Abstract
Recently, our laboratory reported that secondary CD8+ T-cell mediated anti-tumor responses were impaired following successful initial anti-tumor responses using various immunotherapeutic approaches. While immunotherapy stimulated significant increases in CD8+ T cell numbers, the number of CD4+ T cells remained unchanged. The current investigation revealed a marked differential expansion of CD4+ T-cell subsets. Successful immunotherapy surprisingly resulted in an expansion of CD4+ Foxp3+ T-regulatory (Treg) cells concurrent with a reduction of conventional CD4+ T cells (Tconv), despite the marked anti-tumor responses. Following immunotherapy, we observed differential upregulation of PD-1 on the surface of CD4+ Foxp3+ regulatory T cells (Treg cells) and CD4+ Foxp3− T cells (Tconv cells). Interestingly, it was the ligand for PD-1, B7-H1 (PDL-1) that correlated with Tconv loss after treatment. Furthermore, interferon gamma knockout (IFNγ−/−) and interferon gamma receptor knockout (IFNγR−/−) animals lost upregulation of surface B7-H1 even though PD-1 expression of Tconv cells was not changed and this correlated with CD4+ Tconv increases. These results suggest that subset specific-expansion may contribute to marked shifts in the composition of the T-cell compartment, potentially influencing the effectiveness of some immunotherapeutic approaches that rely on interferon gamma (IFNγ).
Keywords: T Cells, Apoptosis, Memory, Tolerance/Suppression/Anergy, Tumor Immunity
Introduction
Immunotherapeutic use of an agonist CD40 mAb in combination with IL-2 has been shown to have synergistic anti-tumor effects in mouse models of advanced renal cell carcinoma (RCC) and lung carcinoma (1). More recently, we reported that treatment of mice after immunization combined with this and other immunotherapeutic regimens can lead to an interferon-gamma (IFN-γ) dependent loss of CD4+ T cells and subsequent inability to mount an effective memory response after a delayed live tumor challenge despite successful initial anti-tumor responses (2). Additionally, other investigators using a viral antigen challenge model have shown similar effects after administration of anti-CD40 alone. Administration of anti-CD40 to LCMV-infected mice was associated with loss of virus specific CD8+ T cells upon secondary challenge in vitro. While a loss of CD4+ T cells was also observed, the dominant effector outcome was due to the loss of CD8+ T cells and was mediated by the Fas-FasL pathway (3). The majority of tumor models focus on CD8+ T cell effector pathways. However, in addition to helping generate tumor-antigen specific CD8+ T-cell memory, recent studies suggest a more direct role for CD4+ T cells in some anti-tumor responses (4). While we previously reported a loss in CD4+ T cell numbers after anti-CD40 and IL-2 immunotherapy despite increases in CD8+ T cells, the mechanism underlying this lack of CD4+ T-cell expansion was not clear.
CD4+ T cells are a very diverse lymphocyte population with respect to the cytokines they can produce and understanding their polarization toward stimulatory or inhibitory activity is important for understanding how they can affect treatment in a disease setting (5). Regulatory CD4+ T cells expressing the hallmark forkhead transcription factor 3 (Foxp3) (Treg cells) are of particular interest with respect to cancer immunotherapy due to their potent immunosuppressive effects. It has therefore been suggested that their presence should be evaluated with all immunotherapeutic regimens since increases in Treg cells can be counterproductive to the desired outcome (6). We therefore examined the effects of CD40-based immunotherapeutic regimens on CD4+ T cell subsets and key markers correlating with their expansion or loss. Our current observations presented herein report a differential expression patterns of the cell surface marker Programmed Death-1 (PD-1, CD279) in response to anti-CD40 and IL-2 immunotherapy on the surface of conventional CD4+ T cells (Tconv) and Treg cells. PD-1 is found on most cells of hematopoietic origin and its surface expression has been associated with programmed cell death of thymocytes after TCR ligation (7, 8). PD-1 upregulation after T-cell activation has been implicated as being important for the peripheral tolerance of CD8+ T cells to tissue antigens, as well as self antigens early in their development (9-11).
We observed markedly increased expression of PD-1 on the surface of CD4+ Tconv cells, but not Treg cells after treatment with anti-CD40 and IL-2. Additionally, B7-H1 was upregulated in an IFN-γ dependent fashion, consistent with previous reports, (12) and we found this upregulation of B7-H1 to correlate with the observed loss of CD4+ T cells. These findings caused us to look more closely at CD4+ T cell subsets in the context of immunotherapy-induced alterations of CD4+ T cell subsets and overall changes in the composition of the T-cell compartment. The results reported herein led us to the hypothesis that IFN-γ dependent upregulation of B7-H1 after immunotherapy is met with a differential expression of PD-1 on conventional CD4+ T cell versus Treg cells. From these results, we suggest that differential expression pattern of the regulatory marker PD-1 following immunotherapy contributes to the loss of Tconv cells while simultaneously allowing Treg cells to expand. This may have ramifications in the length and extent of anti-tumor effects after immunotherapy.
Materials and Methods
Mice
Female C57BL/6 and BALB/c mice were purchased from the Animal Production Area of the National Cancer Institute (Frederick, MD). B6.129S7-Ifngtm1Ts (IFNγ−/−) and B6129S7IfngR (IFNγR−/−) as well as some aged matched control WT C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were between 8 and 12 weeks at the start of experiments and housed in microisolator cages or in the case of genetically engineered and aged matched control mice, on a Hepa filtered vent rack. Under all settings, mice were housed under specific pathogen free conditions. All experiments were in accordance with IACUC guidelines.
Reagents and cell Lines
Agonist rat anti-mouse CD40 (FGK115B3) was purified by ammonium sulfate precipitation from ascites. The endotoxin level of the anti-mouse CD40 antibody was <1endotoxin unit/mg antibody as determined by quantitative chromogenic limulus amebocyte lysate kit (QCL-1000, Bio Whittaker, Cambrex, Walkersville, MD). Recombinant human Interleukin-2 (IL-2; TECIN. Teceleukin) was provided by the National Cancer Institute. Recombinant human IL-15 (IL-15) was purchased from Peprotech (Rocky Hill, NJ). Purified rat IgG was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Cell Preparations
Spleen and lymph node cells were prepared by gentle dissociation and filtered to remove excess debris followed by washing two times in DPBS containing 5% FBS (Hyclone, Logan UT) and 1% Penicillin/Streptomycin (Mediatech, Herndon, VA). Cell counts were determined by a lyse/no wash procedure with known concentration of fluorescent beads or on a Coulter Z1 particle counter (Coulter Electronics, Arlington, TX). Blood was collected in tubes containing EDTA, Red blood cells were lysed in blood samples with FACSLyse (BD Biosciences, San Jose, CA).
Antibodies and Flow Cytometry
Cell suspensions from lymph node or spleen or whole blood were incubated with antibodies labeled with fluorescein (FITC) R-Phycoerythrin (PE), PE-cyanine 5 (PC5) and/or PE-Cyanine 7 (PC7) and PE-Texas Red (PE-TXred) followed by wash and resuspension in PBS + 5%FBS (Hyclone, Logan UT) + 1% Penicillin/Streptomycin (Mediatech, Herndon, VA.). Intracellular Foxp3 labeling was completed using the Ready Set Go, Foxp3 labeling Kit (eBiosciences, San Diego, CA) and all samples were resuspended in 1% formaldehyde (Sigma) in 1X Dulbeccos Phosphate Buffered Saline (Mediatech, Herndon, VA). Antibodies were purchased from either eBiosciences or BD Biosciences. Listmode data files were collected on a three color FACScan flow cytometer using Cell Quest software (Becton Dickinson, San Jose, CA), on a four-color Beckman Coulter XL/MCL using system II software or on a 5-color FC 500/MPL (Beckman Coulter, Fullerton CA). All data sets were analyzed using FlowJo software (Treestar, Ashland, OR).
Treatment Protocol
Agonist rat anti-mouse CD40 (FGK115B3) was administered i.p. at 65ug (IFNγ−/−or IFNγR−/−) or 80ug per dose for 5 consecutive days. Recombinant human IL-2(IL-2), 0.5 − 1.0 X106 IU/dose was administered i.p. four times per week in two sets of two injections; the second injection in a set being 8−20hrs from the previous one. In experiments where IL-15 was used in combination with anti-CD40, 2.5ug of recombinant human IL-15 (IL-15) was administered i.p. twice daily in place of IL-2 injections.
Statistics
Statistical analysis was performed using Prism software (Graphpad Software Inc.) Flow cytometry data were analyzed using student's t test; a Welch's correction was applied to data sets with significant differences in variance. Survival data were analyzed using a Log Rank Test. A minimum of 3 mice per group was used and all experiments. Experiments using C57BL/6 mice were repeated at least 3 times. BALB/c experiments were performed once with 3 mice per group to support the observations made using C57BL/6 mice. Data were tested for normality and variance, a p-value of <0.05 was considered significant.
Results
Systemic Immunotherapy Results in a Selective Loss of Conventional CD4+ T cells but not Regulatory CD4+ T cells
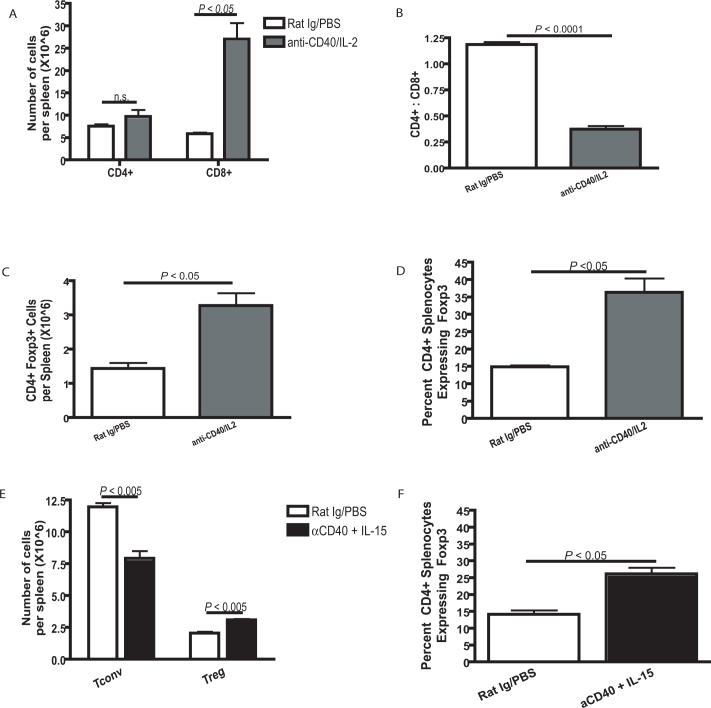
Evaluation of splenic CD4+ T cell percentages 11 days after the start of immunotherapy shows a marked lack of expansion of CD4+ T cells compared with CD8+ T cells (Fig. 1A). Despite reported initial anti-tumor effects (1) this was found to be due to cell death as CD4+ T cells were shown to be entering into the cell cycle after immunotherapy even though no expansion was taking place. Similar to our previous observations in multiple immunotherapeutic models (2), evaluation of CD4+ and CD8+ T cells shortly after immunotherapy administration resulted in an alteration of the normal ratio between CD4+ and CD8+ T cells (Fig. 1B). We next examined the effect of immunotherapy on the different CD4+ T cell subsets. Surprisingly, while CD4+ T cells did not expand as a whole population, the regulatory subset of CD4+ T cells defined by the expression of Foxp3 (Treg Cells) significantly (P < 0.05) expanded following administration of immunotherapy (Fig. 1C). In addition to total cell number, Treg cell expansion concurrent with the lack of Tconv cell expansion resulted in Treg cells making up a larger percentage of the CD4+ T cell compartment (Fig. 1D). Since IL-2 and not IL-15 is reported to be a strong promoter of Treg cells in vivo, anti-CD40 was combined with IL-15 to determine if this combination would also result in a significant expansion of Treg cells. In addition to anti-CD40 and IL-2, anti-CD40 and IL-15 combined immunotherapy resulted in similar preferential expansion of Treg cells and not Tconv (Fig. 1E and F). This appears to be due to a dominant effect of CD40 as IL-15 alone did not promote Treg expansion (data not shown). These results suggest that administration of immunotherapy results in an early loss of Tconv cells and simultaneous expansion of Treg cells, despite the occurrence of marked anti-tumor effects.
Figure 1. Anti-CD40 and IL-2 combination immunotherapy results in skewing of normal lymphocyte ratios.
Spleens from C57BL/6 mice harvested on day 11 of treatment with anti-CD40 and IL-2 showed expansion of CD4+ Foxp3+ regulatory T cells. (A) Animals that had received immunotherapy showed a significant (P < 0.05) increase in the number of splenic CD8+ T cells but not in CD4+ T cells as determined by flow cytometry. (B) After treatment with anti-CD40 and IL-2, animals showed a significant (P < 0.0001) decrease in the ratio of CD4+ to CD8+ T cells in the spleen. (C and D) Despite the lack of expansion of splenic CD4+ T cells after immunotherapy, we observed a significant (P < 0.05) increase in Treg cells in animals that had been treated with anti-CD40 and IL-2. This increase in Treg cells was determined to be in total CD4+ Foxp3+ cell numbers (C) and as a percentage (D) of the total CD4+ T cell population. (E and F) IL-15 was used in combination with anti-CD40 in place of IL-2 and Treg cells were analyzed by flow cytometry for expansion (E) in cell numbers and (F) as a percentage of all CD4+ T cells. Data in A-D was repeated at least three times with similar results, the data in E and F was repeated two times. Analysis for all parts of figure 3 were analyzed using an unpaired student t test, a Welch's correction was applied for any set of data with significantly different variances.
Systemic Immunotherapy Results in a Differential Expression of PD-1 on the Surface of Conventional and Regulatory T cells in Conjunction with B7-H1 Upregulation on all CD45+ splenocytes
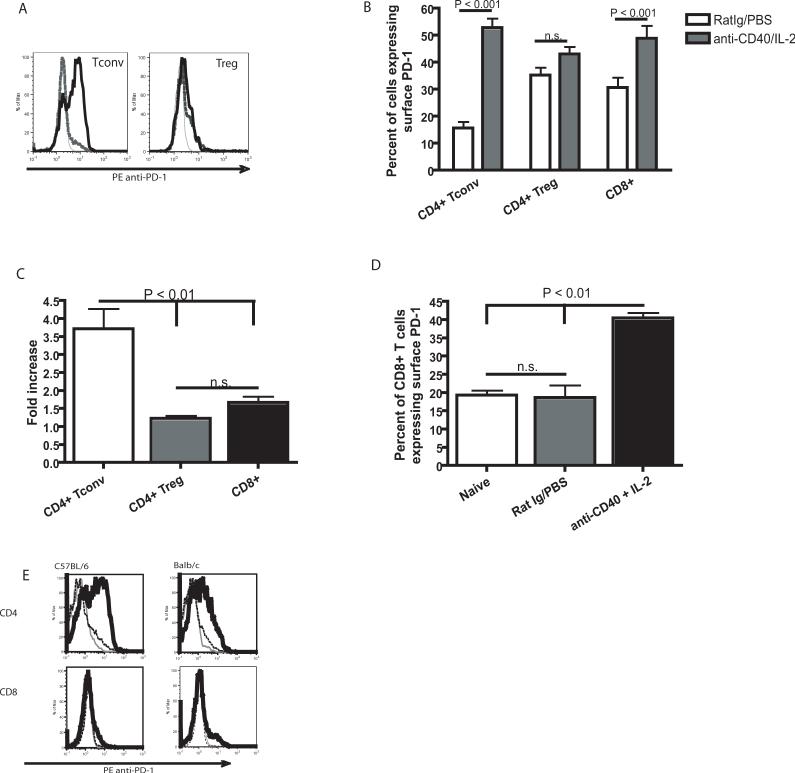
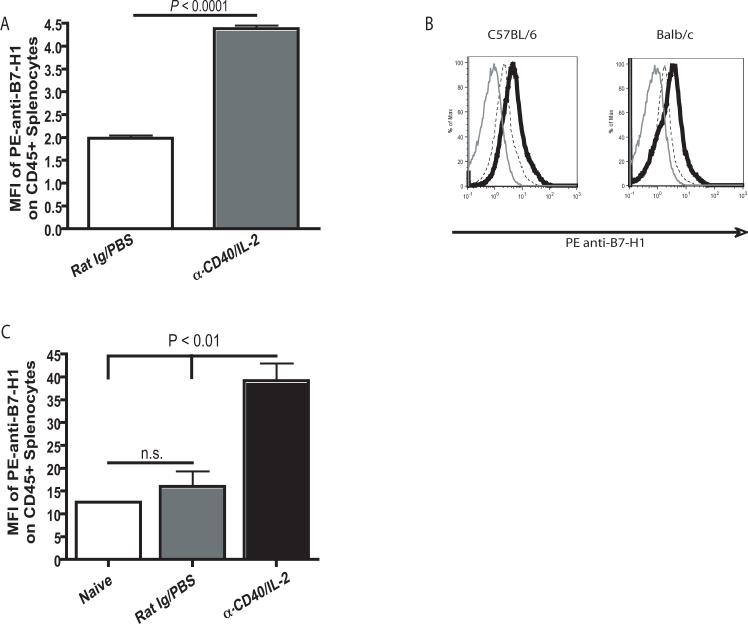
PD-1/B7-H1 ligation has been shown to have inhibitory and even pro-apoptotic effects on CD8+ T cells (7, 13). However, the effect of immunotherapy on this pathway with regard to CD4+ T cells has not previously been investigated. Therefore, we assessed surface PD-1 expression on CD4+ Tconv cells and CD8+ T cells as well as CD4+ Treg cells by flow cytometry immediately following administration of an anti-CD40 and IL-2 regimen. Following immunotherapy, we observed a significant increase of the percentage of Tconv cells expressing PD-1 on the cell surface (P < 0.001) which was not observed in the Treg cell subset (P > 0.05). We also observed a significant increase in the percentage of CD8+ T cells that expressed surface PD-1 after treatment with anti-CD40 and IL-2 (Fig. 2A and B), however the fold increase in the percentage of CD8+ T cells expressing surface PD-1 was significantly (P < 0.01) lower than CD4+ Tconv cells (Fig. 2C). The percentage of CD8+ T cells from control treated animals did not significantly differ from naïve animals (Fig. 2D). While we observed a higher baseline percentage of CD8+ T cells that were also PD-1+, CD8+ T cells had a consistently lower (P < 0.0001) level of receptor expression than CD4+ T cells as determined by median fluorescence intensity (data not shown). Further studies performed in both C57BL/6 mice and BALB/c mice showed that administration of anti-CD40 and IL-2 induced similar expression patterns of PD-1 on the surface of CD4+ T cells in both strains (Fig. 2E). In addition to PD-1, we also examined the expression of the PD-1 ligand, B7-H1. B7-H1 is widely expressed on most hematopoietic cell types (12), therefore we evaluated its expression by flow cytometric analysis on all CD45+ splenocytes. Following anti-CD40 and IL-2 administration, we observed a significant (P < 0.0001) increase in the median fluorescence intensity of surface B7-H1 on CD45+ splenocytes (Fig. 3A and B). B7-H1 expression was also significantly (P< 0.05) higher on the surface of the CD11c+ population of leukocytes (data not shown) however, the expression was not limited to myeloid or lymphoid cells therefore we evaluated surface B7-H1 expression on all hematopoietic (CD45+) cells. We did observe some variation in the baseline level of B7-H1 in our control treated animals between experiments however a comparison between naïve and control treated animals did not show an effect of the rat Ig and PBS treatment in the relative levels of B7-H1 on CD45+ cells (Fig. 3C). These data show that anti-CD40 and IL-2 results in the upregulation of B7-H1 on CD45+ cells, while simultaneous increasing surface PD-1 on conventional CD4+ T cells and not on Treg cells. These changes correlate directly with the observed loss in CD4+ T cell numbers suggesting that changes in the expression of B7-H1 and PD-1 may contribute to the decrease in conventional CD4+ T cells in the absence of similar effects on CD4+ Treg cells.
Figure 2. Regulatory T cells fail to upregulate PD-1 as a result of anti-CD40 and IL-2 immunotherapy.
Relative levels of PD-1 on the surface of Foxp3+ CD4+ Treg cells were compared with conventional Foxp3− CD4+ T cells 11 day after initiation of immunotherapy by flow cytometry. (A) Representative histograms of PD-1 labeling show unlabeled control (gray line), Rat Ig/PBS treated animals (dashed black line) and anti-CD40 and IL-2 treated animals (solid black line). (B) The relative percentage of Treg cells expressing surface PD-1 was not significantly (P > 0.05) greater in animals which had received anti-CD40 and IL-2 immunotherapy. However, surface PD-1 expression was significantly (P < 0.01) higher on conventional CD4+ T cells and CD8+ T cells from animals that had received immunotherapy. (C) The increase in the percentage of cells expressing surface PD-1 as a fold increase in animals treated with anti-CD40 and IL-2 over control treated animals. (D) The percent of CD8+ T cells expressing surface PD-1 is not significantly (P > 0.05) increased when animals are treated with the control therapy. (E) Representative histograms show similar upregulation of surface PD-1 on CD4+ T cells from C57BL/6 mice as well as BALB/c mice; unlabeled control (gray line), Rat Ig/PBS treated animals (dashed black line) and anti-CD40 and IL-2 treated animals (solid black line). The data in A and B were repeated at least three times with similar results, the data in C was collected one time. A student t-test, with a Welch's correction for any data sets with significant differences in variance was used in B, a one way ANOVA was used in parts C and D.
Figure 3. Anti-CD40 and IL-2 immunotherapy results in an increased surface expression of B7-H1 on all hematopoietic cells.
Mice were treated with anti-CD40 and IL-2 according to the standard regimen listed in the Materials and Methods section. 11 days after treatment initiation, spleen were harvested and analyzed by flow cytometry for surface B7-H1 expression. (A) B7-H1 (PDL-1) expression was determined by median fluorescence intensity analysis and is significantly (P < 0.0001 by unpaired student t test) higher on splenocytes from animals which received anti-CD40 and IL-2. (B) Representative histograms show a similar pattern in both C57BL/6 mice as well as BALB/c mice; unlabeled control (gray line) and labeling with anti-B7-H1 antibody (clone MIH5) on CD45+ splenocytes from control treated animals (dashed black line) and animals treated with anti-CD40 and IL-2 (solid black line). (C) A comparison of the MFI of B7-H1 on CD45+ cells in control treated animals versus anti-CD40 and IL-2 treated animals, a one way ANOVA was used for statistical analysis. Each experiment was made up of three mice per group. The data shown in B and C were collected on two different flow cytometers (Beckman Coulter XL and FacScan, respectively) and the Y axes are representative of the MFI as reported by the two instruments. Experiments shown in A were completed at least three times with similar results, data in B and C was completed one time (n=3 for each).
Surface PD-1 Expression on CD4+ Tconv Cells is not Changed in the Absence of IFNγ After Immunotherapy
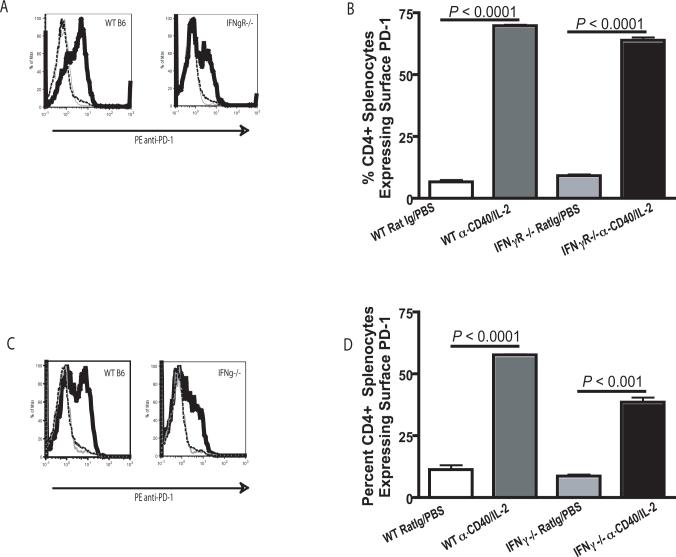
Previous data from our laboratory indicated that the selective loss of CD4+ T cells following anti-CD40 and IL- 2 was dependent on IFNγ (2). Therefore, we evaluated the relative levels of PD-1 on the surface of CD4+ T cells from wild type mice versus mice lacking either IFNγ (IFNγ−/−) or the IFNγ receptor (IFNγR−/−). After treatment with anti-CD40 and IL-2, we found surface expression of PD-1 on CD4+ T cells was still significantly (P < 0.001) upregulated in IFNγR−/− mice (Fig. 4A and B) and IFNγ−/−mice (Fig. 4C and D). Surface upregulation of PD-1 on CD4+ T cells occurred in the absence of IFNγ signaling despite increases in CD4+ T cell numbers following treatment (2). These data suggest that IFNγ is not influencing the observed reduction in CD4+ T cells through direct alteration of the surface expression of PD-1 on CD4+ T cells.
Figure 4. Surface expression of PD-1 on CD4+ T cells is not affected by IFN-γ.
Surface PD-1 expression on CD4+ T cells was evaluated by flow cytometry 11 days after immunotherapy initiation in animals lacking either IFN-γ (IFNγ−/−) or the IFN-γ Receptor (IFNγR−/−). (A) Representative histograms of PD-1 in IFNγR−/− mice show unlabeled control (gray line), Rat Ig/PBS treated animals (dashed black line) and anti-CD40 and IL-2 treated animals (solid black line). (B) Cells isolated from the spleens of IFNγR−/− and WT C57BL/6 animals showed a significantly (P < 0.001) higher percentage of CD4+ T cells in the spleen expressing surface PD-1 from animals which had been administered immunotherapy. (C) Histograms of PD-1 labeling in IFNγ−/− mice show unlabeled control (gray line), Rat Ig/PBS treated animals (dashed black line) and anti-CD40 and IL-2 treated animals (solid black line). (D) Evaluation of splenocytes from IFNγ−/− mice 11 days after the start of immunotherapy showed a higher (P < 0.001) percentage of CD4+T cells expressing surface PD-1 in animals that had been administered anti-CD40 and IL-2. Experiments using IFNγR−/− animals were completed one time with three animals per group and were supported by IFNγ−/− data which was completed three times with three animals per group. An unpaired student t-test was used to determine significant differences between animals which had received immunotherapy and the control immunotherapy, a Welch's correction was used for data sets with significant differences in variance.
IFN-γ Dependent B7-H1 Expression on Hematopoietic Cells Correlates with CD4+ T cell Loss
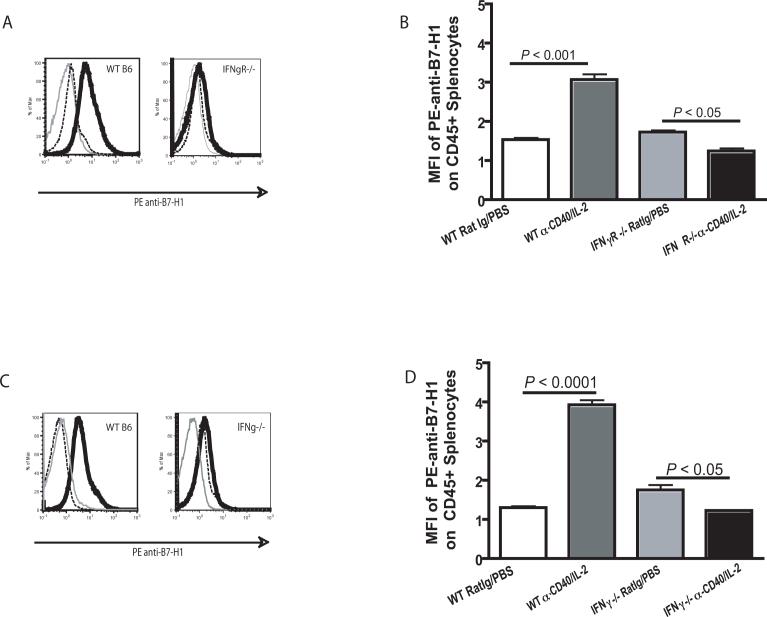
Since surface expression of PD-1 on CD4+ T cells in IFNγR−/− and IFNγ−/−mice did not directly correlate with IFNγ dependent loss of CD4+ T cells despite restoration of CD4+ T cell expansion, we evaluated the relative levels of B7-H1 expression following immunotherapy. Surface expression of B7-H1 and not PD-1, is reported to be dependent on IFNγ (12). To examine a possible correlation between B7-H1 expression and immunotherapy induced CD4+ T cell loss, flow cytometric analysis was used to determine the relative levels of B7-H1 on CD45+ cells from IFNγR−/− mice and IFNγ−/− mice after administration of anti-CD40 and IL-2. As expected, CD45+ splenocytes from wild type mice showed a significant (P < 0.001) upregulation of surface B7-H1 expression after treatment with anti-CD40 and IL-2. In contrast, CD45+ splenocytes from both IFNγR−/− mice (Fig. 5A and B) and IFNγ−/−mice (Fig. 5C and D) did not show elevated cell surface B7-H1 expression after treatment with anti-CD40 and IL-2. These data suggest that direct effects of IFNγ on B7-H1 expression patterns correlated with the observed loss of CD4+ T cells following anti-CD40 and IL-2. As selective immunotherapy induced expression of PD-1 on CD4+ Tconv cells and not Treg cells this allowed for Treg cells to avoid the inhibitory effects of anti-CD40 and IL-2 via upregulation of B7-H1. This selective upregulation results in a marked alteration of the CD4+ Tconv: CD4+ Treg: CD8+ T cell ratio which may contribute to the loss of secondary responses at a later time, after immunotherapy.
Figure 5. B7-H1 expression correlates with IFN-γ.
B7-H1 expression on all CD45+ leukocytes was evaluated in wild type animals and animals deficient in either interferon-gamma (IFNγ−/−) or the interferon-gamma receptor (IFNγR−/−). Unlike WT mice where a significantly (P < 0.001) higher level of B7-H1 was observed on cells from animals which had received immunotherapy, B7-H1 was not higher after immunotherapy in either IFNγR−/− (A and B) or IFNγ−/− (C and D) mice. An unpaired student t test was used to determine significant differences between animals which had received immunotherapy and the control immunotherapy. (A and C) Representative histograms of IFNγR−/− mice and IFNγ−/− mice respectively show control labeling (gray line), labeling of splenocytes from animals which received Rat Ig and PBS (black dashed line) or anti-CD40 and IL-2 (black solid line).
Discussion
In this manuscript, we show that tumor immunotherapy regimens that can lead to successful initial anti-tumor responses paradoxically result in a lack of CD4+ Tconv cell expansion concurrent with CD4+ Treg cell expansion and this correlates with PD-1/B7-H1 expression patterns following immunotherapy. Specifically, we present evidence that upregulation of the inhibitory molecule PD-1 on Tconv cells following immunotherapy is a likely mechanism that contributes substantively to an imbalance between potentially beneficial Tconv cells and deleterious Treg cells. Treg cells are important mediators of the inflammatory immune response through their inhibitory actions on CD4+ and CD8+ T cells as well as NK cells (14, 15). Their presence has been shown to hinder the promotion of an effective immune-mediated anti-tumor response (6). The selective expansion of Treg cells after immunotherapy described here may present a mechanism by which the immune system attempts to down-regulate itself after being exposed to such a powerful stimulus such as anti-CD40 and IL-2. Therefore, these cells may be a critical determining factor in the outcome of at least some immunotherapeutic approaches to cancer treatment.
In a previous publication, we reported substantial effects of anti-CD40 and IL-2 on the ratio of CD4+ to CD8+ T cells. In tumor-bearing mice, we showed long term effects, which changes in this ratio can have on memory CD8+ T cell responses (2). Based on our current observations, we cannot rule out the possibility that PD-1 ligation is also having an effect on CD8+ T cells. However we observed higher increases in PD-1 expression on CD4+ T cells in terms of MFI and the percent of PD-1 positive cells. Therefore, we think that in our model PD-1 is having a more pronounced effect on CD4+ Tconv cells than on CD8+ T cells. The data presented in this publication extend our previous observations to suggest that when subsets of CD4+ T cells are carefully evaluated, substantial differences in important subsets can be detected. For example, our findings suggest that the ratio of CD4+ Tconv cells to CD8+ T cells may be lower than was originally thought. This is due to a preferential expansion of Treg cells in the CD4+ T cell compartment after treatment with either anti-CD40 and IL-2 or anti-CD40 and IL-15. While the consequences of such a preferential expansion following immunotherapy have not been previously described, this may contribute to the loss of secondary responses, and also may shorten the duration of the initial anti-tumor response. It is therefore reasonable to suggest that combination immunotherapy in conjunction with Treg cell depletion may further enhance the effectiveness of this approach. One potential way to reduce the induction of Treg cells would be to use IL-15 instead of IL-2 (16), as IL-2, and not IL-15, is known to be a strong promoter of Treg cell expansion (17-19). However, we did not observe a difference in the expansion of Treg cells between the two immunotherapeutic regimens, and IL-15 alone did not induce Treg cell expansion (data not shown). This suggests Treg cell expansion may rely more on the administration of anti-CD40 than on the administration of IL-2.
Agonist CD40 mAb administration has been shown to suppress the immune response to LCMV infection resulting in an increase in viral titers after treatment. In this LCMV model, loss of antigen specific CD8+ T cells was observed. Interestingly, in this model a significant decrease in CD4+ T cells was also observed after treatment with anti-CD40 alone, however any potential correlation with PD-1 expression was not discussed (3).
The use of anti-CD40 and IL-2 provides a model for investigating the disadvantages and benefits of potent immune stimulation. This model magnifies differences that may occur in the effectiveness of initial versus long term immune-mediated tumor responses. In this regard, our recent studies indicate that strong immunotherapeutic regimens such as anti-CD40 and IL-2 combined therapy can hamper long term responses to antigen through deleterious changes in the CD4+:CD8+ T cell balance (2). Alterations in this balance have been of great interest for some time (20, 21). Most recently there has been a debate about potentially “helpless” CD8+ T cells being incapable of responding to secondary antigenic challenge, which can occur even when primary response capabilities function with complete normalcy and strength (22, 23).
Surface expression patterns of PD-1 on human CD4+ T cells can be used as an indication of disease outcome in various human disease settings such as rheumatoid arthritis, schistosomiasis and Hodgkin's lymphoma (24-26). Similarly, PD-1 expression occurs on the CD8+ T-cells of patients infected with HIV who show long term progressor status (27). However, few reports have discussed the relative expression patterns on CD4+ T cell subsets, and to our knowledge, no reports have discussed a differential response of CD4+ T cell subsets to immunotherapy dependent on this pathway. While we observed a differential expression of PD-1 on the surface of Tconv cells and Treg cells after anti-CD40 and IL-2, we did not however find a difference in the expression pattern of other immune markers such as Fas or DR5 after treatment (data not shown).
PD-1 has two known ligands, B7-H1 (PDL-1, CD274) and B7-DC (PDL-2, CD273) (12). B7-H1 is found on many cell types including lymphocytes and myeloid cells as well as cells that are not of hematopoietic origin (8). B7-DC is primarily found on dendritic cells and is not upregulated in response to IFN-γ; therefore, we have focused on effects of B7-H1. Ligation of PD-1 by B7-H1 is capable of eliciting either apoptosis or senescence (7, 12). B7-H1 is inducibly upregulated on tumor cells both in vivo and in vitro, and is therefore thought to be important in tumor evasion of immune responses (28). PD-1 engagement by B7-H1 has been shown to have potent inhibitory effects on immune stimulation (29, 30) resulting in promotion of CD8+ T cell tolerance to self antigens in the periphery (9). Therefore, the PD-1/B7-H1 pathway is currently under intense investigation since manipulating it has the potential to modulate immune responses in a positive or negative manner (31-33).
Given our data presented here, further studies in tumor bearing mice could address the question of whether selective upregulation of PD-1 on Tconv cells and not Treg cells following immunotherapy might allow the tumor to dampen the effectiveness of tumor infiltrating lymphocytes, while not affecting the immune inhibiting function of Treg cells. B7-H1 upregulation may additionally promote immune suppression by supporting cell conversion to suppressive phenotype. In addition to Tconv cells being negatively affected through ligation of PD-1 by B7-H1, Treg cells may benefit from this interaction (34). In H. Pylori infection, T-cell anergy at the site of infection has been shown to be dependent on PD-1 ligation by B7-H1. It was shown that the presence of B7-H1 promoted an increase in Treg cell frequency when CD4+ T cells from H. Pylori infected donors were co-cultured with H. Pylori infected epithelial cells in vitro. This Treg cell expansion was abrogated when anti-B7-H1 antibody was included (34).
Blockade of PD-1 and/or B7-H1 as well as other inhibitory markers such as CTLA-4 has recently been of interest when attempting to break tolerance (11, 35), (36). Combined PD-1 and B7-H1 blockade, but not B7-DC is reported not only to enhance CD8+ T cell mediated anti-tumor responses, but also to reverse anergy in CD8+ T cells (11). It is important to note, however that blockade of the PD-1/B7-H1 pathway usually only results in a partial removal of its inhibitory effect. Studies in our model aimed at the blockade of PD-1 or B7-H1 singly with anti-CD40 and IL-2 had no effect in vivo possibly due to the massive expansion of PD-1+ and B7-H1+ cells which would require very high levels of blocking antibody to obtain results (data not shown). This demonstrates one of the potential problems when exerting very strong stimulatory signals to amplify immune responses. Studies using PD-1 or B7-H1 knockout mice may be the best way to determine if anti-tumor responses after immunotherapy are enhanced by the removal of PD-1 or B7-H1 signaling.
Relieving immune responses from the strict control that is mediated through PD-1/B7-H1 may be beneficial for the development of more effective anti-tumor immunotherapeutic approaches. Herein we report a previously undescribed preferential expansion of Treg cells which occurs in parallel to the loss of effector cells after administration of anti-CD40 and IL-2. It is of interest that this preferential Treg expansion still resulted in marked initial anti-tumor effects (1, 2). Because of its potent immunomodulating capabilities, the PD-1 and B7-H1 receptor/ligand interactions provide a potentially important component that should be further considered with regard to immune changes and overall responses that can be induced by different forms of immunotherapy. Our observations highlight the strong opposing force that the immune system has to potent stimuli. The different regulatory mechanisms utilized to protect from over stimulation may hinder efforts toward more effective anti-tumor immunotherapies.
Acknowledgements
We thank Myriam Bouchlaka and William Hallett for help with reviewing the manuscript as well as Weihong Ma and Megan Whitaker for their technical help.
Footnotes
Grant Support: R01 A134495; R01 CA72669; R37; HL56067; P01 AI0562991, P20 RR-016464
Contribution: W.J.M, D.R, L.A.W, B.R.B and R.W.H contributed research design and experimental oversight as well as data interpretation and help with writing the manuscript. K.L.A, Q.Z., V.B., D.E.C.W and J.M.W. conducted experiments as well as helped with data analysis and writing the manuscript.
Conflict-of-Interest Disclosure: W.J.M and B.R.B are members of the Scientific Advisory Board for Seattle Genetics, Inc. Bothell, Wa, 98021.
References
- 1.Murphy WJ, Welniak L, Back T, Hixon J, Subleski J, Seki N, Wigginton JM, Wilson SE, Blazar BR, Malyguine AM, Sayers TJ, Wiltrout RH. Synergistic anti-tumor responses after administration of agonistic antibodies to CD40 and IL-2: coordination of dendritic and CD8+ cell responses. J Immunol. 2003;170:2727–2733. doi: 10.4049/jimmunol.170.5.2727. [DOI] [PubMed] [Google Scholar]
- 2.Berner V, Liu H, Zhou Q, Alderson KL, Sun K, Weiss JM, Back TC, Longo DL, Blazar BR, Wiltrout RH, Welniak LA, Redelman D, Murphy WJ. IFN-gamma mediates CD4+ T-cell loss and impairs secondary antitumor responses after successful initial immunotherapy. Nat Med. 2007;13:354–360. doi: 10.1038/nm1554. [DOI] [PubMed] [Google Scholar]
- 3.Bartholdy C, Kauffmann SO, Christensen JP, Thomsen AR. Agonistic anti-CD40 antibody profoundly suppresses the immune response to infection with lymphocytic choriomeningitis virus. J Immunol. 2007;178:1662–1670. doi: 10.4049/jimmunol.178.3.1662. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, Matzinger P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109:5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148:32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. Embo J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okazaki T, Iwai Y, Honjo T. New regulatory co-receptors: inducible co-stimulator and PD-1. Curr Opin Immunol. 2002;14:779–782. doi: 10.1016/s0952-7915(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 9.Martin-Orozco N, Wang YH, Yagita H, Dong C. Cutting Edge: Programmed death (PD) ligand-1/PD-1 interaction is required for CD8+ T cell tolerance to tissue antigens. J Immunol. 2006;177:8291–8295. doi: 10.4049/jimmunol.177.12.8291. [DOI] [PubMed] [Google Scholar]
- 10.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg MV, Maris CH, Hipkiss EL, Flies AS, Zhen L, Tuder RM, Grosso JF, Harris TJ, Getnet D, Whartenby KA, Brockstedt DG, Dubensky TW, Jr., Chen L, Pardoll DM, Drake CG. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–192. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flies DB, Chen L. The new B7s: playing a pivotal role in tumor immunity. J Immunother (1997) 2007;30:251–260. doi: 10.1097/CJI.0b013e31802e085a. [DOI] [PubMed] [Google Scholar]
- 13.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picca CC, Larkin J, 3rd, Boesteanu A, Lerman MA, Rankin AL, Caton AJ. Role of TCR specificity in CD4+ CD25+ regulatory T-cell selection. Immunol Rev. 2006;212:74–85. doi: 10.1111/j.0105-2896.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 15.Barao I, Hanash AM, Hallett W, Welniak LA, Sun K, Redelman D, Blazar BR, Levy RB, Murphy WJ. Suppression of natural killer cell-mediated bone marrow cell rejection by CD4+CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:5460–5465. doi: 10.1073/pnas.0509249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antony PA, Restifo NP. CD4+CD25+ T regulatory cells, immunotherapy of cancer, and interleukin-2. J Immunother (1997) 2005;28:120–128. doi: 10.1097/01.cji.0000155049.26787.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato N, Patel HJ, Waldmann TA, Tagaya Y. The IL-15/IL-15Ralpha on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc Natl Acad Sci U S A. 2007;104:588–593. doi: 10.1073/pnas.0610115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 21.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 23.Badovinac VP, Messingham KA, Griffith TS, Harty JT. TRAIL deficiency delays, but does not prevent, erosion in the quality of “helpless” memory CD8 T cells. J Immunol. 2006;177:999–1006. doi: 10.4049/jimmunol.177.2.999. [DOI] [PubMed] [Google Scholar]
- 24.Chemnitz JM, Eggle D, Driesen J, Classen S, Riley JL, Debey-Pascher S, Beyer M, Popov A, Zander T, Schultze JL. RNA-fingerprints provide direct evidence for the inhibitory role of TGF{beta} and PD-1 on CD4+ T cells in Hodgkin's lymphoma. Blood. 2007 doi: 10.1182/blood-2006-12-064360. [DOI] [PubMed] [Google Scholar]
- 25.Colley DG, Sasser LE, Reed AM. PD-L2+ dendritic cells and PD-1+ CD4+ T cells in schistosomiasis correlate with morbidity. Parasite Immunol. 2005;27:45–53. doi: 10.1111/j.1365-3024.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 26.Hatachi S, Iwai Y, Kawano S, Morinobu S, Kobayashi M, Koshiba M, Saura R, Kurosaka M, Honjo T, Kumagai S. CD4+ PD-1+ T cells accumulate as unique anergic cells in rheumatoid arthritis synovial fluid. J Rheumatol. 2003;30:1410–1419. [PubMed] [Google Scholar]
- 27.Zhang JY, Zhang Z, Wang X, Fu JL, Yao J, Jiao Y, Chen L, Zhang H, Wei J, Jin L, Shi M, Gao GF, Wu H, Wang FS. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 28.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 29.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Grakoui A, John Wherry E, Hanson HL, Walker C, Ahmed R. Turning on the off switch: regulation of anti-viral T cell responses in the liver by the PD-1/PD-L1 pathway. J Hepatol. 2006;45:468–472. doi: 10.1016/j.jhep.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Khoury SJ, Sayegh MH. The roles of the new negative T cell costimulatory pathways in regulating autoimmunity. Immunity. 2004;20:529–538. doi: 10.1016/s1074-7613(04)00116-5. [DOI] [PubMed] [Google Scholar]
- 32.Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, Nishimura H, Taylor PA. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171:1272–1277. doi: 10.4049/jimmunol.171.3.1272. [DOI] [PubMed] [Google Scholar]
- 33.Hori J, Wang M, Miyashita M, Tanemoto K, Takahashi H, Takemori T, Okumura K, Yagita H, Azuma M. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J Immunol. 2006;177:5928–5935. doi: 10.4049/jimmunol.177.9.5928. [DOI] [PubMed] [Google Scholar]
- 34.Beswick EJ, Pinchuk IV, Das S, Powell DW, Reyes VE. B7-H1 Expression on Gastric Epithelial Cells after Helicobacter pylori Exposure Promotes the Development of CD4+ CD25+ FoxP3+ Regulatory T Cells. Infect Immun. 2007 doi: 10.1128/IAI.00553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsushima F, Yao S, Shin T, Flies A, Flies S, Xu H, Tamada K, Pardoll DM, Chen L. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110:180–185. doi: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175:7746–7754. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]