Abstract
Derivatives of oleanolic acid, ursolic acid and glycyrrhetinic acid substituted with electron withdrawing groups at the 2-position in the A-ring which also contains a 1-en-3-one structure are potent inhibitors of cancer cell growth. In this study, we have compared the effects of several 2-substituted analogs of triterpenoid acid methyl esters derived from ursolic and glycyrrhetinic acid on proliferation of KU7 and 253JB-V bladder and Panc-1 and Panc-28 pancreatic cancer cells. The results show that the 2-cyano and 2-trifluoromethyl derivatives were the most active compounds. The glycyrrhetinic acid derivatives with the rearranged C-ring containing the 9(11)-en-12-one structure were generally more active than the corresponding 12-en-11-one isomers. However, differences in growth inhibitory IC50 values were highly variable and dependent on the 2- substitutent (CN vs. CF3) and cancer cell context.
Keywords: glycyrrhetinate analogs, growth inhibition, bladder cancer, pancreatic cancer
Pentacyclic triterpenoid acids such as betulinic acid, oleanolic acid, ursolic acid, and glycyrrhetinic acid are phytochemicals that have been extensively used in traditional medicines for treatment of a wide variety of human ailments.1–4 Most of these compounds exhibit anti-inflammatory and anticarcinogenic activities as well as a large number of compound-specific effects. For example, the major bioactive component of licorice extracts is glycyrrhizic acid glycoside which is readily hydrolyzed to glycyrrhetinic acid and these extracts/compounds possess anti-inflammatory, antiviral and endocrine activities.4 All of these triterpenoid acids have been used as building blocks for the synthesis of more active analogs. Oleanolic acid has been converted into 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO), the corresponding methyl ester (CDDO-Me) and other structurally-related analogs, and these compounds are potent anticancer agents.5–9 These synthetic triterpenoids activate the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ)9 and also induce several other responses including apoptosis in various cancer cell lines. The cytotoxicity and anti-inflammatory activity of CDDO-Me and related compounds are due to the 2-cyano group and the 1-en-3-one and 9-en-12-one functionalities in the A- and C-rings, respectively.7, 8 We also showed that the glycyrrhetinic acid analog methyl 2-cyano-3,11-dioxo-18β-olean-1,12-dien-30-oate (β-CDODA-Me) and the corresponding 18α derivative (α-CDODA-Me) which contain 2-cyano-1-en-3-one and 12-en-11-one functionalities in their A- and C-rings, respectively, activated PPARγ and were also highly cytotoxic in colon cancer cells.10 Similar results were obtained for the betulinic acid derivatives containing a 2-cyano-1 en-3-one function.11 In this study, we have further investigated the structure-dependent growth inhibitory effects of β-CDODA-Me and analogs containing different electronegative 2-substituents and a selected number of the corresponding ursolic acid 2-substituted-1-ene-3-one derivatives.12 In addition, we also show that for the glycyrrhetinic acid derivatives growth inhibitory differences of the 9-en-12-one and 12-en-11-one isomers are lower than previously reported for anti-inflammatory activity of CDDO-Me and the 12-en-11-one isomers.8 Growth inhibition by the 2-substituted glycyrrhetinic acid and ursolic acid methyl ester analogs is dependent on the nature of the 2-substituent and cancer cell context among two bladder (KU7 and 253JB-V) and two pancreatic (Panc-1 and Panc-28) cancer cell lines.13
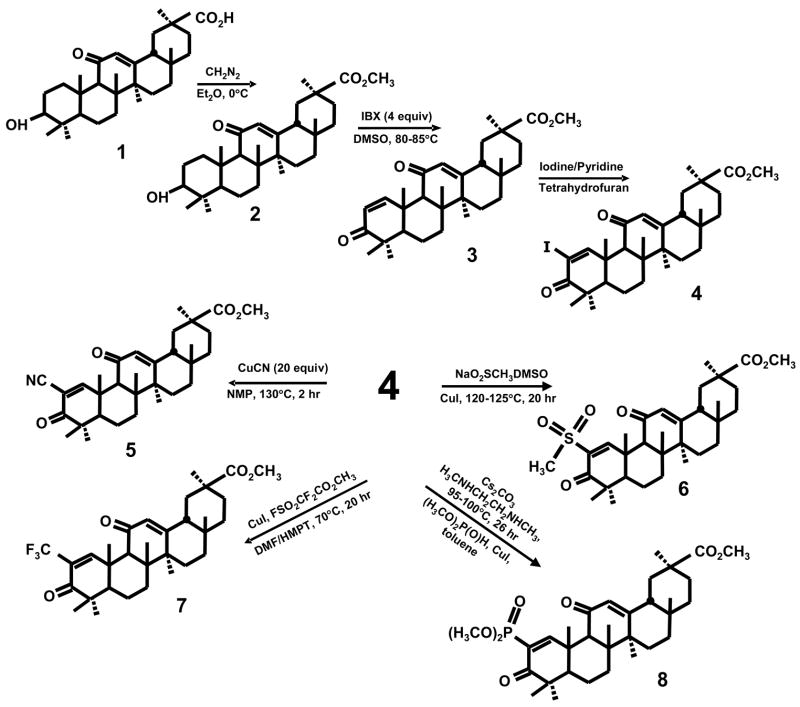
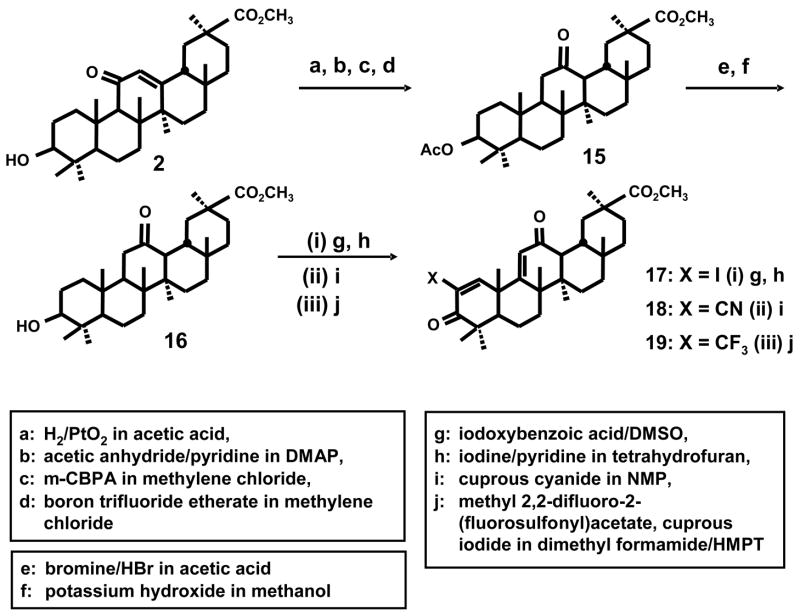
The synthesis of a series of 2-substituted glycyrrhetinic acid derivatives is summarized in Scheme 1. The free acid (1) was esterified at 0°C with ethereal diazomethane to give methyl glycyrrhetinate (2) which was then treated with 4 equivalents of 2-iodoxybenzoic acid (IBX) in DMSO at 85°C for 21 hours. The resulting methyl 3,11-dioxo-18β-oleana-1,12-dien-30-oate product (3) was then converted into the 2-iodo derivative (4) by treating with 2 equivalents of iodine and 3 equivalents of pyridine in tetrahydrofuran (refluxing). The methyl 2-iodo-3,11-dioxo-18β-oleana-1,12-dien-30-oate derivative (4) was used as the precursor for the synthesis of the 2-cyano (5), 2-methanesulfonyl (6), 2-trifluoromethyl (7) and 2-dimethylphosphonyl (8) analogs (Scheme 1). The 2-cyano derivative was prepared by treating 4 with 2 equivalents of cuprous cyanide in N-methylpyrrolidinone (NMP) for 2 hours at 130°C. The 2-methanesulfonyl derivative was obtained by treating 4 with sodium methanesulfinate and cuprous iodide in DMSO at 120–125°C for 20 hours. The 2-dimethylphosphonyl analog was synthesized by treating 4 with dimethylphosphite, cesium carbonate, N,N-dimethylethylenediamine in toluene at 95–100°C for 26 hours. Finally the 2-trifluoromethyl derivative was obtained by treating 4 with cuprous iodide and methyl 2,2-difluoro-2-(fluorosulfonyl) acetate in dimethylformamide/HMPT at 70°C for 20 hours. The synthetic scheme used for conversion of ursolic acid (9) into the 2-iodo derivative (12) and the corresponding 2-cyano (13) and 2-trifluoromethyl (14) derivatives (Scheme 2) involved intermediates (10) and (11) and was identical to that carried out for conversion of methyl glycyrrhetinate into the analogous 2-substituted compound as illustrated in Scheme 1. Previous studies with oleanolic acid derivatives reported the synthesis of several analogs including CDDO which contain the 1-en-3-one A-ring and a 9-en-12-one C-ring8, and Scheme 3 outlines an analogous route for conversion of methyl glycyrrhetinate into the rearranged C-ring analogs of CDODA-Me. Methyl glycyrrhetinate was reduced with H2/platinum oxide catalyst in acetic acid, then acetylated with acetic anhydride/pyridine in dimethylaminopyridine (DMAP) to give the acetylated ester (15) in which the 9-oxo group had been reduced. Treatment with m-chloroperbenzoic acid (m-CPBA) in methylene chloride gave the 12,13-epoxide which was converted into the 12-oxo derivative by boron trifluoride etherate in methylene chloride. Bromination of the 12-oxo derivative followed by dehydrobromination in acetic anhydride followed by basic hydrolysis of the acetate group gave the rearranged C-ring derivative of methyl glycyrrhetinate (16). Subsequent treatment with 2-iodoxybenzoic acid and iodine/pryridine in tetrahydrofuran gave methyl 2-iodo-3,12-dioxo-oleana-1,9-dien-30-oate (17) which is converted into the C-ring rearranged cyano (18) and trifluoromethyl (19) derivatives as outlined in Schemes 1 and 2.
Scheme 1.
Synthesis of 2-substituted-1-en-3-one derivative of methyl glycyrrhetinate.
Scheme 2.
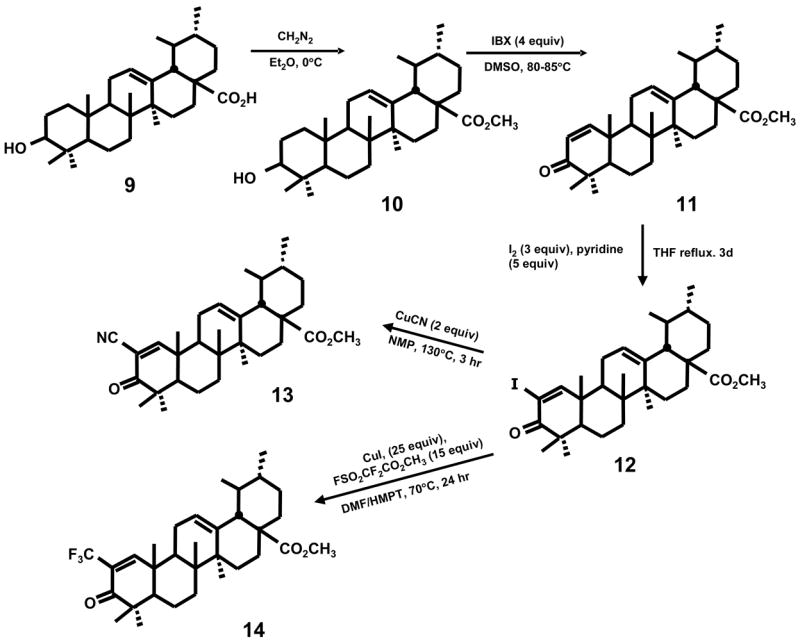
Synthesis of 2-substituted-1-en-3-one derivatives of methyl ursolate.
Scheme 3.
Synthesis of C-ring rearranged analogs of CDODA-Me.
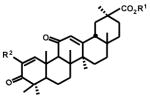
Previous studies with CDDO-Me and related compounds showed that the 1-en-3-one structure containing electronegative 2-substituent groups such as cyano were highly cytotoxic5–9 and similar results were observed for β-CDODA-Me, the corresponding 2-cyano analog of glycyrrhetinic acid.10 In this study, we used 253JB-V and KU7 bladder and Panc-1 and Panc-28 pancreatic cancer cells to investigate the growth inhibitory effects14 of CDODA-Me analogs containing several electronegative 2-substituents including iodo, cyano, trifluoromethyl, dimethylphosphonyl and methanesulfonyl groups (Table 1). β-CDODA, the parent free acid derivative of β-CDODA-Me is used as a control for this group of compounds, and IC50 values for inhibition of bladder and pancreatic cancer cell growth varied from 5.9 – 7.3 μM. The corresponding range of IC50 values for β-CDODA-Me was 0.25 to 1.80 μM indicating that in cell culture studies the methyl ester derivatives were more potent than the free acid (β-CDODA) as previously reported for growth inhibition of colon cancer cells.10 The effects of compounds unsubstituted at C-2 were >7 fold less active than the 2-cyano derivatives (data not shown) and these results were similar to those observed for these compounds in colon cancer cells.10 Based on the synthetic scheme which used the 2-iodo derivative (4) as a precursor, we synthesized the 2-trifluoromethyl, dimethylphosphonyl and methanesulfonyl derivatives and determined the effects of different electronegative substituents on their inhibition of bladder and pancreatic cancer cell growth (Table 1). The IC50 values were lowest for 2-cyano and 2-trifluoromethyl (β-CF3DODA-Me) derivatives; however, their relative potencies were dependent on cell context; β-CF3DODA-Me was more active than β-CDODA-Me in KU7 (IC50: 0.38 vs. 1.59 μM), Panc-1 (IC50: 0.82 vs. 1.22 μM) and Panc-28 (IC50: 1.14 vs. 1.80 μM) cells, whereas β-CDODA-Me was more active in 253JB-V cells (IC50: 0.25 vs. 0.67 μM). Both the 2-dimethylphosphonyl and 2-methanesulfonyl analogs were relatively inactive with IC50 values ranging from 3.34–12.0 μM over the four cell lines.
Table 1.
Cytotoxicity of 2-substituted compounds derived from methyl glycyrrhetinate.
| IC50(μM) | |||||
|
253JB-V | KU7 | PANC 1 | PANC 28 | |
| R1 = H : R2 = CN | 6.10 | 5.88 | 3.81 | 7.32 | |
| R1 = CH3 : R2 = I (4) | 2.67 | 3.04 | 4.08 | 12.75 | |
| R1 = CH3 : R2 = CN (5) | 0.25 | 1.59 | 1.22 | 1.80 | |
| R1 = CH3 : R2 = CF3 (6) | 0.67 | 0.38 | 0.82 | 1.14 | |
| R1 = CH3 : R2 = CH3SO2 (7) | 11.97 | 3.34 | 7.69 | 9.75 | |
| R1 = CH3 : R2 = CH3O)2PO (8) | 7.90 | 3.73 | 6.11 | 8.14 | |
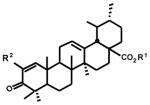
Since the 2-cyano and 2-trifluoromethyl derivatives were the most active of the glycyrrhetinic acid group of 2-substituted 1-en-3-one compounds, we synthesized a similar series of analogs from methyl ursolate and compared their growth inhibitory IC50 values to the 2-iodo analog and methyl ursolate (Table 2). IC50 values for methyl ursolate (9) and the 2-iodo-1-en-3-one analog (12) varied from 6.13–11.75 and 4.90–13.50 μM, respectively, in the bladder and pancreatic cancer cell lines and there was less than a 2-fold difference in their IC50 values. IC50 values for the 2-cyano (13) and 2-trifluoromethyl (14) analogs varied from 0.17–0.97 and 0.17–1.13 μM, respectively, and were at least an order of magnitude more potent in the growth inhibition assay than the 2-iodo compound or methyl ursolate. These data confirm that 2-cyano and 2-trifluoromethyl groups coupled with introduction of a 1-en-3-one functionality into the A-ring of methyl ursolate resulted in enhanced growth inhibition with comparable IC50 values in KU7, 253JB-V, Panc-1 and Panc-28 cancer cells.
Table 2.
Cytotoxicity of 2-substituted compounds derived from methyl ursolate.
| IC50(μM) | |||||
|
253JB-V | KU7 | PANC 1 | PANC 28 | |
| Methyl Ursolate (a) | 6.13 | 8.95 | 11.75 | 10.58 | |
| R1 = CH3 : R2 = I (12) | 4.90 | 6.02 | 6.91 | 13.49 | |
| R1 = CH3 : R2 = CN (13) | 0.17 | 0.30 | 0.53 | 0.97 | |
| R1 = CH3 : R2 = CF3 (14) | 0.17 | 0.47 | 0.65 | 1.13 | |
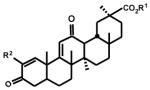
Previous studies with CDDO-Me and related compounds showed that the functionality of the C-ring markedly influenced the activity of oleanolic acid derivatives with 2-cyano-1-en-3-one functionality.9 For example, CDDO-Me which contains a 9(11)-en-12-one C-ring structure is 200 times more potent as an inhibitor of nitric oxide production in mouse macrophages than the corresponding 12-en-11-one isomer.9 We therefore synthesized a series of 2-iodo, 2-cyano, and 2-trifluoromethyl-1-en-3-one analogs of glycyrrhetinic acid which also contain a 9(11)-en-12-one C-ring functionality (Table 3) and compared their growth inhibitory effects to the 12-en-11-one isomers (Table 1). The effects of the C-ring en-one functionality on the activity of these isomers was dependent not only on the 2-substituent but also on cell context. IC50 values were similar for both 2-iodo isomers in 253JB-V, KU7 and Panc-1 cells, whereas IC50 values for the 12-en-11-one and 9(11)-en-12-one isomers in Panc-28 cells were 12.75 and 3.66 μM, respectively. Thus, for the 2-iodo derivative, the C-ring structure had minimal effects on growth inhibitory activity, except for one cell line where IC50 differences were < 4-fold.
Table 3.
Cytotoxicity of 2-substituted compounds derived from methyl glycyrrhetinate with C-ring rearrangement.
| IC50(μM) | |||||
|
253JB-V | KU7 | PANC 1 | PANC 28 | |
| CDDO-Me | 0.03 | 0.12 | 0.27 | 0.29 | |
| R1 = CH3 : R2 = I (17) | 3.62 | 2.61 | 4.45 | 3.66 | |
| R1 = CH3 : R2 = CN (17) | 0.11 | 0.12 | 0.07 | 0.05 | |
| R1 = CH3 : R2 = CF3 (18) | 0.36 | 1.37 | 0.68 | 1.13 | |
Differences in IC50 values for the 2-cyano methyl-glycyrrhetinate isomers with 12-en-11-one and 9(11)-en-12-one functionalities were more dramatic and varied from a 2.2- (253JB-V cells) to a 39.1-fold (Panc-28) lower IC50 values for isomers containing the former C-ring structure. These differences for the 2-cyano derivatives of methyl glycyrrhetinate were significantly lower than differences observed for CDDO-Me and the corresponding 12-en-11-one isomer where IC50 values for inhibition of nitric oxide production in mouse macrophages was 0.1 nM and 20 nM, respectively, a 200-fold difference.9 We also directly compared the effects of the rearranged 2-cyano methyl glycyrrhetinate derivative (18) with CDDO-Me in the four cell lines; the former compound (18) was more active than CDDO-Me in Panc-1 and Panc-28 cells, whereas IC50 values were similar in KU7 cells and CDDO-Me (IC50 = 0.03 μM) was more active in 253JB-V cells. Thus, a direct comparison of the 2-cyano derivative of methyl glycyrrhetinate (18) and CDDO-Me (derived from methyl oleanolate) on their inhibition of cancer cell proliferation indicates that both synthetic triterpenoids are highly potent and differences in their IC50 values in four cell lines were less than an order of magnitude.
The antiproliferative activity of the trifluoromethyl derivative (6) (Table 1) was <2 fold lower than the 9(11)-en-12-one isomer in 253JB-V and Panc-1 cells, whereas similar IC50 values were observed in Panc-28 cells. In contrast the 12-en-11-one (unrearranged) trifluoromethyl analog was more active (IC50 = 0.38 μM) in KU7 cells than the corresponding 9(11)-en-12-one isomer (IC50 = 1.37 μM). Figure 1 illustrates the structure-dependent differences in growth inhibitory activities in KU7 cells of the 2-cyano and 2− trifluoromethyl analogs containing the unrearranged 12-en-11-one (5 and 7) and rearranged 9(11)-en-12-one (18 and 19) C-ring in the methyl glycyrrhetinate series of isomers.
Figure 1.
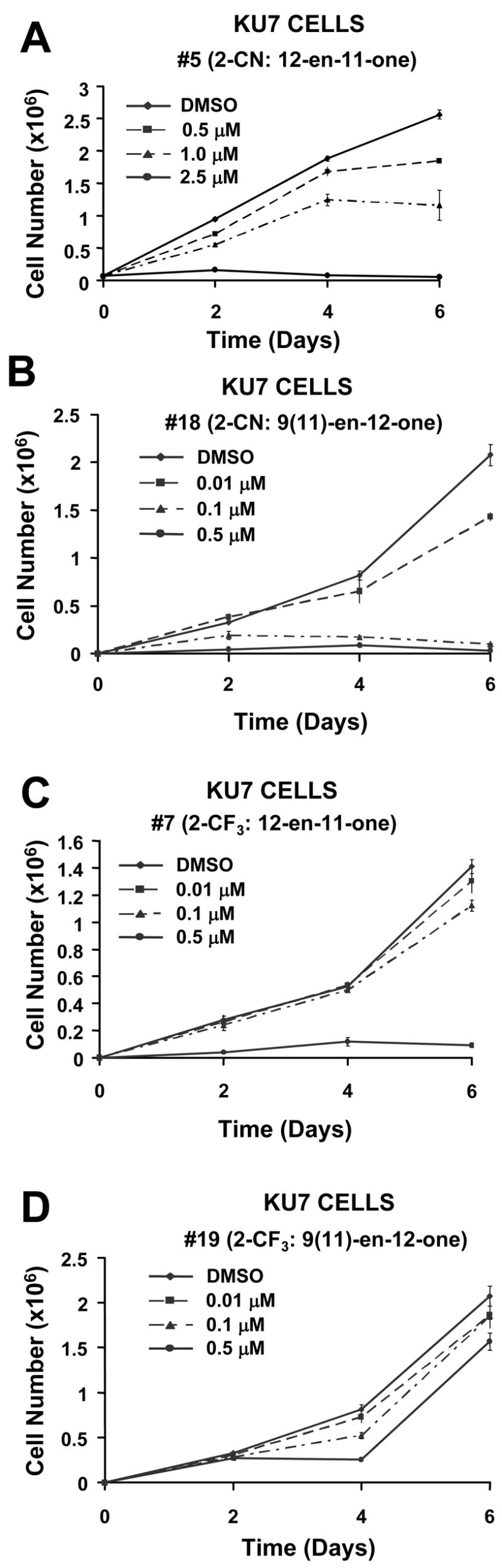
Effects of 2-CN- and 2-CF3-1-en-3-one analogs containing 12-en-11-one or 9(11)-en-12-one functionality in the C-ring.
In summary, the results of this study show that the antiproliferative activities of several 2-substituted glycyrrhetinic acid and ursolic acid derivatives containing the 1-en-3-one functionality in their A-rings were maximal for both the cyano and trifluoromethyl derivatives. The corresponding 2-iodo substituted analogs were less active (Tables 1 and 2). The 2-dimethylphosphonyl and 2-methanesulfonyl derivatives of glycyrrhetinic acid (Table 1) also exhibited higher IC50 values than the corresponding 2-cyano and 2-trifluoromethyl derivatives. The rationale for the observed differences in the activity of the different 2-substituted analogs containing electronegative substituents is unclear and is currently being investigated using other assay systems. Compared to previous studies with oleanolic acid derivatives, 5–9 the effects of the C-ring functionality [9(11)-en-12-one vs. 12-en-11-one] on inhibition of cancer cell growth were minimal (Tables 1 and 3) and dependent on the 2-substituent (cyano vs. trifluoromethyl) (Figure 1) and cancer cell context. This suggests that for the glycyrrhetinic acid derivatives it may not be necessary to carry out the additional synthetic steps to convert the 12-en-11-one to the 9(11)-en-12-one functionality in the C-ring. This is confirmed, in part, by ongoing in vivo studies in mouse xenograft experiments where significant inhibition of tumor growth by β-CDODA-Me is observed at doses of 10 to 15 mg/kg/day (data not shown). Ongoing work includes further investigation of structure-dependent anticarcinogenic potencies of the cyano and trifluoromethyl glycyrrhetinic acid analogs, and studies of their underlying mechanism of action and clinical applications.
Chemicals
The ursolic acid and glycyrrhetinic acid starting materials were purchased from Sigma-Aldrich (St. Louis, MO) all chemical reagents used for synthesis were purchased from Sigma-Aldrich. Dulbecco’s modified/Ham’s F-12 and RPMI media and the antibiotic/antimycotic solution were also obtained from Sigma Aldrich.
Cell lines, chemicals and other materials
KU7 human bladder cancer and Panc-1 pancreatic cancer cells were obtained from the American Type Culture Collection (Manassas, VA) and 253JB-V cells were provided by Dr. A. Kamat, M.D Anderson Cancer Center, Houston, TX. The Panc-28 cell line was a generous gift from Dr. Paul Chiao, The University of Texas M.D. Anderson Cancer Center (Houston, TX).
Both 253JB-V and KU7 cell lines were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 0.15% sodium bicarbonate, 0.011% sodium pyruvate, 0.24% HEPES and 10 ml/L of antibiotic/antimycotic cocktail solution. The pancreatic cancer cells were maintained in Dulbecco’s modified/Ham’s F-12 with phenol red supplemented with 0.22% sodium bicarbonate, 0.011% sodium pyruvate, 5% fetal bovine serum and 10 ml/L 100x antibiotic/antimycotic solution. The cells were grown in 150 cm2 culture plates in an air/CO2 (95:5) atmosphere at 37°C and passaged approximately every 3 days.
Cell proliferation assays
All four cancer cell lines (3 × 104 cells per well) were plated using DMEM:Ham’s F-12 medium containing 2.5% charcoal-stripped FBS in 12-well plates and left to attach for 24 hours. Cells were then treated with either vehicle (DMSO) or the indicated concentrations of different compounds. Fresh medium and test compounds were added every 48 hours and cells were then counted at the indicated times using a Coulter Z1 particle counter. Each experiment was done in triplicate, and results are expressed as means ± SE for each determination. The IC50 values were calculated using linear regression method and were expressed in μM.
Acknowledgments
The financial assistance of the National Institutes of Health (ES09106 and CA108718) and the Texas Agricultural Experiment Station is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sami A, Taru M, Salme K, Jari YK. Eur J Pharmacol Sci. 2006;29:1. [Google Scholar]
- 2.Yogeeswari P, Sriram D. Curr Med Chem. 2005;12:657. doi: 10.2174/0929867053202214. [DOI] [PubMed] [Google Scholar]
- 3.Liu J. J Ethnopharmacol. 1995;49:57. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 4.Fiore C, Eisenhut M, Ragazzi E, Zanchin G, Armanini D. J Ethnopharmacol. 2005;99:317. doi: 10.1016/j.jep.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honda T, Finlay HJ, Gribble GW, Suh N, Sporn MB. Bioorg Med Chem Lett. 1997;7:1623. [Google Scholar]
- 6.Honda T, Rounds BV, Gribble GW, Suh N, Wang Y, Sporn MB. Bioorg Med Chem Lett. 1998;8:2711. doi: 10.1016/s0960-894x(98)00479-x. [DOI] [PubMed] [Google Scholar]
- 7.Honda T, Gribble GW, Suh N, Finlay HJ, Rounds BV, Bore L, Favaloro FG, Jr, Wang Y, Sporn MB. J Med Chem. 2000;43:1866. doi: 10.1021/jm000008j. [DOI] [PubMed] [Google Scholar]
- 8.Honda T, Rounds BV, Bore L, Finlay HJ, Favaloro FG, Jr, Suh N, Wang Y, Sporn MB, Gribble GW. J Med Chem. 2000;43:4233. doi: 10.1021/jm0002230. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Porter WW, Suh N, Honda T, Gribble GW, Leesnitzer LM, Plunket KD, Mangelsdorf DJ, Blanchard SG, Willson TM, Sporn MB. Mol Endocrinol. 2000;14:1550. doi: 10.1210/mend.14.10.0545. [DOI] [PubMed] [Google Scholar]
- 10.Chintharlapalli S, Papineni S, Jutooru I, McAlees A, Safe S. Mol Cancer Therap. 2007;6:1588. doi: 10.1158/1535-7163.MCT-07-0022. [DOI] [PubMed] [Google Scholar]
- 11.Chintharlapalli S, Papineni S, Liu S, Jutooru I, Chadalapaka G, Cho SD, Murthy R, You YJ, Safe S. Carcinogenesis. 2007;28:2337. doi: 10.1093/carcin/bgm189. [DOI] [PubMed] [Google Scholar]
- 12.Chemicals: The ursolic acid and glycyrrhetinic acid starting materials were purchased from Sigma-Aldrich (St. Louis, MO) all chemical reagents used for synthesis were purchased from Sigma-Aldrich. Dulbecco’s modified/Ham’s F-12 and RPMI media and the antibiotic/antimycotic solution were also obtained from Sigma Aldrich.
- 13.Cell lines, chemicals and other materials: KU7 human bladder cancer and Panc-1 pancreatic cancer cells were obtained from the American Type Culture Collection (Manassas, VA) and 253JB-V cells were provided by Dr. A. Kamat, M.D Anderson Cancer Center, Houston, TX. The Panc-28 cell line was a generous gift from Dr. Paul Chiao, The University of Texas M.D. Anderson Cancer Center (Houston, TX). Both 253JB-V and KU7 cell lines were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 0.15% sodium bicarbonate, 0.011% sodium pyruvate, 0.24% HEPES and 10 ml/L of antibiotic/antimycotic cocktail solution. The pancreatic cancer cells were maintained in Dulbecco’s modified/Ham’s F-12 with phenol red supplemented with 0.22% sodium bicarbonate, 0.011% sodium pyruvate, 5% fetal bovine serum and 10 ml/L 100x antibiotic/antimycotic solution. The cells were grown in 150 cm2 culture plates in an air/CO2 (95:5) atmosphere at 37°C and passaged approximately every 3 days.
- 14.Cell proliferation assays: All four cancer cell lines (3 × 104 cells per well) were plated using DMEM:Ham’s F-12 medium containing 2.5% charcoal-stripped FBS in 12-well plates and left to attach for 24 hours. Cells were then treated with either vehicle (DMSO) or the indicated concentrations of different compounds. Fresh medium and test compounds were added every 48 hours and cells were then counted at the indicated times using a Coulter Z1 particle counter. Each experiment was done in triplicate, and results are expressed as means ± SE for each determination. The IC50 values were calculated using linear regression method and were expressed in μM.