Abstract
Accumulation of oxidative stress-induced damage in brain tissue plays an important role in the pathogenesis of normal aging and neurodegenerative diseases. Neuronal oxidative damage typically increases with age in humans, and also in the invertebrate and vertebrate model species most commonly used in aging research. By use of quantitative immunohistochemistry and Western blot, we show that this aspect of brain senescence is largely decoupled from chronological age in the honey bee (Apis mellifera). The bee is a eusocial insect characterized by the presence of a reproductive queen caste and a caste of functionally sterile female workers that performs various alloparental tasks such as nursing and foraging. We studied patterns of oxidative nitration and carbonylation damage in the brain of worker bees that performed nurse tasks as 8- and 200-day-olds and foraging tasks as 20- and 200-day-olds. In addition, we examined 180-day-old diutinus bees, a stress-resistant temporal worker form that survives unfavorable periods. Our results indicate that nitration damage occurs only at low levels in vivo, but that a 60-kDa protein from honey bee brain is selectively nitrated by peroxynitrite in vitro. Oxidative carbonylation is present at varying levels in the visual and chemosensory neuropiles of worker bees, and this inter-individual variation is better explained by social role than by chronological age.
Keywords: Honey bee, Oxidative stress, Brain senescence, Stage-dependent aging
1. Introduction
The honey bee worker is an established model for studies of learning and memory formation (Menzel and Müller, 1996; Scheiner et al., 2001; Giurfa, 2003; Sandoz et al., 2003), behavioral ecology (Robinson et al., 1992; Pankiw et al., 1998), and social evolution (Page and Erber, 2002; Amdam and Omholt, 2003; Amdam et al., 2004a). Recently, this eusocial insect caste has also started to contribute to our understanding of aging regulation (Omholt and Amdam, 2004; Amdam, 2005; Amdam and Page, 2005; Amdam et al., 2005a). Worker bees display a flexible pattern of longevity that is interlinked with the alloparental tasks they perform (Amdam and Page, 2005). Typically, a worker engages in nest activities (such as nursing larvae), before she switches to more risky exterior hive activities like guarding and foraging as a 18–28-day-old (Winston, 1987). This behavioral progression leads to life spans of 6–7 weeks because workers normally survive only 1–3 weeks of active foraging (Visscher and Dukas, 1997).
Worker bees, however, can become foragers as early as 4–7 days after adult emergence (reviewed by Amdam and Omholt, 2002), and they can also enter the stress-resistant diutinus stage formerly referred to as the “winter bee” phenotype (Omholt and Amdam, 2004) that enables them to survive for 8–10 months (Maurizio, 1950; Sekiguchi and Sakagami, 1966). Adult worker bees of the North European A. mellifera spp. carnica and mellifera enter the diutinus stage when brood (eggs, larvae, and pupae) are removed from their colonies (Maurizio, 1950; Fluri et al., 1982; Amdam et al., 2004b, 2005b). Thus, in temperate regions this survival stage develops in late fall when brood-reading comes to a natural halt (Fluri and Imdorf, 1989). The long-lived diutinus phenotype is absent in tropical and sub-tropical A. m. scutellata and scutellata hybrids, and it is a possible adaptation to survival in temperate climates (Amdam and Omholt, 2002; Amdam et al., 2005b; Seehuus et al., 2006). Diutinus workers do not nurse brood or forage, but they maintain the colony core temperature at about 30 °C. Heat is generated by resting metabolism and thoracic muscle contraction, and the energy flux from a colony of about 15,000 diutinus bees is about 520 and 2500 kJ daily when ambient temperature is 5–10 and −20 °C, respectively (Omholt, 1987).
The plasticity of worker longevity has led to the hypothesis that senescence is not a simple function of chronological age in the bee. Stage- rather than age-dependent patterns of aging have been suggested to emerge in part through an interplay between the yolk precursor gene vitallogenin and the systemic juvenile hormone (JH) titer (Amdam et al., 2004c). The vitellogenin protein has an antioxidant function (Seehuus et al., 2006), and in addition vitellogenin activity suppresses the intrinsic JH level of worker bees (Guidugli et al., 2005). In Drosophila melanogaster, JH negatively affects longevity and oxidative stress resistance (Tatar and Yin, 2001; Tu and Tatar, 2003), and accumulated oxidative stress-induced damage is an established biomarker of aging (Sohal et al., 1995; Radak et al., 2002; Sohal, 2002; Soreghan et al., 2003; Nystrom, 2005). It has been proposed that foragers in response to low vitellogenin protein levels and high JH titers age more rapidly than nurse bees and diutinus workers that are characterized by high vitellogenin levels and low intrinsic JH titers (reviewed by Amdam, 2005). Yet, it has not been confirmed that putative stage-dependent modulation of somatic maintenance translates into corresponding patterns of oxidative stress-induced damage in free-flying bees.
Here we address this pending question through a study of cellular senescence in the brain of foragers, nurses, and diutinus bees. The brain was chosen for examination because it is sensitive to highly reactive oxygen (hROS) and nitrogen species (hRNS) (Bowling and Beal, 1995; Tyurin et al., 2000; Rival et al., 2004). If not counteracted by free radical defense mechanisms, reactive oxygen (ROS) and nitrogen species (RNS) cause damage to cellular components via protein oxidation and nitration (Sohal and Weindruch, 1996; Sohal, 2002).
2. Materials and methods
2.1. Bees
Bees were obtained from host colonies in the apiary of the Norwegian University of Life Sciences, Aas. From each host colony, collected subsets of bees represented the different social task groups (or temporal stages) of honey bee workers. One hundred and eighty day-old diutinus workers (“winter bees”), 200-day-old post-wintering nurse bees and 200-day-old post-wintering pollen foragers were collected in March and April 2004 and 2006. Bees of known age were obtained by marking newly emerged bees on the thorax with a spot of paint (Uni Posca) before introduction into the host colonies. Three colony sources were used to obtain newly emerged bees, and the bees were mixed before marking and introduction so that each host colony received roughly equal numbers of workers from each source.
As described by Maurizio (1950) and Omholt and Amdam (2004), workers were characterized as diutinus workers in the absence of brood (i.e., egg, larvae, and pupae), whereas post-wintering nurses and post-wintering foragers were obtained within 2 weeks after the colonies initiated brood rearing after the winter dearth. To control for effects of absolute age and season, 8-day-old nurse bees and 20-day-old foragers were collected from the same host colonies in June 2004. Nurse bees and pollen foragers were collected in the brood nests and at the hive entrances, respectively.
2.2. Immunohistochemistry
For each experimental worker group, 30–50 individuals were assayed with immunohistochemistry. Protein oxidation and nitration can be detected by the presence of protein carbonyls and tyrosine nitration, respectively (Sohal and Weindruch, 1996; Sohal, 2002). Putative oxidative stress damage to proteins, therefore, was visualized by staining with anti-dinitrophenyl (anti-DNP) against oxidative carbonylation, and anti-nitrotyrosine (anti-Ntyr) for detection of nitration. Bee heads were fixed in 4% methanol-free formaldehyde solution. After fixation, tissues for cryosectioning were infiltrated with a graded series of sucrose in a phosphate-buffered saline (PBS) solution (0.8, 1.0, 1.4 M), thoroughly washed in PBS, and mounted in Tissue-Tek, O.C.T.™ compound (Tamro Medlab) over dry ice. The cryomounts were sectioned in 15–25-µm slices, captured on SuperFrost®Plus (Chemi-Teknik) slides and kept cold before staining. Tissues for embedding in resin were washed in 0.1 M PIPES buffer, dehydrated in a graded series of alcohol (70, 90, 95%, and 4 × 100%), each step for 15 min. After dehydration, tissues were infiltrated with a graded series of LR-White (Electron Microscopy Sciences) (1) three parts ethanol:one part LR-White overnight at room temperature (RT), (2) one part ethanol:one part LR-White overnight at RT, (3) one part ethanol:three parts LR-White overnight at RT, and (4) 100% LR-White overnight at RT. Tissues were placed in Teflon beds filled with LR-White and polymerized overnight at 60 °C. The resulting resin blocks were cut in semi-thin sections, captured on SuperFrost®Plus (Chemi-Teknik), and allowed to dry before staining.
Sections for anti-DNP staining were washed (3 × 15 min) in PBS, incubated with 0.2% DNP-hydrazine (Intergen) for 1 h at RT, and etched in 0.6% H2O2 in methanol (30 min, RT). Etched slices were washed thoroughly (4 × 15 min) in PBS supplemented with 1% bovine serum albumin (BSA) before blocking with PBS w. 2% BSA for 1 h (RT). The slices were then washed (4 × 15 min) in PBS w. 1% BSA and incubated with a monoclonal antibody (1:150) to DNP (Intergen) overnight at 4 °C.
Sections for anti-Ntyr staining were washed (3 × 15 min) in PBS and etched in 0.6% H2O2 in methanol for 30 min (RT) washed (4 × 15 min) in PBS w.1% BSA before blocking with PBS w. 2% BSA for 1 h (RT). The sections were washed (4 × 15 min) in PBS w. 1% BSA followed by incubation with a monoclonal antibody (1:150) to Ntyr (Chemicon) at 4 °C overnight. Controls for both Ntyr and DNP staining were treated with secondary antibody only. Primary antibody-labeled sections were washed (4 × 15 min) in PBS w. 1% BSA and (4 × 15 min) in PBS before incubation with HRP-conjugated goat anti-rabbit IgG (1:300) overnight at 4 °C. Labeled sections were washed (4 × 15 min) in PBS before incubation for 1 h (RT) with HRP (1:200) (Sigma–Aldrich). Sections were then washed (4 × 15 min) in PBS and (4 × 15 min) in aqua dest and subsequently developed in a diaminobenzidine-nickel solution (DAB) (Sigma–Aldrich) until dark blue in color, washed in aqua dest, and coverslipped. Stained sections were examined by light microscopy.
2.3. Immunoblot detection of nitrotyrosine damage
Because sectioned tissues showed uniformly low levels of anti-Ntyr staining, an alternative detection method with positive controls for nitrotyrosine nitration was established by Western blot. Bees were anesthetized on ice before brains were dissected out in insect saline. Tissues were transferred to Tris buffer (20 mM Tris–HCl, 150 mM NaCl, 5 mM EDTA pH 7.5, 1 mM phenylmethylsulfonyl fluoride, 5 mM benzamidin, 0.7 µM pepstatin, 8 µM chymostatin, 10 µM leupeptin, and 0.8 µM aprotinin, Sigma–Aldrich). Within group, three brains were pooled in duplicate and directly processed for detection of nitrotyrosine damage using the Nitrotyrosine Immunoblot Kit (Cell Biolabs, Inc.). In brief, the brains were homogenized in Tris buffer (see above) and centrifuged for 5 min at 14,000g. The supernatant was collected and protein concentration adjusted to 2 µg/µl. Twenty microliters of sample, 30 µl of H2O, and 10 µl of reducing loading buffer were boiled 5 min. For each sample, 15 µl corresponding to 20 µg protein was run on 7.5% SDS–PAGE gels. Nitrotyrosine immunoblot control (nitrated BSA, 1 µg protein) and brain samples pretreated with 31.3 mM peroxynitrite in 0.3 M NaOH (Sigma–Aldrich) (20 µg protein) were used as positive controls. Separated proteins were transferred to nitro-cellulose membrane, blocked with 5% BSA, washed, and incubated with anti-Ntyr. The membrane was washed repeatedly and incubated with goat anti-rabbit IgG, HRP-conjugated, washed, and developed with DAB until the nitrated BSA control marker was stained specifically as described by the manufacturer (Cell Biolabs, Inc.).
2.4. Semi-quantitative estimation of carbonylation damage
Initial analysis of the sectioned and stained material (see above) showed that areas positive for carbonylation damage primarily were located in the optical lobes. Therefore images of these regions, taken at 16× with a Leica DFC320 camera, were converted to 8-bit gray scale and densitometrically quantified using QantiOne software (Bio-Rad). From each sample group (180-day-old diutinus bees, 200-day-old post-wintering foragers, 200-day-old post-wintering nurse bees, 8-day-old nurses, and 20-day-old foragers), pictures from three to six individuals were selected by an operator that was blind to the social task identity of the bees. The operator ensured that the general quality of the images and section depth from the optical lobes were similar. Subsequently, 60 areas were randomly marked throughout each image, and positive staining intensity measured densitometrically. After background correction, the mean score of the 60 areas was used as a semi-quantitative measurement of individual carbonylation damage.
2.5. Data analysis
The data from the densitometric quantification of carbonylation damage passed Levene’s test of homogeneity of variances. As a first step, therefore, multivariate ANOVA was used. Age, task, and host colony were used as categorical factors. Data from diutinus workers were initially omitted because task (diutinus) and age (180 days) were confounded. The multivariate ANOVA showed that only task explained a significant proportion of variance (see below), and thus the diutinus stage was included in an LSD post hoc test on the effect of task on carbonylation damage. Proc GLM in SAS, release 8.02 and Statistica 6.0 were used for the analysis.
3. Results
Our immunohistochemical analysis did not identify distinct patterns of nitration damage in the honey bee worker brain (Fig. 1). Therefore, brain tissue samples were also analyzed for tyrosine nitration by Western blot. Positive controls for nitration damage were included (Fig. 2). Brain protein samples from social task groups showed very low nitrotyrosine levels compared to brain tissue nitrated with 31.3 mM peroxynitrite (Fig. 2, lanes 1–6 vs. 7); and only one protein at about 60 kDa was strongly nitrated in this positive control (Fig. 2, lane 7). Limited positive staining was found for a protein of similar size in one pooled sample of diutinus bees (Fig. 2, lane 1) and one pooled sample of nurse bees (Fig. 2, lane 4). The immunoreactive response of the 60-kDa product after peroxynitrite treatment (Fig. 2, lane 7) demonstrates that this unidentified protein can be selectively nitrated. Similar selective nitration has been reported previously for p130(cas) (130 kDa) from human neuroblastoma SH-SY5Y cells (Saeki and Maeda, 1999), alpha-tubulin (50–55 kDa) from human gliomas and rat brain (Dremina et al., 2005; Fiore et al., 2006), alpha-actinin (100 kDa) from human myocardium (Borbely et al., 2005), and prostaglandin endoperoxide synthase-2 (72 kDa) from RAW 264.7 macrophages (Schildknecht et al., 2006). Yet, although our data indicate that selective nitration by peroxynitrite targets one protein product also in honey bee brain, the cumulative data from the immunohistochemical (Fig. 1) and Western blot analyses (Fig. 2, lanes 1–6) suggest that worker bees are characterized by low levels of brain nitration damage in vivo.
Fig. 1.
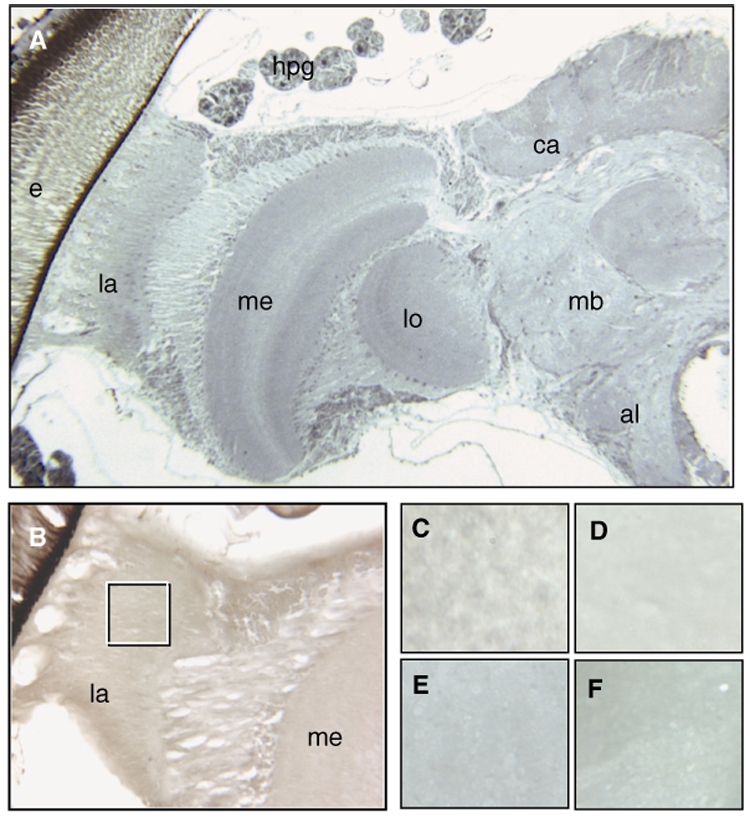
Overview of one-half of a honey bee brain (A) at 5×. Immunolocalization of nitrated proteins in cryosections (B–F) did not detect positive signals for nitration damage. The inserted box in (B) (16×, diutinus worker) refers to the area depicted in (C–F) (40×). (C) Nurse bee, (D) diutinus worker, (E) post-wintering forager, and (F) forager collected in summer. The differences in color between the pictures are optical artifacts and not due to positive staining. Abbreviations in (A and B): e, compound eye; la, lamina; me, medulla; lo, lobula; al, antennal lobe; mb, mushroom bodies; ca, calyx; hpg, hypopharyngeal gland (brood-food-producing glands). The images are representative for the full sample set (n = 30–50 per experimental group).
Fig. 2.
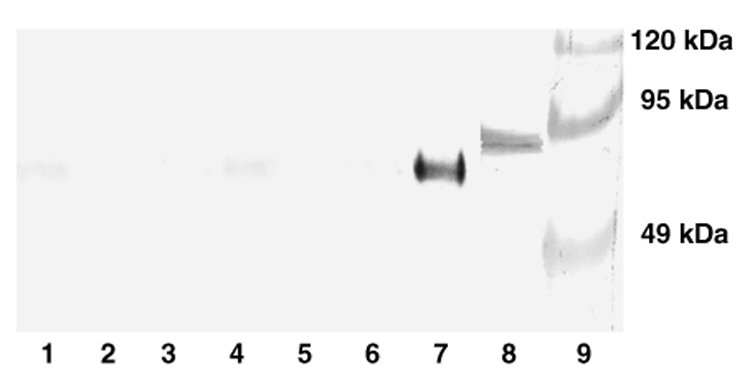
Nitrotyrosine levels in bee brain visualized by Western blot. The amount of nitrotyrosine nitration damage is low compared to positive controls. Lanes 1 and 2: diutinus bees. Lanes 3 and 4: nurse bees. Lanes 5 and 6: foragers. Lane 7: positive control; honey bee brain tissue homogenized with 31.3 mM peroxynitrite. Lane 8: positive control: 1 µg nitrated BSA from Cell Biolabs, Inc. Lane 9: Prestained molecular marker. Each sample from worker bees (lanes 1–7) contains 20 µg protein.
Oxidative carbonyls were not found in the antennal lobes (as exemplified by Fig. 3), and only scattered oxidative carbonylation damage was detected in the mushroom bodies and the calyx regions (data not shown). However, consistent positive staining for protein carbonylation was documented in the optic lobes (Fig. 3 and Fig. 4). In particular, the lamina region and the transition zone between the lamina and the medulla were affected in all the examined bees (n = 30–50 per experimental group) (Figs. 3A–C). This carbonylation damage was estimated semi-quantitatively in bees from three different host colonies. We found that social role (task) had a significant effect on the level of protein carbonylation in worker brain (multivariate ANOVA: F1,6 = 20.82, P < 0.005). Chronological age and host colony, however, did not affect the level of carbonylation damage (multivariate ANOVA: F2,6 = 3.64, P = 0.10 and F2,6 = 0.49, P = 0.63, respectively). Post hoc comparisons between social task groups showed that nurse bees and diutinus workers are characterized by correspondingly low levels of protein carbonylation in the brain (LSD: P = 0.28). In comparison, the amount of carbonylation damage is significantly higher in foragers (LSD: P < 0.000005) (Fig. 5). These data show that oxidative carbonylation damage in the brain of honey bees is more strongly linked to social role than to chronological age.
Fig. 3.
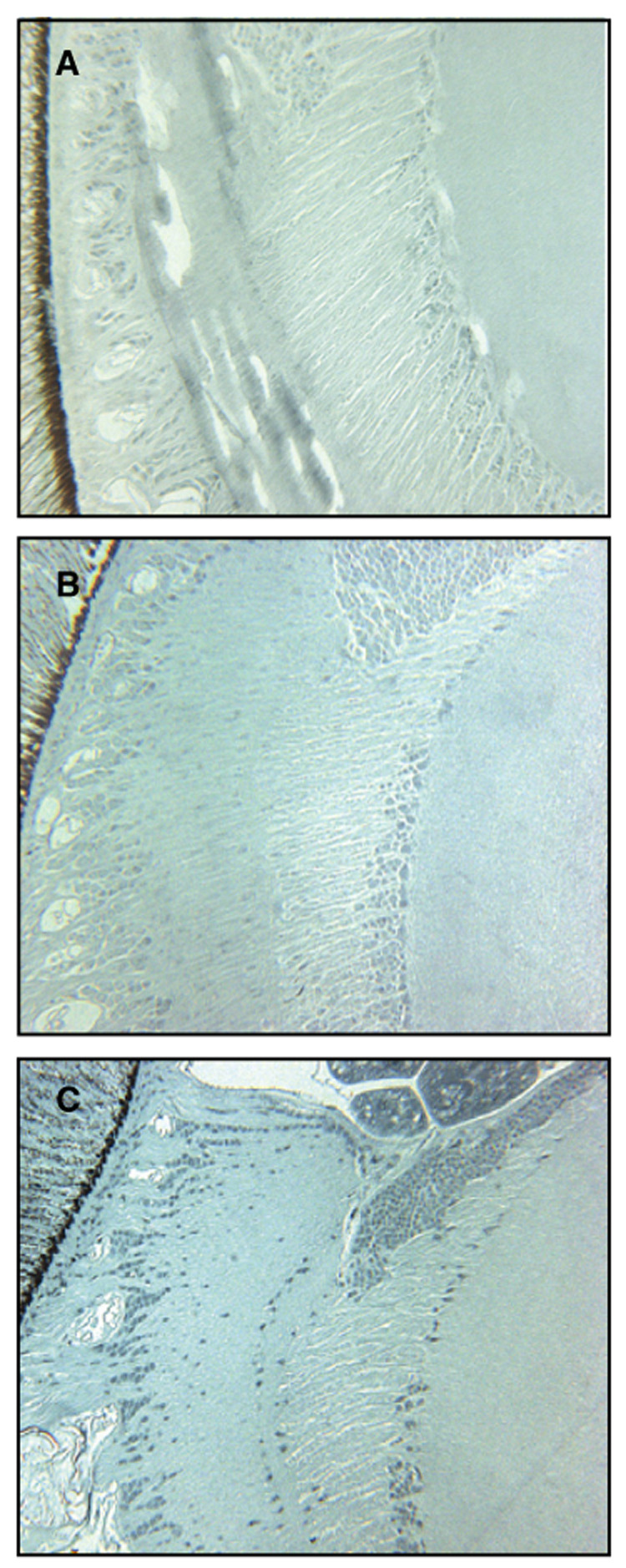
Immunolocalization of carbonylated proteins in semi-thin (A–C) sections. The differences in oxidative carbonylation of proteins were consistent between groups in the lamina region and transition zone between lamina and medulla. Eight-day-old nurse bees (A, 16×) show very low levels of protein carbonylation, 180-day-old diutinus workers (B, 16×) are characterized by some positive staining, mainly in the lamina, whereas 20-day-old foragers (C, 16×) are characterized by intense labeling of carbonylated proteins both in the laminar area and the medulla.
Fig. 4.
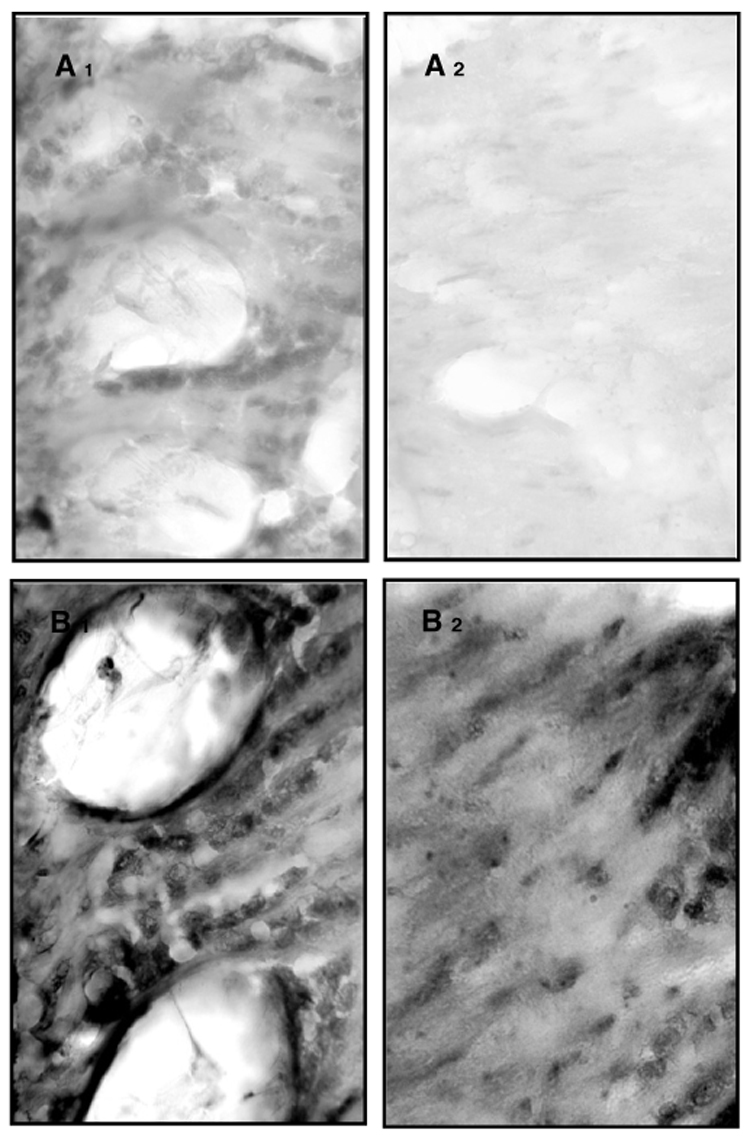
Immunolocalization of carbonylated proteins in cryosections (A and B). Close ups (40×) of lamina (1) and medulla (2) show that between-group differences in protein carbonylation are linked to social role rather than chronological age. The close up of 200-day-old nurse bees show some staining, mainly in the lamina (A1), with scattered staining in the medulla (A2), whereas 200-day-old foragers have high amounts of carbonylated proteins in both the lamina (B1) and medulla regions (B2).
Fig. 5.
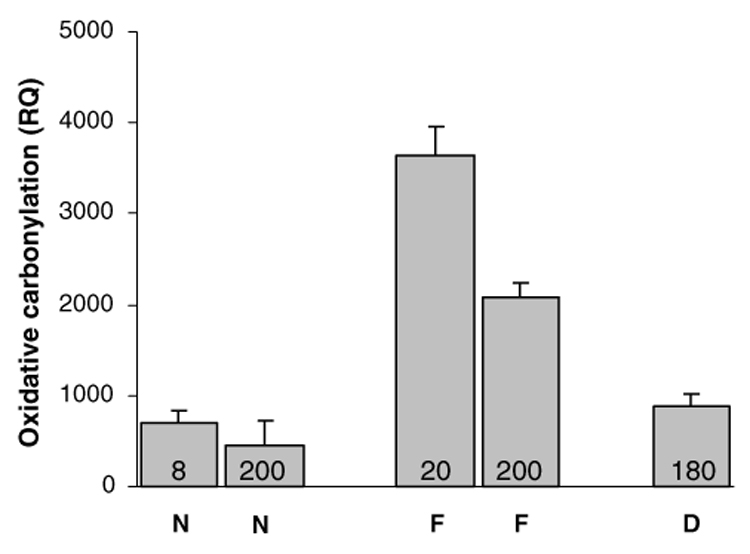
Densitometrical intensities as relative quantities (RQ) in images of brain sections stained for oxidative carbonylation damage. N, nurse bees; F, foragers; D, diutinus bees. Numbers within bars are chronological age in days. Social role (P < 0.005) but not age (P = 0.10) had a significant effect on the level of carbonylation damage. Bars are means with standard errors (n = 3–6).
4. Discussion
The oxidative stress hypothesis of aging states that senescence is caused by an accumulation of oxidative stress-induced damage (Sohal, 2002). The presence of protein carbonyl derivatives in cells reflects injury from multiple forms of ROS, and is used as a measure of oxidative stress-induced cellular damage that is associated with aging and age-related disease (Wang et al., 2001; Stadtman and Levine, 2003). Here, we have demonstrated the presence of such damage in honey bee workers. In principle, this physiological deterioration is similar to observations in other species, in which the oxidative insult to macromolecules, such as lipids, proteins, and DNA, has been shown to increase with age (Sohal, 2002). Contrary to these findings, our data indicate that cellular senescence is not a simple function of chronological age in the bee: 8- and 200-day-old nurse bees and 180-day-old diutinus bees show low levels of carbonylation damage in brain, whereas 20- and 200-day-old foragers show elevated levels of brain carbonylation damage (Fig. 5). These results are in accordance with the hypothesis that foragers age more rapidly than other workers (Omholt and Amdam, 2004), a proposition that is further supported by recent data on cellular immunity (Amdam et al., 2004c) and oxidative stress resistance (Seehuus et al., 2006).
Nitration damage occurs in brain when nitric oxide is produced at so high levels that it changes from a physiological neuromodulator to a neurotoxic factor. This condition occurs in association with pathologies that affect the amount of nitric oxide in neuronal tissue, such as inflammation or extracellular aggregation of amyloid β-peptide (reviewed by Guix et al., 2005). The low levels of nitration damage detected in our study may be due to an absence of these, or similar, disease states. However, future biochemical identification of the 60-kDa protein product selectively nitrated by peroxynitrite (Fig. 2) could provide higher-resolution information based on distribution and putative function in brain. Oxidative carbonylation damage was mainly restricted to the optic lobes of the worker bees. This pattern may emerge because optic lobes are more sensitive to oxidative stress than other parts of the brain, or because oxidative load per se is higher in this region. Lack of more excessive oxidative stress damage in foragers, the task group with highest accumulation of carbonylated proteins (Fig. 5), may be explained by our sampling of free-flying bees. Forager mortality in part is due to predation, to adverse ambient conditions, and to failure of finding the colony after flight (reviewed by Gary, 1992). Navigation and nest-site recognition is a complex cognitive process (Menzel et al., 1996). Foragers with a more severely damaged brain, therefore, may not survive. The observed pattern of accelerated cellular senescence in the optic lobes of foragers, furthermore, can be explained by one or more physiological, behavioral, and environmental factors that distinguish foraging bees from nurse bees and diutinus workers. These factors are discussed in the following sections.
Foragers may show an accumulation of carbonylated proteins because they produce more ROS than nurse bees and diutinus workers. Flying insects achieve the highest mass-specific rates of aerobic metabolism in the animal kingdom (Crailsheim et al., 1996; Suarez et al., 1996). In the housefly Musca domestica, prevention of flight activity decreases metabolic rate and results in reduced oxygen consumption and ROS production; it also decreases levels of carbonylation of mitochondrial proteins, reduces loss of protein function, and increases life span (Ryan et al., 2000). Elevated levels of oxygen consumption and mass-specific metabolic rate have been documented in honey bee foragers (Neukirch, 1982; Crailsheim et al., 1996; Suarez et al., 1996), and it has previously been hypothesized that the progression of aging in worker bees is driven by intense flight (Neukirch, 1982). Yet, diutinus bees may also perform considerable metabolic work as they metabolize the colony’s honey stores to produce heat over several months (Omholt, 1987). An additional explanation for the diverging patterns of oxidative carbonylation damage, therefore, is that foragers differ from the other social task groups in their relative investment in free radical defense (Warner, 1993). One such defense mechanism is the circulating level of the vitellogenin protein, which acts as free radical scavenger in honey bees (Seehuus et al., 2006). The vitellogenin titer is downregulated in foragers (5–10 µg/µl) (Pinto et al., 2000), but is high in diutinus bees (60–90 µg/µl) and nursing workers (20–50 µg/µl) (Amdam et al., 2004c). The pattern of oxidative protein carbonylation observed in our study, therefore, correlates with previously established differences in oxidative stress resistance between the social task groups of honey bees.
Gene expression patterns in brain also diverge between foragers and nurse bees (Whitfield et al., 2003). Transcription of several genes encoding neurotransmitter transporters, ion channels, protein kinases, and phosphatases, e.g., is enhanced in the foraging bee (Tsuchimoto et al., 2004). Oxidative stress inactivates particular proteins preferentially (Sohal et al., 1990, 1995; Sohal and Weindruch, 1996; Sohal, 2002). This phenomenon has been verified for protein carbonylation and nitration, and may lead to inactivation of enzymatic activity and kinase signaling pathways (Sohal, 2002; Stadtman and Levine, 2003; Yang et al., 2005). State-dependent changes in gene expression, and thus protein product patterns, in brain thereby can be a contributing characteristic that causes foragers to be more susceptible to oxidative stress damage than other workers.
Another perspective is that factors in the milieu of foragers vs. nurse bees and diutinus workers may translate directly into different patterns of oxidative damage in the bees. Foraging is a risky task that is associated with a high rate of mechanical senescence (Cartar, 1992), and frequent exposure to pathogens and ultraviolet (UV) light (Kefuss and Nye, 1970). Mechanical senescence, such as wing wear, has been found to reduce life expectancy in the honey bee (Neukirch, 1982), possibly because it induces an increased work load and, thereby, a higher metabolic rate (Neukirch, 1982; Cartar, 1992). UV light, moreover, constitutes a major environmental insult to all exposed tissues, including the visual system, and exposure can potentially damage a wide variety of macromolecular components, ranging from DNA to proteins and lipids, through the generation of ROS (Podhaisky et al., 2002; Parker et al., 2004; Linsenmayer et al., 2005). The lamina is vulnerable although chromophores are especially developed to absorb UV radiation (Miguel et al., 2002), and in humans oxidative stress-induced damage has been found to increase in retinal tissue with age (Parker et al., 2004). It is thus important to note that foragers depend on UV light for guidance on foraging flights, whereas other worker groups predominantly perform tasks within the hive and rely on chemosensory cues rather than visual cues for task performance (Kefuss and Nye, 1970).
In conclusion, we have documented a stage-dependent pattern of oxidative damage in the brain of honey bee workers that can be explained by several factors independently or in concert. An interesting aspect for future studies aimed at understanding these dynamics in further detail is that honey bees can be triggered to revert from foraging to nurse tasks (Robinson et al., 1992; Huang and Robinson, 1996; Amdam et al., 2005a). This reversion causes an increase in the vitellogenin protein titer and a reversal of immunosenescence (Amdam et al., 2005a), possibly implying that a remission of brain aging can occur as well (S.-C. Seehuus, K.A. Nilsen, M.K. Fondrk, and G.V. Amdam, unpublished results). Also, although chronological age did not significantly affect oxidative carbonylation levels in this study, our data show a trend towards lower levels of carbonylation damage in 200-day-old post-wintering nurse bees and foragers (collected in spring) compared with 8- and 20-day-old nurse bees and foragers (collected in summer), respectively (Fig. 5). Unfavorable ambient conditions that restrict foraging opportunities (Sekiguchi and Sakagami, 1966; Finch, 1990) and low air temperatures that may suppress flight metabolic rate and oxygen radical formation (Harrison and Fewell, 2002) could explain this putative relationship in foragers. Further research is needed to address these questions. Overall, the data presented here parallel physiological events that lead to more severe stages of neurodegeneration in diseases like Alzheimer and Parkinson (Aksenov et al., 2001; Tsang and Soong, 2003). Thus, our findings indicate that the bee may emerge as a valuable model in neurogerontological research.
Acknowledgements
We thank Ulrike Gimsa and Julie Mustard for helpful reviews of the manuscript and Ingunn Berget for assistance with the statistical analysis. S.C.S. and G.V.A. were supported by Norwegian Research Council (Grant Nos. 171958 and 175413). The authors declare that they have no competing financial interests.
References
- Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR. Protein oxidation in the brain in Alzheimer’s disease. Neuroscience. 2001;103:373–383. doi: 10.1016/s0306-4522(00)00580-7. [DOI] [PubMed] [Google Scholar]
- Amdam GV, Omholt SW. The regulatory anatomy of honeybee lifespan. J. Theor. Biol. 2002;216:209–228. doi: 10.1006/jtbi.2002.2545. [DOI] [PubMed] [Google Scholar]
- Amdam GV, Omholt SW. The hive bee to forager transition in honeybee colonies: the double repressor hypothesis. J. Theor. Biol. 2003;223:451–464. doi: 10.1016/s0022-5193(03)00121-8. [DOI] [PubMed] [Google Scholar]
- Amdam GV, Norberg K, Fondrk MK, Page RE. Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proc. Natl. Acad. Sci. USA. 2004a;101:11350–11355. doi: 10.1073/pnas.0403073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Hartfelder K, Norberg K, Hagen A, Omholt SW. Altered physiology in worker honey bees (Hymenoptera: Apidae) infested by the mite Varroa destructor (Acari: Varroidae): a factor in colony loss during over-wintering? J. Econ. Entomol. 2004b;97:741–747. doi: 10.1093/jee/97.3.741. [DOI] [PubMed] [Google Scholar]
- Amdam GV, Simões ZLP, Hagen A, Norberg K, Schrøder K, Mikkelsen O, Kirkwood TBL, Omholt SW. Hormonal control of the yolk precursor vitellogenin regulates immune function and longevity in honeybees. Exp. Gerontol. 2004c;39:767–773. doi: 10.1016/j.exger.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Amdam GV. Social control of aging and frailty in bees. In: Carey JR, Robine J-M, Michel J-P, Christen Y, editors. Longevity and Frailty. Berlin: Springer; 2005. pp. 17–26. [Google Scholar]
- Amdam GV, Page RE. Intergenerational transfers may have decoupled physiological and chronological age in a eusocial insect. Aging Res. Rev. 2005;4:398–408. doi: 10.1016/j.arr.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Aase ALTO, Seehuus S-C, Norberg K, Hartfelder K, Fondrk MK. Social reversal of immunosenescence in honey bee workers. Exp. Gerontol. 2005a;40:939–947. doi: 10.1016/j.exger.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Norberg K, Omholt SW, Kryger P, Lourenco AP, Bitondi MMG, Simões ZLP. Higher vitellogenin concentrations in honey bee workers may be an adaptation to life in temperate climates. Insects Soc. 2005b;52:316–319. [Google Scholar]
- Borbely A, Toth A, Edes I, Virag L, Papp JG, Varro A, Paulus WJ, van der Velden J, Stienen GJM, Papp Z. Peroxynitrite-induced alpha-actinin nitration and contractile alterations in isolated human myocardial cells. Cardiovasc. Res. 2005;67:225–233. doi: 10.1016/j.cardiores.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Bowling AC, Beal MF. Bioenergetic and oxidative stress in neurodegenerative diseases. Life Sci. 1995;56:1151–1171. doi: 10.1016/0024-3205(95)00055-b. [DOI] [PubMed] [Google Scholar]
- Cartar RV. Morphological senescence and longevity – an experiment relating wing wear and life-span in foraging wild bumble bees. J. Anim. Ecol. 1992;61:225–231. [Google Scholar]
- Crailsheim K, Hrassnigg N, Stabentheiner A. Diurnal behavioural differences in forager and nurse honey bees (Apis mellifera carnica Pollm) Apidologie. 1996;27:235–244. [Google Scholar]
- Dremina ES, Sharov VS, Schoneich C. Protein tyrosine nitration in rat brain is associated with raft proteins, flotillin-1 and alpha-tubulin: effect of biological aging. J. Neurochem. 2005;93:1262–1271. doi: 10.1111/j.1471-4159.2005.03115.x. [DOI] [PubMed] [Google Scholar]
- Finch CE. Chicago: University of Chicago Press; 1990. Longevity, Senescence and the Genome; pp. 67–72. [Google Scholar]
- Fiore G, Di Cristo C, Monti G, Amoresano A, Columbano L, Pucci P, Cioffi FA, Di Cosmo A, Palumbo A, d’Ischia M. Tubulin nitration in human gliomas. Neurosci. Lett. 2006;394:57–62. doi: 10.1016/j.neulet.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Fluri P, Lüscher M, Wille H, Gerig L. Changes in weight of the pharyngeal gland and haemolymph titres of juvenile hormone, protein and vitellogenin in worker honey bees. J. Insect Physiol. 1982;28:61–68. [Google Scholar]
- Fluri P, Imdorf A. Brutstopp im August/September – Auswirkungen auf die Ein- und Auswinterung der Völker. Schwiz. Bienen-Zeitung. 1989;112:452–455. [Google Scholar]
- Gary NE. Activities and behavior of honey bees. In: Graham JM, editor. The Hive and the Honey Bee. Hamilton, IL: Dadant & Sons; 1992. pp. 269–371. [Google Scholar]
- Giurfa M. Cognitive neuroethology: dissecting non-elemental learning in a honeybee brain. Curr. Opin. Neurobiol. 2003;13:726–735. doi: 10.1016/j.conb.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Guidugli KR, Nascimento AM, Amdam GV, Barchuk AR, Angel R, Omholt SW, Simões ZLP, Hartfelder K. Vitellogenin regulates hormonal dynamics in the worker caste of a eusocial insect. FEBS Lett. 2005;579:4961–4965. doi: 10.1016/j.febslet.2005.07.085. [DOI] [PubMed] [Google Scholar]
- Guix FX, Uribesalgo I, Coma M, Munoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog. Neurobiol. 2005;76:126–152. doi: 10.1016/j.pneurobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Harrison JF, Fewell JH. Environmental and genetic influences on flight metabolic rate in the honey bee, Apis mellifera. Comp. Biochem. Physiol. 2002;133:323–333. doi: 10.1016/s1095-6433(02)00163-0. [DOI] [PubMed] [Google Scholar]
- Huang Z-Y, Robinson GE. Regulation of honey bee division of labor by colony age demography. Behav. Ecol. Sociobiol. 1996;39:147–158. [Google Scholar]
- Kefuss JA, Nye WP. The influence of photoperiod on the flight activity of honeybees. J. Apic. Res. 1970;9:133–139. [Google Scholar]
- Linsenmayer TF, Cai CX, Millholland JM, Beazley KE, Fitch JM. Nuclear ferritin in corneal epithelial cells: tissue-specific nuclear transport and protection from UV-damage. Prog. Retin. Eye Res. 2005;24:139–159. doi: 10.1016/j.preteyeres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Maurizio A. The influence of pollen feeding and brood rearing on the length of life and physiological condition of the honeybee preliminary report. Bee World. 1950;31:9–12. [Google Scholar]
- Menzel R, Müller U. Learning and memory in honeybees: from behavior to neural substrates. Rev. Neurosci. 1996;19:379–404. doi: 10.1146/annurev.ne.19.030196.002115. [DOI] [PubMed] [Google Scholar]
- Menzel R, Geiger K, Chittka L, Joerges J, Kunze J, Muller U. The knowledge base of bee navigation. J. Exp. Biol. 1996;199:141–146. doi: 10.1242/jeb.199.1.141. [DOI] [PubMed] [Google Scholar]
- Miguel NCD, Meyer-Rochow VB, Allodi S. Ultrastructural study of first and second order neurons in the visual system of the crab Ucides cordatus following exposure to ultraviolet radiation. Micron. 2002;33:627–637. doi: 10.1016/s0968-4328(02)00030-6. [DOI] [PubMed] [Google Scholar]
- Neukirch A. Dependence of the life span of the honeybee (Apis mellifera) upon flight performance and energy consumption. J. Comp. Physiol. 1982;146:35–40. [Google Scholar]
- Nystrom T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005;24:1311–1317. doi: 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omholt SW. Thermoregulation in the winter cluster of the honeybee, Apis mellifera. J. Theor. Biol. 1987;128:219–231. [Google Scholar]
- Omholt SW, Amdam GV. Epigenic regulation of aging in honeybee workers. Sci. Aging Knowledge Environ. 2004;26:pe28. doi: 10.1126/sageke.2004.26.pe28. [DOI] [PubMed] [Google Scholar]
- Page RE, Erber J. Levels of behavioral organization and the evolution of division of labor. Naturwissenschaften. 2002;89:91–106. doi: 10.1007/s00114-002-0299-x. [DOI] [PubMed] [Google Scholar]
- Pankiw T, Page RE, Fondrk MK. Brood pheromone stimulates pollen foraging in honey bees (Apis mellifera) Behav. Ecol. Sociobiol. 1998;44:193–198. [Google Scholar]
- Parker NR, Jamie JF, Davies MJ, Truscott RJW. Protein-bound kynurenin is a photosentitizer of oxidative damage. Free Rad. Biol. Med. 2004;37:1479–1489. doi: 10.1016/j.freeradbiomed.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Pinto LZ, Bitondi MMG, Simões ZLP. Inhibition of vitellogenin synthesis in Apis mellifera workers by a juvenile hormone analogue, pyriproxyfen. J. Insect Physiol. 2000;46:153–160. doi: 10.1016/s0022-1910(99)00111-0. [DOI] [PubMed] [Google Scholar]
- Podhaisky HP, Riemschneder S, Wohlrab W. UV light and oxidative damage of the skin. Pharmazie. 2002;57:30–33. [PubMed] [Google Scholar]
- Radak Z, Takahashi R, Kumiyama A, Nakamoto H, Ohno H, Ookawara T, Goto S. Effect of aging and late onset dietary restriction on antioxidant enzymes and proteasome activities, and protein carbonylation of rat and skeletal muscle and tendon. Exp. Gerontol. 2002;37:1423–1430. doi: 10.1016/s0531-5565(02)00116-x. [DOI] [PubMed] [Google Scholar]
- Rival T, Soustelle L, Strambi C, Besson MT, Iche M, Birman S. Decreasing glutamate buffering capacity triggers oxidative stress and neuropil degeneration in the Drosophila brain. Curr. Biol. 2004;17:599–605. doi: 10.1016/j.cub.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Robinson GE, Page RE, Strambi C, Strambi A. Colony integration in honey bees: mechanisms of behavioral reversion. Ethology. 1992;90:336–348. [Google Scholar]
- Ryan KM, Ernst MK, Rice NR, Vousden KH. Role of NF-kappa B in p53-mediated programmed cell death. Nature. 2000;404:892–897. doi: 10.1038/35009130. [DOI] [PubMed] [Google Scholar]
- Saeki M, Maeda S. p130(cas) is a cellular target protein for tyrosine nitration induced by peroxynitrite. Neurosci. Res. 1999;33:325–328. doi: 10.1016/s0168-0102(99)00019-x. [DOI] [PubMed] [Google Scholar]
- Sandoz JC, Galizia CG, Menzel R. Side-specific olfactory conditioning leads to more specific odor representation between sides but not within sides in the honeybee antennal lobes. Neuroscience. 2003;120:1137–1148. doi: 10.1016/s0306-4522(03)00384-1. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Page RE, Erber J. The effects of genotype, foraging role, and sucrose responsiveness on the tactile learning performance of honey bees (Apis mellifera L.) Neurobiol. Learn. Mem. 2001;76:138–150. doi: 10.1006/nlme.2000.3996. [DOI] [PubMed] [Google Scholar]
- Schildknecht S, Heinz K, Daiber A, Hamacher J, Kavakli C, Ullrich V, Bachschmid M. Autocatalytic tyrosine nitration of prostaglandin endoperoxide synthase-2 in LPS-stimulated RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2006;340:318–325. doi: 10.1016/j.bbrc.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Seehuus S-C, Norberg K, Gimsa U, Krekling T, Amdam GV. Reproductive protein protects sterile honey bee workers from oxidative stress. Proc. Natl. Acad. Sci. USA. 2006;103:962–967. doi: 10.1073/pnas.0502681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi K, Sakagami SF. Structure of foraging population and related problems in the honeybee, with considerations on division of labour in bee colonies. Hakkaido Natl. Agric. Exp. Stat. Rep. 1966;69:1–58. [Google Scholar]
- Sohal RS, Arnold LA, Sohal BH. Age-related-changes in antioxidant enzymes and prooxidant generation in tissues of the rat with special reference to parameters in 2 insect species. Free Rad. Biol. Med. 1990;9:495–500. doi: 10.1016/0891-5849(90)90127-5. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Sohal BH, Orr WC. Mitochondrial superoxide and hydrogen peroxide generation, protein oxidative and longevity in different species of flies. Free Rad. Biol. Med. 1995;19:499–504. doi: 10.1016/0891-5849(95)00037-x. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS. Role of oxidative stress and protein oxidation in the aging process. Free Rad. Biol. Med. 2002;33:37–44. doi: 10.1016/s0891-5849(02)00856-0. [DOI] [PubMed] [Google Scholar]
- Soreghan BA, Yang F, Thomas SN, Hsu J, Yang AJ. High-throughput proteomic-based identification of oxidatively induced protein carbonylation in mouse brain. Pharm. Res. 2003;20:1713–1720. doi: 10.1023/b:pham.0000003366.25263.78. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- Suarez RK, Lighton JRB, Joos B, Roberts SP, Harrison JF. Energy metabolism, enzymatic flux capacities, and metabolic flux rates in flying honeybees. Proc. Natl. Acad. Sci. USA. 1996;93:12616–12620. doi: 10.1073/pnas.93.22.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Yin CM. Slow aging during insect reproductive diapause: why butterflies, grasshoppers and flies are like worms. Exp. Gerontol. 2001;36:723–738. doi: 10.1016/s0531-5565(00)00238-2. [DOI] [PubMed] [Google Scholar]
- Tsang F, Soong TW. Interactions between environmental and genetic factors in the pathophysiology of Parkinson’s disease. IUBMB Life. 2003;55:323–327. doi: 10.1080/1521654031000153058. [DOI] [PubMed] [Google Scholar]
- Tsuchimoto M, Aoki M, Takada M, Kanou Y, Sasagawa H, Kitagawa Y, Kadowaki T. The changes of gene expression in honeybee (Apis mellifera) brains associated with ages. Zool. Sci. 2004;21:23–28. doi: 10.2108/0289-0003(2004)21[23:TCOGEI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Tu MP, Tatar M. Juvenile diet restriction and the aging and reproduction of adult Drosophila melanogaster. Aging Cell. 2003;2:327–333. doi: 10.1046/j.1474-9728.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- Tyurin VA, Tyurina YY, Borisenko GG, Sokolova TV, Ritov VB, Quinn PJ, Rose M, Kochanek P, Graham SH, Kagan VE. Oxidative stress following traumatic brain injury in rats: quantitation of biomarkers and detection of free radical intermediates. J. Neurochem. 2000;75:2178–2189. doi: 10.1046/j.1471-4159.2000.0752178.x. [DOI] [PubMed] [Google Scholar]
- Visscher PK, Dukas R. Survivorship of foraging honey bees. Insects Soc. 1997;44:1–5. [Google Scholar]
- Wang Y, Oberley LW, Murhammer DW. Antioxidant defense systems of two lepidopteran insect cell lines. Free Rad. Biol. Med. 2001;30:1254–1262. doi: 10.1016/s0891-5849(01)00520-2. [DOI] [PubMed] [Google Scholar]
- Warner HR. Mechanisms of antioxidant action on life-span—overview. Toxicol. Ind. Health. 1993;9:151–161. doi: 10.1177/0748233793009001-211. [DOI] [PubMed] [Google Scholar]
- Whitefield CW, Cziko AM, Robinson GE. Gene expression profiles in the brain predict behavior in individual honey bees. Science. 2003;302:296–299. doi: 10.1126/science.1086807. [DOI] [PubMed] [Google Scholar]
- Winston ML. The Biology of the Honey Bee. Cambridge, MA,: Harvard University Press; 1987. p. 296. [Google Scholar]
- Yang YF, Gehrke S, Haque ME, Imai Y, Kosek J, Yang LC, Beal MF, Nishimura I, Wakarnatsu K, Ito S, Takahashi R, Lu BW. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc. Natl. Acad. Sci. USA. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]


