months of age in wild-type mice. Furthermore, by 20 months of age, 87% of transgenic mice had entered a state of constant diestrus, compared with only 6% of wild-type mice. In addition to exhibiting an early onset of constant diestrus, some transgenic mice also displayed common signs of ovarian failure including the presence of large fluid-filled ovarian cysts or hemorrhagic cysts. Ovaries of transgenic mice that had entered constant diestrus were also characterized by a lack of developing follicles.
Based on these findings, we hypothesize that although there are more secondary follicles during the FSH-independent stages in transgenic mice, many of the developing follicles undergo atresia due to the decreased level of FSHR during the FSH-dependent stages. Thus, comparable numbers of dominant follicles reach the preovulatory stage in wild-type and transgenic mice. However, due to the accelerated follicle development and increased atresia, ovarian reserve is exhausted earlier in transgenic mice, resulting in a premature onset of acyclicity and constant diestrus.
In summary, we have confirmed two of the most important functions of BMP-15 in vivo, the ability to stimulate granulosa cell mitosis and inhibit FHSR mRNA expression. However, mice overexpressing BMP-15 suffer from an early onset of acyclicity. This indicates that the lack of BMP-15 in wild-type mice during early folliculogenesis is important in restraining follicle development to prevent a premature decline in the ovarian follicle pool.
Acknowledgments
We thank Dr. G. F. Erickson for helpful discussion; Dr. I. Saito for the pCALNL5 vector; Dr. J. Marth for ZP3-Cre mice; the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core supported by National Institute of Child Health and Human Development, National Institutes of Health, through a cooperative agreement as part of the Specialized Cooperative Centers Program in Reproduction Research (U54-HD28934) for serum FSH analysis; and Ms. A. Hartgrove for excellent editorial assistance.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant R01 HD41494 (to S.S.), University of California, San Diego, Academic Senate Grant RG008B (to S.S.), and NIH Grants U54 HD012303 (to P.L.M. and S.S.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online February 28, 2008
Abbreviations: BMP, Bone morphogenetic protein; Cre, cAMP response element; GDF, growth and differentiation factor; FSHR, FSH receptor; hCG, human chorionic gonadotropin; hhm, human proregion, human cleavage site, and mouse mature region; PMSG, pregnant mare serum gonadotropin; pn, pyknotic nuclei.
References
- Moore RK, Erickson GF, Shimasaki S 2004 Are BMP-15 and GDF-9 primary determinants of ovulation quota in mammals? Trends Endocrinol Metab 15:356–361 [DOI] [PubMed] [Google Scholar]
- Galloway SM, McNatty KP, Cambridge LM, Laitinen MPE, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, Beattie AE, Davis GH, Ritvos O 2000 Mutations in an oocyte-derived growth factor gene (bmp15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet 25:279–283 [DOI] [PubMed] [Google Scholar]
- Hanrahan JP, Gregan SM, Mulsant P, Mullen M, Davis GH, Powell R, Galloway SM 2004 Mutations in the genes for oocyte-derived growth factors gdf9 and bmp15 are associated with both increased ovulation rate and sterility in cambridge and belclare sheep (ovis aries). Biol Reprod 70:900–909 [DOI] [PubMed] [Google Scholar]
- McNatty KP, Juengel JL, Wilson T, Galloway SM, Davis GH 2001 Genetic mutations influencing ovulation rate in sheep. Reprod Fertil Dev 13:549–555 [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Beck-Peccoz P, Persani L 2004 Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (bmp15) gene. Am J Hum Genet 75:106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk M 1996 Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 383:531–535 [DOI] [PubMed] [Google Scholar]
- Ledig S, Ropke A, Haeusler G, Hinney B, Wieaker P 2008 Bmp15 mutations in xx gonadal dysgenesis and premature ovarian failure. Am J Obstet Gynecol 198:84.e1–e5 [DOI] [PubMed] [Google Scholar]
- Zhang P, Shi YH, Wang LC, Chen ZJ 2007 Sequence variants in exons of the bmp15 gene in Chinese patients with premature ovarian failure. Acta Obstet Gynecol Scand 86:585–589 [DOI] [PubMed] [Google Scholar]
- Chand AL, Ponnampalam AP, Harris SE, Winship IM, Shelling AN 2006 Mutational analysis of bmp15 and gdf9 as candidate genes for premature ovarian failure. Fertil Steril 86:1009–1012 [DOI] [PubMed] [Google Scholar]
- Laissue P, Christin-Maitre S, Touraine P, Kuttenn F, Ritvos O, Aittomaki K, Bourcigaux N, Jacquesson L, Bouchard P, Frydman R, Dewailly D, Reyss A-C, Jeffery L, Bachelot A, Massin N, Fellous M, Veitia RA 2006 Mutations and sequence variants in gdf9 and bmp15 in patients with premature ovarian failure. Eur J Endocrinol 154:739–744 [DOI] [PubMed] [Google Scholar]
- Dixit H, Rao LK, Padmalatha VV, Kanakavalli M, Deenadayal M, Gupta N, Chakrabarty B, Singh L 2006 Missense mutations in the bmp15 gene are associated with ovarian failure. Hum Genet 119:408–415 [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Rossetti R, Marozzi A, Bodega B, Borgato S, Cavallo L, Einaudi S, Radetti G, Russo G, Sacco M, Wasniewska M, Cole T, Beck-Peccoz P, Nelson LM, Persani L 2006 Identification of new variants of human bmp15 gene in a large cohort of women with premature ovarian failure. J Clin Endocrinol Metab 91:1976–1979 [DOI] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM 2001 Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol 15:854–866 [DOI] [PubMed] [Google Scholar]
- Otsuka F, Yao Z, Lee TH, Yamamoto S, Erickson GF, Shimasaki S 2000 Bone morphogenetic protein-15: identification of target cells and biological functions. J Biol Chem 275:39523–39528 [DOI] [PubMed] [Google Scholar]
- Otsuka F, Shimasaki S 2002 A negative feedback system between oocyte bone morphogenetic protein 15 and granulosa cell kit ligand: its role in regulating granulosa cell mitosis. Proc Natl Acad Sci USA 99:8060–8065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M, Sonntag B, Hwang S, Byerly T, Hourvitz A, Adashi EY, Shimasaki S, Erickson GF 2004 Pregnancy-associated plasma protein-a (PAPP-A) production in rat granulosa cells: stimulation by follicle stimulating hormone and inhibition by the oocyte-derived bone morphogenetic protein-15. Endocrinology 145:3686–3695 [DOI] [PubMed] [Google Scholar]
- Moore RK, Otsuka F, Shimasaki S 2003 Molecular basis of bone morphogenetic protein-15 signaling in granulosa cells. J Biol Chem 278:304–310 [DOI] [PubMed] [Google Scholar]
- Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, O'Brien MJ, Matzuk MM, Shimasaki S, Eppig JJ 2007 Oocyte-derived BMP-15 and FGFs cooperate to promote glycolysis in cumulus cells. Development 134:2593–2603 [DOI] [PubMed] [Google Scholar]
- Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM 1998 The bone morphogenetic protein-15 gene is X-linked and expressed in oocytes. Mol Endocrinol 12:1809–1817 [DOI] [PubMed] [Google Scholar]
- Hashimoto O, Moore RK, Shimasaki S 2005 Posttranslational processing of mouse and human BMP-15: potential implication in the determination of ovulation quota. Proc Natl Acad Sci USA 102:5426–5431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino O, McMahon HE, Sharma S, Shimasaki S 2006 A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse. Proc Natl Acad Sci USA 103:10678–10683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueripel X, Brun V, Gougeon A 2006 Oocyte bone morphogenetic protein-15, but not growth differentiation factor-9, is increased during gonadotropin-induced follicular development in the immature mouse and is associated with cumulus oophorus expansion. Biol Reprod 75:836–843 [DOI] [PubMed] [Google Scholar]
- Lewandoski M, Wassarman KM, Martin GR 1997 ZP3-Cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr Biol 7:148–151 [DOI] [PubMed] [Google Scholar]
- Kanegae Y, Lee G, Sato Y, Tanaka M, Nakai M, Sakaki T, Sugano S, Saito I 1995 Efficient gene activation in mammalian cells by using recombinant adenovirus expressing site-specific Cre recombinase. Nucleic Acids Res 23:3816–3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatsuka T, Kaneto H, Kajimoto Y, Hirota S, Arakawa Y, Fujitani Y, Umayahara Y, Watada H, Yamasaki Y, Magnuson MA, Miyazaki J, Hori M 2003 Ectopically expressed PDX-1 in liver initiates endocrine and exocrine pancreas differentiation but causes dysmorphogenesis. Biochem Biophys Res Commun 310:1017–1025 [DOI] [PubMed] [Google Scholar]
- Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB 2004 Methods for quantifying follicular numbers within the mouse ovary. Reproduction 127:569–580 [DOI] [PubMed] [Google Scholar]
- Sadrkhanloo R, Hofeditz C, Erickson GF 1987 Evidence for widespread atresia in the ovaries of the hypophysectomized estrogen-treated rat. Endocrinology 120:146–155 [DOI] [PubMed] [Google Scholar]
- Going JJ 2006 Counting cells made easier. Histopathology 49:309–311 [DOI] [PubMed] [Google Scholar]
- Erickson GF, Kokka S, Rivier C 1995 Activin causes premature superovulation. Endocrinology 136:4804–4813 [DOI] [PubMed] [Google Scholar]
- Otsuka F, Moore RK, Wang X, Sharma S, Miyoshi T, Shimasaki S 2005 Essential role of the oocyte in estrogen amplification of follicle-stimulating hormone signaling in granulosa cells. Endocrinology 146:3362–3367 [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL 2007 The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol 80:84–97 [DOI] [PubMed] [Google Scholar]
- Otsuka F, Yamamoto S, Erickson GF, Shimasaki S 2001 Bone morphogenetic protein-15 inhibits follicle-stimulating hormone (FSH) action by suppressing FSH receptor expression. J Biol Chem 276:11387–11392 [DOI] [PubMed] [Google Scholar]
- Thrung PJ, Boot LM, Muhlbock O 1956 Senile changes in the oestrous cycle and in ovarian structure in some inbred strains of ice. Acta Endocrinol (Copenh) 23:8–32 [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE 1982 A longitudinal study of estrous cyclicity in aging c57bl/6j mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod 27:327–339 [DOI] [PubMed] [Google Scholar]