Abstract
The physiology and regulation of steroid synthesis in the brain have emerged as important for understanding brain function. Neurosteroids, those steroids synthesized de novo in nervous tissue, have been associated with numerous central nervous system functions, including myelination, mental retardation, and epilepsy. Central regulation of reproduction was thought to depend on steroids of peripheral origin. Only recently has the role of neurosteroids in reproduction been appreciated. This minireview describes our work trying to understand how circulating estradiol modulates the synthesis of neuroprogesterone. The synthesis of neuroprogesterone occurs primarily in astrocytes, and requires the interaction of membrane-associated estrogen receptor with metabotropic glutamate receptor and the release of intracellular calcium stores. The newly synthesized neuroprogesterone acts on estradiol-induced progesterone receptors in nearby neurons to initiate the LH surge.
IT IS DIFFICULT to overstate the importance of steroid hormones. These compounds are critical mediators of homeostasis, and an organism’s response to various stressors and injury. In addition to the massive quantities of steroid hormones produced by peripheral steroidogenic organs, the gonads and the adrenal glands, it is now well accepted that the brain synthesizes steroids de novo (neurosteroids), and converts circulating steroids to neuroactive steroids (e.g. dehydroepiandrosterone, estradiol, allopregnanolone). Both neurosteroid and neuroactive steroids affect brain function through actions at their cognate receptors, estrogen receptor (ER), progesterone receptor (PR), or by modulating receptors whose primary transmitter is not a steroid (e.g. the GABAA receptor).
A distinction based on the origin of the steroids is useful. Steroids of peripheral origin are hormones, released into the circulation to act on distal target sites, including nervous tissue. Thus, the actions of these steroid hormones affect a wide variety of tissues and influence numerous physiological properties. Neurosteroids, more properly could be considered neurotransmitters. They are made in the brain, their release is regulated, and they are neuroactive as demonstrated by their modulation of intracellular signaling pathways, channels, and transcription.
Neurosteroids are not isolated from peripheral steroids. Mutual interactions modulate levels in the brain and periphery. In mammalian species, levels of neurosteroids are very low and do not appear to “spill” into circulating pools of hormonal steroids. Peripheral, hormonal, or circulating steroids are made in large concentrations by both the gonads and adrenal cortex. One of the hallmarks of steroid hormones is that they are carried in the blood, either free or bound to a carrier protein. Free steroids are capable of diffusing across the blood-brain barrier into cells of the nervous system to encounter both membrane-associated steroid receptors and intracellular receptors. Thus, the concentration of steroids in the brain is a mixture of peripherally derived steroids, converted peripheral steroids, and neurosteroids. Intracellular steroid receptors are either ligand-activated transcription factors, or they stabilize other transcription factors such as fos and jun (1,2,3). These transcriptional actions of steroids are very dramatic and have been extensively studied (4,5). Membrane steroid receptors are either coupled to intracellular pathways directly or interact with growth factor receptors (e.g. IGF-I receptor) or G protein-coupled receptors (e.g. glutamate receptors).
In addition to providing a reservoir of steroids, circulating hormonal steroids also modulate the site-specific synthesis of neurosteroids (6,7) and their cognate receptors (8,9,10). This dual regulation of neurosteroidogenesis and postsynaptic receptor expression has profound implications for neurosteroid function (Fig. 1). Interactions of peripheral steroids with neurosteroid synthesis are involved in regulating reproduction in the hypothalamus.
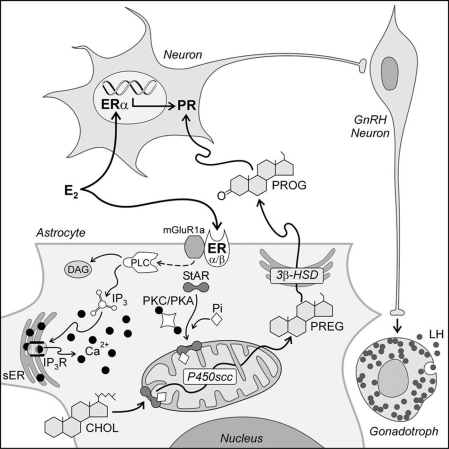
Figure 1.
A model of proposed estradiol (E2) actions on hypothalamic cells. In astrocytes, circulating estradiol acts on a membrane-associated ER to increase [Ca2+]i through the mGluR1a, which activates the PLC/IP3 pathway, releasing Ca2+ from the smooth endoplasmic reticulum (sER). Protein kinase C (PKC) or protein kinase A (PKA) mediates StAR phosphorylation, activating the rate-limiting step of neurosteroidogenesis. In the mitochondrion, P450scc converts cholesterol (CHOL) to pregnenolone (PREG), which is further converted to progesterone (PROG) by 3β-HSD. Progesterone activates estradiol-induced PR in neurons that project to and stimulate GnRH neurons initiating the LH surge (60). DAG, Diacylglycerol; Pi, inorganic phosphate.
Neurosteroidogenesis
The knowledge that the brain makes steroids de novo (i.e. neurosteroids) dates back over a quarter century (11). Studies indicate that astrocytes are the primary steroidogenic cells, and their primary steroid product is neuroprogesterone, but oligodendrocytes and neurons are also steroidogenic (12,13,14,15,16,17,18,19,20,21,22,23). Steroidogenesis begins when the C-27 cholesterol side chain is removed by the cytochrome side chain cleavage enzyme P450 (P450scc; Fig. 1) along the inner mitochondrial membrane. 3β-Hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase (3β-HSD) converts pregnenolone to progesterone (for review, see Refs. 24 and 25). The overall rate-limiting step in steroidogenesis is the delivery of cholesterol to the inner mitochondrial membrane. At least three proteins mediate the transport of cholesterol from the outer to the inner mitochondrial membrane: steroid acute regulatory protein (StAR); peripheral type benzodiazepine receptor; and its endogenous ligand endozepine, also called diazepam binding inhibitor (26,27,28,29,30) (for review, see Refs. 31 and 32). Both StAR and peripheral type benzodiazepine receptor have been localized in astrocytes (33,34,35).
Circulating estradiol stimulates neuroprogesterone synthesis in the adult female brain by regulating the levels of steroidogenic proteins and their activity (6). In vivo, estradiol significantly increased hypothalamic 3β-HSD mRNA levels and 3β-HSD enzyme activity with a time course that accounts for the increase in hypothalamic neuroprogesterone seen before the LH surge (6,10). In vitro, estradiol rapidly stimulates the synthesis of neuroprogesterone in cultured female astrocytes (19,20). Estradiol acts through membrane ERs to rapidly increase free cytoplasmic calcium ([Ca2+]i), leading to increase in progesterone synthesis (19,36).
Rapid estradiol signaling in astrocytes is mediated by metabotropic glutamate receptor (mGluR) 1a
In peripheral steroidogenic tissues, steroid production is regulated by G protein-coupled receptors that induce the phosphorylation of protein kinases (37,38,39,40). In the brain, estradiol stimulates neuroprogesterone synthesis that involves the phospholipase C (PLC)-inositol 1,4,5-trisphosphate (IP3)-[Ca2+]i pathway (Fig. 1). Membrane ERs activate the PLC-IP3 pathway through an interaction with mGluRs (41). Moreover, ERα-mGluR interactions have regulated sexual receptivity (42) and mediated antinociceptive estradiol actions in dorsal root ganglion neurons (43,44). In our astrocyte culture system, antagonizing mGluR1a prevents the estradiol-induced [Ca2+]i flux (Kuo, J., O. Hariri, G. Bondar, J. Ogi, and P. Micevych, submitted for publication). These data are consistent with the hypothesis that estradiol can act via a membrane-associated ERα that signals through mGluR1a and the PLC/IP3 pathway, releasing [Ca2+]i in astrocytes to increase neuroprogesterone synthesis (Fig. 1).
Neuroprogesterone and the LH Surge
During each estrous cycle, FSH stimulates the growth, maturation of, and steroidogenesis in ovarian follicles that produce a rapid increase in circulating, hormonal estradiol. When the circulating concentration of estradiol reaches a critical level, the negative feedback onto the hypothalamus and anterior pituitary becomes positive. This estrogen-positive feedback initiates the surge release of GnRH and stimulates the release of LH from the anterior pituitary. Under the influence of LH, ovulation is stimulated, the ruptured follicle forms a corpus luteum and secretes progesterone.
In addition to estradiol (45,46,47), preovulatory progesterone is essential for the LH surge (6,48,49,50,51). For example, ovariectomized rats treated with only estradiol show a physiological but blunted LH surge. Progesterone treatment increases the magnitude and duration of the surge (52). Although the ovary and adrenal have been proposed as sources of preovulatory progesterone (53,54,55,56), ovariectomized and adrenalectomized rats continue to produce an LH surge in response to estradiol priming (6,57). The estradiol-induced LH surge is blocked by RU486, a PR antagonist, or progesterone synthesis inhibitors (trilostane or epostane) (6,48,50,51,58,59).
Estradiol induction of neuroprogesterone synthesis
In the adult brain, neuroprogesterone is synthesized by astrocytes (15,19,20). Thus, neuroprogesterone needed to trigger the LH surge can be produced locally in the hypothalamus (6). Moreover, hypothalamic neuroprogesterone concentrations were correlated with surge levels of plasma LH (r2 = 0.77), indicating that an LH surge is triggered by hypothalamic neuroprogesterone. Blocking hypothalamic neurosteroidogenesis prevents estrogen positive feedback. Intact animals injected with aminoglutethimide (AGT) (a P450scc inhibitor) into the third ventricle on the morning of proestrus had significantly reduced hypothalamic neuroprogesterone levels (49.8 ± 16.7 vs. 18.4 ± 12.2 pg/mg; P < 0.05). Although plasma levels of estradiol were similar between control and AGT rats (28.4 ± 12.3 vs. 17.7 ± 5.0 pg/ml), progesterone levels (27.9 ± 7.6 vs. 10.4 ± 4.4 ng/ml; P < 0.05) were attenuated. These data demonstrate that peripheral steroidogenesis was not blocked but suggested that the LH surge did not occur. This was confirmed by low LH levels on the day of the expected LH surge in AGT rats, and by a lack of a cornified vaginal cytology (estrus), and corpora lutea. AGT rats had atrophied fluid-filled uteri demonstrating the importance of neuroprogesterone for initiating the LH surge.
Summary
The idea that neurosteroids and, in particular, neuroprogesterone are important components of normal central nervous system functions is emerging. We reviewed the mechanisms of neuroprogesterone synthesis, its relationship to peripheral steroids, and the importance of neuroprogesterone to estrogen positive feedback and reproduction (Fig. 1). Initiation of the LH surge requires that circulating estradiol stimulates neuroprogesterone synthesis and induces PR in the hypothalamus. The locally synthesized neuroprogesterone acts on these PRs to initiate the release of GnRH and stimulates the surge release of LH needed for ovulation.
Acknowledgments
We thank our collaborators Drs. Dewing, Kuo, and Bondar for their contributions.
Footnotes
This work was supported by National Institutes of Health Grant HD042635.
Disclosure Statement: The authors have nothing to disclose.
First Published Online February 28, 2008
Abbreviations: AGT, Aminoglutethimide; ER, estrogen receptor; [Ca2+]i, free cytoplasmic calcium; 3β-HSD, 3β-hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase; IP3, inositol 1,4,5-trisphosphate; mGluR, metabotropic glutamate receptor; PLC, phospholipase C; PR, progesterone receptor; StAR, steroid acute regulatory protein.
References
- Matthews J, Wihlen B, Tujague M, Wan J, Strom A, Gustafsson JA 2006 Estrogen receptor (ER) β modulates ERα-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol Endocrinol 20:534–543 [DOI] [PubMed] [Google Scholar]
- Uht RM, Anderson CM, Webb P, Kushner PJ 1997 Transcriptional activities of estrogen and glucocorticoid receptors are functionally integrated at the AP-1 response element. Endocrinology 138:2900–2908 [DOI] [PubMed] [Google Scholar]
- Webb P, Lopez GN, Uht RM, Kushner PJ 1995 Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol 9:443–456 [DOI] [PubMed] [Google Scholar]
- Beato M, Klug J 2000 Steroid hormone receptors: an update. Hum Reprod Update 6:225–236 [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Katzenellenbogen JA 2000 Estrogen receptor transcription and transactivation: estrogen receptor α and estrogen receptor β: regulation by selective estrogen receptor modulators and importance in breast cancer. Breast Cancer Res 2:335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK 2003 The luteinizing hormone surge is preceded by an estrogen-induced increase of hypothalamic progesterone in ovariectomized and adrenalectomized rats. Neuroendocrinology 78:29–35 [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I 2007 Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J Neurosci 27:2155–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell PE, Levine JE 2000 Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinology 141:1477–1485 [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, McEwen BS 1978 Oestrogen modulates progestin receptor concentrations in some rat brain regions but not in others. Nature 274:276–278 [DOI] [PubMed] [Google Scholar]
- Soma KK, Sinchak K, Lakhter A, Schlinger BA, Micevych PE 2005 Neurosteroids and female reproduction: estrogen increases 3β-HSD mRNA and activity in rat hypothalamus. Endocrinology 146:4386–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu EE 1981 Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci USA 78:4704–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robel PB, EE 1995 Neurosteroids and neuroactive steroids. In: Micevych PE, Hammer RP, eds. Neurobiological effects of sex steroid hormones. Cambridge: Cambridge University Press; 281–296 [Google Scholar]
- Jung-Testas I, Hu ZY, Baulieu EE, Robel P 1989 Neurosteroids: biosynthesis of pregnenolone and progesterone in primary cultures of rat glial cells. Endocrinology 125:2083–2091 [DOI] [PubMed] [Google Scholar]
- Jung-Testas I, Renoir JM, Gasc JM, Baulieu EE 1991 Estrogen-inducible progesterone receptor in primary cultures of rat glial cells. Exp Cell Res 193:12–19 [DOI] [PubMed] [Google Scholar]
- Zwain IH, Yen SS 1999 Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology 140:3843–3852 [DOI] [PubMed] [Google Scholar]
- Guennoun R, Fiddes RJ, Gouezou M, Lombes M, Baulieu EE 1995 A key enzyme in the biosynthesis of neurosteroids, 3β-hydroxysteroid dehydrogenase/δ 5-δ 4-isomerase (3β-HSD), is expressed in rat brain. Brain Res Mol Brain Res 30:287–300 [DOI] [PubMed] [Google Scholar]
- Kohchi C, Ukena K, Tsutsui K 1998 Age- and region-specific expressions of the messenger RNAs encoding for steroidogenic enzymes p450scc, P450c17 and 3β-HSD in the postnatal rat brain. Brain Res 801:233–238 [DOI] [PubMed] [Google Scholar]
- Sanne JL, Krueger KE 1995 Expression of cytochrome P450 side-chain cleavage enzyme and 3β-hydroxysteroid dehydrogenase in the rat central nervous system: a study by polymerase chain reaction and in situ hybridization. J Neurochem 65:528–536 [DOI] [PubMed] [Google Scholar]
- Micevych PE, Chaban V, Ogi J, Lakhter A, Lu JKH, Sinchak K 2007 Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology 148:782–789 [DOI] [PubMed] [Google Scholar]
- Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK, Micevych P 2003 Estrogen induces de novo progesterone synthesis in astrocytes. Dev Neurosci 25:343–348 [DOI] [PubMed] [Google Scholar]
- Furukawa A, Miyatake A, Ohnishi T, Ichikawa Y 1998 Steroidogenic acute regulatory protein (StAR) transcripts constitutively expressed in the adult rat central nervous system: colocalization of StAR, cytochrome P-450SCC (CYP XIA1), and 3β-hydroxysteroid dehydrogenase in the rat brain. J Neurochem 71:2231–2238 [DOI] [PubMed] [Google Scholar]
- Ukena K, Usui M, Kohchi C, Tsutsui K 1998 Cytochrome P450 side-chain cleavage enzyme in the cerebellar Purkinje neuron and its neonatal change in rats. Endocrinology 139:137–147 [DOI] [PubMed] [Google Scholar]
- Rune GM, Frotscher M 2005 Neurosteroid synthesis in the hippocampus: role in synaptic plasticity. Neuroscience 136:833–842 [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB 2004 Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 25:947–970 [DOI] [PubMed] [Google Scholar]
- Mensah-Nyagan AG, Do-Rego JL, Beaujean D, Luu-The V, Pelletier G, Vaudry H 1999 Neurosteroids: expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacol Rev 51:63–81 [PubMed] [Google Scholar]
- Kallen CB, Arakane F, Christenson LK, Watari H, Devoto L, Strauss 3rd JF1998 Unveiling the mechanism of action and regulation of the steroidogenic acute regulatory protein. Mol Cell Endocrinol 145:39–45 [DOI] [PubMed] [Google Scholar]
- Granot Z, Silverman E, Friedlander R, Melamed-Book N, Eimerl S, Timberg R, Hales KH, Hales DB, Stocco DM, Orly J 2002 The life cycle of the steroidogenic acute regulatory (StAR) protein: from transcription through proteolysis. Endocr Res 28:375–386 [DOI] [PubMed] [Google Scholar]
- King SR, Manna PR, Ishii T, Syapin PJ, Ginsberg SD, Wilson K, Walsh LP, Parker KL, Stocco DM, Smith RG, Lamb DJ 2002 An essential component in steroid synthesis, the steroidogenic acute regulatory protein, is expressed in discrete regions of the brain. J Neurosci 22:10613–10620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco DM 2001 StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol 63:193–213 [DOI] [PubMed] [Google Scholar]
- Stocco DM, Clark BJ 1996 Role of the steroidogenic acute regulatory protein (StAR) in steroidogenesis. Biochem Pharmacol 51:197–205 [DOI] [PubMed] [Google Scholar]
- Niswender GD 2002 Molecular control of luteal secretion of progesterone. Reproduction 123:333–339 [DOI] [PubMed] [Google Scholar]
- Papadopoulos V 1993 Peripheral-type benzodiazepine/diazepam binding inhibitor receptor: biological role in steroidogenic cell function. Endocr Rev 14:222–240 [DOI] [PubMed] [Google Scholar]
- Lamacz M, Tonon MC, Smih-Rouet F, Patte C, Gasque P, Fontaine M, Vaudry H 1996 The endogenous benzodiazepine receptor ligand ODN increases cytosolic calcium in cultured rat astrocytes. Brain Res Mol Brain Res 37:290–296 [DOI] [PubMed] [Google Scholar]
- Young JK 1994 Immunoreactivity for diazepam binding inhibitor in Gomori-positive astrocytes. Regul Pept 50:159–165 [DOI] [PubMed] [Google Scholar]
- Karri S, Dertien JS, Stocco DM, Syapin PJ 2007 Steroidogenic acute regulatory protein expression and pregnenolone synthesis in rat astrocyte cultures. J Neuroendocrinol 19:860–869 [DOI] [PubMed] [Google Scholar]
- Chaban VV, Lakhter AJ, Micevych P 2004 A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology 145:3788–3795 [DOI] [PubMed] [Google Scholar]
- Shalem Z, Izhar M, Shore LS, Shemesh M, Hansel W, Strauss 3rd JF 1988 Control of bovine placental progestin synthesis: calcium dependent steroidogenesis is modulated at the site of the cholesterol side chain cleavage enzyme. J Steroid Biochem 31:835–838 [DOI] [PubMed] [Google Scholar]
- Shemesh M, Hansel W, Strauss 3rd JF 1984 Calcium-dependent, cyclic nucleotide-independent steroidogenesis in the bovine placenta. Proc Natl Acad Sci USA 81:6403–6407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MC, Palfrey HC, Nash NT, Greisman A, Jayatilak PG, Gibori G 1987 Effects of estradiol on calcium-specific protein phosphorylation in the rat corpus luteum. Endocrinology 120:1010–1018 [DOI] [PubMed] [Google Scholar]
- Alila HW, Davis JS, Dowd JP, Corradino RA, Hansel W 1990 Differential effects of calcium on progesterone production in small and large bovine luteal cells. J Steroid Biochem 36:687–693 [DOI] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG 2005 Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci 25:5066–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P 2007 Membrane estrogen receptor-α interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci 27:9294–9300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban VV, Mayer EA, Ennes HS, Micevych PE 2003 Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience 118:941–948 [DOI] [PubMed] [Google Scholar]
- Chaban V, Li J, McDonald J, Rapkin A, Micevych P, Estradiol attenuates ATP-induced increase of intracellular calcium through group II metabotropic glutamate receptors in rat DRG neurons. Proc Society for Neuroscience, San Diego, CA, 2007 (Abstract 519.7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brom GM, Schwartz NB 1968 Acute changes in the estrous cycle following ovariectomy in the golden hamster. Neuroendocrinology 3:366–377 [DOI] [PubMed] [Google Scholar]
- Ferin M, Tempone A, Zimmering PE, Van de Wiele RL 1969 Effect of antibodies to 17β-estradiol and progesterone on the estrous cycle of the rat. Endocrinology 85:1070–1078 [DOI] [PubMed] [Google Scholar]
- Labhsetwar AP 1970 Role of estrogens in ovulation: a study using the estrogen-antagonist, I.C.I. 46,474. Endocrinology 87:542–551 [DOI] [PubMed] [Google Scholar]
- Hibbert ML, Stouffer RL, Wolf DP, Zelinski-Wooten MB 1996 Midcycle administration of a progesterone synthesis inhibitor prevents ovulation in primates. Proc Natl Acad Sci USA 93:1897–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remohi J, Balmaceda JP, Rojas FJ, Asch RH 1988 The role of pre-ovulatory progesterone in the midcycle gonadotrophin surge, ovulation and subsequent luteal phase: studies with RU486 in rhesus monkeys. Hum Reprod 3:431–435 [DOI] [PubMed] [Google Scholar]
- DePaolo LV 1988 Attenuation of preovulatory gonadotrophin surges by epostane: a new inhibitor of 3β-hydroxysteroid dehydrogenase. J Endocrinol 118:59–68 [DOI] [PubMed] [Google Scholar]
- Mahesh VB, Brann DW 1992 Interaction between ovarian and adrenal steroids in the regulation of gonadotropin secretion. J Steroid Biochem Mol Biol 41:495–513 [DOI] [PubMed] [Google Scholar]
- DePaolo LV, Barraclough CA 1979 Dose dependent effects of progesterone on the facilitation and inhibition of spontaneous gonadotropin surges in estrogen treated ovariectomized rats. Biol Reprod 21:1015–1023 [DOI] [PubMed] [Google Scholar]
- Putnam CD, Brann DW, Mahesh VB 1991 Acute activation of the adrenocorticotropin-adrenal axis: effect on gonadotropin and prolactin secretion in the female rat. Endocrinology 128:2558–2566 [DOI] [PubMed] [Google Scholar]
- Buckingham JC, Deohler KD, Wilson CA 1978 Activity of the pituitary-adrenocortical system and thyroid gland during the oestrous cycle of the rat. J Endocrinol 78:359–366 [DOI] [PubMed] [Google Scholar]
- Shaikh AA, Shaikh SA 1975 Adrenal and ovarian steroid secretion. Endocrinology 96:37–44 [DOI] [PubMed] [Google Scholar]
- Mahesh VB, Brann DW 1998 Neuroendocrine mechanisms underlying the control of gonadotropin secretion by steroids. Steroids 63:252–256 [DOI] [PubMed] [Google Scholar]
- Mann DR, Korowitz CD, Barraclough CA 1975 Adrenal gland involvement in synchronizing the preovulatory release of LH in rats. Proc Soc Exp Biol Med 150:115–120 [DOI] [PubMed] [Google Scholar]
- Snyder BW, Beecham GD, Schane HP 1984 Inhibition of ovulation in rats with epostane, an inhibitor of 3β-hydroxysteroid dehydrogenase. Proc Soc Exp Biol Med 176:238–242 [DOI] [PubMed] [Google Scholar]
- Sanchez-Criado JE, Hernandez G, Bellido C, Gonzalez D, Tebar M, Diaz-Cruz MA, Alonso R 1994 Periovulatory LHRH, LH and FSH secretion in cyclic rats treated with RU486: effects of exogenous LHRH and LHRH antagonist on LH and FSH secretion at early oestrus. J Endocrinol 141:7–14 [DOI] [PubMed] [Google Scholar]
- Micevych P, Soma KK, Sinchak K 3 August 2007 Neuroprogesterone: key to estrogen positive feedback? Brain Res Rev [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]