Abstract
Objective
Little information about the disposition of individual antidepressant drugs during pregnancy has been published. We examined the dose requirements and level-to-dose (L/D) ratios of citalopram, escitalopram, and sertraline during pregnancy and after birth.
Method
Women aged from 32 to 43 years with major depressive disorder according to the Structured Clinical Interview for DSM-IV Axis I Disorders participated in the study. Doses were charted across each week of gestation and postpartum. Samples were collected at 20, 30, and 36 weeks’ gestation; delivery; and at 2 and 12 weeks postpartum. Plasma trough levels were obtained 8 to 15 hours after dose intake. Across pregnancy and postpartum, the mean dose-corrected plasma concentrations (L/D ratios) of S- and R-citalopram and S-sertraline, and the corresponding primary chiral metabolites S- and R-desmethylcitalopram and N-desmethylsertraline were assessed. The samples were analyzed for concentrations of stereospecific parent drug and metabolites. The study was conducted from 2003 to 2006.
Results
Three women received citalopram, 2 women were treated with escitalopram, and 6 women received sertraline. In 4 of 5 subjects who received citalopram or escitalopram and 5 of 6 subjects who received sertraline, the L/D ratios for the stereoisomers of the parent compound and primary metabolite decreased between 20 weeks gestation and delivery, which reflects increased drug metabolism. By 12 weeks postpartum the L/D ratios were similar to those detected at 20 weeks gestation.
Conclusions
Our cases illustrate that dose requirements frequently increase during the second half of pregnancy to offset increased drug turnover and maintain optimal pharmacotherapy. These findings replicate and extend earlier published data with other antidepressants.
Trial Registration
clinicaltrials.gov Identifier: NCT00279370
In the United States, 2.8% of pregnant women reported the use of a serotonin reuptake inhibitor (SRI) in the 3 months preconception and during gestation. With 4 million pregnancies that result in live births, more than 90,000 women will be exposed to an SRI yearly (National Birth Defects Prevention Study, 1997–20021). Despite these facts, few data about the dosing of antidepressant drugs across pregnancy and early postpartum have been published.2–5
The dose requirements of many drugs increase during pregnancy due to increased metabolism. Examples include the β blocker metoprolol and the tricyclic antidepressants, which are substrates of cytochrome 2D63,6–8; the calcium channel blocker nifedipine, a substrate of cytochrome 3A49,10; and the antiepileptic agent lamotrigine, which mainly undergoes glucuronidation.11,12 In contrast, the antimalarial drug proguanil accumulates in the second half of pregnancy as the result of diminished 2C19 enzymatic activity.11,13 The physiologic changes of pregnancy that explain altered dose requirements include steroid hormone effects on cytochrome activities14; plasma volume expansion; reduction in plasma protein (albumin) levels that leads to decreased drug binding; diminished hepatic blood flow15,16; and increased glomerular filtration rate and renal excretion.17,18 The increases in plasma volume and total body water, which contribute to weight gain across pregnancy, may increase the volume of distribution and thereby increase the dose requirements that are necessary to sustain therapeutic drug levels.19
The pharmacologic activity of citalopram arises mainly from the S-stereoisomers of the parent drug and the first metabolite desmethylcitalopram.20,21 Although the concentration of desmethylcitalopram reaches half that of the parent compound,20 the bioactivity of desmethylcitalopram is 10 times lower than the parent drug.21 The half-lives of the parent isomers are 35 hours (S-citalopram) and 47 hours (R-citalopram),22 and steady-state concentrations are reached within 1 week.23 The linear correlation between single and multiple dosing and steady-state concentrations suggests linear pharmacokinetics at the recommended dose range of 10 to 60 mg daily.23–25 Citalopram is metabolized via N-demethylation by cytochromes 2C19 (37%), 3A4 (34%), and 2D6 (28%) into desmethylcitalopram.20,26–28 Polymorphisms of cytochrome 2C19 can affect the metabolism of some drugs; however, measures of urinary metabolites indicated no phenotypic differences in the metabolism of citalopram.20,29 During pregnancy, the activity of cytochrome 2C19 decreases by up to 50%,13 whereas the activity of cytochromes 3A4 (the most abundant cytochrome in humans30) and 2D6 increases. 11,31
Sertraline is produced solely as the S-isomeric compound and N-demethylated into the primary metabolite N-desmethylsertraline. The parent drug sertraline contributes predominantly to the serotonergic effect.32 Linear pharmacokinetics are found when sertraline is prescribed at the recommended doses of 50 to 200 mg daily.32 The cytochromes P450 2D6, 2C9, 2B6, 2C19, and 3A4 metabolize sertraline and contribute 35%, 29%, 14%, 13%, and 9%, respectively, to the drug’s metabolism.33 Sertraline induces drug-drug interactions infrequently due to the contribution of multiple cytochrome P450 in the drug’s metabolism.33
We examined the disposition of the parent compounds and the primary metabolites of sertraline, escitalopram, and the stereoisomers of citalopram at 20, 30, and 36 weeks gestation; delivery; and 2 and 12 weeks postpartum. This longitudinal approach was advantageous because subjects act as their own controls. The comparison of changes within each subject is important because of the extensive interindividual variability in the pharmacokinetics of these drugs. For example, the coefficients of variation were 55% for the level-to-dose (L/D) ratios of citalopram and desmethylcitalopram,24 and the L/D ratios varied between subjects by 88- and 29-fold for sertraline and desmethylsertraline, respectively.34 We hypothesized that the metabolism of the stereoisomers of citalopram and sertraline would increase during pregnancy and decline after birth.
METHOD
The study protocol was approved and reviewed annually by the University of Pittsburgh Institutional Review Board. All subjects provided written informed consent.
Subjects
Eleven women who were enrolled in the National Institute of Mental Health–sponsored study Antidepressant Drug Use in Pregnancy (principal investigator, K.L.W.) received treatment with citalopram, escitalopram, or sertraline from their own prescribing physicians. They ranged from 32 to 43 years of age and were of white background with the exception of 1 African American woman who took sertraline. The diagnosis of major depressive disorder was confirmed with the Structured Clinical Interview for DSM-IV Axis I Disorders).35 Women with active alcohol or substance dependence or abuse (based on interview and urine drug screen) or those with medical illnesses that could affect outcomes (such as twin gestation, preexisting type I diabetes) were excluded. Symptom severity was defined with the Structured Interview Guide for the Hamilton Depression Rating Scale-Atypical Depression Symptoms Version (SIGH-ADS).36 The SIGH-ADS is a 29-item instrument that incorporates the Hamilton Rating Scale for Depression37 and a set of questions designed to assess the atypical neurovegetative symptoms of depression. Atypical symptoms characterize reproductive-related depression, acute onset nonseasonal and seasonal depression, and chronic forms of depression. The scale has high intraclass reliability.38,39
Procedure
The Timeline technique40 was adapted to chart psychiatric episodes; mood severity on the SIGH-ADS; exposure to citalopram, escitalopram, sertraline, and other drugs; cigarette smoking; total body weight; and body mass index (BMI) across time. Updates of these measures were plotted across time at 20, 30, and 36 weeks gestation; delivery; and at 2 and 12 weeks postpartum. The dose information was corroborated with the treating physician and/or pharmacy records for accuracy. Only subjects who received monotherapy or concurrent drugs that did not interfere with the metabolism of the antidepressant were included.
Laboratory Procedure
For women of reproductive age, the study drugs had the following expected mean ± SD half lives: 34.8 ± 4.3 hours for S-citalopram, 46.9 ± 10.6 hours for R-citalopram, 50.6 ± 17.7 hours for S-desmethylcitalopram, and 69.8 ± 18.8 hours for R-desmethylcitalopram22; the mean half lives for sertraline and desmethylsertraline were 32 and 71 to 200 hours, respectively.41 All subjects had been taking stable doses for 2 weeks or more prior to obtaining the plasma level. The peak concentrations of citalopram, escitalopram, and sertraline are, respectively, 4, 5, and 6 hours after drug intake.22,32 The steady-state drug concentrations, which were obtained at 8 to 15 hours after the previous dose, represented trough levels and were used for comparisons within each individual and among the subjects.
Plasma samples were analyzed for total chiral drug levels in the Clinical Pharmacology Program at Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center,4 in the laboratory of J.M.P. The analytic method for citalopram and escitalopram was adapted from that described by Rochat and colleagues42; the method of analysis for sertraline was described in Wisner et al.43 A Cyclobond I 2000 AC, 5μm, 25 cm × 4.6 mm (length × inside diameter) column (Astec, Inc.; Whippany, N.J.) was used. The most significant methodological changes were as follows. The extraction was shortened considerably by reextracting from the isoamyl alcohol/heptane layer into a small volume of 0.1 molar hydrochloric acid, which was dried in a centrifuge evaporator and reconstituted. The detection was changed to filter emission fluorescence spectroscopy with a deuterium source (excitation at 240 nm) and with the photomultiplier tube window as the cut-off filter (295 nm). The internal standard was S-propranolol, and the mobile phase was 10/90 volume to volume acetonitrile/(12 mL/L aqueous diethyl-amine adjusted to pH 5.3 with acetic acid). The limit of quantitated detection was 3.0 ng/mL. The day-to-day coefficients of variation were between 2.6% and 8.2% for the medium and high controls and between 5.2% and 10.0% for the low control.
The drug dose data and plasma chiral drug levels were used to calculate chiral L/D ratios. The L/D ratio is a measure of the drug plasma level corrected for the dose. Since the pharmacokinetics of sertraline, citalopram, and escitalopram are linear in adults, we may use the L/D ratios for comparisons at different dosages during therapeutic treatment.
RESULTS
Citalopram
Three women received citalopram at doses from 20 to 40 mg daily and 2 women received escitalopram from 10 to 20 mg daily (Table 1). None of these women smoked during pregnancy; subject 5 resumed smoking one half to 1 pack per day by the second postnatal week. Subject 1, who had mild depression at 20 weeks gestation (SIGH-ADS = 14), developed increased depressive symptoms at 30 weeks’ gestation (SIGH-ADS = 23). She responded to a citalopram dose increase from 40 to 50 mg daily, and her depression improved (SIGH-ADS = 7) by 36 weeks’ gestation. Subject 2 had moderate depression (SIGH-ADS = 18) with citalopram 20 mg daily at 20 weeks pregnancy. At 36 weeks’ gestation, her symptoms worsened (SIGH-ADS = 25). She increased the dose to citalopram 40 mg daily and by 12 weeks postpartum, she had responded fully (SIGH-ADS = 11). Subject 4 was euthymic throughout pregnancy and postpartum and remained on escitalopram therapy 10 mg daily; her L/D ratios were also stable. In contrast, subject 5 was euthymic at 20 weeks’ gestation (SIGH-ADS = 10) and developed moderate depression at 30 and 36 weeks’ pregnancy that persisted to 12 weeks postpartum (SIGH-ADS = 20, 21, and 19, respectively). She received the same dose of escitalopram (20 mg daily) through pregnancy and after delivery. Increased depression levels and dose requirements occurred in 3 mothers receiving citalopram or escitalopram. Two mothers who increased their dose responded with full restoration of euthymic mood. The mother who refused dose increases developed persistent depression that extended from pregnancy to postchildbirth.
Table 1.
Drug Dose and Plasma Levels in Pregnancy and Postpartum
| 20 Weeks
|
30 Weeks
|
36 Weeks
|
Delivery
|
Postpartum 2 Weeks |
Postpartum 4 to 6 Weeksa |
Postpartum 12 Weeks |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Dose, mg/d |
Plasma Level, ng/mL |
Dose, mg/d |
Plasma Level, ng/mL |
Dose, mg/d |
Plasma Level, ng/mL |
Dose, mg/d |
Plasma Level, ng/mL |
Dose, mg/d |
Plasma Level, ng/mL |
Dose, mg/d |
Plasma Level, ng/mL |
Dose, mg/d |
Plasma Level, ng/mL |
| 1 | ||||||||||||||
| S-citalopram | 40 | 32 | 40 | 40 | 50 | 25 | 50 | 15 | NA | NA | NA | NA | 50 | 18 |
| R-citalopram | 62 | 64 | 41 | 24 | NA | NA | NA | NA | 36 | |||||
| 2 | ||||||||||||||
| S-citalopram | 20 | 14 | 20 | 14 | 40 | 18 | NA | NA | 40 | 54 | NA | NA | 40 | 27 |
| R-citalopram | 24 | 24 | 36 | NA | NA | 101 | NA | NA | 75 | |||||
| 3b | ||||||||||||||
| S-citalopram | 30 | 19 | 30 | 9 | 20 | 5 | … | … | … | … | … | … | … | … |
| R-citalopram | 41 | 22 | 14 | … | … | … | … | … | … | … | … | |||
| 4 | ||||||||||||||
| S-citalopram | 10 | 17 | 10 | 10 | 10 | 13 | 10 | 14 | 10 | 24 | NA | NA | NA | NA |
| 5 | ||||||||||||||
| S-citalopram | 20 | 58 | 20 | 70 | 20 | 67 | NA | NA | 20 | 95 | NA | NA | 20 | 63 |
| 6 | ||||||||||||||
| S-sertraline | NA | NA | 50 | 27 | NA | NA | 50 | 11 | NA | NA | 50 | 27 | 50 | 21 |
| N-desmethylsertraline | NA | 44 | NA | 26 | NA | 52 | 50 | |||||||
| 7 | ||||||||||||||
| S-sertraline | 50 | 10 | 50 | 20 | 100 | 16 | 50 | 7 | 50 | 22 | 50 | 15 | NA | NA |
| N-desmethylsertraline | 18 | 40 | 66 | 32 | 41 | 29 | NA | |||||||
| 8 | ||||||||||||||
| S-sertraline | 50 | 4 | 75 | 10 | 100 | 39 | NA | NA | 200 | 21 | NA | NA | 200 | 21 |
| N-desmethylsertraline | 16 | 17 | 74 | NA | 59 | NA | 29 | |||||||
| 9 | ||||||||||||||
| S-sertraline | NA | NA | 150 | 17 | 200 | 43 | 200 | 32 | 200 | 39 | NA | NA | NA | NA |
| N-desmethylsertraline | NA | 141 | 147 | 120 | 149 | NA | NA | |||||||
| 10 | ||||||||||||||
| S-sertraline | 100 | 93 | 100 | 45 | NA | NA | 100 | 28 | NA | NA | 100 | 62 | NA | NA |
| N-desmethylsertraline | 72 | 92 | NA | 59 | NA | 74 | NA | |||||||
| 11 | ||||||||||||||
| S-sertraline | 50 | 32 | 75 | 18 | 75 | 23 | 100 | 32 | NA | NA | 100 | 83 | 125 | 99 |
| N-desmethylsertraline | 52 | 77 | 73 | 97 | NA | 128 | 183 | |||||||
Values were available at 4 to 6 weeks postpartum for breast-feeding mother-infant pairs only.
Subject discontinued medication at delivery.
Abbreviation: NA = not available.
Sertraline
Six women received sertraline at 50 to 200 mg daily (Table 1). Subject 9 smoked 1 pack daily during pregnancy and postpartum. Increased depressive symptoms and dose requirements across pregnancy were evident in 3 mothers who received sertraline. Subject 8 developed increased depression levels at 30 and 36 weeks gestation (SIGH-ADS = 24 and 19, respectively) and required gradual dose elevations from 75 mg daily to a final dose of 200 mg daily. By 2 weeks postpartum, she had mild symptoms (SIGH-ADS = 13). Subject 9 developed moderate depression at 30 weeks gestation (SIGH-ADS = 21) with sertraline 150 mg daily. Following a dose increase to 200 mg, her symptoms improved and she remained euthymic at 36 weeks’ gestation and 2 weeks postpartum (SIGH-ADS = 14 and 12, respectively). Subject 11 had depressive symptoms (SIGH-ADS = 24) with sertraline 50 mg daily at 20 weeks gestation. With a dose increase to 75 mg daily, her mood improved (SIGH-ADS = 14) by 30 weeks gestation.
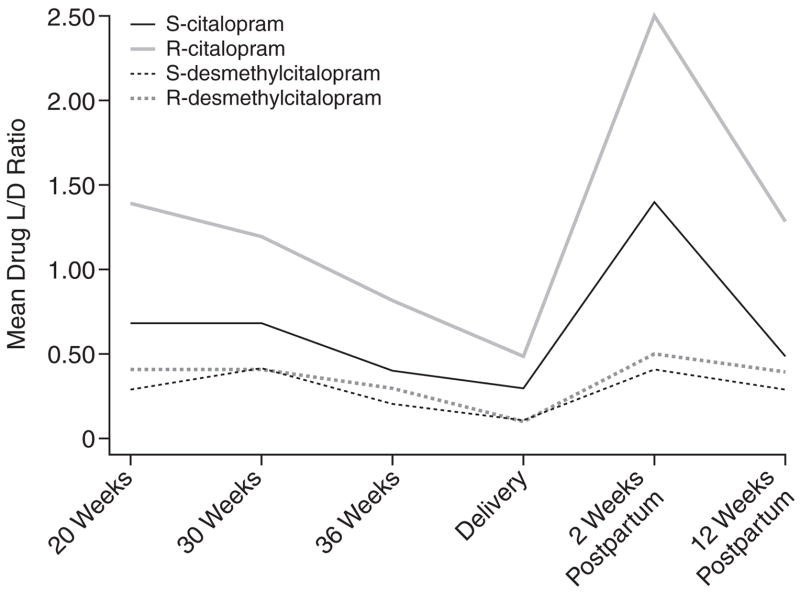
The chiral L/D ratios of citalopram, escitalopram, and sertraline decreased in the second half of pregnancy (Table 2, Figures 1–3). By the 12th postpartum week, the values were similar to those detected at 20 weeks’ gestation. Reductions in L/D ratios corresponded with increased severity of depression or minimal symptomatic improvement. The antenatal and postnatal changes in the chiral L/D ratios persisted after adjustments for total body weight (kg) and BMI (kg/m2) were made to address intra-individual variations24 and pregnancy-related changes in drug processing.19,44,45 Therefore, we report the unadjusted L/D values. The drug levels and L/D ratios were consistent with those observed in age-matched women in the general population.32,42,46–48
Table 2.
Mean ± SD (Range) of Drug Level-to-Dose Ratiosa
| Drug | 20 weeks | 30 weeks | 36 weeks | Delivery | Postpartum 2 weeks | Postpartum 4 to 6 weeks | Postpartum 12 weeks |
|---|---|---|---|---|---|---|---|
| Citalopram | |||||||
| S-citalopram | 0.7 ± 0.1 (0.6–0.8) | 0.7 ± 0.4 (0.3–1.0) | 0.4 ± 0.1 (0.3–0.5) | 0.3 | 1.4 | NA | 0.5 ± 0.2 (0.4–0.7) |
| R-citalopram | 1.4 ± 0.2 (1.2–1.5) | 1.2 ± 0.4 (0.7–1.6) | 0.8 ± 0.1 (0.7–0.9) | 0.5 | 2.5 | NA | 1.3 ± 0.8 (0.7–1.8) |
| S-desmethylcitalopram | 0.3 ± 0.1 (0.3–0.4) | 0.4 ± 0.2 (0.2–0.5) | 0.2 ± 0.02 (0.21–0.24) | 0.1 | 0.4 | NA | 0.3 ± 0.1 (0.2–0.3) |
| R-desmethylcitalopram | 0.4 ± 0.1 (0.3–0.5) | 0.4 ± 0.1 (0.2–0.5) | 0.3 ± 0.03 (0.2–0.3) | 0.1 | 0.5 | NA | 0.4 ± 0.2 (0.2–0.5) |
| Escitlopram | |||||||
| S-citalopram | 2.3 ± 0.8 | 2.2 ± 1.8 | 2.3 ± 1.5 | 1.4 | 3.6 ± 1.6 | NA | 3.2 |
| S-desmethylcitalopram | 0.5 ± 0.2 | 0.2 ± 0.1 | 0.5 ± 0.01 | 0.3 | 0.4 ± 0.1 | NA | 0.3 |
| Sertaline | |||||||
| S-Sertaline | 0.5 ± 0.4 (0.07–0.9) | 0.3 ± 0.2 (0.1–0.5) | 0.3 ± 0.1 (0.2–0.4) | 0.2 ± 0.1 (0.1–0.3) | 0.2 ± 0.2 (0.1–0.4) | 0.6 ± 0.2 (0.3–0.8) | 0.4 ± 0.3 (0.1–0.8) |
| N-demethylsertaline | 0.6 ± 0.3 (0.3–1.1) | 0.8 ± 0.3 (0.2–1.0) | 0.8 ± 0.1 (0.7–1.0) | 0.7 ± 0.2 (0.5–1.0) | 0.6 ± 0.3 (0.3–0.8) | 0.9 ± 0.3 (0.6–1.3) | 0.9 ± 0.7 (0.1–1.5) |
The level-to-dose raito is a measure of the drug plasma level corrected for the dose.
Abbreviation: NA = not available.
Figure 1.
Mean Drug Level-to-Dose (L/D) Ratios Across Childbearing for Citalopram-Treated Women
Figure 3.
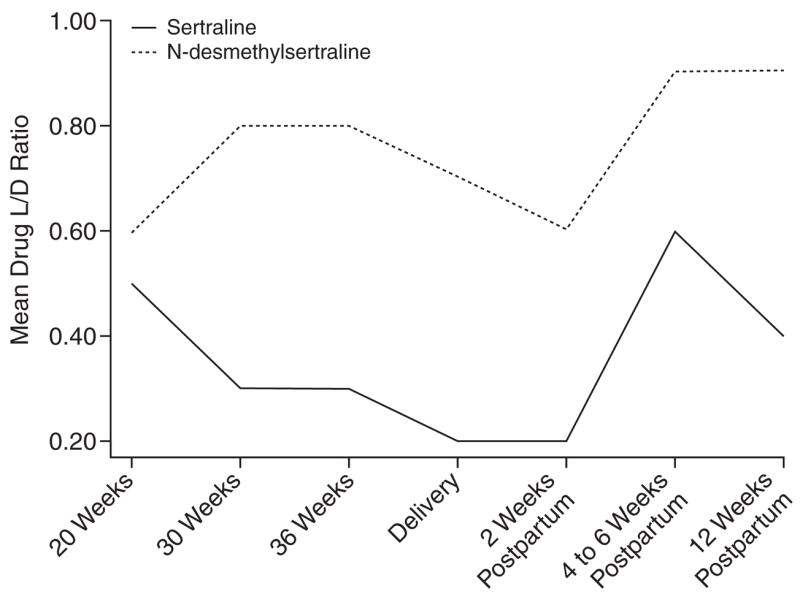
Mean Drug Level-to-Dose (L/D) Ratios Across Childbearing for Sertraline-Treated Women
DISCUSSION
Our data indicate that antidepressant drug disposition changed across pregnancy. In 4 of 5 subjects who received citalopram or escitalopram and 5 of 6 subjects who received sertraline, the L/D ratios for the stereoisomers of the parent compound and primary metabolite decreased between 20 weeks’ gestation and delivery, which reflects increased drug metabolism. The dose requirements of citalopram, escitalopram, and sertraline increased in the second half of gestation. These findings replicated reports that pregnant women who received fluoxetine, paroxetine, or sertraline required dose increases by 24 to 28 weeks’ gestation to alleviate recurrent depression.5,49 Our observations suggested that the decline in L/D ratios often corresponded with lowered drug efficacy. These findings were consistent with the induction of cytochrome 3A4 and 2D6 enzymes during pregnancy31,50 that overrides the pregnancy-induced inhibition of 2C19 activity.51 Heikkinen et al.50 detected diminished plasma citalopram concentrations during pregnancy without increased depressive symptoms. Our study adds a systematic assessment of mood symptoms in addition to plasma drug levels.
The volume of distribution (Vd) represents the ratio of the total quantity of drug in the body to the total concentration of drug in the plasma.45 The Vd of individual drugs may change across the gestational period. During pregnancy, reduction in plasma proteins may result in an increased distribution of unbound drug.19 In a review of 23 articles, 9 studies (39%) suggested increased Vd; 11 (48%) did not indicate change in Vd; and 3 (12%) suggested reduction in Vd.11,19 The complexity of estimating the hypothetical Vd values could explain the lack of reporting.45 Without direct measures of Vd, total body weight is an appropriate actual descriptor for drug distribution.44 After adjusting for total body weight and BMI, the L/D ratios of the isomers of citalopram, S-sertraline, and the primary metabolites did not vary from the unadjusted values. Thus, the changes in Vd likely contributed minimally to the decline in L/D ratios across pregnancy.
The dramatic rise in chiral L/D ratios at 2 weeks postpartum reflects a brief postpartum refractory metabolic state (Table 2, Figures 1–3). Other investigators have provided evidence of a postpartum drop in drug metabolic capacity for citalopram,50 nortriptyline (a substrate of cytochrome 2D6),4 and nelfinavir (substrates of 3A4 and 2C19).51 Although some researchers have not detected variable biotransformation of citalopram based on genetic polymorphisms of the 2C19 isoenzyme,20,29 significant reduction in N-demethylation has been described in poor metabolizers.52–54 The frequency of poor metabolism differs across racial groups; for example, 2% of the white compared to 15% to 20% of the Asian populations are homozygous for the alleles that encode deficient 2C19 activity.55 The individuals who received citalopram or escitalopram were white. The range of plasma concentration and L/D ratios was uniform and indicated no poor metabolizers in the group. The induction of 3A4 and 2D6 enzyme activity could mask impairment in 2C19 activity during pregnancy.55 On the other hand, the refractory period in the first 6 postnatal weeks could result in elevated plasma drug concentrations and increased side effects in selected women with the mutated alleles.
Preclinical data suggest that the R-isomer of citalopram could antagonize the antidepressant effects of S-citalopram.56–61 We found that changes in depression severity and dose requirements across pregnancy and the postnatal period were similar in mothers who received citalopram (subjects 1–3) and escitalopram (subjects 4 and 5). To establish gestational differences in antidepressant responses between citalopram and escitalopram, we require additional subjects.
The optimal management of depression across pregnancy is to quickly identify recurrent depressive symptoms3 through a systematic exploration of symptom levels at each trimester or ideally at each gestational month in the latter half of pregnancy. If symptoms worsen or recur, a dose increase should be considered. In office settings, depression severity could be monitored with the following instruments: (1) the Center for Epidemiological Studies-Depression Scale, which is a 20-item screening tool that is highly sensitive for detecting depression in women during pregnancy and postpartum (score of ≥ 16 indicates depression)62,63; (2) the Montgomery-Asberg Depression Rating Scale,64 which is a brief 10-item clinician-rated scale that is highly sensitive to drug-related changes in depression severity; or (3) the Clinical Global Impressions scale,65 which is a clinical rating on a single item, 7-point scale (1 = normal, not ill; 7 = very severely ill) to assess depression severity, change across time, and overall efficacy that takes into account both clinical improvement and severity of side effects.
After delivery, the antidepressant should be restarted immediately at the nonpregnant dose of response or two thirds of the final dose in pregnancy.4 Since hepatic metabolism undergoes a period of refractory activity in the first several weeks postdelivery as suggested by our data and others,4,50,51 side effects should be monitored for indication of elevated plasma levels. By 12 weeks post-delivery, women may require a dose increase as the prepregnancy rate of metabolism is restored. Our cases represent the steady-state L/D ratios in a small sample of women. Therefore, replication studies to investigate the antidepressant drug L/D ratios and efficacy across pregnancy and early postpartum are essential to inform physicians and patients of the possible changes in dose requirements across the gestational period.
Figure 2.
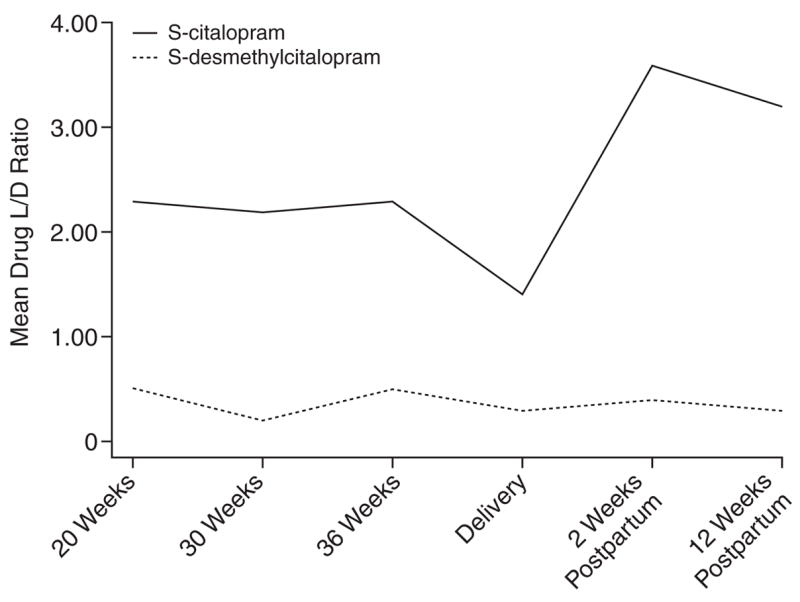
Mean Drug Level-to-Dose (L/D) Ratios Across Childbearing for Escitalopram-Treated Women
Acknowledgments
Dr. Wisner has received research/grant support from the National Institute of Mental Health, Stanley Medical Research Foundation, New York–Mid-Atlantic Consortium for Genetics and Newborn Screening Services, State of Pennsylvania, American Society for Bariatric Surgery, Pfizer, and Wyeth and serves on the speakers bureau of GlaxoSmithKline. Drs. Sit and Perel and Mr. Helsel report no additional financial or other relationships relevant to the subject of this article.
Funding for this article was provided by National Institute of Mental Health grant R01 MH60335 for the study Antidepressant Drug Use in Pregnancy and by Junior Faculty Scholars R25 Program 5 R25 MH060473-08.
Drug names
- Celexa and others
citalopram
- Lexapro and others
escitalopram
- Prozac and others
fluoxetine
- Lamictal and others
lamotrigine
- Viracept
nelfinavir
- Procardia
Adalat, and others, nifedipine
- Pamelor
Aventyl, and others, nortriptyline
- Paxil
Pexeva, and others, paroxetine
- Inderide
Innopran, and others, propranolol
- Zoloft and others
sertraline
Footnotes
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Women’s Mental Health section. Please contact Marlene Freeman, M.D., at mfreeman@psychiatrist.com.
References
- 1.Yoon PW, Rasmussen SA, Lynberg MC, et al. The National Birth Defects Prevention Study. Public Health Rep. 2001;116(suppl 1):32–40. doi: 10.1093/phr/116.S1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Food and Drug Administration. Pharmacokinetics in Pregnancy: Study Design, Data Analysis, and Impact on Dosing and Labeling. US Dept Health Human Services; 2004. October Issue, Guidance for Industry; pp. 1–14. [Google Scholar]
- 3.Wisner KL, Perel JM, Wheeler SB. Tricyclic dose requirements across pregnancy. Am J Psychiatry. 1993;150:1541–1542. doi: 10.1176/ajp.150.10.1541. [DOI] [PubMed] [Google Scholar]
- 4.Wisner KL, Perel JM, Peindl KS, et al. Effects of the postpartum period on nortriptyline pharmacokinetics. Psychopharmacol Bull. 1997;33:243–248. [PubMed] [Google Scholar]
- 5.Hostetter A, Stowe ZN, Strader JR, Jr, et al. Dose of selective serotonin uptake inhibitors across pregnancy: clinical implications. Depress Anxiety. 2000;11:51–57. [PubMed] [Google Scholar]
- 6.Wadelius M, Darj E, Frenne G, et al. Induction of CYP2D6 in pregnancy. Clin Pharmacol Ther. 1997;62:400–407. doi: 10.1016/S0009-9236(97)90118-1. [DOI] [PubMed] [Google Scholar]
- 7.Hogstedt S, Lindberg B, Peng DR, et al. Pregnancy-induced increase in metoprolol metabolism. Clin Pharmacol Ther. 1985;37:688–692. doi: 10.1038/clpt.1985.114. [DOI] [PubMed] [Google Scholar]
- 8.Zanger UM, Raimundo S, Eichelbaum M. Cytochrome P450 2D6: overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:23–37. doi: 10.1007/s00210-003-0832-2. [DOI] [PubMed] [Google Scholar]
- 9.Prevost RR, Akl SA, Whybrew WD, et al. Oral nifedipine pharmacokinetics in pregnancy-induced hypertension. Pharmacotherapy. 1992;12:174–177. [PubMed] [Google Scholar]
- 10.Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 11.Johannessen SI, Tomson T. Pharmacokinetic variability of newer antiepileptic drugs: when is monitoring needed? Clin Pharmacokinet. 2006;45:1061–1075. doi: 10.2165/00003088-200645110-00002. [DOI] [PubMed] [Google Scholar]
- 12.Pennell PB. Antiepileptic drug pharmacokinetics during pregnancy and lactation. Neurology. 2003;61:S35–S42. doi: 10.1212/wnl.61.6_suppl_2.s35. [DOI] [PubMed] [Google Scholar]
- 13.McGready R, Stepniewska K, Seaton E, et al. Pregnancy and use of oral contraceptives reduces the biotransformation of proguanil to cycloguanil. Eur J Clin Pharmacol. 2003;59:553–557. doi: 10.1007/s00228-003-0651-x. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka E. Gender-related differences in pharmacokinetics and their clinical significance. J Clin Pharm Ther. 1999;24:339–346. doi: 10.1046/j.1365-2710.1999.00246.x. [DOI] [PubMed] [Google Scholar]
- 15.Stika C, Frederiksen M. Drug therapy in pregnant and nursing women. In: Atkinson AJ Jr, Daniels CE, Dedrick RL, et al., editors. Principles of Clinical Pharmacology. New York, NY: Academic Press; 2001. pp. 277–291. [Google Scholar]
- 16.Kalra S, Born L, Sarkar M, et al. The safety of antidepressant use in pregnancy. Expert Opin Drug Saf. 2005;4:273–284. doi: 10.1517/14740338.4.2.273. [DOI] [PubMed] [Google Scholar]
- 17.Keller F, Griesshammer M, Haussler U, et al. Pregnancy and renal failure: the case for application of dosage guidelines. Drugs. 2001;61:1901–1920. doi: 10.2165/00003495-200161130-00003. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein M. Lithium Treatment of Women During Pregnancy and in the Post-Delivery Period. Lancaster, England: MTP Press; 1980. [Google Scholar]
- 19.Little B. Pharmacokinetics during pregnancy: evidence-based maternal dose formulation. Obstet Gynecol. 1999;93:858–868. doi: 10.1016/s0029-7844(98)00444-x. [DOI] [PubMed] [Google Scholar]
- 20.von Moltke LL, Greenblatt DJ, Giancarlo GM, et al. Escitalopram (S-citalopram) and its metabolites in vitro: cytochromes mediating biotransformation, inhibitory effects, and comparison to R-citalopram. Drug Metab Dispos. 2001;29:1102–1109. [PubMed] [Google Scholar]
- 21.Hyttel J, Bogeso KP, Perregaard J, et al. The pharmacological effect of citalopram residues in the (S)-(+)-enantiomer. J Neural Transm Gen Sect. 1992;88:157–160. doi: 10.1007/BF01244820. [DOI] [PubMed] [Google Scholar]
- 22.Sidhu J, Priskorn M, Poulsen M, et al. Steady-state pharmacokinetics of the enantiomers of citalopram and its metabolites in humans. Chirality. 1997;9:686–692. doi: 10.1002/(SICI)1520-636X(1997)9:7<686::AID-CHIR9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Baumann P, Larsen F. The pharmacokinetics of citalopram. Rev Contemp Pharmacother. 1995;6:287–295. [Google Scholar]
- 24.Reis M, Lundmark J, Bengtsson F. Therapeutic drug monitoring of racemic citalopram: a 5-year experience in Sweden, 1992–1997. Ther Drug Monit. 2003;25:183–191. doi: 10.1097/00007691-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Bezchlibnyk-Butler K, Aleksic I, Kennedy SH. Citalopram—a review of pharmacological and clinical effects. J Psychiatry Neurosci. 2000;25:241–254. [PMC free article] [PubMed] [Google Scholar]
- 26.Rochat B, Amey M, Gillet M, et al. Identification of three cytochrome P450 isozymes involved in N-demethylation of citalopram enantiomers in human liver microsomes. Pharmacogenetics. 1997;7:1–10. doi: 10.1097/00008571-199702000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez C, Bergqvist PB, Brennum LT, et al. Escitalopram, the S-(+)-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with potent effects in animal models predictive of antidepressant and anxiolytic activities. Psychopharmacology. 2003;167:353–362. doi: 10.1007/s00213-002-1364-z. [DOI] [PubMed] [Google Scholar]
- 28.Hyttel J. Citalopram—pharmacological profile of a specific serotonin uptake inhibitor with antidepressant activity. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6:277–295. doi: 10.1016/s0278-5846(82)80179-6. [DOI] [PubMed] [Google Scholar]
- 29.Herrlin K, Yasui-Furukori N, Tybring G, et al. Metabolism of citalopram enantiomers in CYP2C19/CYP2D6 phenotyped panels of healthy Swedes. Br J Clin Pharmacol. 2003;56:415–421. doi: 10.1046/j.1365-2125.2003.01874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimada T, Yamazaki H, Mimura M, et al. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- 31.Tracy TS, Venkataramanan R, Glover DD, et al. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A activity) during pregnancy. Am J Obstet Gynecol. 2005;192:633–639. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 32.Ronfeld RA, Tremaine LM, Wilner KD. Pharmacokinetics of sertraline and its N-demethyl metabolite in elderly and young male and female volunteers. Clin Pharmacokinet. 1997;32(suppl 1):22–30. doi: 10.2165/00003088-199700321-00004. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi K, Ishizuka T, Shimada N, et al. Sertraline N-demethylation is catalyzed by multiple isoforms of human cytochrome P-450 in vitro. Drug Metab Dispos. 1999;27:763–766. [PubMed] [Google Scholar]
- 34.Lundmark J, Reis M, Bengtsson F. Therapeutic drug monitoring of sertraline: variability factors as displayed in a clinical setting. Ther Drug Monit. 2000;22:446–454. doi: 10.1097/00007691-200008000-00014. [DOI] [PubMed] [Google Scholar]
- 35.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 36.Williams JBW, Terman M. Structured Interview Guide for the Hamilton Depression Rating Scale With Atypical Depression Supplement (SIGH-ADS) New York, NY: New York State Psychiatric Institute; 2003. [Google Scholar]
- 37.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams JBW, Link MJ, Rosenthal NE, et al. Structured Interview Guide for the Hamilton Depression Scale–Seasonal Affective Disorder Version (SIGH-SAD) Revised. New York, NY: New York State Psychiatric Institute; 2002. [Google Scholar]
- 39.Terman M, Terman JS, Williams JBW. Seasonal affective disorder and its treatments. J Pract Psychiatry Behav Health. 1998;5:287–303. [Google Scholar]
- 40.Post RM, Roy-Byrne PP, Uhde TW. Graphic representation of the life course of illness in patients with affective disorder. Am J Psychiatry. 1988;145:844–848. doi: 10.1176/ajp.145.7.844. [DOI] [PubMed] [Google Scholar]
- 41.Warrington SJ. Clinical implications of the pharmacology of sertraline. Int Clin Psychopharmacol. 1991;6(suppl 2):11–21. doi: 10.1097/00004850-199112002-00004. [DOI] [PubMed] [Google Scholar]
- 42.Rochat B, Amey M, Baumann P. Analysis of enantiomers of citalopram and its demethylated metabolites in plasma of depressive patients using chiral reverse-phase liquid chromatography. Ther Drug Monit. 1995;17:273–279. doi: 10.1097/00007691-199506000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;26:353–360. doi: 10.1097/01.jcp.0000227706.56870.dd. [DOI] [PubMed] [Google Scholar]
- 44.Green B, Duffull SB. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol. 2004;58:119–133. doi: 10.1111/j.1365-2125.2004.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berezhkovskiy LM. Determination of volume of distribution at steady state with complete consideration of the kinetics of protein and tissue binding in linear pharmacokinetics. J Pharm Sci. 2004;93:364–374. doi: 10.1002/jps.10539. [DOI] [PubMed] [Google Scholar]
- 46.Leinonen E, Lepola U, Koponen H, et al. The effect of age and concomitant treatment with other psychoactive drugs on serum concentrations of citalopram measured with a nonenantioselective method. Ther Drug Monit. 1996;18:111–117. doi: 10.1097/00007691-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Nikisch G, Mathe AA, Czernik A, et al. Stereoselective metabolism of citalopram in plasma and cerebrospinal fluid of depressive patients: relationship with 5-HIAA in CSF and clinical response. J Clin Psychopharmacol. 2004;24:283–290. doi: 10.1097/01.jcp.0000125680.89843.a6. [DOI] [PubMed] [Google Scholar]
- 48.Bondolfi G, Chautems C, Rochat B, et al. Non-response to citalopram in depressive patients: pharmacokinetic and clinical consequences of a fluvoxamine augmentation. Psychopharmacology. 1996;128:421–425. doi: 10.1007/s002130050152. [DOI] [PubMed] [Google Scholar]
- 49.Suri R, Altshuler L, Hellemann G, et al. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry. 2007;164:1206–1213. doi: 10.1176/appi.ajp.2007.06071172. [DOI] [PubMed] [Google Scholar]
- 50.Heikkinen T, Ekblad U, Kero P, et al. Citalopram in pregnancy and lactation. Clin Pharmacol Ther. 2002;72:184–191. doi: 10.1067/mcp.2002.126181. [DOI] [PubMed] [Google Scholar]
- 51.van Heeswijk RP, Khaliq Y, Gallicano KD, et al. The pharmacokinetics of nelfinavir and M8 during pregnancy and post partum. Clin Pharmacol Ther. 2004;76:588–597. doi: 10.1016/j.clpt.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Rudberg I, Hendset M, Uthus LH, et al. Heterozygous mutation in CYP2C19 significantly increases the concentration/dose ratio of racemic citalopram and escitalopram (S-citalopram) Ther Drug Monit. 2006;28:102–105. doi: 10.1097/01.ftd.0000189899.23931.76. [DOI] [PubMed] [Google Scholar]
- 53.Yu BN, Chen GL, He N, et al. Pharmacokinetics of citalopram in relation to genetic polymorphism of CYP2C19. Drug Metab Dispos. 2003;31:1255–1259. doi: 10.1124/dmd.31.10.1255. [DOI] [PubMed] [Google Scholar]
- 54.Sindrup SH, Brosen K, Hansen MG, et al. Pharmacokinetics of citalopram in relation to the sparteine and the mephenytoin oxidation polymorphisms. Ther Drug Monit. 1993;15:11–17. doi: 10.1097/00007691-199302000-00002. [DOI] [PubMed] [Google Scholar]
- 55.Desta Z, Zhao X, Shin JG, et al. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet. 2002;41:913–958. doi: 10.2165/00003088-200241120-00002. [DOI] [PubMed] [Google Scholar]
- 56.Sánchez C. R-citalopram attenuates anxiolytic effects of escitalopram in a rat ultrasonic vocalisation model. Eur J Pharmacol. 2003;464:155–158. doi: 10.1016/s0014-2999(03)01376-1. [DOI] [PubMed] [Google Scholar]
- 57.Mørk A, Kreilgaard M, Sánchez C. The R-enantiomer of citalopram counteracts escitalopram-induced increase in extracellular 5-HT in the frontal cortex of freely moving rats. Neuropharmacology. 2003;45:167–173. doi: 10.1016/s0028-3908(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 58.Stórustovu S, Sánchez C, Pörzgen P, et al. R-citalopram functionally antagonises escitalopram in vivo and in vitro: evidence for kinetic interaction at the serotonin transporter. Br J Pharmacol. 2004;142:172–180. doi: 10.1038/sj.bjp.0705738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El Mansari M, Sánchez C, Chouvet G, et al. Effects of acute and long-term administration of escitalopram and citalopram on serotonin neurotransmission: an in vivo electrophysiological study in rat brain. Neuropsychopharmacology. 2005;30:1269–1277. doi: 10.1038/sj.npp.1300686. [DOI] [PubMed] [Google Scholar]
- 60.Mnie-Filali O, El Mansari M, Espana A, et al. Allosteric modulation of the effects of the 5-HT reuptake inhibitor escitalopram on the rat hippocampal synaptic plasticity. Neurosci Lett. 2006;395:23–27. doi: 10.1016/j.neulet.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 61.El Mansari M, Wiborg O, Mnie-Fifali O, et al. Allosteric modulation of the effect of escitalopram, paroxetine and fluoxetine: in-vitro and in-vivo studies. Int J Neuropsychopharmacol. 2007;10:31–40. doi: 10.1017/S1461145705006462. [DOI] [PubMed] [Google Scholar]
- 62.Beeghly M, Weinberg MK, Olson KL, et al. Stability and change in level of maternal depressive symptomatology during the first postpartum year. J Affect Disord. 2002;71:169–180. doi: 10.1016/s0165-0327(01)00409-8. [DOI] [PubMed] [Google Scholar]
- 63.Beeghly M, Olson KL, Weinberg MK, et al. Prevalence, stability, and socio-demographic correlates of depressive symptoms in Black mothers during the first 18 months postpartum. Matern Child Health J. 2003;7:157–168. doi: 10.1023/a:1025132320321. [DOI] [PubMed] [Google Scholar]
- 64.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 65.Guy W. US Dept Health, Education, and Welfare Publication (ADM) Rockville, Md: National Institute of Mental Health; 1976. ECDEU Assessment Manual for Psychopharmacology; pp. 76–338.pp. 218–222. [Google Scholar]