Summary
Ant colony mortality has not been sufficiently studied, even though it is crucial for understanding social insect population biology and can serve as an important model for general aging and mortality processes. Particularly, studies on proximate mechanisms on mortality and stress resistance of ant colonies are lacking. This study explores the long-term colony starvation resistance of the small myrmecine ant Temnothorax rugatulus. We report extraordinary starvation resistance in the 21 colonies investigated, as most survived the eight months of total starvation. Furthermore, we studied demographic and behavioral changes over the experimental period. Brood decline began first (after two months) and mortality was highest, worker decline was intermediate, and queen mortality started latest and remained lowest. We found brood (its relative change during the first four months and the level of brood relative to colony size) to be the only significant predictor of colony starvation resistance, but not the degree of polygyny. As expected, rates of trophallaxis increased during the starvation period while colony activity bouts occurred more frequently but were much shorter, leading to an overall decrease in activity levels. This study is the first to comprehensively study mechanisms of starvation resistance in ant colonies, linking demography and behavior.
Keywords: Activity cycles, adaptive demography, mortality, starvation stress, superorganism
Introduction
Ant colony mortality has not been studied in great detail, despite considerable interest in ant population dynamics and their major importance in a variety of ecosystems (Hölldobler and Wilson, 1990). Quantitative data on colony mortality are lacking for most species (Kaspari and Vargo, 1995) but some notable exceptions have revealed a large inter-specific variability from one year in Harpegnathos saltator (Liebig and Poethke, 2004) to about 17 years in Pogonomyrmex owyheei (Porter and Jorgensen, 1988). Apart from comparisons of mortality between young and older colonies (Gordon, 1991) no studies address intraspecific variability in colony mortality. Particularly, the proximate causes of colony mortality have not been studied in any ant species, except in the specific context of pathogens (Schmid-Hempel, 1998), social parasitism and intraspecific competition (Hölldobler and Wilson, 1990).
However, ant colony mortality is not only important for understanding social insect biology, but its study can also serve as a model for more general aging and mortality processes (Rueppell et al., 2004). The organization and functioning of social insect colonies have repeatedly been compared to that of multi-cellular organisms in demography, ecology, and life-history analysis (Wheeler, 1928; Wilson, 1971; Oster and Wilson, 1978; Hölldobler and Wilson, 1990; Bourke and Franks, 1995). According to the superorganism concept, the functionally sterile workers can be compared to somatic tissue and the reproductive queens to the germ line (Oster and Wilson, 1978; Moritz and Fuchs, 1998). Like multicellular organisms, social insect colonies display trade-offs in resource allocation between growth, defense, and reproduction (Sudd and Franks, 1987; Bourke and Franks, 1995; Kaspari and Byrne, 1995). Colony mortality and resource allocation trade-offs are fundamentally different between colonies with one queen (monogynous), in which colony survival is determined by queen survival (Franks et al., 1990a; Pamilo, 1991), and polygynous colonies (with several queens) in which colonies are potentially immortal through serial polygyny (Seppa, 1994). Enhanced colony survival has been suggested as one important factor for the evolution of polygyny (Hölldobler and Wilson, 1977). On the other hand, additional queens place an energetic burden on their colony and may inhibit each others’ reproduction (Vargo and Fletcher, 1989).
Ant colonies are perennial (Bourke and Franks, 1995) and many species’ colonies can live over several decades (Wilson, 1971). Furthermore, the value of the nest structure makes ant colonies quasi-sessile “organisms” (but see Thomas (2002) and references therein) with little opportunity to evade unfavorable conditions (Bourke and Franks, 1995). Thus, ant colonies have to represent efficient homeostatic systems to survive to reproductive age (Hölldobler and Wilson, 1990), and their size may be of crucial importance (Kaspari and Vargo, 1995). In seasonal environments, ant colonies must be highly resistant to climate and starvation stress. Under environmental stress, the ant colony as a super-organism is expected to sacrifice somatic tissue (brood and workers) to ensure queen and hence colony survival (Wilson, 1971; Taylor, 1978; Sorensen et al., 1983; Chapuisat et al., 1997). The individuals are relatively autonomous components, and considerable losses of workers can be tolerated without the risk of colony extinction. This low level of integration of ant colonies also facilitates its experimental manipulation and the detailed study of its components and their interactions in response to environmental changes.
Ants lack a general mechanism for food storage but some species have lasting resources in the form of stored grain, fungus-gardens, or replete workers (Hölldobler and Wilson, 1990). Resources in “regular” workers (Brian, 1983; Amdam et al., 2003) may also serve to ensure against seasonal food shortages and consequently seasonality has been suggested to be a major determinant of worker number or colony size (Kaspari and Vargo, 1995; Cassill, 2002). A number of storage proteins have been isolated from ants (Wheeler and Martínez, 1995; Martínez et al., 2000). The modularity of ant colonies also allows for the production of excess brood items to preserve food resources (Bourke and Franks, 1995). Trophic eggs exist in many ant species (Gobin and Ito, 2000) but excess larvae can likewise be a means of food preservation over longer periods (Nonacs, 1991). However, we are not aware of any direct tests of the hypothesis that food is stored by raising excess larvae in ants.
Another ubiquitous means of starvation resistance in ant colonies is resource preservation, which has been suggested to be the ultimate reason for the occurrence of periodic activity cycles in ants (Franks et al., 1990b). In Leptothorax acervorum, colony members showed synchronized activity rhythms with over 70% of their time spent inactive, a typical proportion for myrmicine ants (Herbers, 1981). The coordinated activity patterns allow for long periods of inactivity, while maximizing task performance, including information transfer among colony members, during short synchronous activity bouts (Franks et al., 1990b). One important process for information transfer is trophallaxis, the exchange of liquid food among colony members, which does not only serve the distribution of food in the colony but also allows individuals to integrate information on the colony food level and to recruit nestmates to food resources (Hölldobler and Wilson, 1990). Short-term starvation (Howard and Tschinkel, 1980) and resource uncertainty (De Marco and Farina, 2003) increase the frequency of trophallaxis among workers. However, queens as the dominant individuals are the main recipients of trophallactic acts under normal circumstances (Liebig et al., 1997). If ant colonies are acting as cooperative superorganisms, the rates of trophallaxis should significantly increase under severe starvation stress because information exchange and food channeling toward the queens become more critical.
The presented study is the first long-term experiment to address these important life-history issues by investigating the demographic and behavioral responses to long-term starvation in the ant Temnothorax (formerly Leptothorax) rugatulus. The experiment was designed to obtain quantitative data on the starvation resistance and mortality in relation to colony size and social structure. Furthermore, we link the colony-level demographic processes during starvation to behavioral changes in activity rhythms and trophallaxis rates. We demonstrate an extraordinary starvation resistance of T. rugatulus colonies, and relate this resistance to colony demography. Furthermore, we investigate the activity patterns and trophallaxis over the duration of the experiment and correlate those behavioral variables to the demographic changes in four focal colonies.
Materials and methods
Temnothorax rugatulus is a North American ant with a wide distribution in the forests of the western United States (Creighton, 1950). It is a typical formicoxenine ant with small individuals and has small colonies that nest mostly in rock crevices. It displays a queen size dimorphism (co-occurrence of micro- and macrogynes) that is linked to social structure and dispersal tactics (Rüppell et al., 2001). Twenty-six entire colonies of T. rugatulus were randomly collected from a polygynous population containing macro- and microgyes (“T” population in Rüppell et al. (2002a) in the Manzano Mountains, Torrance County, New Mexico) during June 2003. In the laboratory, these colonies were housed in plaster nests at room temperature (approx. 20°C) throughout the experiment. From June to November all colonies were fed weekly with diluted honey and cut-up cricket parts. Feeding frequency was increased to 2–3 times per week for four weeks in December, before 21 colonies were subjected to a total starvation regime (water only) while five control colonies were continued to be fed twice per week (see Fig. 1 for details on initial colony conditions and starvation period).
Figure 1.
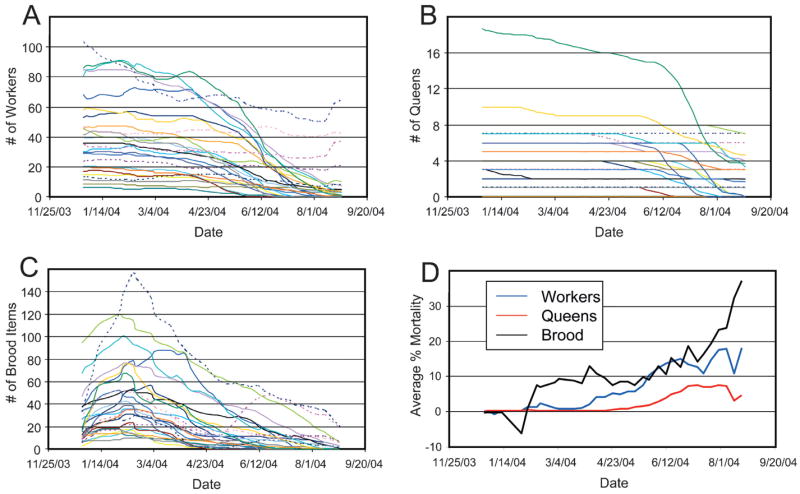
The temporal change of worker (a), queen (b) and brood (c) numbers in 26 laboratory colonies of T. rugatulus over an experimental eight months starvation period. Dashed lines indicate control colonies. (d) indicates the average proportional net mortality (“deaths” – “births”) of workers, queens and brood items in all starved colonies
Colony censuses were performed regularly (3–14 day intervals) during the course of the experiment under a stereomicroscope with a tally counter. Worker, queen, and brood number (larvae and pupae) were recorded separately. During regular repeat counts the counting error was estimated to vary between zero and eight percent and the two counts were averaged if they were different from each other. Data were smoothed (five-census wide gliding window). Prior to smoothing, the queen counts were additionally corrected because queen pupae were never observed during the experiment, therefore no increase in queen number was allowed.
From the census data, we calculated the proportional change in worker, queen and brood item number after 4 and 8 months. Statistical analyses were performed on arcsine transformed data but means and standard deviations (SD) are given after back-transformation. To compare means between control and starved colonies, we used t-tests according to a prior Levene’s test for homogeneity of variances. At the individual level, we compared survival between micro- and macrogynes by Cox’s F-test.
Within the group of starved colonies, we assessed starvation resistance in two different ways: 1) as final colony size (number of individuals alive at the end of the experiment), and 2) as the experimental duration until the colony population had declined to 90% of its original size (LD90). For four colonies that had not reached this threshold by the end of the experiment, LD90 was set to the total duration of the starvation. LD90 was significantly more variable (hence informative about the starvation resistance of individual colonies) than the final colony size, which had to be transformed (ln(x + 0.5)) to comply with parametric assumptions. All statistical analyses were performed with SPSS® for Windows 11.5. To further assess the relationship between brood/worker ratio and worker mortality in all starved colonies throughout the experiment, we performed a correlation between the “time × colony” matrices of these two factors. Significance was determined by 1000 bootstraps, permuting rows (Manly, 1997).
The behavior of four randomly selected colonies (#13, 17, 22, 23) under starvation was recorded with time-lapse video for 24 hours every two weeks. At the end of the experiment, tapes were randomized and analyzed at 12× recording speed. The following behavioral traits were recorded: 1) number of food exchanges (trophallaxis) among workers; 2) number of food exchanges (trophallaxis) involving queens; 3) overall colony activity bouts (defined as >50% of adult colony members active). From the latter behavioral variable we computed number, mean length and SD of length of the activity bouts and proportion of time spent active.
In combination with the demographic results, we also computed the trophallaxis rates for workers and queens in absolute (per individual) and relative (per possible pair-wise interaction) terms. The variables did not differ significantly from a normal distribution (Kolmogorov-Smirnov Z = 0.56 to 1.3, p = 0.060 to 0.913), except for the SD of activity bout length (Z = 1.4, p = 0.036), relative queen trophallaxis (Z = 2.3, p < 0.001), and relative worker trophallaxis (Z = 2.3, p < 0.001). These three were log-transformed to correspond better to normality (SD of activity bout length: Z = 0.69, p = 0.737; relative queen trophallaxis: Z = 0.78, p = 0.578; relative worker trophallaxis: Z = 1.4, p = 0.042).
Outliers were detected with Grubb’s test using Quickcals© (Graph-pad: http://www.graphpad.com/quickcalcs/) and removed. Subsequently, linear time trends over the course of the experiment for these variables were evaluated by simple ANCOVAs, using experimental duration as covariate and colony as main factor. Effects within single colonies were evaluated by simple regression analyses. Additionally, we tested the dependency of single behavioral variables (proportion of time spent active, # of activity bouts, and individual queen and worker trophallaxis rates) on multiple demographic variables (queen number, worker number, brood number, worker mortality, queen mortality) independent of experimental duration by ANCOVAs of the behavior (colony as main factor) with the demographic variables and the experimental duration as covariates.
Results
Demographic data
The first colony died after 158 days (slightly over 5 months) of starvation and by the end of the experiment (after exactly 8 months) 15 of 21 starved colonies were still alive, although all were clearly in the process of extinction. All five control colonies survived and maintained a sustainable colony size, even though they shrank on average by a third of their size. The proportional loss of workers, queens and brood items in the starved colonies was significantly higher than in the control colonies at the end of the experiment (Table 1). After half of the experimental duration (4 months) similar trends for workers and brood were apparent, but the decrease in workers, queens, or brood items did not differ significantly between control and starved colonies (Table 1).
Table 1.
Average colony population development (mean and back-transformed S.D.
| Control (n = 5) | Starvation (n=21) | Difference | |
|---|---|---|---|
| Workers loss (4 month) | 11.1% (1.0 – 30.1) | 23.0% (10.4 – 38.8) | t = 1.735, df = 24, p = 0.096 |
| Queen loss (4 month) | 0.4% (−0.5 – 3.7) | 0.5% (−0.9 – 5.8) | t = 0.177, df = 24, p = 0.861 |
| Brood loss (4 month) | 16.6% (−4.5 – 75.5) | 32.5% (2.3 – 76.1) | t = .767, df = 24, p = 0.450 |
| Workers loss (8 month) | 31.5% (16.7 – 46.8) | 97.7% (88.3 – 99.8) | t = 8.676, df = 24, p < 0.001 |
| Queen loss (8 month) | 0.6% (−0.9 – 6.2) | 48.7% (2.8 – 96.2) | t = 4.548, df = 22.7, p < 0.001 |
| Brood loss (8 month) | 33.3% (0.0 – 87.8) | 99.3% (94.8 – 99.6) | t = 3.234, df = 4.1, p = 0.031 |
The dynamics of the different colony members in the starved colonies differed over the experimental period (Fig. 1). While the amount of brood increased considerably during the first two months and then continuously declined (Fig. 1c), worker numbers decreased very slowly over the first 4 months and more strongly over the second half of the experiment (Fig. 1a). Except for 3 deaths in the most polygynous colony, significant queen deaths were only apparent in the second half of the experiment (Fig. 1b). Thus, the brood began to decline first, then workers, then queens and this order is also reflected in the average degree of decline (Fig. 1d). At the end of the experiment, the number of workers did not exceed the number of queens by more than two in any colony, and in eight colonies the only remaining survivors were queens. There was no significant difference in survival probability between macrogynes and microgynes (F(78,42) = 1.46, p = 0.091).
Multiple regressions of the LD90 on the initial demographic colony parameters revealed that LD90 was only influenced by the initial amount of brood (β = 0.491, r2 =0.241, F(1,19) = 6.0, p = 0.024; excluded: “queens”, “workers”, and “brood/worker ratio”). The final colony size showed significant relations to the number of workers and the brood/worker ratio (βworkers = 0.347, βb/w ratio = 0.570, r2 = 0.348, F(2,18) = 4.8, p = 0.021; excluded: “queens” and “brood”). We repeated these analyses using the “brood/worker ratio” after four months and the relative change of the three basic demographic variables (amount of brood, number of queens, number of workers) over the first four months as predictors for LD90 and final colony size. We expected these trends to contain more information than the initial demographic variables, specifically on the physiological state of individuals that potentially differed among colonies at the beginning of the experiments. LD90 was influenced by the change in the amount of brood and the brood/worker ratio (βbrood = −0.579, βb/w ratio = 0.375, r2 = 0.643, F(2,18) = 16.2, p < 0.001; excluded: “queens” and “workers”). Final colony size was only influenced by the brood/worker ratio (βb/w ratio = 0.675, r2 = 0.456, F(1,19) = 15.9, p = 0.001; excluded: “brood”, “queens” and “workers”). Incorporation of the initial demographic variables (initial queen, worker, and brood number, initial brood/worker ratio) into these analyses did not change their results because the initial variables were all excluded. Throughout the experiment, across all starved colonies, we found that the worker mortality risk was significantly negatively correlated to the concurrent brood/worker ratio (r = −0.380, p < 0.001).
Behavioral data
The proportion of time spent active (in colony activity bouts) declined significantly (F(1,61) = 10.4, p = 0.002) over the experimental period from an average of 0.19 to 0.10. This decrease was apparent in all four colonies but, analyzed separately, it was only significant in colony 22 (β = −0.526, r2 = 0.276, F(1,16) = 6.1, p = 0.025) and colony 23 (β = −0.708, r2 = 0.502, F(1,11) = 11.1, p = 0.007). The overall decrease in activity was not due to the number of activity cycles, which in contrast increased significantly (F(1,62) = 7.7, p = 0.007) from an average of 14 to 22 (Fig. 2a), but due to shorter duration of activity cycles (F(1,61) = 33.8, p < 0.001). This shortening of activity cycles was significant in colony 13 (β = −0.692, r2 = 0.479, F(1,16) = 14.7, p = 0.001), colony 22 (β = −0.699, r2 = 0.489, F(1,16) = 15.3, p = 0.001), and colony 23 (β = −0.874, r2 = 0.763, F(1,11) = 32.3, p < 0.001), but not colony 17 (Fig. 2b). The decrease in mean length of activity bouts was accompanied by a significant decrease in SD of the length of activity cycles (F(1,62) = 9. 9, p = 0.003).
Figure 2.
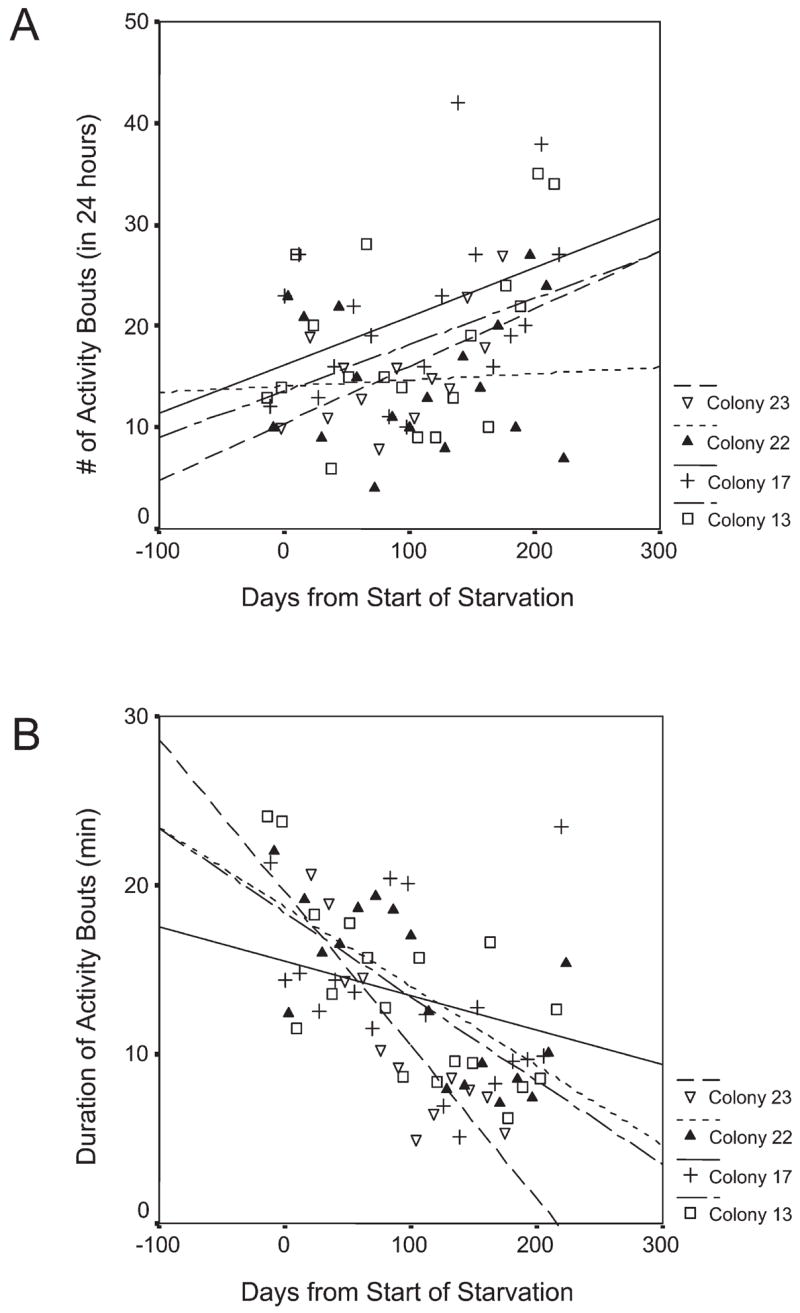
The decline of overall activity over the starvation period was not due to the number of activity bouts, which actually increased (a), but due to the shorter duration of activity bouts (b)
The absolute number of trophallaxis events among workers decreased significantly (F(1,59) = 10.8, p = 0.002) over the experimental period from an average of 265 to 128. In contrast, trophallaxis events involving queens increased from an average of 81 to 97, but this increase was not significant (F(1,62) = 3.2, p = 0.078). The individual trophallaxis rates of workers significantly increased (F(1,59) = 30.0, p < 0.001) from seven to seventeen events per day on average. The trend was apparent in all colonies (Fig. 3a), but only significant in colony 13 (β = 0.899, r2 = 0.807, F(1,16) = 67.0, p < 0.001) and colony 17 (β = 0.670, r2 = 0.449, F(1,14) = 11.4, p = 0.005). The individual trophallaxis rates of queens also increased significantly (F(1,62) = 20.9, p < 0.001) from an average of 16 to 30 during the starvation period. Again the trend was apparent in all colonies (Fig. 3b), but analyzed separately, only the increase in colony 13 was significant (β = 0.674, r2 = 0.454, F(1,16) = 13.3, p = 0.002). Trophallaxis rate per possible inter-action increased for workers significantly (F(1,60) = 217.1, p < 0.001) from 0.3 to 9, and for queens from 0.4 to 6 (F(1,62) = 206.2, p < 0.001). Both trends were also significant in each colony analyzed separately (workers: β from 0.795 to 0.956, r2 from 0.632 to 0.913, F from 25.76 to 168.23, p < 0.001; queens: β from 0.804 to 0.897, r2 from 0.647 to 0.805, F from 20.15 to 66.23, p < 0.001).
Figure 3.
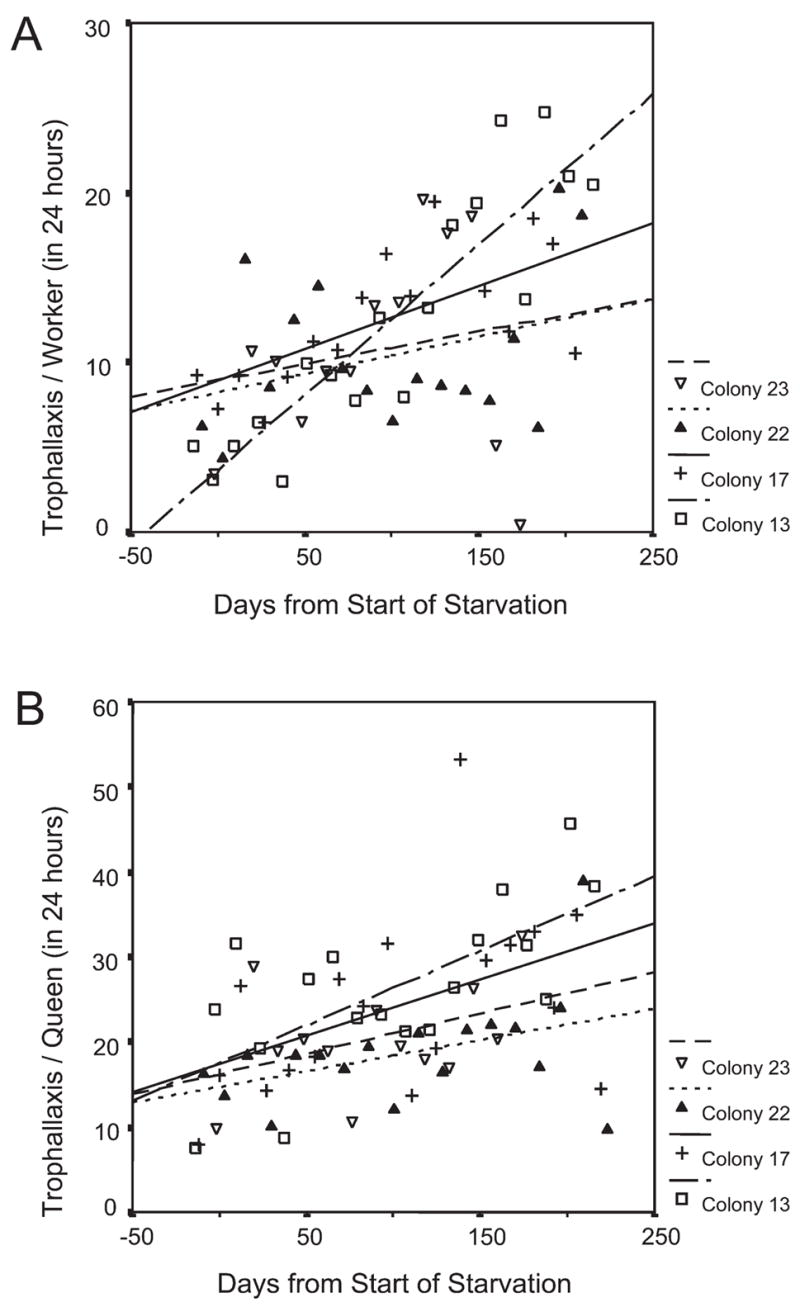
Individual rates of both worker-worker trophallaxis (a) and trophallaxis involving queens (b) increased significantly over the experimental period
The ANCOVAs investigating the effect of multiple demographic variables and experimental duration in the four focal colonies simultaneously revealed more detailed statistical relationships (Table 2). In these multivariate models, the proportion of time spent active was negatively related to the number of workers and the experimental duration, and positively related to worker mortality. Likewise, the number of activity bouts was negatively related to the number of workers and the experimental duration, and positively related to worker mortality. The individual trophallaxis rates among workers were positively related to the number of queens and worker mortality, and negatively related to the amount of brood. Individual trophallaxis rates of queens were negatively related to worker mortality and positively related to queen mortality (see also Fig. 4).
Table 2.
Relation* between demographic variables, starvation duration, and behavioral variables
| Behavioral Variable (Colony differentiation) | Demographic Covariates | |
|---|---|---|
| % time spent active (F(3,54) = 4.7, p = 0.005) | # of workers | F(1,54) = 6.5, p = 0.014; B = −0.004 |
| Worker mortality | F(1,54) = 27.4, p < 0.001; B = 0.006 | |
| Experimental duration | F(1,54) = 38.8, p < 0.001; B = −0.002 | |
| # of activity bouts (F(3,54) = 6.1, p = 0.001) | # of workers | F(1,54) = 12.4, p = 0.001; B = −0.535 |
| Worker mortality | F(1,54) = 5.0, p = 0.029; B = 0.006 | |
| Experimental duration | F(1,54) = 5.1, p = 0.028; B = −0.002 | |
| Worker trophallaxis (F(3,54) = 6.7, p = 0.001) | # of queens | F(1,54) = 6.9, p = 0.012; B = 27.225 |
| # of brood | F(1,54) = 7.2, p = 0.010; B = −1.466 | |
| Worker mortality | F(1,54) = 6.7, p = 0.013; B = 1.982 | |
| Queen trophallaxis (F(3,54) = 1.6, p = 0.195) | Worker mortality | F(1,54) = 6.6, p = 0.013; B = −0.326 |
| Queen mortality | F(1,54) = 5.7, p = 0.021; B = 0.708 |
Results of ANCOVAs (colony as main factor, experimental duration and the four demographic variables as covariates) are displayed. Only the results of significant covariates are listed.
Figure 4.
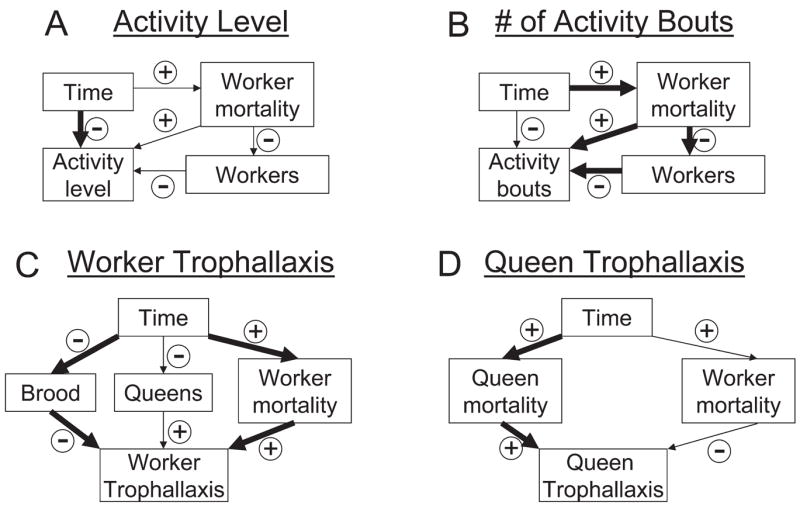
Associations between the experimental duration and several demographic variables to the proportion of time spent in activity bouts (activity level), the number of activity bouts during 24 hours, and the individual rates of trophallaxis in workers and queens in the four focal colonies, as suggested by our ANCOVAs with multiple covariates. Associations (arrows) that determine the overall trend of the behavioral variable are printed in bold
Discussion
This study documents an extraordinary resistance to starvation by Temnothorax rugatulus colonies in the laboratory. Under the laboratory conditions most colonies survived four months of total starvation without a significant decline (relative to the control colonies) in colony population. Furthermore, most starved colonies were still alive at the end of the eight month starvation period, although the numbers of brood items and workers were dramatically reduced at this point. However, eight months exceed the annual activity period in most habitats of T. rugatulus. The average temperature, an important determinant of metabolism (Lighton et al., 1993), probably does not significantly exceed room temperature in the natural habitat of T. rugatulus. Thus, we conclude that most well-fed colonies are theoretically able to survive a whole season without food. Starvation resistance can vary between closely related species (Toda and Kimura, 1997) and it would be interesting to investigate the taxonomic distribution of starvation resistance of ant colonies. Solenopsis invicta colonies declined in size by about 85% during six weeks of starvation at 30°C (Porter, 1989) which indicates a larger dependency on incoming resources of this ecologically dominant species. However, comparable data from other ant species that would allow for broad comparisons are not available to date.
The high resistance to starvation may be important for a small, subdominant ant species, such as T. rugatulus (Rüppell et al., 1998). Although we do not know much about the dietary habits of the genus in general, and T. rugatulus in particular, scavenging in the leaf litter in a restricted foraging territory seems most likely. Local food supplementation experiments show clear effects on colony life history (Foitzik et al., 2004) indicating that food is a limiting factor. A restricted activity area in T. rugatulus is also suggested by significant population viscosity over small distances in polygynous populations (Rüppell et al., 2003). Food in these small foraging areas is presumably clumped in time which makes food storage by colonies highly beneficial.
T. rugatulus has no specialized food storing mechanism as found in other ants (Hölldobler and Wilson, 1990) but the results of this study suggest that internal resources and brood are effective mediators of starvation resistance. The initial amount of brood did ultimately not predict the colonies’ resistance to starvation because the amount of brood continued to increase to various degrees in most colonies over the first two months of starvation. This indicates that workers and/or queens were able to use internal resources to produce additional brood without access to food for a prolonged period of time (Børgesen, 2000). The trend in absolute amount of brood over the first four months and the brood-to-worker ratio after four months were the only significant predictors of the colony resistance to starvation over the entire experimental period. This suggests the ultimate importance of brood items as food resource under long-term starvation for T. rugatulus and possibly in general. Colonies with more brood items per adult colony member suffered less mortality throughout the experiment and we found no indication that this is related to other colony demographic parameters. Surprisingly, the number of queens and workers did not have a direct effect in any of the analyses. In several species, it has been demonstrated that brood is also an active component of the food flow in ant colonies under regular conditions (Taylor, 1978; Hölldobler and Wilson, 1990) but this study is to our knowledge the first to directly demonstrate that the relative amount of brood is important for colony survival.
Our results suggest that colony size does not confer a survival advantage under starvation stress. This finding is in contrast to the major assumption of the “fasting endurance” hypothesis for Bergman’s rule (Kaspari and Vargo, 1995) and the yoyo-bang hypothesis (Cassill, 2002). The brood emerged as the single most important factor for colony survival, and it appears that workers and super-numerous queens of T. rugatulus can not be efficiently sacrificed to keep the reminder of the colony alive under the experimental circumstances. Colony size may indirectly increase because of excess brood rearing as an adaptation to seasonal environments and it is interesting to note in this regard that many temperate ant species have remarkably long larval developmental times and rear significant amounts of brood towards the end of the season, in contrast to honey bees for example which have other means of resource storage for overwintering (Wilson, 1971).
Due to brood cannibalism (pers. obs.), the amount of brood started to decline two months earlier than workers or queens and brood mortality remained relatively constant throughout the remainder of the experiment. Queens suffered later and less mortality than workers, which could indicate different robustness of these castes but more likely it reflects priorities in colony-level resource allocation (Wilson, 1971; Amdam et al., 2004). Body size is an important factor for starvation resistance (Heinze et al., 2003). However, body size seems not to be as important as caste because microgynes (who are more similar in size to workers than macrogynes: Rüppell et al., 1998) did not differ in mortality from macrogynes but from workers. Some brood was maintained in most colonies as long as workers were present, and several queens died before the last workers, particularly in polygynous colonies. The queens may not have a total survival advantage in T. rugatulus because they are doomed without workers (Rüppell et al., 2002b).
The recording of focal colonies allowed us to align behavioral changes with increased starvation over the experimental period and with specific demographic parameters. Overall activity levels decreased while individual trophallaxis rates increased over the course of the experiment. The decline in activity was due to the much shorter duration of an increased number of activity bouts. Proximally, this can be explained by more frequently incoming foragers, triggering activity (Boi et al., 1999), under starvation. Food shortage commonly stimulates the foraging effort in ants (Hölldobler and Wilson, 1990; Haack et al., 1995). However, with no food available, and decreasing amounts of brood the duration of these activity bouts may decline (Franks et al., 1990b; Boi et al., 1999). In general, the activity bouts that we recorded in T. rugatulus are longer and less frequent than previously reported for L. acervorum (Franks et al., 1990b) and L. allardycei (Cole, 1991). This may be the result of our different recording technique and not reflect biologically significant species differences. However, our methods were consistent throughout the experiment and do not bias the conclusions of this paper.
The ultimate reason for reducing activity under starvation conditions may be energy preservation (Lighton et al., 1993; Porter and Tschinkel, 1993), while frequent information gathering becomes more valuable and thus short, numerous activity bouts can be regarded as adaptive. The information gathering hypothesis is corroborated by the increase of the individual trophallaxis rates throughout the experiments. Even though the absolute number of trophallactic events among workers declined in parallel to the declining number of workers in the colonies, trophallaxis rates per individual increased for workers and queens, and this increase is even more dramatic when trophallaxis rates were calculated relative to all possible pair-wise interactions in the respective colony. Proximally, starvation increases individual begging (Liebig et al., 1997; Schulz et al., 2002) and the individual encounter rates among colony members increase in smaller colonies. It will be important to establish the degree of food- versus information-transfer over the course of colony starvation in future experiments and to relate behavioral and demographic changes to the time course of changes in physiological variables, such as crop content (Heinze et al., 1996), fat body (Amdam et al., 2003), and propharyngeal glands (Hölldobler and Wilson, 1990).
The ANCOVAs considering the effects of multiple demographic variables simultaneously to the effects of the colony and the duration of the experiment are more difficult to interpret, and the statistical associations can not be translated into biological causation without further experimental verification. Overall, we recorded a decline of activity levels, in contrast to the effect of short-term starvation (Franks et al., 1990b). The multivariate analysis showed that the negative relation of activity level to starvation was stronger than the combined effect of its two other significant associations with worker mortality and worker number (Fig. 4a). These associations both weaken the overall decrease of the activity level over the experimental duration because worker mortality is positively associated with the experimental duration and activity level, while the number of workers has a negative correlation with both factors (Fig. 4a). The analysis of the number of activity bouts revealed the same underlying associations. However, the associations differed in their relative magnitude, which explains the overall increase in the number of activity bouts over the duration of the experiment (Fig. 4b).
Dying workers may be associated with increased activity levels because searching/begging for food is the only possibly successful strategy given their physiological state (McNamara and Houston, 1989). Consistent with this, worker mortality also showed a positive relation to the individual worker trophallaxis rate. Alternatively, if workers are dying inside the colony, they trigger corpse removal and thus activity (Hölldobler and Wilson, 1990). Both hypotheses may also explain the positive association of worker mortality and the number of activity bouts. Additionally, the number of workers in colonies had a negative effect on the number of activity bouts (Fig. 4b). This contrasts with the predictions of theoretical models of the cyclic ant nest activity (Hemerik et al., 1990) and may only be explained on the basis of increased task efficiency with more workers (Anderson and McShea, 2001): Tasks may get done faster, shortening the activity cycles and increasing the individual thresholds to become active (Hemerik et al., 1990), especially under starvation conditions.
Both, individual worker and queen trophallaxis rates increased over the experimental period but no significant effect of the time was found independent of the demographic variables. The overall increase in individual worker trophallaxis can be explained by its negative association with the amount of brood (which in turn decreases over the duration of the experiment) and its positive association with worker mortality (which increases over the duration of the experiment). These two trends seem to outweigh its positive correlation with the number of queens (which slightly declines in time) (Fig. 4c). In contrast, the individual queen trophallaxis rates are only significantly positively associated with queen mortality (increasing) and negatively associated with worker mortality (increasing) (Fig. 4d).
The opposite effect of worker mortality on worker and queen trophallaxis rates is particularly interesting because it indicates some degree of self-preservation in the workers. The reduced trophallaxis under acute mortality may reflect the tendency of selfishly refusing to engage in trophallaxis, especially in polygynous, genetically heterogeneous colonies (Heinze et al., 1994). In contrast, queen mortality seems to trigger an increase of trophallaxis towards queens (Liebig et al., 1997), as predicted by the superorganism concept (Hölldobler and Wilson, 1990; Amdam et al., 2004).
Strikingly, the most important demographic determinant of starvation resistance, the brood, did only have one small effect on the investigated behavioral variables. It has been shown that brood makes activity cycles more pronounced (Cole and Hoeg, 1996) but in our experiment no significant correlation of the amount of brood with the number of activity cycles or the proportion of time spent active was detected. After the initial production of brood from presumably internal body reserves of workers (Wheeler and Martínez, 1995), the brood suffered a constant decline over the experimental period. Thus, although the brood represents an important food reserve, it is used up at a constant rate and does not influence the behavior of adults independently of the level of starvation (experimental duration).
Even though our results make evolutionary sense in an ultimate and proximate context, this study was not designed to test causal relations between demography, behavior, and resistance to starvation stress. However, this first, descriptive study reveals some remarkable results that are of interest in biodemography, sociobiology, and life-history evolution. The discovered patterns provide intriguing hypotheses for future research. New experiments will have to address the causes and consequences of the interacting behavioral, physiological, and demographic mechanisms of extreme starvation resistance in ant colonies, arisen by social evolution.
Acknowledgments
We would like to thank all lab members for their practical support over the long course of this experiment, members of the NIA-funded program project “Biodemographic Determinant of Lifespan” (PO1 AG22500) for advice, and J. Heinze and two anonymous reviewers for comments that helped to improve the manuscript. Financial support came from the University of North Carolina, Greensboro. The work described does comply with all applicable laws of the United States of America.
References
- Amdam GV, Norberg K, Hagen A, Omholt SW. Social exploitation of vitellogenin. Proc Nat Acad Sci USA. 2003;100:1799–1802. doi: 10.1073/pnas.0333979100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Simoes ZLP, Hagen A, Norberg K, Schroder K, Mikkelsen O, Kirkwood TBL, Omholt SW. Hormonal control of the yolk precursor vitellogenin regulates immune function and longevity in honeybees. Exp Gerontol. 2004;39:767–773. doi: 10.1016/j.exger.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Anderson C, McShea DW. Individual versus social complexity, with particular reference to ant colonies. Biol Rev. 2001;76:211–237. doi: 10.1017/s1464793101005656. [DOI] [PubMed] [Google Scholar]
- Boi S, Couzin ID, DelBuono N, Franks NR, Britton NF. Coupled oscillators and activity waves in ant colonies. Proc R Soc London B. 1999;266:371–378. [Google Scholar]
- Børgesen LW. Nutritional function of replete workers in the pharaoh’s ant, Monomorium pharaonis (L.) Insect Soc. 2000;47:141–146. [Google Scholar]
- Bourke AFG, Franks NR. Social Evolution in Ants. Princeton University Press; Princeton: 1995. p. 529. [Google Scholar]
- Brian MW. Social Insects: Ecology and Behavioral Biology. Chapman and Hall; New York: 1983. p. 377. [Google Scholar]
- Cassill D. Yoyo-bang: a risk-aversion investment strategy by a perennial insect society. Oecologia. 2002;132:150–158. doi: 10.1007/s00442-002-0928-2. [DOI] [PubMed] [Google Scholar]
- Chapuisat M, Sundström L, Keller L. Sex-ratio regulation: the economics of fratricide in ants. Proc R Soc London B. 1997;264:1255–1260. [Google Scholar]
- Cole BJ. Short-term activity cycles in ants: generation of periodicity by worker interaction. Am Nat. 1991;137:244–259. [Google Scholar]
- Cole BJ, Hoeg L. The influence of brood type on activity cycles in Leptothorax allardycei (Hymenoptera: Formicidae) J Insect Behav. 1996;9:539–547. [Google Scholar]
- Creighton WS. The ants of North America. Bull Mus Comp Zool. 1950;104:1–585. [Google Scholar]
- De Marco RJ, Farina WM. Trophallaxis in forager honeybees (Apis mellifera): Resource uncertainty enhances begging contacts? J Comp Physiol A. 2003;189:125–134. doi: 10.1007/s00359-002-0382-y. [DOI] [PubMed] [Google Scholar]
- Foitzik S, Backus VL, Trindl A, Herbers JM. Ecology of Leptothorax ants: impact of food, nest sites, and social parasites. Behav Ecol Sociobiol. 2004;55:484–493. [Google Scholar]
- Franks N, Ireland B, Bourke AFG. Conflicts, social economics and life history strategies in ants. Behav Ecol Sociobiol. 1990a;27:175–181. [Google Scholar]
- Franks NR, Bryant S, Griffiths R, Hemerik L. Synchronization of the behaviour within nests of the ant Leptothorax acervorum (Fabricius). I Discovering the phenomenon and its relation to the level of starvation. Bull Math Biol. 1990b;52:597–612. [Google Scholar]
- Gobin B, Ito F. Queens and major workers of Acanthomyrmex ferox redistribute nutrients with trophic eggs. Naturwissenschaften. 2000;87:323–326. doi: 10.1007/s001140050731. [DOI] [PubMed] [Google Scholar]
- Gordon DM. Behavioral flexibility and the foraging ecology of seed-eating ants. Am Nat. 1991;138:379–411. [Google Scholar]
- Haack KD, Vinson SB, Olson JK. Food distribution and storage in colonies of Monomorium pharaonis (L) (Hymenoptera, Formicidae) J Entomol Sci. 1995;30:70–81. [Google Scholar]
- Heinze J, Foitzik S, Fischer B, Wanke T, Kipyatkov VE. The significance of latitudinal variation in body size in a holarctic ant, Leptothorax acervorum. Ecography. 2003;26:349–355. [Google Scholar]
- Heinze J, Hölldobler B, Peeters C. Conflict and cooperation in ant societies. Naturwissenschaften. 1994;81:489–497. [Google Scholar]
- Heinze J, Stahl M, Hölldobler B. Ecophysiology hibernation in boreal Leptothorax ants (Hymenoptera: Formicidae) Ecoscience. 1996;3:429–435. [Google Scholar]
- Hemerik L, Britton NF, Franks NR. Synchronization of the behavior within nests of the ant Leptothorax acervorum (Fabricius) 2. Modeling the phenomenon and predictions from the model. Bull Math Biol. 1990;52:613–628. [Google Scholar]
- Herbers JM. Time resources and laziness in animals. Oecologia. 1981;49:252–262. doi: 10.1007/BF00349198. [DOI] [PubMed] [Google Scholar]
- Hölldobler B, Wilson EO. The number of queens: an important trait in ant evolution. Naturwissenschaften. 1977;64:8–15. [Google Scholar]
- Hölldobler B, Wilson EO. The Ants. Harvard University Press; Cambridge, Mass: 1990. p. 732. [Google Scholar]
- Howard DF, Tschinkel WR. The effect of colony size and starvation on food flow in the fire ant, Solenopsis invicta (Hymenoptera: Formicidae) Behav Ecol Sociobiol. 1980;7:293–300. [Google Scholar]
- Kaspari M, Byrne MM. Caste allocation in litter Pheidole: lessons from plant defense theory. Behav Ecol Sociobiol. 1995;37:255–263. [Google Scholar]
- Kaspari M, Vargo EL. Colony size as a buffer against seasonality: Bergmann’s rule in social insects. Am Nat. 1995;145:610–632. [Google Scholar]
- Liebig J, Heinze J, Hölldobler B. Trophallaxis and aggression in the ponerine ant, Ponera coarctata: Implications for the evolution of liquid food exchange in the Hymenoptera. Ethology. 1997;103:707–722. [Google Scholar]
- Liebig J, Poethke HJ. Queen lifespan and colony longevity in the ant Harpegnathos saltator. Ecol Entom. 2004;29:203–207. [Google Scholar]
- Lighton JRB, Weier JA, Feener DH. The energetics of locomotion and load carriage in the desert harvester ant Pogonomyrmex rugosus. J Exp Biol. 1993;181:49–61. [Google Scholar]
- Manly BFJ. Randomization, Bootstrap and Monte Carlo Methods in Biology. 2. Chapman and Hall; London, UK: 1997. p. 424. [Google Scholar]
- Martínez T, Burmester T, Veenstra JA, Wheeler DE. Sequence and evolution of a hexamerin from the ant Camponotus festinatus. Insect Mol Biol. 2000;9:427–431. doi: 10.1046/j.1365-2583.2000.00204.x. [DOI] [PubMed] [Google Scholar]
- McNamara JM, Houston AI. State-dependent contests for food. J Theor Biol. 1989;137:457–479. [Google Scholar]
- Moritz RFA, Fuchs S. Organization of honeybee colonies: characteristics and consequences of a superorganism concept. Apidologie. 1998;29:7–21. [Google Scholar]
- Nonacs P. Less growth with more food: how insect-prey availability changes colony demographics in the ant, Camponotus floridanus. J Insect Physiol. 1991;37:891–898. [Google Scholar]
- Oster GF, Wilson EO. Caste and Ecology in the Social Insects. Princeton University Press; Princeton: 1978. p. 352. [PubMed] [Google Scholar]
- Pamilo P. Life-span of queens in the ant Formica execta. Insect Soc. 1991;38:111–119. [Google Scholar]
- Porter SD. Effects of diet on the growth of laboratory fire ant colonies (Hymenoptera: Formicidae) J Kansas Entomol Soc. 1989;62:288–291. [Google Scholar]
- Porter SD, Jorgensen CD. Longevity of harvester ant colonies in southern Idaho. J Range Management. 1988;41:104–107. [Google Scholar]
- Porter SD, Tschinkel WR. Fire ant thermal preferences behavioral control of growth and metabolism. Behav Ecol Sociobiol. 1993;32:321–329. [Google Scholar]
- Rueppell O, Amdam GV, Page RE, Jr, Carey JR. From genes to society: Social insects as models for research on aging. Sci Aging Knowl Environ 2004. 2004 http://sageke.sciencemag.org/cgi/content/full/2004/5/pe5.
- Rüppell O, Heinze J, Hölldobler B. Size-dimorphism in the queens of the North American ant Leptothorax rugatulus (Emery) Insect Soc. 1998;45:67–77. [Google Scholar]
- Rüppell O, Heinze J, Hölldobler B. Alternative reproductive tactics in the queen-size-dimorphic ant Leptothorax rugatulus (Emery) and their consequences for genetic population structure. Behav Ecol Sociobiol. 2001;50:189–197. [Google Scholar]
- Rüppell O, Heinze J, Hölldobler B. Intracolonial patterns of reproduction in the queen-size dimorphic ant Leptothorax rugatulus. Behav Ecol. 2002a;13:239–247. [Google Scholar]
- Rüppell O, Schäffler L, Hölldobler B. Lack of plasticity in the behavior of queens of the ant Leptothorax rugatulus Emery (Formicidae: Hymenoptera) J Insect Behav. 2002b;15:447–454. [Google Scholar]
- Rüppell O, Strätz M, Baier B, Heinze J. Mitochondrial markers in the ant Leptothorax rugatulus reveal the population genetic consequences of female philopatry at different hierarchical levels. Mol Ecol. 2003;12:795–801. doi: 10.1046/j.1365-294x.2003.01769.x. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P. Parasites in Social Insects. Princeton University Press; Princeton, NJ: 1998. p. 392. [Google Scholar]
- Schulz DJ, Vermiglio MJ, Huang ZY, Robinson GE. Effects of colony food shortage on social interactions in honey bee colonies. Insect Soc. 2002;49:50–55. [Google Scholar]
- Seppa P. Sociogenetic organization of the ants Myrmica ruginodis and Myrmica lobicornis – number, relatedness and longevity of reproducing individuals. J Evol Biol. 1994;7:71–95. [Google Scholar]
- Sorensen AA, Busch TM, Vinson SB. Factors affecting brood cannibalism in laboratory colonies of the imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae) J Kansas Entomol Soc. 1983;56:140–150. [Google Scholar]
- Sudd JH, Franks NR. The Behavioural Ecology of Ants. Chapman & Hall; New York: 1987. p. 206. [Google Scholar]
- Taylor RW. Nothomyrmecia macrops: a living-fossil ant rediscovered. Science. 1978;201:979–985. doi: 10.1126/science.201.4360.979. [DOI] [PubMed] [Google Scholar]
- Thomas ML. Nest site selection and longevity in the ponerine ant Rhytidoponera metallica (Hymenoptera, Formicidae) Insect Soc. 2002;49:147–152. [Google Scholar]
- Toda MJ, Kimura MT. Life-history traits related to host selection in mycophagous drosophilids. J Anim Ecol. 1997;66:154–166. [Google Scholar]
- Vargo EL, Fletcher DJC. On the relationship between queen number and fecundity in polygyne colonies of the fire ant Solenopsis invicta. Physiol Entomol. 1989;14:223–232. [Google Scholar]
- Wheeler DE, Martínez T. Storage proteins in ants (Hymenoptera: Formicidae) Comp Biochem Physiol B. 1995;112:15–19. doi: 10.1016/0305-0491(95)00035-7. [DOI] [PubMed] [Google Scholar]
- Wheeler WM. The Social Insects: Their Origin and Evolution. Harcourt, Brace and Co; New York: 1928. p. 378. [Google Scholar]
- Wilson EO. The Insect Societies. Harvard University Press; Cambridge, Mass: 1971. p. 548. [Google Scholar]


