Summary
Monocytes are circulating mononuclear phagocytes with a fundamental capacity to differentiate into macrophages. This differentiation can, in the presence of the right environmental cues, be re-directed instead to dendritic cells (DCs). Recent advances have been made in understanding the role of monocytes and their derivatives in presenting antigen to drive immune responses, and we review this topic herein. We briefly discuss the heterogeneity of monocytes in the blood and subsequently raise the possibility that one of the major monocyte phenotypes in the blood corresponds with a population of “blood DCs” previously proposed to drive T-independent antibody reactions in the spleen. Then we evaluate the role of monocytes in T-dependent immunity, considering their role in acquiring antigens for presentation prior to exiting the bloodstream and their ability to differentiate into macrophages versus antigen-presenting DCs. Finally, we review recent literature on the role of monocyte-derived cells in cross-presentation and discuss the possibility that monocyte-derived cells participate critically in processing antigen for cross-priming, even if they do not present that antigen to T cells themselves.
Just recently, we participated in a group discussion with other colleagues whose research interests includes the biology of monocytes, and the discussion moved to the point of whether one can define the term “monocyte.” Not all of our colleagues believed that such a definition could be clearly made in the current molecular age where cell types are readily divided up and defined by the presence and absence of an array of cell surface markers. However, we cannot approach research on the subject of monocytes without drawing a definition for these cells. Though universal agreement on this definition may not be possible, as we learned during this recent collegial exchange, the definition we adhere to is practical and a product of our training and discussion with other experts on monocytes over more than 15 years. For decades, a monocyte was identifiable morphologically and functionally as the mononuclear phagocyte in the blood [1–3]. Corresponding with this definition was the understanding that the cell readily differentiated into macrophages. Thus, our research on monocytes operates under the premise that monocytes are (a) mononuclear cells in the circulation with (b) the capacity for phagocytosis and (c) propensity to differentiate to macrophages at least in some settings. Some cell types, often termed circulating dendritic cells (DCs), have been identified in human blood that fit one or both of the first two parts of the definition but so far not the third [4–6]. So, fittingly, these latter cells are not included in the definition of monocytes. We also uphold the view that the term “monocyte” defines a cell that is in the bloodstream. Once a monocyte extravasates into tissue, a differentiation program toward macrophages or DCs begins, and the cell should no longer be referred to as a monocyte, but rather as a monocyte-derived cell.
DCs are specialized antigen-presenting cells that can efficiently initiate immune responses [7,8], and there has been a long interest in the role of monocytes as potential precursors for these cells. A growing body of evidence indicates that many DC populations are not typically derived from monocytes [9–11]. Nonetheless, monocytes do have a role as precursors for important antigen-presenting cells, including DCs. In this review, we will discuss the role of monocytes and their derivatives in presenting antigen and driving immune responses in vivo.
Monocyte subsets
The working definition of monocytes outlined above fits well with definitions based on molecular markers. Human monocytes, at least as it appears in all studies so far, can be unequivocally identified by the expression of CD14. However, the magnitude of expression of CD14 on the cell surface can vary, and this variation corresponds with the overall recognition, led by Löms Ziegler-Heitbrock approximately 20 years ago, that monocytes in the blood are heterogeneous [12]. In humans, CD14hi monocytes comprise the major subset in the circulation, and CD14int monocytes make up the typically far more infrequent subset. This less frequent subset is readily identified not only by expression of lower surface levels of CD14, but also by de novo expression of CD16 [12], the FcγRIII receptor, which plays a key role in recognizing immunocomplexes. By comparison, the high affinity immunoglobulin receptor FcγRI, or CD64, which can bind monomeric IgG, is elevated on CD14hi monocytes that are CD16− [13–15].
Close counterparts to these populations exist in other species, and have been studied particularly well in mice [13,16–20]. Since CD14 levels are very low on the cell surface of mouse monocytes, CD14 does not serve as a practical marker for identifying blood monocytes, as in humans. Instead, the expression of the macrophage colony-stimulating factor receptor CD115, a key factor in driving the development of macrophages [3], selectively delineates monocytes in the blood of mice. Use of CD115 alone or in combination with F4/80 identifies the same subsets of monocytes in wild-type C57BL/6 mice as does the use of GFP knocked into the CX3CR1 locus [19], a popular model for tracing monocytes through an endogenous fluorescent tag [16,17,21]. CD115 staining is far superior to methods that involve gating on CD11bhi mononuclear cells as a means for identifying monocytes, since the latter contains a variety of other mononuclear cells, including natural killer cells. In mice, staining for Ly-6C, a molecule of unknown functional significance at present, delineates monocyte subsets that resemble human monocyte subsets in patterns of chemokine receptor expression, like differential expression of CCR2 (Figure 1), and at least some adhesion molecules [13]. Ly-6Chi CD115+ monocytes in mice are considered to be the counterparts of human CD14hiCD16− monocytes, whereas Ly-6Clo CD115+ mouse monocytes serve as counterparts to CD14intCD16+ human monocytes. Due to the fact that a mAb that recognizes “Gr-1” (traditionally thought to be selective for Ly-6G) reacts with Ly-6C, the mouse monocyte subsets are often also referred to as Gr-1hi (interchangeable with Ly-6Chi) and Gr-1lo (interchangeable with Ly-6Clo), with Ly-6C being the preferable, more accurate designation. Our recent Affymetrix gene expression analysis indicates that the analogy between species is vast, though not fully overlapping, and there is unrecognized conservation of molecules like the pattern of FcR expression between the human and mouse monocyte subsets that are deemed to be counterparts (unpublished observations made in collaboration with Andreas J. Habenicht and Rainer Spänbroeck in Jena, Germany). Thus, it would be appropriate to refer to Ly-6Clo mouse monocytes as “CD16+ monocytes ” (Figure 1), the most popular term for the CD14int monocytes in humans. A major difference between the mouse and human is the frequency of the CD16+ monocyte subset: this subset represents about half of circulating monocytes in mice [17], but less than 15% in healthy humans [12].
Figure 1.
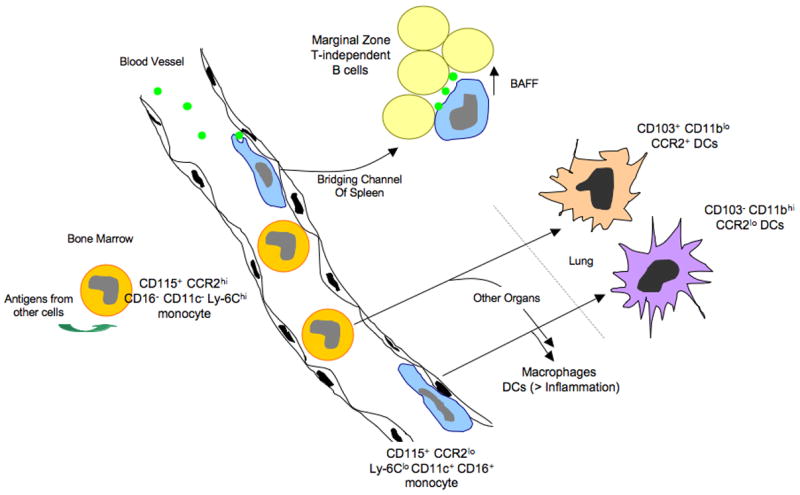
Monocyte subsets, access to antigen, and possible relationships to DCs and antigen-presenting cells that drive T-independent antibody production from innate B cells: model from the mouse. Monocytes emerge from the bone marrow as a CCR2hi Ly-6Chi population. In the bone marrow, they can take up antigens from at least some other neighboring cells, and these antigens can be presented later in the life-cycle of the monocyte. Once mobilized to the bloodstream, some CCR2hi monocytes convert to a subset of monocytes that expresses CD16 and CD11c and has low levels of CCR2 and Ly-6C. These monocytes crawl along the endothelial surfaces of the vessel wall and are able to preferentially acquire particulates that enter the bloodstream, possibly due to their marginated positioning that would give them an advantage in capturing particles. CCR2loLy-6Clo CD11c+ monocytes fit the description of DCs that have been reported to promote T-independent antibody responses by picking up particulate antigens in the blood and presenting them to B cells in the bridging channels of the spleen. Support of T-dependent immunity by monocyte-derived cells occurs in part when they differentiate into dendritic cells, a step that is facilitated by inflammatory signals. Both subsets of monocytes have the ability to become DCs, in addition to macrophages, in various body organs. In the lung, the two subsets of monocytes respectively give rise to DCs with distinct characteristics.
The two subsets of monocytes do not arise from distinct lineages but instead the Ly-6Clo monocyte subset is a product of the Ly-6Chi monocyte subset [11,18,22]. However, this conversion is not obligatory. Ly-6Chi monocytes often extravasate from the bloodstream before they convert to Ly-6Clo monocytes, and their differentiation pathway in tissues does not necessarily recapitulate the development of Ly-6Clo monocytes in the bloodstream. Befitting the definition for monocytes, there is evidence that both human monocyte subsets readily differentiate to macrophages [23,24]. In mice, Ly6Chi monocytes contribute to macrophage pools in vivo during inflammation, such as in atherosclerosis [25–27] or other inflammatory settings [28], and Ly-6Clo monocytes have also been proposed to contribute to macrophage populations [29,30]. When the antigen-presenting capacity of the monocyte subsets is tested through mixed lymphocyte reaction in vitro, human CD16+ monocytes [13] and their mouse counterparts [19] respectively show enhanced capacity for driving allogeneic T cell proliferation, compared with the other subset of monocytes.
The elusive Ly-6Clo / CD16+ monocytes: possible drivers of T independent antigen-presentation to innate B cells?
The functional roles of CD16+ monocytes or Ly-6Clo monocytes in mice, have been more elusive than those of CD16− Ly6Chi monocytes. As discussed previously [31], one surprise was that Ly-6Clo monocytes in vivo did not accumulate at sites of inflammation in large numbers, in contrast to the CCR2hi Ly-6Chi monocytes that readily respond to inflammatory chemokines like CCL2. Consequently, Ly-6Clo monocytes were proposed to serve as precursors of macrophages or DCs during homeostasis [17]. However, more recently Ly-6Clo monocytes were observed to patrol blood vessels continuously by crawling on the endothelial lining, allowing them to rapidly extravasate during inflammation, and now they are proposed to coordinate further recruitment of inflammatory cells [30]. Thus, the role of Ly-6Clo monocytes during inflammation should not be discounted, though there is ample evidence that these monocytes figure quantitatively less significantly in inflammation than their Ly-6Chi monocyte counterparts [17–19,25,26,32–35]. Beyond these studies, where much remains to be resolved, another body of work raises the possibility that Ly-6Clo monocytes may have a key role in presenting antigen for the purposes of driving T-independent antibody production by B cells, as we now discuss.
CD11c is often considered to be a reliable marker for DCs in vivo, such that identification of CD11c expression on an antigen-presenting cell can sometimes cause investigators to categorize that cell as a “DC.” In that vein, a few years ago, J. Kearney and colleagues described a circulating DC that could readily and selectively take up heat-killed bacteria introduced in the blood and mobilize to the bridging channels of the spleen [36]. There, these putative DCs interacted with marginal zone B cells that produce IgM antibodies independent of T cell help. This interaction, in the presence of appropriate antigen, led to survival, expansion, and antibody production by marginal T-independent, cognate B cell populations due to the provision of ligands for TACI on B cells [37]. In the spleen, these DCs remained distinct from other DCs, as they maintained, for example, lower levels of CD11c than conventional DCs.
In examining this work in relation to the current knowledge on monocyte subsets in the mouse, it appears to be quite likely that the “DCs” that drive T independent immunity are actually Ly-6Clo monocytes. All characteristics of the circulating DC described by Kearney’s team are features now known to be associated with Ly-6Clo monocytes. First, the cell surface markers studied entirely overlap. CD11c is expressed on Ly-6Clo monocytes at low levels [18,26], and they co-express CD43 [18], but are negative for Gr-1, CD8α, and B220, just as described for the “DCs” that support T-independent immunoglobulin responses [36]. Moreover, introduction of particulate bacteria into the blood of mice led to a preferential uptake by the CD11clo cells putative DCs. Similarly, Ly-6Clo monocytes are preferentially capable of engulfing particulates like latex beads when they are introduced i.v. [22] (Figure 1), suggesting a functional link between Ly-6Clo monocytes and the circulating DCs previously described. The propensity of this subset of monocytes to acquire particulate antigen in the blood may stem from preferential margination and crawling within the vessels [30] (Figure 1), as this position that would give them a competitive advantage in acquisition of particles. Given the phenotypic and functional similarities between the putative circulating DCs and Ly-6Clo monocytes, we propose a model in which Ly-6Clo (CD16+) monocytes in vivo may drive T-independent immune responses by providing ligands for TACI in the context of presenting the highly repetitive epitopes of antigen that characterize T-independent reactions (Figure 1).
If this model is correct, a lingering question is whether Ly-6Clo monocytes actually engulf the bacteria or particles that they encounter in the bloodstream with full efficiency. It would seem necessary that these cells would bind the particles on their surface in order to present them to B cells in a manner that would allow extensive cross-linking of the B cell receptor. If this subset of monocytes completely internalizes the particles they acquire, it is difficult to understand how they could be involved in presenting antigen to marginal zone B lymphocytes.
Many of the naturally occurring IgM antibodies produced in T-independent reactions recognize oxidation epitopes that are not necessarily linked to microbial exposure, but also can be generated endogenously [38]. An example is the class of T-independent antibodies that react with phosphatidylcholine-containing epitopes in the cell wall of Streptococcus pneumoniae. These antibodies cross-react with oxidatively modified low density lipoprotein and promote the clearance of the lipoprotein from the bloodstream, protecting the host from the inflammatory damage that can be mediated by modified LDL. Understanding the pathways and cell types that are involved in regulating the titers of T-independent antibodies has the potential not only to impact resistance to infection but also protection against oxidative insult in the cardiovascular system and in aging.
Monocytes can acquire exogenous antigens in the bone marrow for presentation after further differentiation in the periphery
Returning to a consideration of T-dependent immunity, though it is known that CD16+ monocytes drive expansion of T cells in a mixed lymphocyte reaction better than CD16− monocytes [13,19], the role of monocytes per se as antigen-presenting cells is rarely considered. Typically, their role as antigen-presenting cells is mainly confined to discussions and studies that attempt to determine if they serve as precursors for particular populations of macrophages or DCs. Recently, however, we have found evidence that antigens that monocytes encounter before they extravasate into tissues are sufficiently retained by monocytes such that those antigens can become available for presentation long after they were acquired [22]. At least in experimental settings, monocytes took up exogenous cell-associated antigens in the bone marrow (Figure 1) and were able to present peptides derived from these antigens on MHC II molecules much later in their life cycle. This feature of retaining antigen for presentation later, after appropriate maturation, is a characteristic that has been ascribed mainly to DCs and is thought to distinguish them from macrophages [39], which have little capacity to retain antigens for delayed presentation. Monocytes may be less proteolytic than macrophages [40], allowing for retention rather than degradation of antigens.
If monocytes are able to capture antigen normally within the bone marrow or during their transit through blood, they may be critical vehicles for the supply of antigen to other organ systems. For example, it has been argued that antigen-presenting cells that transit through blood can supply tissue-restricted antigens to the thymus [41,42], possibly filling in gaps of self-antigen that may be poorly expressed by thymic epithelium, even though the latter possess elegant mechanisms to express a wide-range of tissue-restricted antigens [43]. Studies using mice that are surgically joined and therefore share a common circulatory system (parabiosis) indicate that antigen-presenting cells from the blood enter the thymus on an ongoing basis [42]. Monocytes that differentiate into DCs may provide a source of antigen to be presented to thymocytes within the thymus, and if so, the capacity of monocytes to capture antigen from other cells prior to extravasation from blood would extend the range of antigens that they might present after differentiating into thymic DCs.
Monocytes as precursors for antigen-presenting DC populations
DCs are the most potent antigen-presenting cells in the immune system for promoting primary activation of naïve T cells [8], so if monocytes are significant precursors for DCs, then they would be central to antigen presentation that drives primary T-dependent immune responses. However, several recent publications collectively agree that monocytes do not serve as precursors for the major splenic CD11chi DC populations in the steady state [9–11], including in particular the classical CD8α+ and CD8α− DC subsets described in mice [44]. One study indicated that Ly-6Clo monocytes might make a minor contribution to splenic DCs in the steady state [9], but this is unlikely since elegant parabiosis studies are inconsistent with this possibility [45]. It is unclear whether these studies would have included in their analysis the CD11clo cells that Kearney et al. [36] indicated contributed to antigen presentation to B cells during T-independent responses. In contrast, DCs in peripheral organs like lung and intestine do appear to arise from monocytes on an ongoing basis [11](our unpublished data), raising the interesting and unexpected possibility that the origin of DCs in nonlymphoid tissues is different than in spleen. Thus, at least some DCs in lymph nodes—those that come into the lymph node from upstream lymphatics—may very well derive from monocytes.
The phenotype of Ly-6Clo monocytes is more akin to DCs than that of Ly-6Chi monocytes and we have found that the human counterparts of Ly-6Clo monocytes possessed migratory behavior in vitro reminiscent of DCs [46][15]. Thus, we have wondered whether the two subsets of monocytes have differential capacity to become DCs or macrophages. Since both Ly-6Clo and Ly-6Chi monocyte subsets migrate into the lung on a continuous basis [17,29](CJ, GJR, unpublished observations), tracking the fate of the two monocyte subsets in the lung provides a good model to compare their differentiation potentials.
Macrophages in the lung are not replenished by monocytes in the steady state [47,48], so monocytes that emigrate into lung do not participate in replacing macrophages. However, they do contribute to replenishing pulmonary DCs, which are known to rapidly turnover [49,50]. There are two distinct MHC IIhi DC phenotypes in the alveolar space and lung interstitium, which are readily divided up by differential surface staining: one is CD103+CD11blo and the other is CD103−CD11bhi. The latter DC population expresses CX3CR1 and thus fluoresces green in CX3CR1gfp/+ mice, but the former is GFP- in this knock-in mouse and apparently lacks expression of CX3CR1, but instead expresses higher levels of CCR2 (CJ, GJR, unpublished observations). Functionally, CD11bhi DCs have been associated with maintenance of LACK-specific memory T cells in the lung after appropriate sensitization, because these DCs bear MHC complexes on their surface loaded with LACK peptides for weeks after infection [51]. Both DC populations migrate to the downstream mediastinal lymph node, and when purified from that source and studied in vitro, CD103+ DCs standout as preferentially activating CD8+ T cell responses over the other DC population [52]. When we traced the fate of monocyte subsets in the lung, we observed that Ly-6Chi CCR2hi monocytes were the precursors of CD103+ DCs in particular, whereas Ly-6Clo monocytes developed exclusively into CD11bhi DCs (CJ, GJR, unpublished observations) (Figure 1). Thus, it seems incorrect to envisage that only one monocyte subset has the potential to become a DC and the other perhaps a macrophage. Instead, at least in lung, both subsets of monocytes become DCs (Figure 1), but they retain distinctive characteristics and become DCs that may carry out disparate functions in presenting antigen to T cells. Progress in this area can be expected in the near future.
The role of monocytes in repopulating DCs and macrophages during inflammation is very different than in homeostasis. When the lung is injured with radiation or other insults or pulmonary macrophages are depleted so that they cannot maintain themselves locally, blood monocytes do play an important role in replenishing the local macrophage pool [29,48]. This replacement appears to be favored by Ly-6Clo monocytes [29], though this propensity to act as macrophage precursors preferentially over the other subset of monocytes may not extend to other organs. For example, human alveolar macrophages express markers in common with their Ly-6Clo (CD16+) mouse counterparts, CD16+ human monocytes. One common marker is CD16 itself. This marker is not expressed, for example, by peritoneal macrophages [12], though it is expressed by alveolar macrophages. Besides becoming macrophages during inflammation, monocytes are thought to be an important source of DCs during inflammation [32,53–57], giving rise to the idea that monocytes serve as precursors for “emergency DCs” [58]. There is wide-spread support for this concept, though it is important to keep in mind that adoptive transfer of monocytes—a technique employed in many such studies—possesses numerous caveats. Only about 2% of transferred monocytes can be recovered for “fate mapping” studies at time points following transfer [17], heightening the chance that a nonmonocytic contaminant could contribute to the DC replenishment ascribed to monocytes. In some cases, monocytes “purified” for adoptive transfer have been defined very loosely as any cell in the bone marrow that is not a lymphocyte, NK cell, or neutrophil [56], again possibly incorrectly indicating that monocytes have roles that are actually carried out by a minor contaminant that served as the true DC precursor. Nonetheless, studies that attempt to follow endogenous monocytes are in agreement with the conclusion that monocytes become DCs during many inflammatory responses [53](CJ, GJR, unpublished observations), and the two experimental approaches together—tracing monocytes after adoptive transfer and by endogenous tracking—add strength to the conclusion.
Monocytes as a source of cross-presenting DCs
Most infections, adjuvants, and immunizations induce inflammation, so findings that monocytes are a major source of DCs in inflammation indicates that they may be very relevant sources of DCs during most infections or vaccinations. During infection with viruses, for example, viral antigens are best detected and combated within the immune system through cross-priming: a mechanism by which professional antigen-presenting cells, most notably DCs, acquire exogenous antigens for presentation through the MHC I pathway to CD8+ T cells [59,60]. In mice, several groups have linked the subset of DCs that express CD8α and found only within lymphoid organs as the critical DC population that presents antigen during cross-priming [61–63]. These DCs likely do not to arise from monocytes [9–11]. A widely held view is that CD8α+ DCs do not survey peripheral tissues but instead gain access to lymphoid organs like lymph nodes through the vasculature, giving rise to the term “lymph node resident DCs” for this population. In reality, we know almost nothing about the origins or trafficking patterns of these DCs, but if one assumes that CD8α+ DCs do in fact fail to patrol peripheral tissues, then a question is raised as to how they gain access to antigens for cross-presentation. By comparison, other studies, such as the work of Le Borgne et al. [32], indicate that monocytes can mediate cross-presentation, raising another question as to whether more than one source of DC can mediate or is needed for cross-presentation.
A couple of recent studies have brought forward a model that might address these questions. Carbone, Heath, and co-workers show that CD8α+ DCs do not pick up tracers that mark migration from the periphery, but nonetheless they have a dominant role in presenting antigen deposited in peripheral tissue to CD8+ T cells during cross-priming [64,65]. In these studies, they suggest that migratory DCs drive the response by transporting antigen from the periphery and then handing it off in a so-far uncharacterized form to CD8α+ DCs that carry out the presentation. If monocytes were the source of migratory DCs, they might be seen as playing an indirect role in cross-priming, acting mainly as antigen ferries [66]. However, the work of Le Borgne et al. that recently implicated monocytes as pivotal players during cross-priming showed that monocytes played a direct role in the response, because MHC I-deficient monocytes were unable to promote cross-presentation in a host where all other cells express MHC I appropriately [32].
The studies of Carbone et al. mainly involved experiments in which the migratory DC population derived from the periphery had been infected with herpes virus, a virus that readily induces death in DCs [67,68]. Thus, the first model that comes to mind as to how the migratory DC might transfer antigens to CD8α+ DCs is one in which the migratory DC dies and then is engulfed and processed by the CD8α+ DCs [64] (Figure 2). This model is appealing because (a) migratory DCs can be expected to have a short life-span [45,69], (b) death and reprocessing of DCs by DCs has been previously described [70] , and (c) CD8α+ DCs have a high propensity to engulf dying cells [62]. Nonetheless, this model is incompatible with a direct role of monocyte-derived cells and their own MHC I/peptide complexes in driving cross-priming as described [32].
Figure 2.
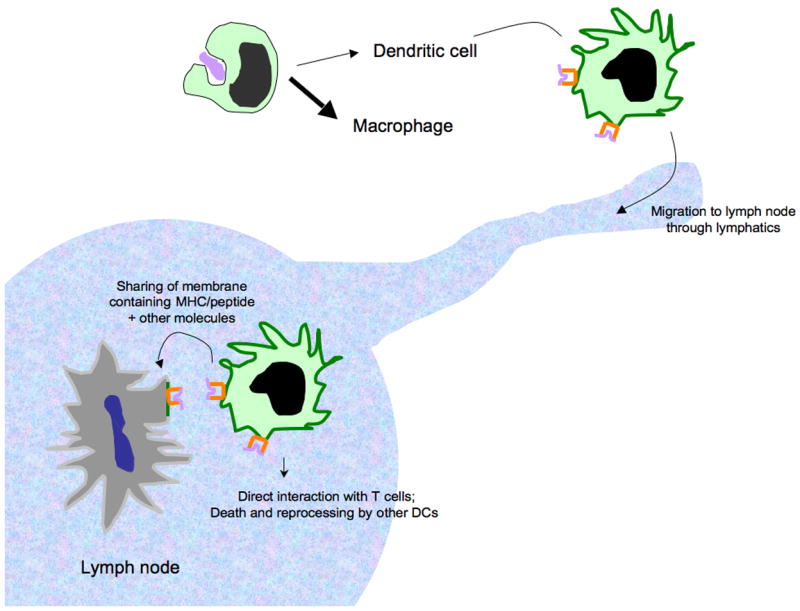
A model that might explain how monocyte-derived DCs and CD8α+ DCs co-operate during cross-priming in a manner that requires MHC/peptide from the monocyte-derived cell. Monocyte-derived cells readily mobilize into the periphery and are proficient at engulfing dying cells that may bear antigens, such as viral antigens, for cross-presentation. Some of these monocytes will differentiate into DCs that display peptide antigens acquired from the dying cells on surface MHC I molecules as they migrate through afferent lymphatics en route to the draining lymph node. In addition to potentially presenting antigen directly to CD8+ T cells, monocyte-derived DCs may transfer portions of membrane that supply MHC/peptide complexes and possibly other molecules, such as costimulatory molecules, to other DCs, including CD8α+ DCs.
A revised model that would account for collaboration between monocyte-derived DCs and CD8α+ DCs but preserves a role for monocyte-derived peptide/MHC I molecules in cross-priming can be generated (Figure 2). Possibly, monocytes serve as the source of peripheral DCs that acquire and initially process antigen for loading onto MHC I molecules, migrate to lymph nodes, and then instead of the monocyte-derived DC being engulfed and subsequently processed by CD8α+ DCs, they transfer intact portions of the plasma membrane including MHC I/peptide itself to CD8α+ DCs. Such a pathway of transfer is plausible based on other in vivo experiments in DCs [71] and the recent description of transfer of MHC/peptide from B cells to macrophages [74]. This pathway would have the advantage over death and reprocessing of the migratory DC by CD8α+ DCs since exchange of plasma membrane, especially if costimulatory molecules were also transferred along with MHC/peptide, would preserve elements of maturation undergone by the migratory DCs. Transfer of intact MHC/peptide complexes along with costimulatory molecules could be mediated through exosomes [72], but not likely through gap junctions, another proposed mechanism of antigen sharing that is restricted mainly to peptide transfer [73].
We are carrying out studies to investigate how human monocytes acquire antigens from other cells and we are employing a three-dimensional model of a vascular connective tissue. Our data indicate that maturation of monocytes to DCs, but not to macrophages, promotes the exchange of MHC molecules between the migratory monocyte-derived DCs that acquire antigens in the connective tissue and “third-party DCs” that are added to the assay later, and this exchange activity is heightened after TLR stimulation of the DC that acquires antigen (CQ and GJR, unpublished observations). The receipt of MHC/peptide complexes from monocyte-derived DCs by “third-party DCs” may mimic the transfer of antigen that occurs in the lymph node.
If migratory DCs from the periphery, such as monocyte-derived DCs, truly transfer whole MHC/peptide complexes to other DCs in the lymph node, then the finding that MHC I-deficient monocytes do not promote cross-presentation [32] could be explained not only by a direct role for monocyte-derived DCs in stimulating CD8+ T cells but also by a mechanism in which monocyte-derived DCs primarily process antigen and generate MHC I/peptide complexes to subsequently transfer them to other DCs (Figure 2). Though this transfer mechanism as a means to achieve cross-presentation may seem rather cumbersome compared with direct presentation to T cells by migratory DCs, there may be immunological wisdom in the approach. For example, the impact of microbial infection and the mechanisms that microbes develop to subvert immune presentation may be minimized, though not eliminated, by sharing of MHC/peptide between DCs, and the number of DCs that may be able to present relevant antigen would increase, as previously discussed [71].
Concluding Remarks
Here, we have highlighted what we see as the leading-edge issues in the area of how monocytes contribute to presentation of antigen. We have not restricted our discussion to T-dependent immunity, which often first comes to mind, but have also pointed out that the literature is compatible with the possibility that one subset of monocytes, the Ly-6Clo monocytes in mice, may foster T-independent antibody reactions in the spleen. As our discussion moved inevitably to how monocytes may participate in presenting antigen during T-dependent immunity, we have highlighted what we view as the major outstanding questions on the subject. Though most monocytes appear to differentiate into relatively sessile macrophages, some become dendritic cells with the specialized capacity to migrate to the T cell zone of lymphoid organs. Current literature suggests that monocytes may mainly serve as precursors for potent antigen-presenting DCs in peripheral organs, and particularly so during inflammation. There they may acquire antigens from parenchymal cells or pathogens that they encounter, and they would process these along with foreign antigens they may have picked up much earlier in the bone marrow or blood for loading onto MHC molecules. Though they migrate with these MHC/peptide complexes to downstream lymph nodes and simultaneously elevate expression of other costimulatory molecules, it remains unknown whether they have a unique role in directly presenting antigen to T cells. Instead, they may share antigen, and likely intact MHC/peptides, with other DCs that then also or alternatively interact with T cells.
Acknowledgments
This work was supported by NIH grants AI049653 and AI061741 and a subcontract from DARPA, W81XWH-04-C-0139, to GJR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Van Furth R. Phagocytic Cells: Development and Distribution of Mononuclear Phagocytes in Normal Steady State and Inflammation. In: Gallin JI, Goldstein IM, Snyderman R, editors. Inflammation: Basic Principles and Clinical Correlates. Raven Press, Ltd; 1988. pp. 281–295. [Google Scholar]
- 2.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. 2006;18:49–53. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 4.O'Doherty U, Steinman RM, Peng M, Cameron PU, Gezelter S, Kopeloff I, Swiggard WJ, Pope M, Bhardwaj N. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J Exp Med. 1993;178:1067–1076. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas R, Davis LS, Lipsky PE. Isolation and characterization of human peripheral blood dendritic cells. J Immunol. 1993;150:821–834. [PubMed] [Google Scholar]
- 6.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 7.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 8.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 9.Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O'Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 10.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 11.Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Passlick B, Flieger D, Ziegler-Heitbrock HW. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. [PubMed] [Google Scholar]
- 13.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 14.Grage-Griebenow E, Zawatzky R, Kahlert H, Brade L, Flad H, Ernst M. Identification of a novel dendritic cell-like subset of CD64(+) /CD16(+) blood monocytes. Eur J Immunol. 2001;31:48–56. doi: 10.1002/1521-4141(200101)31:1<48::aid-immu48>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Randolph GJ, Sanchez-Schmitz G, Liebman RM, Schakel K. The CD16(+) (FcgammaRIII(+)) Subset of Human Monocytes Preferentially Becomes Migratory Dendritic Cells in a Model Tissue Setting. J Exp Med. 2002;196:517–527. doi: 10.1084/jem.20011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, et al. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med. 2001;194:1361–1373. doi: 10.1084/jem.194.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 18.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 19.Qu C, Edwards EW, Tacke F, Angeli V, Llodra J, Sanchez-Schmitz G, Garin A, Haque NS, Peters W, van Rooijen N, et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J Exp Med. 2004;200:1231–1241. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, Randolph GJ. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med. 2006;203:583–597. doi: 10.1084/jem.20052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ancuta P, Autissier P, Wurcel A, Zaman T, Stone D, Gabuzda D. CD16+ monocyte-derived macrophages activate resting T cells for HIV infection by producing CCR3 and CCR4 ligands. J Immunol. 2006;176:5760–5771. doi: 10.4049/jimmunol.176.10.5760. [DOI] [PubMed] [Google Scholar]
- 24.Ancuta P, Kunstman KJ, Autissier P, Zaman T, Stone D, Wolinsky SM, Gabuzda D. CD16+ monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology. 2006;344:267–276. doi: 10.1016/j.virol.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 25.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swirski FK, Pittet MJ, Kircher MF, Aikawa E, Jaffer FA, Libby P, Weissleder R. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol. 2007;178:2000–2007. doi: 10.4049/jimmunol.178.4.2000. [DOI] [PubMed] [Google Scholar]
- 30.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 31.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, Caux C, Ait-Yahia S, Vicari A, Kaiserlian D, et al. Dendritic Cells Rapidly Recruited into Epithelial Tissues via CCR6/CCL20 Are Responsible for CD8(+) T Cell Crosspriming In Vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Drevets DA, Leenen PJ, Greenfield RA. Invasion of the central nervous system by intracellular bacteria. Clin Microbiol Rev. 2004;17:323–347. doi: 10.1128/CMR.17.2.323-347.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu H, Manivannan A, Dawson R, Crane IJ, Mack M, Sharp P, Liversidge J. Differentiation to the CCR2+ inflammatory phenotype in vivo is a constitutive, time-limited property of blood monocytes and is independent of local inflammatory mediators. J Immunol. 2005;175:6915–6923. doi: 10.4049/jimmunol.175.10.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robben PM, LaRegina M, Kuziel WA, Sibley LD. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J Exp Med. 2005;201:1761–1769. doi: 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balazs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 37.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 38.Binder CJ, Silverman GJ. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin Immunopathol. 2005;26:385–404. doi: 10.1007/s00281-004-0185-z. [DOI] [PubMed] [Google Scholar]
- 39.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 40.Schmid H, Sauerbrei R, Schwarz G, Weber E, Kalbacher H, Driessen C. Modulation of the endosomal and lysosomal distribution of cathepsins B, L and S in human monocytes/macrophages. Biol Chem. 2002;383:1277–1283. doi: 10.1515/BC.2002.143. [DOI] [PubMed] [Google Scholar]
- 41.Liu YJ. A unified theory of central tolerance in the thymus. Trends Immunol. 2006;27:215–221. doi: 10.1016/j.it.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 43.Mathis D, Benoist C. Back to central tolerance. Immunity. 2004;20:509–516. doi: 10.1016/s1074-7613(04)00111-6. [DOI] [PubMed] [Google Scholar]
- 44.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 45.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 46.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–483. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- 47.Sawyer RT, Strausbauch PH, Volkman A. Resident macrophage proliferation in mice depleted of blood monocytes by strontium-89. Lab Invest. 1982;46:165–170. [PubMed] [Google Scholar]
- 48.Sawyer RT. The ontogeny of pulmonary macrophages in parabiotic mice. J Leukoc Biol. 1986;40:347–354. doi: 10.1002/jlb.40.4.347. [DOI] [PubMed] [Google Scholar]
- 49.Holt PG, Haining S, Nelson DJ, Sedgwick JD. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J Immunol. 1994;153:256–261. [PubMed] [Google Scholar]
- 50.Holt PG, Stumbles PA. Characterization of dendritic cell populations in the respiratory tract. J Aerosol Med. 2000;13:361–367. doi: 10.1089/jam.2000.13.361. [DOI] [PubMed] [Google Scholar]
- 51.Julia V, Hessel EM, Malherbe L, Glaichenhaus N, O'Garra A, Coffman RL. A restricted subset of dendritic cells captures airborne antigens and remains able to activate specific T cells long after antigen exposure. Immunity. 2002;16:271–283. doi: 10.1016/s1074-7613(02)00276-5. [DOI] [PubMed] [Google Scholar]
- 52.del Rio ML, Rodriguez-Barbosa JI, Kremmer E, Forster R. CD103- and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J Immunol. 2007;178:6861–6866. doi: 10.4049/jimmunol.178.11.6861. [DOI] [PubMed] [Google Scholar]
- 53.Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 54.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, Randolph GJ, Merad M. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 56.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 57.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 58.Villadangos JA. Hold on, the monocytes are coming! Immunity. 2007;26:390–392. doi: 10.1016/j.immuni.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 59.Bevan MJ. Cross-priming. Nat Immunol. 2006;7:363–365. doi: 10.1038/ni0406-363. [DOI] [PubMed] [Google Scholar]
- 60.Heath WR, Carbone FR. Cross-presentation in viral immunity and self-tolerance. Nat Rev Immunol. 2001;1:126–134. doi: 10.1038/35100512. [DOI] [PubMed] [Google Scholar]
- 61.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schulz O, Reis e Sousa C. Cross-presentation of cell-associated antigens by CD8alpha+ dendritic cells is attributable to their ability to internalize dead cells. Immunology. 2002;107:183–189. doi: 10.1046/j.1365-2567.2002.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Belz GT, Smith CM, Eichner D, Shortman K, Karupiah G, Carbone FR, Heath WR. Cutting edge: conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- 64.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 65.Belz GT, Smith CM, Kleinert L, Reading P, Brooks A, Shortman K, Carbone FR, Heath WR. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc Natl Acad Sci U S A. 2004;101:8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Randolph GJ. Migratory dendritic cells: sometimes simply ferries? Immunity. 2006;25:15–18. doi: 10.1016/j.immuni.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 67.Jones CA, Fernandez M, Herc K, Bosnjak L, Miranda-Saksena M, Boadle RA, Cunningham A. Herpes simplex virus type 2 induces rapid cell death and functional impairment of murine dendritic cells in vitro. J Virol. 2003;77:11139–11149. doi: 10.1128/JVI.77.20.11139-11149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mueller SN, Jones CM, Smith CM, Heath WR, Carbone FR. Rapid cytotoxic T lymphocyte activation occurs in the draining lymph nodes after cutaneous herpes simplex virus infection as a result of early antigen presentation and not the presence of virus. J Exp Med. 2002;195:651–656. doi: 10.1084/jem.20012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100:1734–1741. [PubMed] [Google Scholar]
- 70.Inaba K, Turley S, Yamaide F, Iyoda T, Mahnke K, Inaba M, Pack M, Subklewe M, Sauter B, Sheff D, et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp Med. 1998;188:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith AL, Fazekas de St Groth B. Antigen-pulsed CD8alpha+ dendritic cells generate an immune response after subcutaneous injection without homing to the draining lymph node. J Exp Med. 1999;189:593–598. doi: 10.1084/jem.189.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 73.Neijssen J, Herberts C, Drijfhout JW, Reits E, Janssen L, Neefjes J. Cross-presentation by intercellular peptide transfer through gap junctions. Nature. 2005;434:83–88. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- 74.Harvey B, et al. Eur J Immunol. 2007;37:1739–51. doi: 10.1002/eji.200636452. [DOI] [PubMed] [Google Scholar]


