Abstract
Mitochondrial heat shock protein 70 (mtHsp70/Hsp75/Grp75/mortalin/TRAP-1/PBP74) is an essential mitochondrial chaperone and a member of the heat shock protein 70 (HSP70) family. Although many studies have shown the protective properties of overexpression of the cytosolic inducible member of the HSP70 family, Hsp72, few studies have investigated the protective potential of Hsp75 against ischemic injury. Mitochondria are one of the primary targets of ischemic injury in astrocytes. In this study, we analyzed the effects of Hsp75 overexpression on cellular levels of reactive oxygen species (ROS), mitochondrial membrane potential, ATP levels, and viability during the ischemia-like conditions of oxygen–glucose deprivation (OGD) or glucose deprivation (GD) in primary astrocytic cultures. We show that Hsp75 overexpression decreases ROS production and preserves mitochondrial membrane potential during GD, and preserves ATP levels and cell viability during OGD. These findings indicate that Hsp75 can provide protection against ischemia-like in vitro injury and suggest that it should be further studied as a potential candidate for protection against ischemic injury.
Keywords: astrocyte, mitochondria, ischemia, oxidative stress, heat shock protein, mortalin
Introduction
Astrocytes are the most numerous cell type in the brain and they play a critical role in synapse formation, function and plasticity, maintenance of the extracellular milieu, and in the establishment and maintenance of the blood–brain barrier. Proper astrocyte function is important for synaptic transmission, metabolic and ionic homeostasis, anti-oxidant defense, and trophic support of neurons (Kettenmann and Ransom, 2005). Astrocytic functions are particularly important in maintaining neuronal viability under ischemic conditions where energy depletion and metabolic disruption are severe (Lo et al, 2003). Astrocytic impairment or loss can result in neuronal death or dysfunction (Chen and Swanson, 2003; Ouyang et al, 2007). Thus, understanding the response of astrocytes to stress and possible mechanisms of astrocyte protection represents a crucial focus of studies of brain ischemic injury.
Mitochondria are one of the primary targets of ischemic injury in astrocytes. Several interrelated factors—increase in cytoplasmic-free Ca2+, exposure to reactive oxygen species (ROS) and nitric oxide, and ischemia-associated acidosis—have been shown to contribute to mitochondrial impairment during ischemia (Bambrick et al, 2004). Astrocytes derive a substantial amount of ATP from mitochondrial production (Hertz et al, 2007). Because many astrocyte functions, such as ion and neurotransmitter transport, are energy demanding (Dienel and Hertz, 2005), mitochondrial dysfunction can impair the ability of astrocytes to carry out neuroprotective functions. Mitochondrial impairment during stress is associated with the generation of ROS and release of regulatory and signaling molecules from the intermembrane space, which contribute to both apoptotic and necrotic cell death (Fiskum, 2000).
Although multiple studies have been performed on the cytosolic inducible member of the heat shock protein 70 (HSP70) family, Hsp72 (Giffard and Yenari, 2004), to date few studies have investigated the protective potential of Hsp75, another member of HSP70 family. Unlike some members of the HSP70 family, Hsp75 is not heat-inducible, but is upregulated by glucose deprivation (GD), oxidative injury, and ultraviolet A irradiation (Carette et al, 2002; Hadari et al, 1997; Lee, 2001). Studies have indicated that Hsp75 upregulation suppresses apoptosis induced by arsenite in lung epithelial cells (Lau et al, 2004), mercury in renal cells (Stacchiotti et al, 2006), and glucose starvation and ischemia reperfusion in lung cells (Gao et al, 2003). Hsp75 overexpression has been shown to delay apoptotic response to serum deprivation in muscle cells (Taurin et al, 2002), and decrease oxidative stress during GD in a neuronal cell line (Liu et al, 2005). Hsp75 is an important molecular chaperone in mitochondria, which is a crucial part of the mitochondrial protein import machinery (Krimmer et al, 2000). Studies indicate other mitochondrial proteins, including voltage-dependent anion-selective channel, NADH dehydrogenase, and Hsp60 (Bhattacharyya et al, 1995; Schwarzer et al, 2002) as binding partners of Hsp75, thus suggesting its involvement in multiple mitochondrial functions.
The purpose of this study was to investigate possible protective effects of Hsp75 overexpression on the changes in mitochondrial function, ATP levels, oxidative stress, and viability induced by ischemia-like conditions in mouse astrocytes in primary culture.
Materials and methods
Astrocyte Culture
Primary astrocyte cultures were prepared as described previously (Dugan et al, 1995) in accordance with a protocol approved by the Stanford University Animal Care and Use Committee. Briefly, primary astrocyte cultures were prepared from postnatal (days 1 to 3) Swiss Webster mice (Simonsen, Gilroy, CA, USA). The cortices were dissected and treated with 0.05% trypsin for 15 mins, mechanically dissociated, and suspended in plating medium consisting of MEM (minimal essential medium) (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (Hyclone, Logan, UT, USA), 10% equine serum (Hyclone), 2 mmol/L glutamine, and 10 ng/mL epidermal growth factor (Sigma, St Louis, MO, USA). They were plated as a single-cell suspension at a density of two hemispheres per 24-well plate. Cultures were maintained at 37°C in a 5% CO2 incubator. After 3 days, half the medium was removed and replaced with astrocyte growth medium (MEM supplemented with 10% equine serum and 2 mmol/L glutamine) and this was repeated every 3 days. Experiments were performed after 21 to 40 days in culture. We observed previously that astrocyte vulnerability to injury increased until approximately 21 to 25 days in culture (Papadopoulos et al, 1998), and was then relatively stable through 60 days in culture (Xu et al, 2004).
Viral Construct and Production
The human Hsp75 coding sequence (GenBank L15189) in pBluescript (Bhattacharyya et al, 1995) was a generous gift from R Morimoto. The DNA was cut with SalI, the overhang was blunted using T4 DNA polymerase, and a fragment excised with BamHI, yielding the entire Hsp75 coding sequence plus 692 bases of 3′-untranslated region. We cloned this into HpaI–BamHI cut LXSN vector (Miller and Rosman, 1989) to create LXSN-Hsp75. To produce virus, LXSN-Hsp75 or control empty vector LXSN DNA was transfected into the Ψ-2 ecotropic packaging cell line (Mann et al, 1983), using FuGENE-6 (Roche, Pleasanton, CA, USA). Culture supernatants were harvested 48 to 72 h posttransfection and filtered through 45-micron filters before use to transduce astrocyte cultures.
Hsp75 Overexpression
For Hsp75 or control vector, viral transduction sister cultures in the same 24-well plate were used with 4 to 8 replicates each of (1) control nontransduced, (2) Hsp75-transduced, and (3) control vector-transduced astrocytes. Transduced cultures were treated with packaging cell supernatants of either Hsp75 or control virus mixed 1:1 with plating medium and containing 8 μg/mL polybrene (Sigma) for 24 h, 48 to 72 h after plating. Transduction was repeated twice. Successfully transduced astrocytes were selected in 400 μg/mL G418 (Sigma) for 5 days. The viral transduction did not cause any significant astrocyte death. The antibiotic selection process killed nontransduced astrocytes, so experiments were performed on confluent cultures of cells in which essentially all cells stably expressed Hsp75, or contained control vector, approximately 3 weeks after transduction. The transduced cultures were fed every 3 days with astrocyte growth medium supplemented with 10 ng/mL epidermal growth factor for the first 2 weeks after the antibiotic selection. After the 2-week period, half the medium was removed every 3 days and replaced with astrocyte growth medium. Glucose deprivation or oxygen–glucose deprivation (OGD) used the same protocol for transduced and nontransduced cultures.
Western Blot Analysis
Equal amounts of protein as determined by the bicinchoninic acid method (Pierce, Rockford, IL, USA), 30 μg per condition, were separated on a 12.5% polyacrylamide gel and electrotransferred onto Immobilon polyvinylidene fluoride membrane (Millipore Corp., Bedford, MA, USA). Membranes were blocked with 5% nonfat dry milk in water with 0.05% Tween 20 (blocking buffer) for 1 h, incubated overnight with 1:1,000 dilution of primary anti-Hsp75 antibody (Stressgen, Ann Arbor, MI, USA) in blocking buffer, washed three times with 0.05% Tween in phosphate-buffered saline (PBS), and incubated with 1:2,000 anti-mouse HRP (horseradish peroxidase)-linked antibody (Cell Signaling, Danvers, MA, USA) in blocking buffer for 90 mins. Immunoreactive bands were visualized with the enhanced chemiluminescence detection system (Amersham, Piscataway, NJ, USA) according to manufacturer’s protocol. The membranes used for Hsp75 detection were reprobed with 1:1,000 anti-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The Hsp75 band intensity was normalized to the actin band intensity in the same lane.
Glucose Deprivation
All cells had their medium changed to BSS5.5 containing the following (in mmol/L): glucose, 5.5; NaCl, 116; CaCl2, 1.8; MgSO4, 0.8; KCl, 5.4; NaH2PO4, 1; NaHCO3, 14.7, and HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 10 (pH 7.4) 24 h before the start of the experiment. Control cells had their medium changed to BSS5.5, whereas GD was induced by completely exchanging the medium for BSS0, which was identical to BSS5.5 but lacking glucose. BSS0 was added to each well to bring the total volume to 1 mL. This was followed by three washes in which 0.8 mL medium was removed and replaced by 0.8 mL BSS0. After the final wash, the volume was adjusted to 0.5 mL.
Oxygen–Glucose Deprivation
Cultures were transferred to an anaerobic chamber (Coy, Glass Lake, MI, USA) with an atmosphere of 5% CO2, 10% H2 and 85% N2. The culture medium was replaced three times with deoxygenated BSS0 using the same washing scheme described in the GD method above. Cultures were then placed in a humidified 37°C incubator within the anaerobic chamber. Oxygen tension was monitored with an oxygen electrode (Microelectrodes, Bedford, NH, USA) and was kept under 0.02%. Oxygen–glucose deprivation was ended by adding glucose to the culture medium to a final concentration of 5.5 mmol/L and returning the cultures to the normoxic incubator for 24 h before cell death evaluation. Experimental data were combined from different experiments in which OGD caused ~30% cell death in control vector-transduced astrocytes. Depending on the cell isolation, cells from different dissections required 4.5 to 5.5 h OGD followed by 24 h reperfusion to attain this level of injury.
Live Imaging
The live imaging studies were performed as described previously (Ouyang et al, 2006). Briefly, oxygen radical production was monitored using hydroethidine (HEt). Cultures were loaded in the dark with 5 μmol/L HEt for 30 mins at 37°C, and the same concentration of HEt was maintained in the bath throughout each experiment. Cells were excited at 495 nm, and fluorescence emission monitored at 530 nm. Fluorescence measurements for each cell (Ft) were normalized to the initial fluorescence intensity (F0) for that cell. For assessment of changes in mitochondrial membrane potential, astrocytes were incubated for 30 mins with tetramethylrhodamine ethyl ester (TMRE) (50 nmol/L) at 37°C, and the same concentration of TMRE was maintained in all bathing solutions throughout the experiments. Cells were excited at 535 nm, and fluorescence emission was monitored at 590 nm. Changes in fluorescence were quantified by selecting mitochondria-rich perinuclear regions in each cell, and all subsequent fluorescence measurements were normalized to the baseline fluorescence (F0) for the same cell at the start of the experiment.
Immunocytochemistry
Fluorescence immunocytochemistry was performed on cell cultures in 24-well plates. The wells were washed twice in PBS and then fixed in 4% paraformaldehyde for 30 mins at room temperature. The cells were then washed twice with PBS and then in PBS containing 0.3% Triton X-100 for 15 mins. Nonspecific binding sites were blocked in blocking buffer (3% bovine serum albumin and 0.3% Triton X-100 in PBS) for 1 h. The cells were incubated with anti-Hsp75 primary antibody (Stressgen) at 1:300 dilution in blocking buffer overnight at 4°C and then washed three times with blocking buffer, 10 mins per wash. The cells were incubated with FITC-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA) at 1:100 dilution in blocking buffer at RT for 1 h, then washed 2 times in blocking buffer, and one time in PBS, 10 mins per wash. Fluorescence was visualized with an epifluorescence microscope (Zeiss Axiovert 200 mol/L; Carl Zeiss, Göttingen, Germany), and images were obtained on a Macintosh computer using Openlab software from Improvision Inc. (Lexington, MA, USA).
ATP Measurements
Astrocyte intracellular ATP levels were quantitated immediately at the end of 4.5 h of OGD exposure. Cellular ATP concentrations were measured using the CellTiter-Glo luminescent ATP assay kit, based on the luciferase/luciferin reaction (Promega, Madison, WI, USA) according to the manufacturer’s instructions. A Veritas luminescence counter (Turner BioSystems, Sunnyvale, CA, USA) was used to measure the luminescence signal of the samples in opaque white 96-well plates. ATP standards (Amersham) were used to create a calibration curve. Protein concentrations were measured using a bicinchoninic acid protein assay from Pierce per the manufacturer’s instructions.
Cell Viability Assays
Cell death was quantified by microscopic evaluation of Hoechst 33342 (5 μmol/L) and propidium iodide (PI, 5 μmol/L)-labeled cells. PI readily penetrates cells with compromised plasma membranes (dead cells) but does not cross intact plasma membranes. Hoechst is a cell-permeant nucleic acid stain that labels both live and dead nuclei.
Statistics
Statistical differences between two groups were determined using unpaired two-tailed Student’s t-test. Comparisons between multiple groups were performed with analysis of variance followed by Tukey’s multiple comparison test, with the P < 0.05 level considered significant. Data in all plots are presented as mean ± s.d.
Results
Overexpression of Hsp75
Previous studies have revealed endogenous expression of Hsp75 in various tissues including normal and ischemic brain tissue (Massa et al, 1995). Retroviral transduction of primary astrocyte cultures with Hsp75 resulted in markedly higher levels of Hsp75 expression compared to those in vector-transduced astrocytes and to endogenous Hsp75 levels in control cells, as indicated by Western blot (Figure 1A). Hsp75 overexpression in LXSN-Hsp75-transduced astrocytes, along with preferential mitochondrial localization, was also confirmed by immunocytochemistry (Figure 1B).
Figure 1.
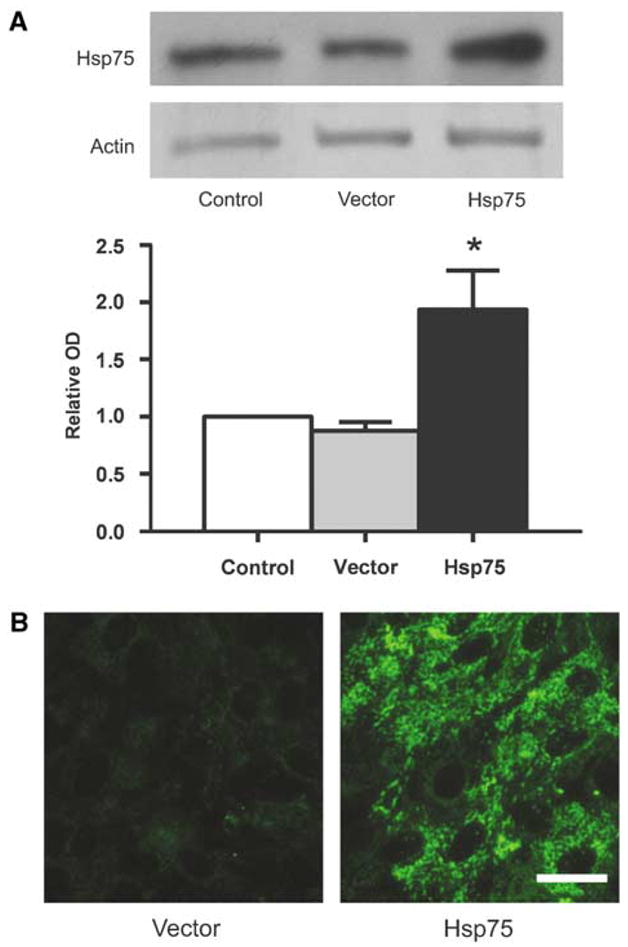
Vector-transduced cells have higher levels of Hsp75. (A) Expression levels of Hsp75 in uninfected control, vector-transduced, and Hsp75-transduced astrocytes as determined by Western blot (*P < 0.05 compared to vector-transduced astrocytes). (B) Immunohistochemical Hsp75 staining of vector- and Hsp75-transduced astrocytes shows typical mitochondrial punctuate perinuclear fluorescence. Scale bar, 25 μm.
Protection from Glucose Deprivation
We previously showed that GD induced increased levels of oxidative stress in primary astrocyte cultures (Ouyang et al, 2006; Papadopoulos et al, 1997). Intracellular ROS levels were evaluated using the ROS-sensitive fluorescent dye HEt (Figure 2). We found that HEt staining was not significantly different between the different groups of astrocytes at 0 h GD. Upon exposure to GD, uninfected and vector-transduced astrocytes have shown immediate and rapid ROS generation, with HEt fluorescence increasing by six-fold in both groups after 2 h of GD. In contrast, Hsp-75-overexpressing astrocytes showed more moderate increases in ROS levels, with the HEt signal increasing approximately twofold after 2 h of GD.
Figure 2.
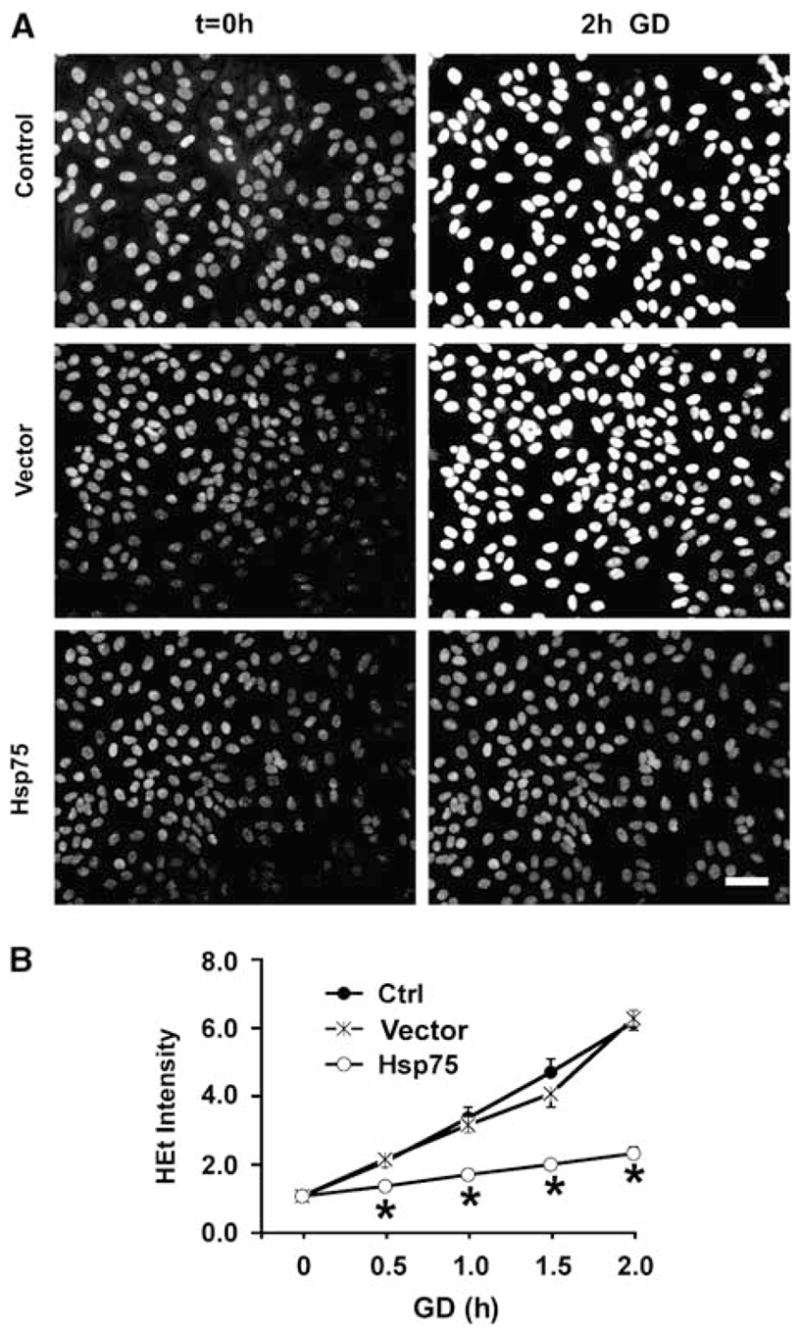
Hsp75 overexpression reduces ROS accumulation induced by glucose deprivation (GD) (A) Examples of changes in hydroethidine (HEt) fluorescence before (t = 0, left column) and after 2 h of GD (right column). Scale bar, 50 μm. (B) Time course of ROS accumulation with GD in uninfected control, vector-transduced, and Hsp75-overexpressing astrocytes. For each cell, the (HEt) fluorescence was normalized to the starting fluorescence for that cell. The traces represent mean ± s.d. of at least 50 astrocytes per condition, derived from at least three experiments (*P < 0.05 compared to both control and vector-transduced under the same condition).
Mitochondria are both targets and sources of cellular oxidative stress (Fiskum et al, 2004). We have also previously reported that GD promoted mitochondrial depolarization in primary astrocyte cultures (Ouyang et al, 2006). Mitochondrial functions including protein import and generation of ATP are dependent on maintenance of the mitochondrial membrane potential (Voisine et al, 1999). To investigate the effect of Hsp75 overexpression on changes in mitochondrial membrane potential induced by GD, we used the fluorescent dye TMRE, which accumulates in mitochondria in a potential-dependent manner. As indicated in Figure 3, at 0 h of GD, all groups showed similar levels of TMRE fluorescence with a typical mitochondrial pattern of perinuclear staining. After 2 h of GD, the TMRE signal of Hsp75-overexpressing astrocytes did not significantly change compared to the 0 h time point. In contrast, TMRE signal in control and vector-transduced astrocytes was reduced by ~15% (P < 0.05) indicating partial mitochondrial depolarization.
Figure 3.
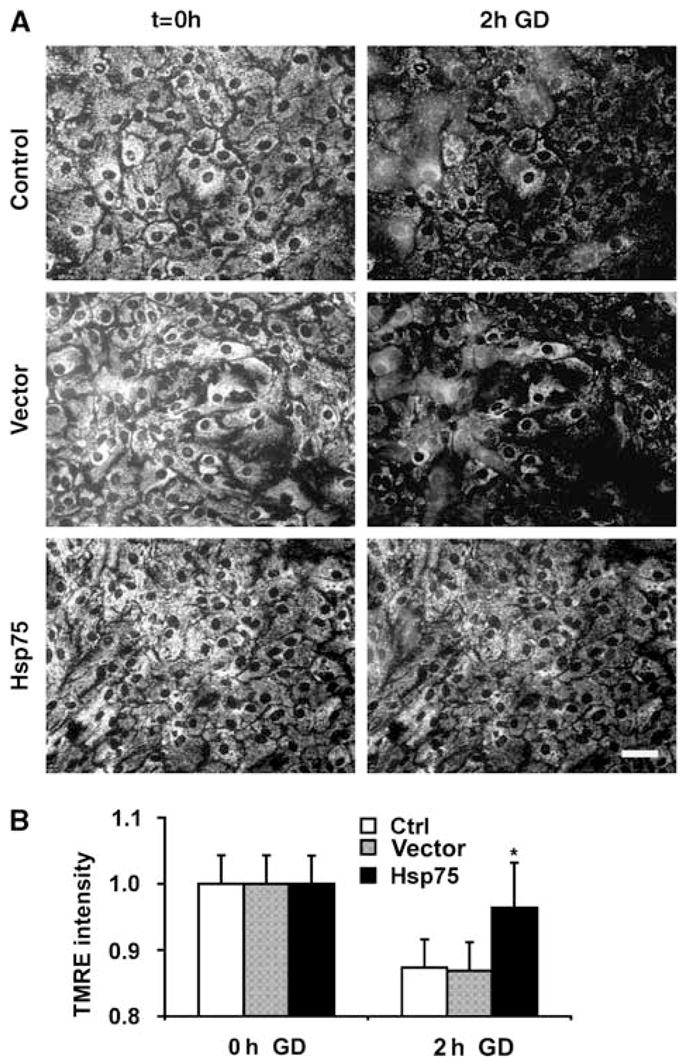
Hsp75 overexpression preserves mitochondrial membrane potential during glucose deprivation (GD). (A) Examples of TMRE staining before (t = 0, left column) and after 2 h of GD (right column). Scale bar, 50 μm. (B) Changes in TMRE mitochondrial fluorescence with GD in uninfected control, vector-transduced, and Hsp75-overexpressing astrocytes. The data are representative of mean ± s.d. of three independent experiments, with at least 50 astrocytes per condition (*P < 0.05 compared to both control and vector-transduced under the same condition).
Protection from Oxygen–Glucose Deprivation
We next investigated whether Hsp75 overexpression protects astrocytes against OGD. ATP depletion is the ultimate consequence of glucose depletion and inhibition of mitochondrial oxidative respiration due to anoxia under OGD conditions. Intracellular ATP levels were quantitated at the end of 4.5 h of OGD exposure. As shown in Figure 4, OGD treatment resulted in similar decreases of 66 and 62% in uninfected and empty vector-transduced astrocytes, respectively. The same OGD exposure caused a 32% decrease in ATP levels of Hsp75-overexpressing astrocytes, significantly less than either control. To investigate the effect of Hsp75 overexpression on astrocyte viability, the extent of astrocyte death was evaluated using PI staining. As shown in Figure 5, the same OGD exposures induced approximately 25% astrocyte death in uninfected and control-transduced cultures, but caused only 5% cell death in Hsp75-overexpressing astrocytes.
Figure 4.
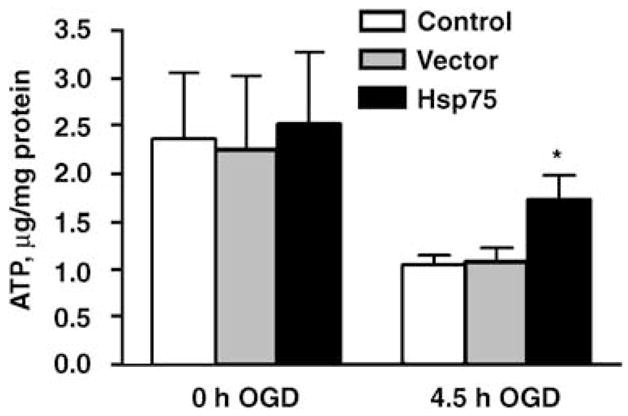
Hsp75 overexpression partially preserves ATP levels in astrocytes subjected to 4.5 h of OGD. The data are representative of mean ± s.d. of three independent experiments, each performed in duplicate (*P < 0.05 compared to both control and vector-transduced under the same condition).
Figure 5.
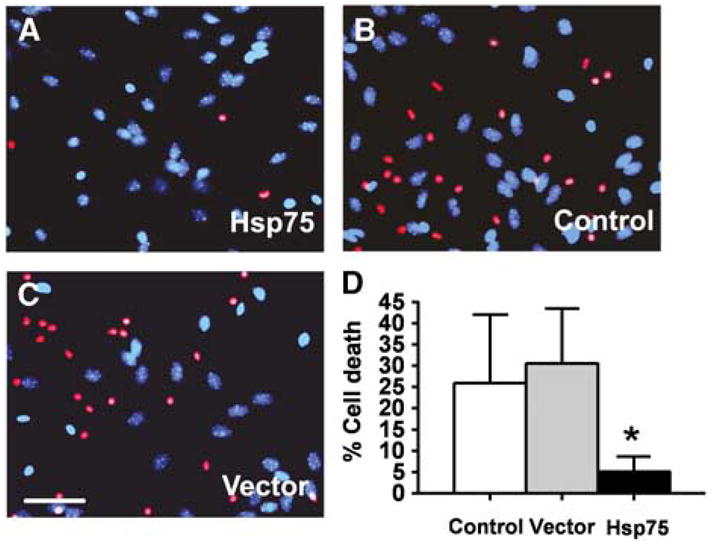
Hsp75 overexpression increases survival of astrocytes subjected to oxygen–glucose deprivation (OGD) and 24 h of recovery. Experimental data from three experiments in which OGD for 4.5 to 5.5 h achieved ~30% cell death in control vector-transduced astrocytes were pooled. Cell death was significantly decreased in Hsp75-overexpressing astrocytes (A) compared to vector-transduced (B) and uninfected control (C), as assessed by PI staining. Scale bar, 50 μm. (D) Quantification of OGD-induced astrocyte death, mean ± s.d. of three independent experiments, 400 to 500 cells for each condition in each experiment (*P < 0.05 compared to both control and vector-transduced under the same condition).
Discussion
Studies have shown that levels of mitochondrial Hsp70 (Hsp75) and cytosolic inducible Hsp70 (Hsp72) are upregulated during ischemic conditions (Kiang and Tsokos, 1998; Sharp et al, 1991). It has been shown that Hsp72 overexpression is neuroprotective both in vitro and in vivo (reviewed in Giffard and Yenari, 2004), inhibits early mitochondrial cytochrome c release after permanent focal ischemia (Tsuchiya et al, 2003), and protects mitochondrial function during GD in vitro (Ouyang et al, 2006). Unlike the case with Hsp72, the protective potential of Hsp75 overexpression against ischemic injury remains relatively unstudied. In this study, we showed protection conferred by Hsp75 overexpression against in vitro ischemic injury in primary astrocyte cultures, reduced ROS generation, and improved mitochondrial function.
Many studies have shown that GD promotes a rapid increase of intracellular ROS (Blackburn et al, 1999; Ouyang et al, 2002; Papadopoulos et al, 1997). This increase can be caused by compromised production of NADPH and pyruvate (Spitz et al, 2000), cellular compounds with antioxidant properties, and reduced intracellular glutathione levels (Papadopoulos et al, 1997). In our experiments, overexpression of Hsp75 protected astrocytes against GD-induced ROS accumulation. This result is consistent with previous studies that showed decreased ROS production in Hsp75-overexpressing PC12 cells subjected to GD (Liu et al, 2005), or a hepatocyte cell line subjected to iron chelation (Im et al, 2007). In addition, a recent study showed that cells with silenced Hsp75 expression are more prone to ROS generation (Hua et al, 2007). The mechanism responsible for Hsp75 suppression of ROS production during GD is not clear. It has been shown recently that, apart from serving as a mitochondrial molecular chaperone and binding partner for several mitochondrial enzymes, Hsp75 is directly involved in suppression of oxidant-induced cytochrome c release from mitochondria (Pridgeon et al, 2007). It is reasonable to suggest that Hsp75 can suppress mitochondrial ROS production through stabilizing cytochrome c and other important components of the electron transport chain, and in addition or alternatively, through enhancing mitochondrial antioxidant mechanisms.
Overexpression of Hsp75 prevented the GD-induced decrease of mitochondrial membrane potential. As under these conditions a reduction in ROS generation was also observed, and because mitochondria are the primary target of intracellular ROS, this may be part of the reason for better preservation of mitochondrial membrane potential. Also, as indicated above, direct interactions with respiratory chain and other mitochondrial proteins may also be important in preserving normal mitochondrial membrane potential during GD.
Oxygen–glucose deprivation is known to induce a marked decrease in ATP levels in cultured astrocytes (Yu et al, 2002). Our data show that Hsp75 overexpression partially prevents OGD-induced ATP depletion. It has been shown that during mitochondrial inhibition, astrocytes are able to maintain mitochondrial membrane potential by reverse operation of mitochondrial complex V (F1F0-ATPase) and consumption of glycolytically produced ATP (Almeida et al, 2001; Voloboueva et al, 2007). The rates of mitochondrial ATP consumption under these conditions depends both on the activity of the mitochondrial ATPase and on the leakiness of the inner mitochondrial membrane (Duchen, 1999). As mitochondrial membrane lipids and proteins are readily damaged by oxidants, both chaperone and antioxidant functions of Hsp75 can contribute to the observed better preservation of ATP levels during OGD. We also found that Hsp75 reduced OGD-induced astrocyte death. The molecular and biochemical basis of this protection likely reflects multiple mechanisms. OGD-induced cell death is promoted by ATP depletion. Mitochondrial cytochrome c release promotes apoptotic signaling and activation of caspases. Reduced ATP loss and suppression of cytochrome c release may both be part of the mechanism of Hsp75 protection from OGD.
Hsp75 plays a central role in protein import into the mitochondrial matrix (Kaul et al, 2007). Both facilitating import, and facilitating and maintaining proper mitochondrial protein conformation likely contribute to protection by Hsp75. As both import and facilitation of folding depend on the ATPase activity of Hsp75 (Moro et al, 2002), it will be interesting to determine the extent to which its neuroprotective ability also requires the ATPase domain and ATPase activity.
The major finding of this study is the demonstration of several cytoprotective effects of Hsp75 against ischemic injury in primary brain cell culture. Although antioxidant effects of Hsp75 overexpression were reported in hepatocytes and PC12 cells (Im et al, 2007; Liu et al, 2005), this is the first evidence, to the best of our knowledge, showing protection against loss of mitochondrial membrane potential and cellular ATP depletion by Hsp75 overexpression. Oxidative stress, mitochondrial dysfunction, and ATP depletion are interconnected during ischemia. Dissection of the mechanisms contributing to the protection by Hsp75 against ischemia-like injury will require further studies to identify the targets and sources of free radicals.
Results of several studies suggest that astrocytes might better preserve their neuroprotective functions if they did not suffer severe metabolic and mitochondrial impairment during ischemic injury (Bambrick et al, 2004; Ouyang et al, 2007; Voloboueva et al, 2007). Thus, strategies aimed at preservation of astrocyte metabolic and mitochondrial function might be useful in developing therapies to attenuate ischemic injury. The results presented here identify Hsp75 as an effective protective protein against ischemia-like in vitro injury. Further studies are required to investigate whether Hsp75 overexpression provides significant protection against ischemic injury in animal models.
Acknowledgments
The work was supported in part by NIH Grants GM49831, NS014543, NS37520 to RGG, and by an American Heart Association fellowship to LAV.
Footnotes
Disclosure/Conflict of interest
The authors state no duality of interest.
References
- Almeida A, Almeida J, Bolanos JP, Moncada S. Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proc Natl Acad Sci USA. 2001;98:15294–9. doi: 10.1073/pnas.261560998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambrick L, Kristian T, Fiskum G. Astrocyte mitochondrial mechanisms of ischemic brain injury and neuroprotection. Neurochem Res. 2004;29:601–8. doi: 10.1023/b:nere.0000014830.06376.e6. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya T, Karnezis AN, Murphy SP, Hoang T, Freeman BC, Phillips B, Morimoto RI. Cloning and subcellular localization of human mitochondrial hsp70. J Biol Chem. 1995;270:1705–10. doi: 10.1074/jbc.270.4.1705. [DOI] [PubMed] [Google Scholar]
- Blackburn RV, Spitz DR, Liu X, Galoforo SS, Sim JE, Ridnour LA, Chen JC, Davis BH, Corry PM, Lee YJ. Metabolic oxidative stress activates signal transduction and gene expression during glucose deprivation in human tumor cells. Free Radic Biol Med. 1999;26:419–30. doi: 10.1016/s0891-5849(98)00217-2. [DOI] [PubMed] [Google Scholar]
- Carette J, Lehnert S, Chow TY. Implication of PBP74/mortalin/GRP75 in the radio-adaptive response. Int J Radiat Biol. 2002;78:183–90. doi: 10.1080/09553000110097208. [DOI] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23:137–49. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Hertz L. Astrocytic contributions to bioenergetics of cerebral ischemia. Glia. 2005;50:362–88. doi: 10.1002/glia.20157. [DOI] [PubMed] [Google Scholar]
- Duchen MR. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J Physiol. 1999;516(Part 1):1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan LL, Bruno VM, Amagasu SM, Giffard RG. Glia modulate the response of murine cortical neurons to excitotoxicity: glia exacerbate AMPA neurotoxicity. J Neurosci. 1995;15:4545–55. doi: 10.1523/JNEUROSCI.15-06-04545.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskum G. Mitochondrial participation in ischemic and traumatic neural cell death. J Neurotrauma. 2000;17:843–55. doi: 10.1089/neu.2000.17.843. [DOI] [PubMed] [Google Scholar]
- Fiskum G, Rosenthal RE, Vereczki V, Martin E, Hoffman GE, Chinopoulos C, Kowaltowski A. Protection against ischemic brain injury by inhibition of mitochondrial oxidative stress. J Bioenerg Biomembr. 2004;36:347–52. doi: 10.1023/B:JOBB.0000041766.71376.81. [DOI] [PubMed] [Google Scholar]
- Gao CX, Zhang SQ, Yin Z, Liu W. Molecular chaperone GRP75 reprove cells from injury caused by glucose deprivation. Shi Yan Sheng Wu Xue Bao. 2003;36:381–7. [PubMed] [Google Scholar]
- Giffard RG, Yenari MA. Many mechanisms for hsp70 protection from cerebral ischemia. J Neurosurg Anesthesiol. 2004;16:53–61. doi: 10.1097/00008506-200401000-00010. [DOI] [PubMed] [Google Scholar]
- Hadari YR, Haring HU, Zick Y. p75, a member of the heat shock protein family, undergoes tyrosine phosphorylation in response to oxidative stress. J Biol Chem. 1997;272:657–62. doi: 10.1074/jbc.272.1.657. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–49. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Hua G, Zhang Q, Fan Z. Heat shock protein 75 (TRAP1) antagonizes reactive oxygen species generation and protects cells from granzyme M-mediated apoptosis. J Biol Chem. 2007;282:20553–60. doi: 10.1074/jbc.M703196200. [DOI] [PubMed] [Google Scholar]
- Im CN, Lee JS, Zheng Y, Seo JS. Iron chelation study in a normal human hepatocyte cell line suggests that tumor necrosis factor receptor-associated protein 1 (TRAP1) regulates production of reactive oxygen species. J Cell Biochem. 2007;100:474–86. doi: 10.1002/jcb.21064. [DOI] [PubMed] [Google Scholar]
- Kaul SC, Deocaris CC, Wadhwa R. Three faces of mortalin: a housekeeper, guardian and killer. Exp Gerontol. 2007;42:263–74. doi: 10.1016/j.exger.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Ransom B. Neuroglia. New York: Oxford University Press; 2005. [Google Scholar]
- Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x. [DOI] [PubMed] [Google Scholar]
- Krimmer T, Rassow J, Kunau WH, Voos W, Pfanner N. Mitochondrial protein import motor: the ATPase domain of matrix Hsp70 is crucial for binding to Tim44, while the peptide binding domain and the carboxy-terminal segment play a stimulatory role. Mol Cell Biol. 2000;20:5879–87. doi: 10.1128/mcb.20.16.5879-5887.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AT, He QY, Chiu JF. A proteome analysis of the arsenite response in cultured lung cells: evidence for in vitro oxidative stress-induced apoptosis. Biochem J. 2004;382:641–50. doi: 10.1042/BJ20040224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–10. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu W, Song XD, Zuo J. Effect of GRP75/mthsp70/PBP74/mortalin overexpression on intracellular ATP level, mitochondrial membrane potential and ROS accumulation following glucose deprivation in PC12 cells. Mol Cell Biochem. 2005;268:45–51. doi: 10.1007/s11010-005-2996-1. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Mann R, Mulligan RC, Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983;33:153–9. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Massa SM, Longo FM, Zuo J, Wang S, Chen J, Sharp FR. Cloning of rat grp75, an hsp70-family member, and its expression in normal and ischemic brain. J Neurosci Res. 1995;40:807–19. doi: 10.1002/jnr.490400612. [DOI] [PubMed] [Google Scholar]
- Miller AD, Rosman GJ. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989;7:980–2. 984–986, 989–990. [PMC free article] [PubMed] [Google Scholar]
- Moro F, Okamoto K, Donzeau M, Neupert W, Brunner M. Mitochondrial protein import: molecular basis of the ATP-dependent interaction of MtHsp70 with Tim44. J Biol Chem. 2002;277:6874–80. doi: 10.1074/jbc.M107935200. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Carriedo SG, Giffard RG. Effect of Bcl-x(L) overexpression on reactive oxygen species, intracellular calcium, and mitochondrial membrane potential following injury in astrocytes. Free Radic Biol Med. 2002;33:544–51. doi: 10.1016/s0891-5849(02)00912-7. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci. 2007;27:4253–60. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Xu LJ, Sun YJ, Giffard RG. Overexpression of inducible heat shock protein 70 and its mutants in astrocytes is associated with maintenance of mitochondrial physiology during glucose deprivation stress. Cell Stress Chaperones. 2006;11:180–6. doi: 10.1379/CSC-182R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos MC, Koumenis IL, Dugan LL, Giffard RG. Vulnerability to glucose deprivation injury correlates with glutathione levels in astrocytes. Brain Res. 1997;748:151–6. doi: 10.1016/s0006-8993(96)01293-0. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Koumenis IL, Yuan TY, Giffard RG. Increasing vulnerability of astrocytes to oxidative injury with age despite constant antioxidant defenses. Neuroscience. 1998;82:915–25. doi: 10.1016/s0306-4522(97)00320-5. [DOI] [PubMed] [Google Scholar]
- Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer C, Barnikol-Watanabe S, Thinnes FP, Hilschmann N. Voltage-dependent anion-selective channel (VDAC) interacts with the dynein light chain Tctex1 and the heat-shock protein PBP74. Int J Biochem Cell Biol. 2002;34:1059–70. doi: 10.1016/s1357-2725(02)00026-2. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Lowenstein D, Simon R, Hisanaga K. Heat shock protein hsp72 induction in cortical and striatal astrocytes and neurons following infarction. J Cereb Blood Flow Metab. 1991;11:621–7. doi: 10.1038/jcbfm.1991.113. [DOI] [PubMed] [Google Scholar]
- Spitz DR, Sim JE, Ridnour LA, Galoforo SS, Lee YJ. Glucose deprivation-induced oxidative stress in human tumor cells. A fundamental defect in metabolism? Ann N Y Acad Sci. 2000;899:349–62. doi: 10.1111/j.1749-6632.2000.tb06199.x. [DOI] [PubMed] [Google Scholar]
- Stacchiotti A, Ricci F, Rezzani R, Li Volti G, Borsani E, Lavazza A, Bianchi R, Rodella LF. Tubular stress proteins and nitric oxide synthase expression in rat kidney exposed to mercuric chloride and melatonin. J Histochem Cytochem. 2006;54:1149–57. doi: 10.1369/jhc.6A6932.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurin S, Seyrantepe V, Orlov SN, Tremblay TL, Thibault P, Bennett MR, Hamet P, Pshezhetsky AV. Proteome analysis and functional expression identify mortalin as an antiapoptotic gene induced by elevation of [Na+]i/[K+]i ratio in cultured vascular smooth muscle cells. Circ Res. 2002;91:915–22. doi: 10.1161/01.res.0000043020.45534.3e. [DOI] [PubMed] [Google Scholar]
- Tsuchiya D, Hong S, Matsumori Y, Shiina H, Kayama T, Swanson RA, Dillman WH, Liu J, Panter SS, Weinstein PR. Overexpression of rat heat shock protein 70 is associated with reduction of early mitochondrial cytochrome C release and subsequent DNA fragmentation after permanent focal ischemia. J Cereb Blood Flow Metab. 2003;23:718–27. doi: 10.1097/01.WCB.0000054756.97390.F7. [DOI] [PubMed] [Google Scholar]
- Voisine C, Craig EA, Zufall N, von Ahsen O, Pfanner N, Voos W. The protein import motor of mitochondria: unfolding and trapping of preproteins are distinct and separable functions of matrix Hsp70. Cell. 1999;97:565–574. doi: 10.1016/s0092-8674(00)80768-0. [DOI] [PubMed] [Google Scholar]
- Voloboueva LA, Suh SW, Swanson RA, Giffard RG. Inhibition of mitochondrial function in astrocytes: implications for neuroprotection. J Neurochem. 2007;102:1383–94. doi: 10.1111/j.1471-4159.2007.4634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Chock VY, Yang EY, Giffard RG. Susceptibility to apoptosis varies with time in culture for murine neurons and astrocytes: changes in gene expression and activity. Neurol Res. 2004;26:632–43. doi: 10.1179/016164104225017587. [DOI] [PubMed] [Google Scholar]
- Yu AC, Lau AM, Fu AW, Lau LT, Lam PY, Chen XQ, Xu ZY. Changes of ATP and ADP in cultured astrocytes under and after in vitro ischemia. Neurochem Res. 2002;27:1663–8. doi: 10.1023/a:1021691112190. [DOI] [PubMed] [Google Scholar]


