Abstract
Paired-like homeodomain transcription factors (PITX) regulate the activity of pituitary hormone-encoding genes. Here, we examined mechanisms through which the family of PITX proteins control murine FSH β-subunit (Fshb) transcription. We observed that endogenous PITX1 and PITX2 isoforms from murine LβT2 gonadotrope cells could bind a highly conserved proximal cis-element. Transfection of PITX1 or PITX2C in heterologous cells stimulated both murine and human Fshb/FSHB promoter-reporter activities, and in both cases, mutation of the critical cis-element abrogated these effects. In homologous LβT2 cells, the same mutation decreased basal reporter activity and greatly reduced activin A-stimulated transcription from murine and human promoter-reporters. Transfecting dominant-negative forms of PITX1 or PITX2C or knocking down PITX1 or -2 expression by RNA interference in LβT2 cells inhibited murine Fshb transcription, confirming roles for endogenous PITX proteins. Both PITX1 and PITX2C interacted with Smad3 (an effector of the activin signaling cascade in these cells) in coprecipitation experiments, and the PITX binding site mutation greatly inhibited Smad2/3/4-stimulated Fshb transcription. In summary, both PITX1 and PITX2C regulate murine and human Fshb/FSHB transcription through a conserved cis-element in the proximal promoter. Furthermore, the data indicate both common and distinct mechanisms of PITX1 and PITX2C action.
FSH REGULATES OVARIAN folliculogenesis and is essential for Sertoli cell proliferation and maintenance of sperm quality in the testis. Gonadotrope cells of the anterior pituitary gland secrete FSH and LH. Like other members of the pituitary glycoprotein hormone family, FSH is produced through the heterodimeric assembly of two subunits, α and β, encoded by separate genes. The glycoprotein hormone α-polypeptide is shared by the gonadotropins (FSH and LH), TSH, and chorionic gonadotropin. Unique β-subunits confer biological specificity to the different hormones, and differential regulation of their transcription constitutes one means to selectively control production of each hormone in physiologically relevant contexts (1).
The development of the murine gonadotrope-derived cell line LβT2 has provided a powerful in vitro model system for the delineation of mechanisms controlling both cell-specific and hormonally regulated transcription of the gonadotropin subunit genes (2). We and others have used this model to investigate transcriptional regulation of FSHβ subunit (Fshb) promoters from a variety of species, including human, rat, mouse, sheep, and pig (3,4,5,6,7,8,9,10,11,12,13,14). Recent studies have implicated proteins in the PITX/RIEG subfamily of homeodomain transcription factors in both basal and hormonally regulated Fshb subunit expression (8,10).
PITX1 (also known as PTX1, BFT, or POTX) is the founding member of the three-gene Paired-like homeodomain transcription factor (PITX) subfamily of bicoid-related proteins. A screen for transcription factors interacting with the pituitary-specific transcription factor Pit-1 (Pou1f1) and binding to a cis-element in the rat proopiomelanocortin (Pomc) promoter (15) led to the cloning of Pitx1. Pitx1 is expressed in the five major hormone-producing cell types of the anterior pituitary in mice and human, although it appears highest in gonadotropes in adulthood (16,17,18,19). Transcription of many pituitary hormone-producing genes, including Cga, Pomc, Gh, Prl, and Tshb, is increased by overexpression of PITX1 in heterologous cell systems (15,20,21,22). These observations suggest a potential role for PITX1 as a pan-pituitary transcriptional regulator. Importantly, transcription of gonadotrope-specific genes, including Lhb, Fshb, and Gnrhr, is also regulated by PITX1 (8,10,22,23,24).
The second PITX subfamily member, PITX2 (also known as PTX2, RIEG1, or ARP1) was originally identified through screening of patients with Axenfeld-Rieger syndrome, an autosomal dominant disease causing craniofacial abnormalities (25,26). Three major mRNA isoforms are derived from the PITX2 gene, encoding proteins of 271, 317, and 324 amino acids. PITX2A and PITX2B mRNAs are produced through alternative splicing, whereas PITX2C is transcribed from an alternative promoter (27,28). All three major PITX isoforms are expressed in the anterior pituitary and craniofacial region (18,28). Like PITX1, PITX2 isoforms were shown to act as pan-pituitary transcriptional regulators of hormone encoding genes and are expressed in gonadotropes in adulthood (16,17,18,22,62). PITX2C was implicated in both basal and activin A-stimulated expression of the rat Fshb subunit gene, although PITX2A and PITX2B appeared to play similar roles (8). The third member of the subfamily, PITX3 (PTX3), is not expressed in the anterior pituitary but rather is restricted to dopaminergic neurons of the substantia nigra and ventral tegmentum and to the developing lens (18,29).
PITX1 and PITX2 are coexpressed in the anterior pituitary (18,22,30). A comparison of the proteins reveals a high degree of sequence conservation. Their homeodomains are 97% identical, differing by only two amino acids. These similarities extend to their C termini, where they share 70% identity (26,27,30). Their N termini, however, are more divergent. PITX proteins contain a lysine at position 50 of their homeodomains (K50). This is a hallmark of the bicoid-related homeodomain transcription factors, including OTX1, OTX2, and goosecoid (GSC) in vertebrates (31,32). Residue 50 is a critical determinant of DNA-binding specificity of homeodomain proteins, and a lysine at this position predicts that these proteins will bind bicoid-like cis-elements TAATCC, TAAGCT (33,34,35). Indeed, PITX1 binds the bicoid-like motifs: TAAGCC (CE3 element) in the rat Pomc promoter (15) and TAATCT in the ovine, bovine, and rat Lhb promoters (21,24,36). Later, both PITX1 and PITX2 were shown to be equally efficient in binding the CE3 element of the POMC promoter (20,21) and in trans-activating promoters of several pituitary hormone-encoding genes (16,20,21,22). Based on the nearly identical homeodomains and C termini of PITX1 and PITX2, it is not surprising that both can bind the same bicoid-related sites and have similar trans-activation properties in vitro.
Recent data suggested a role for PITX1 in basal and GnRH1-stimulated regulation of rat Fshb, acting through a bicoid-like binding site in the proximal promoter, AAATCC (−54/−49) (10). [The PITX1-binding cis-element (AAATCC) identified by Zakaria et al. (10) was indicated to be present between −54/−48 in the rat Fshb promoter. Because the cis-element is 6 bp, we refer to it here as −54/−49.] The involvement of this element in activin-regulated transcription was not investigated, but a second report suggested that PITX2C played a necessary role in both basal and activin A-stimulated transcription of rat Fshb through a cis-element located more distally (−233/−201) (8). [The −230/−199 region of the rat promoter described in Suszko et al. (8) is referred to here as −233/−201. This is based on our identification of a missing base in the sequence reported by Suszko et al. relative to GenBank accession no. M27044 and our mapping of the transcriptional start site using rapid amplification of 5′-cDNA ends.] However, a consensus binding site was not observed in the defined region, nor was an alternative binding sequence mapped. Moreover, whereas gel-shift analyses showed the presence of a specific complex with lysates from HA-PITX2C-transfected cells, the complex was not shifted by an HA antibody making its identity uncertain. Therefore, it is currently unclear whether PITX2C binds directly to DNA in this region to mediate its effects. Nonetheless, a 32-bp deletion encompassing the putative binding site decreased basal and activin A-stimulated transcription in LβT2 cells, suggesting that this promoter region may be required for PITX2C actions, even if it does not bind the protein directly.
The work reported here was designed to 1) clarify the apparent differences in binding sites for PITX1 and PITX2C in the Fshb promoter, 2) assess the extent to which PITX regulatory mechanisms are conserved in the Fshb promoters across species (including humans), 3) examine the roles of both PITX1 and PITX2C proteins in basal and activin A-regulated Fshb expression, and 4) determine how PITX proteins and activin signaling mechanisms might intersect to regulate the Fshb gene.
Materials and Methods
Reagents and constructs
Eagle’s MEM was purchased from American Type Culture Collection (Manassas, VA). DMEM with 4.5 g/liter glucose, l-glutamine, and sodium pyruvate was from Mediatech (Herndon, VA). Ham’s F-12/DME media (1:1) with 1.4 g/liter glucose was from Irvine Scientific (Santa Ana, CA). Lipofectamine/Plus, Lipofectamine 2000, gentamicin, and NuPAGE gels were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) and bovine calf serum were from JRH Biosciences (Lenexa, KS). Horse serum was from Life Technologies, Inc. (Invitrogen, Carlsbad, CA). Human recombinant (rh-) activin A was purchased from R&D Systems (Minneapolis, MN). The anti-PITX1 (SC-18922x) polyclonal antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). SF-1 antirabbit antibody was from Affinity BioReagents (Golden, CO). Anti-Flag M2 monoclonal (F3165), polyclonal anti-Flag antibody (F7425), anti-c-myc (M5546), EZview Red anti-Flag M2 affinity gel, aprotinin, leupeptin, pepstatin, and phenylmethylsulfonyl fluoride were from Sigma Chemical Co. (St. Louis, MO). Deoxynucleotide triphosphates (dNTPs), Taq polymerase, and 5× passive lysis buffer were from Promega (Madison, WI). Protease inhibitor tablets (Complete Mini) was purchased from Roche (Indianapolis, IN). Oligonucleotides were synthesized by IDT (Coralville, IA). Poly(dI)·poly(dC), ECL-plus reagent, and protein marker were purchased from Amersham Biosciences (GE Healthcare, Piscataway, NJ). [γ-32P]ATP was from Perkin-Elmer (Waltham, MA). Pitx1 and Pitx2 SMARTpool small interfering RNAs (siRNAs) were from Dharmacon (Lafayette, CO). The chromatin immunoprecipitation (ChIP) assay reagents and anti-acetyl-histone H3 (Lys9) (07-352) were from Upstate (Lake Placid, NY).
The −1990/+1 mFshb-luc reporter, Flag-human Smad2, Flag-human Smad3, and Flag-murine Smad4 expression constructs were described previously (5,11). The −199/+1 of the murine Fshb promoter was PCR amplified from −1990/+1 mFshb-luc and ligated into pGL3-Basic to make −199/+1 mFshb-luc. The −1028/+7 hFSHB-luc reporter was described previously (11), and PCR was used to make 5′ deletions thereof in pGL3-Basic. The PITX-binding element (PBE) mutation (AAATCC to AggTCg) in murine and human FSHB-luc reporters was produced by site-directed mutagenesis using the QuikChange protocol (Stratagene, La Jolla, CA). Mouse Pitx1, Pitx2a, Pitx2b, and Pitx2c cDNAs were generated by RT-PCR from LβT2 mRNA and ligated into pcDNA3.0 (Invitrogen) by previously described methods (5). The dominant-negative mutants were generated by site-directed mutagenesis. Flag-PITX1 expression vector was constructed by amplifying the PITX1 cDNA and ligating it in-frame with a vector containing the Flag epitope at the N terminus. Myc-PITX1 was generated by adding the myc epitope to the 5′ end of the forward PCR primer. The identities of all constructs were verified by DNA sequencing (Genewiz, South Plainfield, NJ). Primers for cloning and mutagenesis are shown in supplemental Table 1 (published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org).
Cell culture, transfection, and reporter assays
LβT2 cells were provided by Dr. Pamela Mellon (University of California, San Diego, CA) and were cultured as described previously (5,11). HepG2 cells were obtained from American Type Culture Collection and were cultured in MEM/10% FBS. CHO and CV-1 cells were provided by Dr. Patricia Morris (Population Council, New York, NY). CHO cells were cultured in F-12/DME with low glucose, 5% horse serum, and 2.5% bovine calf serum. CV-1 cells were cultured in DMEM/10% FBS. For the reporter experiments, LβT2 and HepG2 cells were plated in 24-well plates at a density of 1–2 × 105 cells per well approximately 36 h before transfection. Cells were transfected with Lipofectamine 2000. Reporter and expression plasmids were transfected at 450 ng and 200–300 ng per well, respectively. In experiments with ligand treatment, cells were washed in serum-free DMEM or 1× PBS and then treated with 1 nm activin A in serum-free DMEM or with DMEM alone (control) for about 24 h. Lysates were collected the following day. Luciferase assays were performed on a Luminoskan Ascent luminometer (Thermo Labsystems, Franklin, MA) using standard reagents. Several vectors typically used for transfection efficiency controls were regulated by the majority of our manipulations (data not shown) and were therefore excluded from the analyses. Measures of protein concentrations showed no differences in cell viability with the different treatments.
To assess knockdown of endogenous PITX1 in LβT2 cells, cells were transfected with the Pitx1 siRNA pool using a reverse transfection protocol (37). Briefly, 10 nm siRNA was combined with Lipofectamine/Plus reagent as per the manufacturer’s protocol. The siRNA-lipid mix was added to 1.5 × 106 cells in suspension and immediately plated into one well of a six-well plate. After 6 h, medium was replaced and cell lysates collected 72 h later in RIPA buffer with protease inhibitor (Roche). CV-1 cells were plated in 12-well plates and transfected using the calcium phosphate method. CHO cells were plated in 10-cm dishes for nuclear protein extraction (for gel shifts) and immunoprecipitation experiments and were transfected when 70–80% confluent with 8 μg of the total plasmid-DNA using Lipofectamine following the manufacturer’s instructions.
EMSA
Nuclear extracts were collected and gel shift experiments performed as described previously (11) with the following modifications: 0.5–1 μg poly(dI)·poly(dC) was used as nonspecific competitor, and gels were run for 2.5–3 h.
DNA precipitation and immunoblot assays
Whole-cell lysates were prepared from LβT2 cells and assays performed as described previously (11) with the following modification: proteins bound to biotinylated probes were isolated using Dynabeads M-280 streptavidin (Dynal; Invitrogen). Briefly, 1/10 of each lysate was used in a DNA precipitation assay with 100 ng biotinylated double-stranded, murine Fshb 61/−40 wild-type (WT) and mutant (Mut) (see supplemental Table 1) probes. After washes, bound proteins were eluted from the beads, separated on 10% NuPAGE Bis-Tris gels, transferred to Protran nitrocellulose (Schleicher & Schuell, Keene, NH), and sequentially probed with PITX1, PITX2 (P2Y4, a gift from Tord Hjalt, Lund University, Lund, Sweden), and anti-SF1. The details of the immunoblot protocol have been described previously (5).
Southwestern blot
Nuclear extracts were separated on a 12% NuPAGE Bis-Tris gel in triplicate and transferred to Protran. The filter was cut into sections for Western blotting or for Southwestern analyses. For the latter, blots were incubated in binding buffer [20 mm HEPES (pH 7.9), 50 mm KCl, 1 mm dithiothreitol, 10% glycerol, and 0.1% Nonidet P-40] for 45 min at room temperature, followed by a 3-h incubation in the same buffer containing 5% nonfat milk. Blots were then hybridized overnight at 4 C with radiolabeled murine −61/−40 or −232/−200 Fshb probes (2 × 106 cpm/ml) in binding buffer plus 0.5% nonfat milk and 1 μg/ml salmon sperm DNA. Blots were washed twice (30 min each) in binding buffer at room temperature and then exposed to x-ray film.
ChIP assay
ChIP assays were performed following the manufacturer’s protocol (Upstate). Briefly, LβT2 cells were grown in 10-cm dishes and either used directly or after transfection. In the latter case, cells were transfected with either −1990 mFshb-luc WT or PBEmut reporter along with Flag-PITX1. In all cases, DNA was cross-linked to protein with 1% formaldehyde for 15 min at 37 C. Cells were washed with PBS, scraped, and resuspended in ChIP lysis buffer. The samples were sonicated six times for 5 sec each at power setting 1.5 (model 100; Fisher Scientific, Ottawa, Ontario, Canada). Sonicated samples were diluted with dilution buffer, precleared with protein G-agarose beads, and then incubated with specific antibodies (PITX1 or Flag) overnight at 4 C. The immune complexes were collected the next day using protein G-agarose beads, and DNA was eluted and cleaned as described in the protocol. For examination of the endogenous gene, −199 to +1 and −505 to −305 of the murine Fshb promoter were amplified using the primers indicated in supplemental Table 1. For analysis of transfected promoters, immunoprecipitated samples were quantified in duplicate by real-time quantitative PCR using SYBR Green qPCR Master Mix (Invitrogen), and the primers are indicated in supplemental Table 1. Input samples were diluted 1/100, whereas undiluted IgG and antibody samples were used for amplification. Quantification was performed by subtracting the calculated chromatin concentration (using the relative standard curve method) of the IgG sample from its corresponding Flag sample and dividing by the concentration of their input sample.
Co-immunoprecipitation assays
CHO cells were cotransfected with the indicated expression constructs. In some experiments, cells were stimulated or not with 1 nm activin A for 1 h before harvesting. Cells were harvested and lysed in 50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1 mm EDTA, and 1% Triton X-100. After the removal of the insoluble material by centrifugation, Bradford assays (Bio-Rad) were performed to determine protein concentrations. Equivalent amounts of protein in each condition were combined with a 40-μl gel volume of anti-FLAG M2 agarose affinity beads [prewashed and equilibrated with Tris-buffered saline: 50 mm Tris-HCl (pH 7.5) and 150 mm NaCl] and incubated overnight at 4 C. Beads were washed three times with Tris-buffered saline and collected by centrifugation. After aspirating the final wash, the affinity beads were resuspended in 3× Flag peptide at a concentration of 150 μg/ml and incubated for 1 h at 4 C. Eluted proteins were collected and run on NuPAGE gels along with whole-cell extracts and probed with the indicated antibodies as described previously (5).
Statistics
The data from three to five replicate experiments were pooled for statistical analyses. Luciferase reporter data are presented as fold change from the control condition (set to 1) in each experiment. Differences between means were compared using one- or two-way ANOVA, followed by post hoc tests where appropriate (Systat 10.2, Richmond, CA). In some experiments, data were log transformed when the variances were unequal between groups. In all the experiments, significance was assessed relative to P < 0.05.
Results
PITX proteins regulate basal Fshb/FSHB transcription through a proximal PITX/bicoid-like binding element
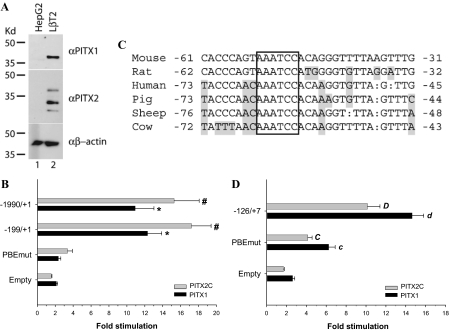
Previous studies using rat, bovine, and porcine Fshb promoter-reporters showed that overexpression of PITX family proteins could stimulate basal transcription in heterologous cell lines (8,9,10,22). Here, we examined the effects of murine PITX1 overexpression on murine −1990/+1 Fshb-luc promoter-reporter (5,11) activity in heterologous HepG2 cells. Western blot analyses confirmed that these cells do not express PITX1 or PITX2 endogenously, whereas these proteins are abundantly expressed in homologous LβT2 cells (Fig. 1A) as reported previously (10,38). PITX1 strongly stimulated promoter activity (∼10.9-fold) and had a relatively modest effect on the promoterless (empty) pGL3-Basic vector (2.1-fold) (Fig. 1B, black bars) (39). We also examined the effects of murine PITX2C transfection (8) and observed comparable results to those with PITX1 (Fig. 1B, gray bars).
Figure 1.
PITX proteins regulate basal murine and human Fshb/FSHB gene transcription through a PITX/bicoid-like binding element in the proximal promoter. A, Western blots for endogenous PITX1 and PITX2 in whole-cell lysates from heterologous HepG2 cells and homologous LβT2 cells. B, HepG2 cells seeded in 24-well plates were transfected with the indicated murine Fshb-luc reporters along with empty, PITX1, or PITX2C expression vectors. Data are presented as fold change in reporter activity in the presence of PITX expression vector relative to empty vector transfected cells with the same reporter. Data represent the mean (±sem) of three experiments performed in triplicate. * and #, Statistically significant differences from the empty pGL3-Basic vector for PITX1 and PITX2C, respectively (Scheffé post hoc test of significant interaction). C, Alignment of proximal Fshb/FSHB promoters in several species. Bases that differ from the mouse are shaded, and gaps are shown by a colon (:). The conserved cis-element, AAATCC, is boxed. D, HepG2 cells were cotransfected with WT or PBE mutant forms of −126/+7 hFSHB-luc along with PITX1 or PITX2C. Data from five replicate experiments are presented as in B. Lowercase (PITX1) and uppercase (PITX2C) letters indicate significant differences from empty vector. Bars with different letters also differed from one another.
To determine specific cis-elements through which these proteins stimulate transcription, we examined the effects of transfected PITX1 and PITX2C on a series of 5′ truncations of the murine Fshb promoter. Although we identified several potential PITX-binding sites in silico (e.g. −1592/−1587, −963/−958, −941/−936, and −53/−48) (21,34,40), truncating the promoter from −1990 to −257 did not significantly affect PITX1- or PITX2C-stimulated transcription in HepG2 cells (data not shown). These results suggested that both proteins mediated their effects principally through a proximal promoter element between −257 and +1. Previous studies indicated that PITX1 acts through a cis-element, AAATCC, at −54/−49 of the rat Fshb promoter (10), whereas PITX2C was reported to exert its effects through a less well-delineated element between −233/−201 in the same gene (8). The promoter is highly conserved between mice and rats (Fig. 1C). To directly assess the functional importance of the −232/−200 region in mouse (equivalent to −233/−201 in rat), we compared the effects of PITX protein overexpression on −1990/+1 mFshb-luc and a truncated reporter, −199/+1 mFshb-luc, which lacks the −232/−200 sequence. Both PITX1 and PITX2C stimulated the two reporters to the same extent (Fig. 1B). We next mutated the proximal PITX cis-element at −53/−48 in the context of −199/+1 mFshb-luc and observed an almost complete abrogation of the PITX1- and PITX2C-stimulated responses (Fig. 1B). These results suggest that PITX1 and PITX2C act through the same proximal cis-element reported previously in the rat Fshb promoter.
We then examined whether the mechanisms observed in mouse extended to the human promoter. We transfected human FSHB promoter-reporter constructs of varying lengths along with PITX1 or PITX2C into HepG2 cells. Both proteins significantly and similarly activated the human reporter, although there was variation in fold stimulation among the different constructs (supplemental Fig. S1). The PITX element identified in rat and mouse is perfectly conserved in human and is contained within the −126/+7 interval (at −65/−60; Fig. 1C). suggesting that PITX proteins might mediate their effects through the same site. Indeed, mutation of this element in the context of the −126/+7 human reporter greatly reduced responses to both PITX1 and PITX2C (Fig. 1D). Interestingly, the mutation did not abolish the response as observed with the murine promoter, suggesting that these proteins may regulate the human gene through additional elements as well. Nonetheless, the bulk of the effect appears to be mediated by the conserved element.
PITX proteins can bind directly to the proximal PITX cis-element
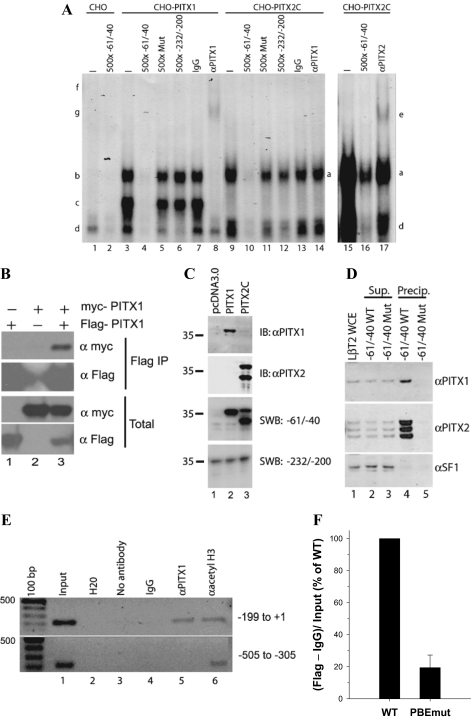
To show that PITX proteins can bind to the proximal cis-element (PBE) in the murine promoter, we first performed gel shift analyses with nuclear protein extracts from CHO cells overexpressing PITX1 or PITX2C. CHO cells do not express PITX proteins endogenously (41,42) (see also Fig. 2C, lane 1). The radiolabeled probe spanned −61/−40 of the murine Fshb promoter. CHO cell nuclear extracts containing overexpressed PITX1 produced two abundant complexes with the −61/−40 probe that were not observed in control CHO lysates (Fig. 2A, complexes b and c, compare lanes 1 and 3). These complexes were specific and required an intact PBE, because they were competed by excess cold homologous probe (lane 4) but not by a probe containing the PBE mutation (lane 5). In contrast, complex formation was not disrupted by excess cold probe corresponding to −232/−200 of the murine promoter (lane 6). Both complexes were supershifted by a PITX1 antibody (lane 8, complexes f and g) but not by control IgG (lane 7) or by a PITX2 antibody (data not shown).
Figure 2.
PITX proteins bind to a proximal cis-element. A, Gel shift analyses were performed with a radiolabeled probe corresponding to −61/−40 of the murine Fshb promoter. The probe was incubated with nuclear extracts from CHO cells transfected with empty (pcDNA3.0) (lanes 1 and 2), PITX1 (lanes 3–8), or PITX2C (lanes 9–17) expression vectors. Complexes associated with PITX1 and PITX2C proteins are labeled on the left (b, c, g, and f) and right (a and e) sides, respectively. Complex d corresponds to an unknown endogenous CHO cell protein complex that specifically interacts with the probe. Lanes 15–17 were run on a separate gel and the gel exposed to film for a longer time to more clearly show that the PITX2 antibody (which is of low titer) could supershift PITX2C bound to the probe. Free (unbound) probe is not pictured, and the lanes are numbered at the bottom. B, CHO cells were cotransfected with Flag-PITX1 and myc-PITX1 alone or in combination. Whole-cell lysates were immunoprecipitated (IP) using anti-Flag M2 affinity gel. Bound proteins were eluted, separated by SDS-PAGE, and immunoblotted with the indicated antibodies. C, Southwestern blot (SWB) analysis performed with CHO cell nuclear extracts used in A: pcDNA3.0 (lane 1), PITX1 (lane 2), and PITX2C (lane 3). The top two panels represent immunoblots (IB) with the indicated antibodies. The bottom two panels are autoradiograms from blots probed with radiolabeled probes corresponding to the indicated base pairs in the murine Fshb promoter. D, DNA precipitation assay with WT −61/−40 (WT) and −61/−40 PBE mutant (Mut) probes. Immunoblots were performed with the indicated antibodies on LβT2 whole-cell extract (WCE) (lane 1), supernatants (Sup.) from extracts incubated with WT and Mut probes bound to magnetic beads (lanes 2 and 3), and proteins eluted from the beads (lanes 4 and 5, Precip.). E, ChIP was performed using LβT2 cells. After cross-linking, IP was performed by incubating with no antibody, IgG, or antibodies against PITX1 or acetylated histone H3 (Lys 9). Input and precipitated DNA were analyzed by PCR using the primers for murine Fshb promoter regions indicated on the right side of the panels. Note that the PCR products in the upper and lower panels were run on separate agarose gels with different sized combs. F, ChIP was performed using LβT2 cells transfected with −1990/+1 mFshb-luc WT or PBEmut together with Flag-PITX1 expression vector. IP was performed by incubating fractions of the same samples with IgG or anti-Flag antibodies. Precipitated DNAs in each condition were quantified using the real-time PCR with primers in the promoter (−119/−98 forward) and the vector (pGL3 reverse). Data are presented as a percentage of binding observed with the WT promoter set to 100%.
PITX proteins have been shown to homodimerize (42,43,44), and we confirmed this here (Fig. 2B), so we reasoned that the two complexes might correspond to PITX1 binding to the probe as a monomer (complex c) or as a dimer (complex b). Alternatively, the two complexes could represent full-length vs. truncated PITX1 protein produced during overexpression. To address this latter possibility, we performed immunoblots with the nuclear extracts used in these analyses and detected a single band of about 36 kDa in PITX1-transfected cells that was absent in control cells (Fig. 2C, top panel, compare lanes 1 and 2). In addition, Southwestern blot analyses of the same proteins using the radiolabeled −61/−40 probe detected a single band of comparable size (Fig. 2C, third panel, lane 2). These data are consistent with our hypothesis that PITX1 may bind the PBE in gel shifts as a monomer or as a dimer, although we have not determined the stoichiometry, nor can we exclude the possibility that complex b reflects PITX1 in association with an endogenous protein in CHO cells.
Gel shift analyses with nuclear extracts from PITX2C-overexpressing CHO cells (Fig. 2A, lanes 9–17) produced comparable results to those with PITX1. Here, a single complex (complex a, lanes 9 and 15) with similar mobility to the larger of the two PITX1 complexes (complex b) was observed that could be competed by the −61/−40 WT (lane 10), but not by mutant −61/−40 (lane 11) nor by WT −232/−200 (lane 12) probes. The complex was supershifted by a PITX2 antibody (complex e, lane 17), but not by control IgG (lane 13) or a PITX1 antibody (lane 14). A longer exposure of the blot in Fig. 2A did not reveal supershifted complexes in lanes 13 or 14 (data not shown). Immunoblot of nuclear extracts from PITX2C-expressing CHO cells using the PITX2 antibody detected two distinct bands (Fig. 2C, second panel, lane 3). Both proteins bound −61/−40 probe in Southwestern blot analyses, although the lower molecular weight species appeared to bind more strongly (Fig. 2C, third panel, lane 3). We determined that the bands corresponded to two forms of PITX2C that are produced through alternative translation initiation, and these data are described elsewhere (62). Southwestern blots using the −232/−200 murine Fshb probe detected a band of approximately 35 kDa that was observed in all lanes and did not bind overexpressed PITX1 or PITX2C (Fig. 2C, bottom panel). These data show that both PITX1 and PITX2C overexpressed in CHO cells can bind the PBE within −60/−41.
To confirm that endogenous PITX proteins can also bind to the PBE, we first performed DNA precipitation assays with LβT2 whole-cell protein lysates and biotinylated −61/−40 probe (−61/−40 WT). A second biotinylated probe containing the PBE mutation (−61/−40 Mut) was included as a control. The precipitated protein complexes were subjected to immunoblot analyses using PITX1- and PITX2-specific antibodies. The results demonstrated that endogenous PITX1 (Fig. 2D, top panel, lane 4) and at least three PITX2 isoforms (Fig. 2D, middle panel, lane 4) could bind to the WT probe. In contrast, the mutated probe failed to precipitate PITX proteins from the same lysates (Fig. 2D, top two panels, lane 5), confirming that the interaction was through the defined cis-element. Another gonadotrope-restricted nuclear protein, steroidogenic factor-1 (SF-1 or NR5A1), is also expressed in LβT2 cells (bottom panel, lanes 1–3) but was not precipitated with the WT probe (bottom panel, lane 4), further showing the specificity of the interaction of the PITX proteins with this element. ChIP assay confirmed that endogenous PITX1 binds to the proximal (−199/+1, Fig. 2E, upper panel, lane 5) but not more distal (−505/−305, lower panel, lane 5) murine Fshb promoter in LβT2 cells. Chromatin from both regions was successfully precipitated by a positive control antibody directed against acetylated histone H3 (Fig. 2E, both panels, lane 6). Unfortunately, the PITX2 antibody available to us was not suitable for ChIP analysis. To confirm that this ex vivo interaction is mediated via the defined PBE, we cotransfected cells with WT or PBEmut reporter constructs and Flag-PITX1. ChIP against the Flag epitope demonstrated that the PBE mutation significantly impaired the PITX1/DNA interaction (Fig. 2F).
A single PBE exists within the −61/−40 promoter region
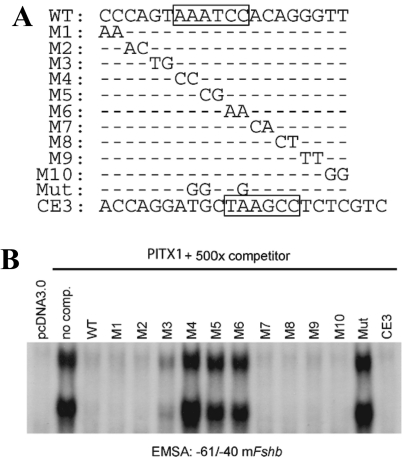
The data above suggested that homodimeric PITX proteins might form complexes with the −61/−40 probe. Although only one PBE appears to be located within this interval, previous data indicate that binding of one member of the dimer to a high-affinity site may facilitate binding of the second protein to an adjacent low-affinity site (45). To assess whether additional sequences outside of the core PBE contributed to higher-order complex binding, we performed gel shifts with the WT −61/−40 probe and nuclear extracts from PITX1-overexpressing CHO cells and competed binding with unlabeled probes containing 2-bp mutations spanning the −61/−40 interval (Fig. 3A). As described above (Fig. 2A), two complexes were detected in the absence of competitor probe (Fig. 3B, no comp.). Complex formation was competed completely by 500-fold excess WT −61/−40 (WT) and mutant probes (M1, M2, M7, M8, M9, and M10) that contained base changes outside of the core 6-bp binding site (AAATCC, boxed in WT sequence in Fig. 3A). In contrast, probes with mutations within the core PBE (M4, M5, and M6) as well as with the PBE mutation used in the reporter assays described above (Mut) failed to inhibit complex formation. One probe containing mutations in the 2-bp 5′ of the PBE also had a partially impaired ability to compete for binding (M3). It has been suggested that the sequence/bases flanking bicoid-like elements can influence binding of PITX proteins (31,46). In Drosophilia, C or T nucleotides are preferentially located 5′ of the bicoid-binding site, TAATCC (47). Collectively, these data indicate that PITX proteins bind to one element within this part of the promoter and therefore that the slower-mobility complex likely contacts DNA through only one of its constituent proteins. Consistent with this interpretation, a competitor containing the closely related PITX1-binding site in the Pomc promoter (CE3; PITX1 element boxed) but otherwise lacking homology to the −61/−40 probe completely inhibited binding of both PITX1 complexes.
Figure 3.
A single PITX binding element exists within −61/−40 of the Fshb promoter. A, Sequences of the probes used in the gel shift analysis in B; − indicates no change in sequence from the WT probe. B, Gel shift assay with radiolabeled −61/−40 probe and nuclear extracts from CHO cells overexpressing PITX1. Complexes were competed with the indicated unlabeled probes (500-fold molar excess). Free probe is not pictured.
PITX proteins do not bind to the putative PITX2C-binding region
The functional data in Fig. 1B and binding data in Figs. 2A (lanes 6 and 12) and 2C (bottom panel) suggest that, contrary to a previous suggestion, −233/−201 of the rat Fshb promoter (8) may not act as a bona fide PITX2C-binding region. To investigate whether this part of the promoter in mouse contains a PITX-binding site, we performed gel shift analyses with the same nuclear proteins used in Fig. 2A and a radiolabeled murine −232/−200 probe. At least three complexes were observed in extracts from control CHO cells (Fig. 4, lane 1, labeled a–c), and all were competed by unlabeled homologous probe (lane 2). Unlike the case with the −61/−40 probe (Fig. 2A), nuclear extracts from CHO cells overexpressing PITX1 (Fig. 4, lanes 3–8) or PITX2C (lanes 9–14) did not produce any additional complexes with the −232/−200 probe when compared with control cell extracts (lane 1). Moreover, the observed complexes were not disrupted by WT or mutated −61/−40 probes (Fig. 4, lanes 4, 5, 10, and 11), nor were they supershifted by PITX antibodies (lanes 8 and 14, and data not shown for PITX2). A similar analysis with a rat −233/−201 probe produced comparable results using our (supplemental Fig. S2, upper panel) or previously described methods (8) (supplemental Fig. S2, lower panel). Collectively, these data suggested that PITX proteins do not bind −232/−200 in mouse or −233/−201 in rat, contrary to the previous report, at least under the experimental conditions investigated.
Figure 4.
PITX proteins do not bind to the putative PITX2C-binding region. Gel shift analyses were performed with a radiolabeled probe corresponding to −232/−200 of the murine Fshb promoter. The probe was incubated with CHO cell nuclear extracts as in Fig. 2. A 500-fold molar excess of unlabeled −232/−200 (lanes 6 and 12), −61/−40 (lanes 4 and 10), and −61/−40 PBE mutant (Mut) (lanes 5 and 11) murine Fshb was used as competitor.
The proximal PBE is required for activin A-mediated transcriptional regulation
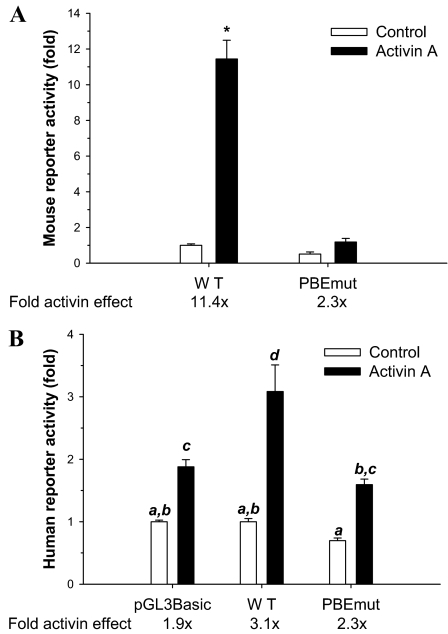
An earlier report indicated a role for the proximal PBE in both basal and GNRH1-stimulated activity of the rat Fshb promoter (10). Because activins are also important positive regulators of Fshb subunit transcription (3,4,5,6,7,8,9,11,12,14,48) and because PITX2C was previously shown to participate in activin A-mediated regulation of the rat Fshb gene (8), we explored the role of the PBE in activin A-stimulated murine Fshb promoter activity. LβT2 cells were transfected with −1990/+1 mFshb-luc or a similar construct containing the PBE mutation [−1990/+1 mFshb PBEmut-luc; see Fig. 3A for the specific mutation (Mut)] and were incubated in the presence or absence of 1 nm activin A for 24 h. The mutation significantly decreased reporter activity and inhibited activin A-stimulated transcription: 11.4-fold for WT vs. 2.3-fold for PBEmut (Fig. 5A).
Figure 5.
The proximal PBE is required for activin A-stimulated transcription. A, LβT2 cells seeded in 24-well plates were transfected with −1990/+1 mFshb-luc (WT) or a similar construct containing PBEmut. The next day, cells were treated with 1 nm activin A or control medium and lysates collected 24 h later. Data from three experiments are presented as fold change in the reporter activity, normalized relative to the untreated WT reporter set to 1. B, As in A, LβT2 cells were transfected with −1028/+7 hFSHB-luc (WT), with the −1028/+7 reporter containing PBEmut, or with empty pGL3-Basic and treated with activin A. Data are derived from three experiments performed in triplicate. Statistical differences are indicated with different letters (Tukey test of significant interaction).
We showed previously that the human −1028/+7 FSHB-luc reporter was activin A responsive in LβT2 cells, although significantly less so than a murine promoter of comparable length (11). Here, we compared basal and activin A-stimulated activity of the WT human reporter with one containing the PBE mutation (Fig. 5B). As with mouse, the mutation decreased basal activity, although to a lesser extent, and this was not statistically significant. Activin A stimulated the WT human promoter 3.1-fold. When controlling for the decline in basal activity, the response was reduced to 2.3-fold for the mutated reporter. The observed level of stimulation did not differ significantly from that observed with the pGL3-Basic (empty) vector assayed at the same time (1.9-fold stimulation by activin A) but both showed significantly less activin A induction than the WT reporter. To our knowledge, this is first report demonstrating that in both mouse and human, the proximal PBE plays a role in basal and activin A-regulated transcription, although the effects of the mutation are more pronounced with the murine reporter.
PITX proteins regulate murine Fshb promoter activity in homologous cells
The data in Fig. 5 confirmed the necessity for the proximal PBE in basal activity (10) and demonstrated for the first time its role in activin A-regulated murine and human Fshb/FSHB promoter activity. However, no study thus far has established a clear role for endogenous PITX proteins in Fshb/FSHB regulation. Therefore, we examined their involvement by functionally impairing endogenous PITX proteins using dominant-negative forms of PITX1 and PITX2C or by depleting endogenous PITX protein levels using Pitx1 and Pitx2 siRNAs in LβT2 cells.
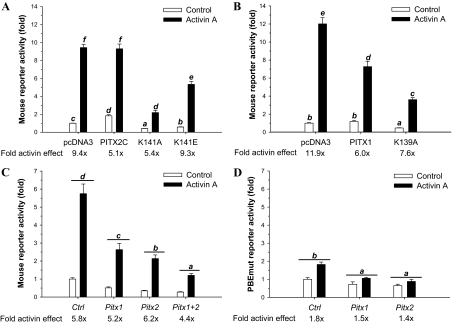
Previous studies showed that mutations of lysine 50 (K50) of the homeodomain in PITX2 produced dominant-negative forms of the protein (41). For example, the DNA-binding-deficient PITX2A mutant, K88E, suppressed prolactin (Prl) promoter activity when overexpressed in homologous GH3 somatolactotrope cells. The mutant protein was argued to produce this effect via dimerization with endogenous PITX proteins bound to PBEs in the Prl promoter, inhibiting their trans-activation function (41,43). With this framework in mind, we produced DNA-binding-deficient forms of murine PITX2C by mutating K141 to glutamic acid (K141E, analogous to the K88E mutation in PITX2A) or to alanine (K141A) and subsequently produced murine PITX1-K139A. Using gel shift analyses with the −61/−40 Fshb probe, we confirmed that these proteins had greatly impaired DNA-binding ability (supplemental Fig. S3C), even although they were expressed at WT levels (Fig. S3, A and B). We also showed that they inhibited WT PITX2C function in heterologous cells (Fig. S3D).
We next transfected the WT or dominant-negative constructs into LβT2 cells along with −1990/+1 mFshb-luc and treated cells with activin A for 24 h. The WT PITX2C mildly increased basal activity, whereas the K141E and K141A mutants impaired basal activity by 43 and 59%, respectively (Fig. 6A). Activin A stimulated a 9.4-fold increase in reporter activity in control cells, and a similar overall level of activation was observed in cells transfected with WT PITX2C. However, the fold activin A effect was reduced when controlling for the small increase in basal activity. The fold activin A effect was also impaired in cells overexpressing the K141A, but not K141E, mutant (Fig. 6A). PITX1-K139A similarly impaired basal (53%) and activin A-regulated Fshb transcription in LβT2 cells (Fig. 6B). Interestingly, overexpression of WT PITX1 did not significantly affect basal promoter activity in these cells but diminished the activin A response by almost 50%. This contrasts with three WT forms of PITX2 (PITX2A, PITX2B, and PITX2C), which only impaired the fold activin A response because of their mild stimulatory effects on basal reporter activity (Fig. 6A and supplemental Fig. S4).
Figure 6.
Dominant-negative PITX proteins and Pitx siRNAs inhibit Fshb transcription. A, LβT2 cells were transfected with −1990/+1 mFshb-luc along with 200 ng/well of PITX2C WT or K50 mutants (either K141A or K141E). The following day, cells were treated with 1 nm activin A for 24 h. Data were normalized to untreated, pcDNA3.0-transfected control cells. Data from three experiments are presented. Bars with different letters differ significantly from each other (Bonferroni). B, LβT2 cells were transfected as in A except with WT and K139A forms of PITX1. C, LβT2 cells were transfected with a murine Fshb reporter and the indicated concentrations of control (Ctrl), Pitx1, or Pitx2 siRNAs either alone (10 nm) or together (5 nm each) and treated the next day with 1 nm activin A for 24 h. Data are presented as fold change in the reporter activity relative to the untreated, 10 nm Ctrl siRNA condition. Bars represent mean (±sem) data derived from four experiments performed in duplicate. Bars with different letters differed significantly (Bonferroni of significant siRNA main effect; note that the ligand × siRNA interaction was not significant). D, LβT2 cells were transfected with a murine Fshb-luc construct with PBEmut along with 10 nm of the indicated siRNAs and treated with 1 nm activin A for 24 h. Data are derived from three experiments and were analyzed as in C.
To confirm the requirement of endogenous PITX proteins in basal and activin A-regulated mFshb transcription, we knocked down protein expression using Pitx1 and Pitx2 siRNA pools in LβT2 cells, after first validating their efficacy and specificity (supplemental Figs. S5 and S6). The Pitx1 and Pitx2 siRNAs inhibited basal Fshb reporter activity by 49 and 65%, respectively (Fig. 6C). When correcting for these declines, there was no apparent effect on the fold activin A response when the two siRNA pools were used alone. However, when used in combination, the siRNA pools also inhibited the activin response by 25%. Unfortunately, the interaction was not statistically significant. We therefore could not make pairwise comparisons to determine whether this decline was significant. We next examined the effects of the siRNAs on the Fshb promoter containing the PBE mutation. Interestingly, reporter activity was decreased by Pitx1 and Pitx2 knockdown (Fig. 6D), although the magnitude of the effects were lower than observed with the WT promoter (Fig. 6C and data not shown). The modest fold activin A stimulation observed with the mutant reporter was not significantly altered by either Pitx siRNA pool. These data suggest that PITX proteins can have some effects on Fshb transcription independent of their actions through the PBE, which is consistent with a previous report (10) and with the data in Fig. 1, B and D.
PITX proteins physically interact with Smad3
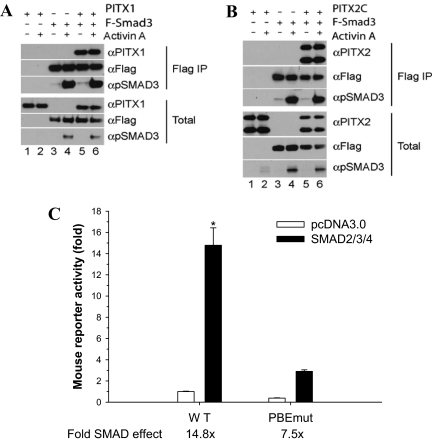
We next explored whether and how PITX proteins interface with the activin signaling transduction cascade. We showed previously that activins stimulate the formation of Smad2/3/4 complexes that bind to a Smad-binding element (SBE) in the murine Fshb promoter at −266/−259 (11). This binding is critical for both the acute and tonic effects of activins on transcription. Therefore, we examined whether PITX proteins interact with Smads, providing a potential link between the PBE and the SBE. CHO cells were cotransfected with PITX1 (Fig. 7A) or PITX2C (Fig. 7B) alone or in combination with Flag-human Smad3 and were treated with or without activin A for 1 h. Western blots of whole-cell lysates confirmed expression of Flag-Smad3 (Fig. 7, A and B, second panel from bottom, lanes 3–6), PITX1 (Fig. 7A, third panel from bottom, lanes 1, 2, 5, and 6), and PITX2C (Fig. 7B, third panel from bottom, lanes 1, 2, 5, and 6). Both PITX1 and PITX2C were coprecipitated with Flag-Smad3 (Fig. 7, A and B, top panels, lanes 5 and 6). Activin A (1 nm) treatment did not affect the Smad3-PITX association (compare lanes 5 and 6), although the cells were clearly activin A responsive as indicated by the increases in Smad3 C-terminal phosphorylation (third panel from top, lanes 4 and 6 in Fig. 7, both A and B). Using the same approach, we failed to observe interactions of PITX1 or PITX2C with either Flag-Smad2 or Flag-Smad4 in the presence or absence of activin A (data not shown).
Figure 7.
Physical and functional interaction between PITX and Smad proteins. A and B, CHO cells were cotransfected with PITX1 (A) or PITX2C (B) alone or in combination with Flag-Smad3 and were treated with activin A or control media for 1 h. Whole-cell lysates were immunoprecipitated (IP) using anti-Flag M2 affinity gel. Bound proteins were eluted and separated by SDS-PAGE and immunoblotted with the indicated antibodies. C, LβT2 cells were seeded in six-well plates and transfected with −1990/+1 mFshb-luc WT (WT) or PBEmut along with expression vectors for Smad2, -3, and -4 (black bars) or empty vector (white bars). The data are from three experiments. *, Smad2/3/4 stimulation of the WT promoter differed from all other groups, which did not differ from one another (Bonferroni).
PITX2C and Smad3 were previously shown to function synergistically and confer activin responsiveness to the rat Fshb promoter in heterologous cells (8). We have been unable to replicate this finding and in fact observe that coexpression of Smad3 slightly inhibits PITX2C stimulation of murine Fshb reporter activity in HepG2 cells (see supplemental Fig. S7B).
The PBE is required for Smad-stimulated promoter activity
Finally, we examined the role of the PBE in Smad-regulated promoter activity. We previously showed that Smads 2, 3, and 4 in combination synergistically stimulate murine Fshb promoter activity (11), and we confirmed that observation again here (Fig. 7C). Mutation of the PBE decreased promoter activity by 62% and significantly impaired Smad2/3/4-stimulated transcription by approximately 50% (a decrease from 14.8- to 7.5-fold). Similar results were observed when we transfected Smad3 alone (data not shown). These data suggest that an intact PBE cis-element is required for maximal Smad-mediated transcription of the murine Fshb promoter in homologous cells.
Discussion
PITX1 and PITX2C were previously shown to regulate basal and hormone-dependent transcription of the Fshb subunit gene, but through distinct mechanisms (8,10). The results presented here clarify these apparently discrepant findings and significantly advance our understanding of how PITX proteins regulate the Fshb gene. We show that both PITX1 and PITX2C mediate their effects through a common cis-element (PBE) in the proximal Fshb promoter. This PBE is conserved in all mammalian species in which the gene has been characterized thus far (Fig. 1C) (49), and it is required for both basal and activin A-regulated murine and human FSHB promoter-reporter activities in LβT2 cells. We further confirm that endogenous PITX1 and three PITX2 isoforms can interact with the PBE. Transfection of dominant-negative PITX proteins or knockdown of PITX expression by RNA interference inhibit murine Fshb reporter activity in LβT2 cells, implicating endogenous PITX proteins in Fshb regulation. Finally, PITX1 and PITX2C can interact with Smad3 in cells, providing a direct link between PITX proteins and the activin signal transduction cascade.
A proximal cis-element mediates regulation of the Fshb by PITX proteins
PITX1 and PITX2 isoforms act as pan-pituitary transcriptional activators but at the same time contribute to the mechanisms of cell-restricted expression of various pituitary hormone-encoding genes (15,21,22,50,51). Our results show that both PITX1 and PITX2C can stimulate murine Fshb and human FSHB promoter activities in heterologous cells. In contrast, overexpression of these proteins in homologous LβT2 cells has little or no effect on basal transcription. These observations are comparable to those from a previous report where PITX1 overexpression stimulated rat Fshb promoter activity in heterologous GGH3-1 cells but not in LβT2 cells (10). However, a second study showed that PITX2 isoforms, especially PITX2C, stimulated basal activity of the rat Fshb promoter in LβT2 cells (8). An explanation for the contrasting results is not immediately obvious; however, several studies using different PITX-sensitive promoters in homologous cell lines have reported little or no potentiation of transcription when endogenous PITX1 and PITX2 protein levels are not limiting (20,41,46,50).
Our data indicate that both PITX1 and PITX2C affect Fshb transcription by direct binding to the cis-element, AAATCC, in the proximal promoter. Although not identical to the consensus binding site for homeodomain proteins within the K50 class, TAATCC (31,34), the element is perfectly conserved in sequence and relative location in the Fshb/FSHB promoters across mammalian species (Fig. 1C) (49). Gel shift analyses clearly show that all 6 bp in the PBE, but not flanking sequences, mediate PITX factor binding (Fig. 3B). Previous studies show that some variation in the binding site is tolerated by PITX proteins (31,52). Although the TAAT core is observed in many homeodomain binding elements, AAAT is clearly sufficient for binding here. Moreover, the structure of the human PITX2 homeodomain bound to DNA shows that the side chain of K50 makes hydrogen bonds to the guanines on the antisense strand complementary to the cytosines at positions 5 and 6 of the binding element (34). This interaction is critical for K50 proteins to bind to DNA, and these base pairs are conserved in the PBE in the Fshb/FSHB promoter.
That both PITX1 and PITX2C (and other PITX2 isoforms; see Ref. 62) bind to the PBE is not surprising given the high conservation (97%) of their homeodomains. However, using 5′ promoter deletions and gel shift analyses, a previous report identified a PITX2C-binding region between −233/−201 in the rat Fshb gene (8). In this case, a well-delineated cis-element was not characterized (49), nor was a consensus PITX-binding site observed therein. Nonetheless, deleting a comparable region, −232/−200, of the mouse promoter did not alter PITX2C (or PITX1) responsiveness in heterologous cells in our analyses. In addition, PITX2C did not bind to this region of the rat or murine promoters in gel shift or Southwestern blot analyses in our hands. This was true whether we used our own or previously described gel shift methods (8). Instead, both exogenous PITX2C and at least three endogenous PITX2 isoforms (likely PITX2A and two isoforms of PITX2C; see Ref. 62) could clearly bind the PBE at −53/−48, and exogenous PITX proteins exerted the bulk of their actions on murine and human promoter activity via binding to this site. Thus, we conclude that the more distal region of the Fshb promoter does not mediate the direct effects of PITX proteins on transcription.
It is possible, however, that the more distal region is important for regulation of Fshb transcription by activins, and this may have impacted previous interpretations (8). Deletion of this region in the context of a −338 rat Fshb luciferase reporter decreased both Smad3 and activin A responsiveness in LβT2 cells (8). A second study similarly showed a role for −230/−194 in follistatin- (and by inference activin B)-mediated regulation of murine Fshb in these cells (6). Thus, whereas we conclude that this part of the promoter does not bind PITX proteins directly, it might be important for activin regulation and could bind proteins that associate with PITX factors binding more proximally.
PITX proteins may bind the Fshb promoter as dimers
Our gel shift assays suggest that PITX proteins may bind to the Fshb promoter as monomers (PITX1) or dimers (PITX1 and PITX2C). Indeed, previous analyses of several promoters show that PITX1 and PITX2 can bind in different multimeric forms (42,43,46,53). Consistent with these observations, previous biochemical analyses showed that PITX1 and PITX2 isoforms can homodimerize (42,44). We confirmed these observations here and also showed for the first time, to our knowledge, that PITX1 and PITX2 proteins can heterodimerize (Fig. 2B and supplemental Fig. S8). In some cases, dimer binding is attributable to binding of one member of the pair to a consensus element and the second to a weaker/cryptic element in close proximity (45). This does not appear to be the case here because gel shift analyses indicate the presence of only one binding site for PITX1 within the −61/−40 interval. Why we detect two protein complexes with PITX1 but only one with PITX2C (and other PITX2 isoforms; see Ref. 62) is not yet clear. We have not definitively determined the stoichiometry of the PITX proteins in the complexes observed by EMSA. Indeed, it is possible that the two PITX1 complexes reflect PITX1 binding alone or with a non-PITX partner expressed endogenously in CHO cells (note that we observe the same two complexes when PITX1 is expressed in COS7 cells, data not shown). However, the results of the Flag-PITX1/myc-PITX1 coprecipitation and the dominant-negative PITX mutant experiments (see below) converge to suggest that two or more PITX protein can coexist within the same complex.
Previous analyses suggest that K50 mutants exert their dominant-negative activity by dimerizing with WT proteins. Whereas the mechanism of inhibition is not completely understood, mutations to K50 appear to increase the association between the homeodomain (DNA-binding domain) and the C terminus (putative activation domain), and the interaction of these two domains has been associated with decreased DNA binding and trans-activation (43). Similarly, we also observe that both PITX1 and PITX2C K50 mutants in heterologous cells have no effect on their own but can dose-dependently antagonize the stimulatory effect of WT. We predict that the Lys to Ala mutants similarly inhibit basal reporter activity in homologous LβT2 cells by dimerizing with endogenous PITX proteins bound to the Fshb promoter. We observed only a minor inhibition of WT PITX1 binding to the PBE in the presence of K139A (data not shown), so we predict that the antagonism is principally through disruption of a trans-activation function of WT proteins. It is also possible that these mutants inhibit basal activity in LβT2 cells by competing for binding to limiting endogenous cofactors, although we currently have no direct evidence of this. Indeed, if this were a mechanism of antagonism, then overexpressed WT PITX proteins might be expected to produce the same inhibitory effects on basal activity, which they did not.
Endogenous PITX proteins regulate Fshb transcription
Although previous reports have shown that overexpressed PITX1 and PITX2 isoforms can stimulate Fshb subunit transcription in heterologous cells, none have confirmed a role for endogenous PITX proteins in this process (8,10,22). Pitx1 or Pitx2 knockout mice have been generated, but both models have developmental defects that preclude an assessment of PITX proteins in FSH regulation in adulthood (54,55,56,57,58). The present and previous work (10) demonstrates that endogenous PITX proteins can bind this site, but this does not preclude the binding of other homeodomain proteins expressed in gonadotrope cells (38). Therefore, based on previous reports, one could not conclude that PITX proteins were endogenous regulators of the Fshb subunit. Here, we show that endogenous PITX1 and three PITX2 isoforms can bind to the PBE and that PITX1 binds the murine Fshb promoter in cells. Moreover, transfection of PITX1 and PITX2C dominant-negative proteins (K50A mutants) or knockdown of endogenous PITX proteins using RNA interference inhibited both basal and activin A-stimulated Fshb transcription. The effect of the Pitx1 and Pitx2 siRNA pools on the activin response was observed only when the two were used in combination, and the overall effect was modest (∼25%) relative to the effects of the PBE mutation (∼80%). These results may reflect an inability of the siRNAs to inhibit completely PITX protein expression in LβT2 cells (e.g. supplemental Fig. S5A). Alternatively, a different homeodomain protein may bind the PBE in the absence of PITX proteins (38), compensating for their loss.
Even though PITX1 and PITX2C were functionally similar in the majority of our analyses, differences in their binding characteristics could have functional consequences. This is most clearly demonstrated in LβT2 cells where WT PITX1 transfection had no effect on basal activity but antagonized the activin A response. In contrast, WT PITX2C affected only the fold activin A response by virtue of its small stimulation of basal activity (this was also the case for PITX2A and PITX2B; Fig. 6A and supplemental Fig. S4). In addition, experiments in heterologous CV-1 cells showed that PITX2C dose-dependently stimulated Fshb reporter activity, whereas PITX1 stimulation had an inverse-U-related effect (Fig. S6). Previously, similar responses were seen with these proteins on the human PRL and salmon Lhb promoters (20,44). These differences may relate to the ability of PITX1, but not PITX2C, to bind as part of at least two different protein complexes (perhaps monomeric and dimeric), although we cannot discount differences in recruitment of cofactors by the different proteins.
PITX proteins regulate activin signaling to the Fshb promoter
Fshb transcription is potently up-regulated by activins in many species including mice (3,4,5,7,8,11). The PBE appears critical for this response. Mutation of this element greatly inhibits activin A responsiveness, particularly of the murine Fshb reporter. This does not reflect a generalized inhibition of reporter responsiveness because fold stimulation of the rat and murine Fshb promoter activity by GNRH1 is unaltered by the same mutation (10) (data not shown). Mutation of the PITX-binding site in the Lhb promoter similarly inhibits basal, but not GNRH1-stimulated, reporter activity in LβT2 cells (24).
We and others have shown that activin A stimulates the formation of Smad2/3/4 complexes that interact with a SBE at −266/−259 in the murine Fshb reporter (4,8,11). Transfection of these Smads in LβT2 cells stimulates reporter activity in ligand-independent fashion, and mutating the SBE blocks this response (11). Here, mutation of the PBE also significantly blunted the effects of transfected Smads on reporter activity. In addition, we observed for the first time that Smad3 could interact with both PITX1 and PITX2C in transfected mammalian cells. A previous report showed direct interactions between Smads 2, 3, and 4 and PITX1 in glutathione-S-transferase pull-down assays (36). Suszko et al. (59) recently showed an interaction between Smad3 and PITX2C in cells and Smad3 and Smad2 with PITX2A and PITX2C in yeast. They did not report any data for Smad4 or PITX1, nor did they show PITX2C/Smad2 interactions in mammalian cells. However, they showed that the homeodomain of PITX2C was required for the interaction with Smads 2 and 3 in yeast (59). We failed to detect interactions between PITX1 or PITX2C with Smads 2 and 4 in CHO cells. The reason for the discrepant results is not clear, although differences in the assay systems used are likely involved. Nonetheless, these data suggest a model in which PITX proteins constitutively bound to the proximal Fshb promoter regulate basal transcription in gonadotrope cells. Upon activin stimulation, Smad2/3/4 complexes bind the SBE and perhaps other promoter elements (6,60). Smad3 in these complexes could directly interact with PITX proteins bound more proximally. This may alter the DNA conformation in such a way as to increase transcriptional activation by Smads or facilitate their interaction with the basal transcriptional machinery. Alternatively, the Smad-PITX interaction may help stabilize the binding of either or both complexes to their respective cis-elements. The SBE is present in rodent Fshb promoters but is absent in other species, including humans (49). Therefore, this model may not fully explain the role of PITX proteins in activin-regulated transcription; however, Smads may interact with alternate promoter elements in other species (6), suggesting that Smad-PITX interactions may be conserved and important in Fshb regulation.
In conclusion, our data suggest that the PITX family of proteins may form part of a cell-restricted transcriptional complex that confers both basal and activin responsiveness to the Fshb promoter in gonadotrope cells. Indeed, PITX proteins have been shown to cooperate with the cell-restricted transcriptional regulators SF-1 and Pit-1 to regulate Lhb and Prl promoter activities in gonadotrope and lactotrope cells, respectively (20,61). The clear delineation of the PBE and proteins binding to it provides the basis for understanding more fully the mechanisms of gonadotrope-restricted expression of the Fshb/FSHB gene.
Supplementary Material
Acknowledgments
We thank Drs. Y. Chen, T. Hjalt, J. Massague, P. Mellon, P. Morris, E. Robertson, and T. Woodruff for generously providing cell lines and reagents. Some of the cell culture work was performed in the Cell and Tissue Culture Core Facility of the Population Council under the direction of Dr. Patricia Morris and with the assistance of Marion Davis and Catherine Rapelje. We are also thankful to Dr. K. Lee for critically reading an earlier version of the manuscript.
Footnotes
This research was supported by National Institute of Child Health and Human Development/National Institutes of Health R01 HD047794 to D.J.B. P.L. was partially supported by the F. M. Kirby Foundation.
Disclosure Statement: The authors have nothing to disclose.
First Published Online March 13, 2008
Abbreviations: ChIP, Chromatin immunoprecipitation; FBS, fetal bovine serum; Mut, mutant; PBE, PITX-binding element; PITX, Paired-like homeodomain transcription factor; SBE, Smad-binding element; siRNA, small interfering RNA; WT, wild type.
References
- Brown P, McNeilly AS 1999 Transcriptional regulation of pituitary gonadotrophin subunit genes. Rev Reprod 4:117–124 [DOI] [PubMed] [Google Scholar]
- Alarid ET, Windle JJ, Whyte DB, Mellon PL 1996 Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development 122:3319–3329 [DOI] [PubMed] [Google Scholar]
- Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang HJ, Miller WL, Mellon PL 2001 Cell-specific transcriptional regulation of follicle-stimulating hormone-β by activin and gonadotropin-releasing hormone in the LβT2 pituitary gonadotrope cell model. Endocrinology 142:2284–2295 [DOI] [PubMed] [Google Scholar]
- Gregory SJ, Lacza CT, Detz AA, Xu S, Petrillo LA, Kaiser UB 2005 Synergy between activin A and gonadotropin-releasing hormone in transcriptional activation of the rat follicle-stimulating hormone-β gene. Mol Endocrinol 19:237–254 [DOI] [PubMed] [Google Scholar]
- Bernard DJ 2004 Both SMAD2 and SMAD3 mediate activin-stimulated expression of the follicle-stimulating hormone β-subunit in mouse gonadotrope cells. Mol Endocrinol 18:606–623 [DOI] [PubMed] [Google Scholar]
- Bailey JS, Rave-Harel N, McGillivray SM, Coss D, Mellon PL 2004 Activin regulation of the follicle-stimulating hormone β-subunit gene involves Smads and the TALE homeodomain proteins Pbx1 and Prep1. Mol Endocrinol 18:1158–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suszko MI, Balkin DM, Chen Y, Woodruff TK 2005 Smad3 mediates activin-induced transcription of follicle-stimulating hormone β-subunit gene. Mol Endocrinol 19:1849–1858 [DOI] [PubMed] [Google Scholar]
- Suszko MI, Lo DJ, Suh H, Camper SA, Woodruff TK 2003 Regulation of the rat follicle-stimulating hormone β-subunit promoter by activin. Mol Endocrinol 17:318–332 [DOI] [PubMed] [Google Scholar]
- West BE, Parker GE, Savage JJ, Kiratipranon P, Toomey KS, Beach LR, Colvin SC, Sloop KW, Rhodes SJ 2004 Regulation of the follicle-stimulating hormone β gene by the LHX3 LIM-homeodomain transcription factor. Endocrinology 145:4866–4879 [DOI] [PubMed] [Google Scholar]
- Zakaria MM, Jeong KH, Lacza C, Kaiser UB 2002 Pituitary homeobox 1 activates the rat FSHβ (rFSHβ) gene through both direct and indirect interactions with the rFSHβ gene promoter. Mol Endocrinol 16:1840–1852 [DOI] [PubMed] [Google Scholar]
- Lamba P, Santos MM, Philips DP, Bernard DJ 2006 Acute regulation of murine follicle-stimulating hormone β-subunit transcription by activin A. J Mol Endocrinol 36:201–220 [DOI] [PubMed] [Google Scholar]
- Safwat N, Ninomiya-Tsuji J, Gore AJ, Miller WL 2005 Transforming growth factor β-activated kinase 1 is a key mediator of ovine follicle-stimulating hormone β-subunit expression. Endocrinology 146:4814–4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HJ, Sebastian J, Strahl BD, Wu JC, Miller WL 2001 The promoter for the ovine follicle-stimulating hormone-β gene (FSHβ) confers FSHβ-like expression on luciferase in transgenic mice: regulatory studies in vivo and in vitro. Endocrinology 142:2260–2266 [DOI] [PubMed] [Google Scholar]
- Huang HJ, Sebastian J, Strahl BD, Wu JC, Miller WL 2001 Transcriptional regulation of the ovine follicle-stimulating hormone-β gene by activin and gonadotropin-releasing hormone (GnRH): involvement of two proximal activator protein-1 sites for GnRH stimulation. Endocrinology 142:2267–2274 [DOI] [PubMed] [Google Scholar]
- Lamonerie T, Tremblay JJ, Lanctot C, Therrien M, Gauthier Y, Drouin J 1996 Ptx1, a bicoid-related homeo box transcription factor involved in transcription of the pro-opiomelanocortin gene. Genes Dev 10:1284–1295 [DOI] [PubMed] [Google Scholar]
- Drouin J, Lamolet B, Lamonerie T, Lanctot C, Tremblay JJ 1998 The PTX family of homeodomain transcription factors during pituitary developments. Mol Cell Endocrinol 140:31–36 [DOI] [PubMed] [Google Scholar]
- Charles MA, Suh H, Hjalt TA, Drouin J, Camper SA, Gage PJ 2005 PITX genes are required for cell survival and Lhx3 activation. Mol Endocrinol 19:1893–1903 [DOI] [PubMed] [Google Scholar]
- Pellegrini-Bouiller I, Manrique C, Gunz G, Grino M, Zamora AJ, Figarella-Branger D, Grisoli F, Jaquet P, Enjalbert A 1999 Expression of the members of the Ptx family of transcription factors in human pituitary adenomas. J Clin Endocrinol Metab 84:2212–2220 [DOI] [PubMed] [Google Scholar]
- Lanctot C, Gauthier Y, Drouin J 1999 Pituitary homeobox 1 (Ptx1) is differentially expressed during pituitary development. Endocrinology 140:1416–1422 [DOI] [PubMed] [Google Scholar]
- Quentien MH, Manfroid I, Moncet D, Gunz G, Muller M, Grino M, Enjalbert A, Pellegrini I 2002 Pitx factors are involved in basal and hormone-regulated activity of the human prolactin promoter. J Biol Chem 277:44408–44416 [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Lanctot C, Drouin J 1998 The pan-pituitary activator of transcription, Ptx1 (pituitary homeobox 1), acts in synergy with SF-1 and Pit1 and is an upstream regulator of the Lim-homeodomain gene Lim3/Lhx3. Mol Endocrinol 12:428–441 [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Goodyer CG, Drouin J 2000 Transcriptional properties of Ptx1 and Ptx2 isoforms. Neuroendocrinology 71:277–286 [DOI] [PubMed] [Google Scholar]
- Jeong KH, Chin WW, Kaiser UB 2004 Essential role of the homeodomain for pituitary homeobox 1 activation of mouse gonadotropin-releasing hormone receptor gene expression through interactions with c-Jun and DNA. Mol Cell Biol 24:6127–6139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk CC, Lozada KL, Keri RA, Nilson JH 2001 A single Pitx1 binding site is essential for activity of the LHβ promoter in transgenic mice. Mol Endocrinol 15:734–746 [DOI] [PubMed] [Google Scholar]
- Hjalt TA, Semina EV 2005 Current molecular understanding of Axenfeld-Rieger syndrome. Expert Rev Mol Med 7:1–17 [DOI] [PubMed] [Google Scholar]
- Semina EV, Reiter R, Leysens NJ, Alward WL, Small KW, Datson NA, Siegel-Bartelt J, Bierke-Nelson D, Bitoun P, Zabel BU, Carey JC, Murray JC 1996 Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet 14:392–399 [DOI] [PubMed] [Google Scholar]
- Arakawa H, Nakamura T, Zhadanov AB, Fidanza V, Yano T, Bullrich F, Shimizu M, Blechman J, Mazo A, Canaani E, Croce CM 1998 Identification and characterization of the ARP1 gene, a target for the human acute leukemia ALL1 gene. Proc Natl Acad Sci USA 95:4573–4578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Camper SA 1997 Pituitary homeobox 2, a novel member of the bicoid-related family of homeobox genes, is a potential regulator of anterior structure formation. Hum Mol Genet 6:457–464 [DOI] [PubMed] [Google Scholar]
- Semina EV, Reiter RS, Murray JC 1997 Isolation of a new homeobox gene belonging to the Pitx/Rieg family: expression during lens development and mapping to the aphakia region on mouse chromosome 19. Hum Mol Genet 6:2109–2116 [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA 1999 The bicoid-related Pitx gene family in development. Mamm Genome 10:197–200 [DOI] [PubMed] [Google Scholar]
- Dave V, Zhao C, Yang F, Tung CS, Ma J 2000 Reprogrammable recognition codes in bicoid homeodomain-DNA interaction. Mol Cell Biol 20:7673–7684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Affolter M, Burglin T 1994 Homeodomain proteins. Annu Rev Biochem 63:487–526 [DOI] [PubMed] [Google Scholar]
- Wilson DS, Sheng G, Jun S, Desplan C 1996 Conservation and diversification in homeodomain-DNA interactions: a comparative genetic analysis. Proc Natl Acad Sci USA 93:6886–6891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaney BA, Clark-Baldwin K, Dave V, Ma J, Rance M 2005 Solution structure of the K50 class homeodomain PITX2 bound to DNA and implications for mutations that cause Rieger syndrome. Biochemistry 44:7497–7511 [DOI] [PubMed] [Google Scholar]
- Baird-Titus JM, Clark-Baldwin K, Dave V, Caperelli CA, Ma J, Rance M 2006 The solution structure of the native K50 bicoid homeodomain bound to the consensus TAATCC DNA-binding site. J Mol Biol 356:1137–1151 [DOI] [PubMed] [Google Scholar]
- Coss D, Thackray VG, Deng CX, Mellon PL 2005 Activin regulates luteinizing hormone β-subunit gene expression through Smad-binding and homeobox elements. Mol Endocrinol 19:2610–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury TB, Binder AK, Grammer JC, Nilson JH 2007 Maximal activity of the luteinizing hormone β-subunit gene requires β-catenin. Mol Endocrinol 21:963–971 [DOI] [PubMed] [Google Scholar]
- Rosenberg SB, Mellon PL 2002 An Otx-related homeodomain protein binds an LHβ promoter element important for activation during gonadotrope maturation. Mol Endocrinol 16:1280–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza HM, Cox CJ, Semina EV, Amendt BA 2002 A molecular basis for differential developmental anomalies in Axenfeld-Rieger syndrome. Hum Mol Genet 11:743–753 [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Qian YQ, Billeter M, Furukubo-Tokunaga K, Schier AF, Resendez-Perez D, Affolter M, Otting G, Wuthrich K 1994 Homeodomain-DNA recognition. Cell 78:211–223 [DOI] [PubMed] [Google Scholar]
- Saadi I, Semina EV, Amendt BA, Harris DJ, Murphy KP, Murray JC, Russo AF 2001 Identification of a dominant negative homeodomain mutation in Rieger syndrome. J Biol Chem 276:23034–23041 [DOI] [PubMed] [Google Scholar]
- Cox CJ, Espinoza HM, McWilliams B, Chappell K, Morton L, Hjalt TA, Semina EV, Amendt BA 2002 Differential regulation of gene expression by PITX2 isoforms. J Biol Chem 277:25001–25010 [DOI] [PubMed] [Google Scholar]
- Saadi I, Kuburas A, Engle JJ, Russo AF 2003 Dominant negative dimerization of a mutant homeodomain protein in Axenfeld-Rieger syndrome. Mol Cell Biol 23:1968–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed P, Koh M, Preklathan P, Bei L, Hew C 2002 Multiple mechanisms for Pitx-1 transactivation of a luteinizing hormone β-subunit gene. J Biol Chem 277:26200–26207 [DOI] [PubMed] [Google Scholar]
- Burz DS, Rivera-Pomar R, Jackle H, Hanes SD 1998 Cooperative DNA-binding by Bicoid provides a mechanism for threshold-dependent gene activation in the Drosophila embryo. EMBO J 17:5998–6009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PD, Hjalt TA, Kirk DE, Sutherland LB, Thomas BL, Sharpe PT, Snead ML, Murray JC, Russo AF, Amendt BA 2001 Antagonistic regulation of Dlx2 expression by PITX2 and Msx2: implications for tooth development. Gene Expr 9:265–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor AP 2005 How to get ahead: the origin, evolution and function of bicoid. Bioessays 27:904–913 [DOI] [PubMed] [Google Scholar]
- Gore AJ, Philips DP, Miller WL, Bernard DJ 2005 Differential regulation of follicle stimulating hormone by activin A and TGFB1 in murine gonadotropes. Reprod Biol Endocrinol 3:73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar TR, Schuff KG, Nusser KD, Low MJ 2006 Gonadotroph-specific expression of the human follicle stimulating hormone β gene in transgenic mice. Mol Cell Endocrinol 247:103–115 [DOI] [PubMed] [Google Scholar]
- Quentien MH, Pitoia F, Gunz G, Guillet MP, Enjalbert A, Pellegrini I 2002 Regulation of prolactin, GH, and Pit-1 gene expression in anterior pituitary by Pitx2: an approach using Pitx2 mutants. Endocrinology 143:2839–2851 [DOI] [PubMed] [Google Scholar]
- Quentien MH, Barlier A, Franc JL, Pellegrini I, Brue T, Enjalbert A 2006 Pituitary transcription factors: from congenital deficiencies to gene therapy. J Neuroendocrinol 18:633–642 [DOI] [PubMed] [Google Scholar]
- Hjalt TA, Amendt BA, Murray JC 2001 PITX2 regulates procollagen lysyl hydroxylase (PLOD) gene expression: implications for the pathology of Rieger syndrome. J Cell Biol 152:545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amendt BA, Sutherland LB, Russo AF 1999 Multifunctional role of the Pitx2 homeodomain protein C-terminal tail. Mol Cell Biol 19:7001–7010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctot C, Moreau A, Chamberland M, Tremblay ML, Drouin J 1999 Hindlimb patterning and mandible development require the Ptx1 gene. Development 126:1805–1810 [DOI] [PubMed] [Google Scholar]
- Szeto DP, Rodriguez-Esteban C, Ryan AK, O'Connell SM, Liu F, Kioussi C, Gleiberman AS, Izpisua-Belmonte JC, Rosenfeld MG 1999 Role of the Bicoid-related homeodomain factor Pitx1 in specifying hindlimb morphogenesis and pituitary development. Genes Dev 13:484–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K, Miura H, Miyagawa-Tomita S, Yanazawa M, Katoh-Fukui Y, Suzuki R, Ohuchi H, Suehiro A, Motegi Y, Nakahara Y, Kondo S, Yokoyama M 1999 Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development 126:5749–5758 [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA 1999 Dosage requirement of Pitx2 for development of multiple organs. Development 126:4643–4651 [DOI] [PubMed] [Google Scholar]
- Lin CR, Kioussi C, O'Connell S, Briata P, Szeto D, Liu F, Izpisua-Belmonte JC, Rosenfeld MG 1999 Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature 401:279–282 [DOI] [PubMed] [Google Scholar]
- Suszko MI, Antenos M, Balkin DM, Woodruff TK 2008 Smad3 and Pitx2 cooperate in stimulation of FSHβ gene transcription. Mol Cell Endocrinol 281:27–36 [DOI] [PubMed] [Google Scholar]
- McGillivray SM, Thackray VG, Coss D, Mellon PL 2007 Activin and glucocorticoids synergistically activate follicle-stimulating hormone β-subunit gene expression in the immortalized LβT2 gonadotrope cell line. Endocrinology 148:762–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay JJ, Marcil A, Gauthier Y, Drouin J 1999 Ptx1 regulates SF-1 activity by an interaction that mimics the role of the ligand-binding domain. EMBO J 18:3431–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba P, Hjalt TA, Bernard DJ 28 March 2008 Novel forms of Paired-like homeodomain transcription factor 2 (PITX2): generation by alternative translation initiation and mRNA splicing. BMC Mol Biol 9:31 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.