Abstract
The transcription factor PPARγ is expressed in endothelium and vascular muscle where it may exert anti-inflammatory and anti-oxidant effects. We tested the hypothesis that PPARγ plays a protective role in the vasculature by examining vascular structure and function in heterozygous knockin mice expressing the P465L dominant negative mutation in PPARγ (L/+). In L/+ aorta, responses to the endothelium-dependent agonist acetylcholine (ACh) were not affected, but there was an increase in contraction to serotonin, PGF2α, and endothelin-1. In cerebral blood vessels both in vitro and in vivo, ACh produced dilation that was markedly impaired in L/+ mice. Superoxide levels were elevated in cerebral arterioles from L/+ mice and responses to ACh were restored to normal with a scavenger of superoxide. Diameter of maximally dilated cerebral arterioles was less, whereas, wall thickness and cross-sectional area was greater in L/+ mice, indicating cerebral arterioles underwent hypertrophy and remodeling. Thus, interference with PPARγ signaling produces endothelial dysfunction via a mechanism involving oxidative stress and causes vascular hypertrophy and inward remodeling. These findings indicate that PPARγ has vascular effects which are particularly profound in the cerebral circulation and provide genetic evidence that PPARγ plays a critical role in protecting blood vessels.
Keywords: endothelial function, dominant negative, hypertension, remodeling, hypertrophy
Introduction
The peroxisome proliferator-activated receptor gamma (PPARγ) is a ligand-activated transcription factor which has gained prominence because of its involvement in complex diseases such as diabetes, obesity, atherosclerosis, and hypertension. Recent interest in the role of PPARγ in the vasculature has substantially increased because of the anti-inflammatory and anti-oxidant effects reported for PPARγ agonists [reviewed by 1]. Naturally occurring mutations in humans, resulting in either constitutive activation or impairment of PPARγ function, strongly support its physiological importance and illustrate the severe consequences for cardiovascular related events when PPARγ signaling is altered 2. Individuals with dominant negative mutations in PPARγ present with early onset hypertension and elements of the metabolic syndrome 2.
Although the importance of PPARγ in adipose tissue is now well documented, its role in the cardiovascular system has only begun to emerge. PPARγ is the molecular target of the thiazolidinediones (TZDs) class of anti-diabetes drugs. These drugs increase insulin sensitivity but also lower blood pressure in patients with type 2 diabetes 3 and in animal models of hypertension 4. TZDs also improve endothelial function and reduce blood pressure in non-diabetic models of hypertension, underscoring the potential protective effects of PPARγ in the vessel wall 5. PPARγ is expressed in endothelium and vascular muscle and there is growing evidence that PPARγ may have direct effects in the vasculature 6-10.
We hypothesize that PPARγ plays an important protective role in the regulation of vascular tone and vascular growth. To address this hypothesis using a genetic approach to avoid potential non-specific effects of synthetic PPARγ ligands 11, we examined vascular function and structure using heterozygous knockin mice carrying a dominant negative mutation in PPARγ (L/+) 12. The P465L mutation is equivalent to the P467L mutation found in humans with insulin resistance, type II diabetes and hypertension 2.
Methods
Experimental Mice
The inbred colony was maintained by breeding heterozygous P465L mice with 129/SvEv mice 12. The experimental colony (5-9 months of age) was maintained by breeding inbred 129/SvEv heterozygous P465L knockin mice with C57BL/6J mice, to produce control and heterozygous P465L mice on an isogenic background. All mice were fed standard chow (LM-485; Teklad Premier Laboratory Diets) and water ad libitum. Care of the mice used in the experiments met the standards set forth by the NIH for the care and use of experimental animals. All procedures were approved by the University Animal Care and Use Committee at the University of Iowa.
Gene Expression Profiling and Computational Analysis
We performed gene expression profiling to gain evidence of dominant negative activity of the PPARγ P465L mutation as detailed in the Supplemental Methods (see http://hyper.ahajournals.org). Data from the microarray studies including CEL files have been submitted to the Gene Expression Omnibus at NCBI (array platform: GPL1261, series accession: GSE8949).
Aortic Ring Preparation
Male and female mice were given a lethal dose of pentobarbital (50 to 100 mg/mouse IP), and the thoracic aorta was quickly removed and prepared for measurements as described in detail 5, 13.
Studies of cerebral arteries in vitro
Male and female mice were given a lethal dose of pentobarbital, the brain removed and the basilar artery isolated and prepared for measurements of vessel diameter in vitro as described 14. At the end of each experiment papaverine (100 mmol/L) was used to produce maximal vasodilation. Vasodilator responses are expressed as percent dilation (% of induced tone) with 100% representing the difference between the resting value and the constricted value with U46619. Agonists used in this study are detailed in the Supplemental Methods (see http://hyper.ahajournals.org).
Studies of cerebral arterioles in vivo
For studies in vivo, female mice were anesthetized with pentobarbital (75—90 mg/kg ip), supplemented at ∼20 mg/kg per hour. Mice were ventilated, arterial blood pressure and blood gases were monitored, and arteriolar diameter was measured using a cranial window 15. Arterial blood gases were maintained within normal limits (pH=7.35±0.01, PCO2=39±1 mm Hg, and PO2=119±4 mm Hg). Body temperature was maintained at approximately 37°C with a heating pad.
Measurements of superoxide and vascular structure
Measurements of superoxide 16 and pressure and diameter in arterioles on the cerebrum was measured in male and female mice as described previously 17. After obtaining baseline values, arterioles were suffused with artificial CSF containing EDTA (67 mmol/L) to deactivate vascular muscle. Pressure-diameter relationships were then obtained in maximally dilated arterioles using controlled hemorrhage. Maximally dilated arterioles were fixed at physiological pressure in vivo by suffusion with glutaraldehyde (2.25% in 0.10 mol/L cacodylate buffer). After the anesthetized animal was killed by injection of KCl, the arteriolar segment studied was removed, processed, and embedded in Spurr's low viscosity resin while cross-sectional orientation was maintained. Cross-sectional area of the arteriolar wall was determined histologically from 1-μm sections.
Statistical Analysis
All data are expressed as mean±SEM. Comparisons were made with 2-way repeated measures ANOVA using a Tukey post-hoc test or t-test where appropriate. P<0.05 was considered significant. Data were analyzed by use of SigmaStat (Systat Software).
Results
To gain evidence for dominant negative activity of PPARγ we performed gene expression profiling of RNA isolated from thoracic aorta comparing those genes regulated by rosiglitazone, a PPARγ agonist, with those altered by the P465L mutation. Evidence of dominant negative activity would be obtained if the same genes up-regulated by rosiglitazone were down-regulated by P465L mutation and vice versa. In aorta, 21 genes up-regulated by rosiglitazone were down-regulated in P465L, half of them significantly (Table S1, http://hyper.ahajournals.org). Half of these genes are involved in lipid or carbohydrate metabolism, consistent with a role of PPARγ, and nearly all had predicted PPARγ binding sites in their promoters. Similarly, 75% of 32 genes in aorta which were down-regulated by rosiglitazone were significantly up-regulated by in P465L mice (Table S2, http://hyper.ahajournals.org). In contrast, these genes were associated with inflammation, cell signaling, or oxidoreductase activity. These data provide evidence for dominant negative activity of the PPARγ P465L mutation in the vasculature.
Aortic rings were pre-contracted with PGF2α and relaxation was measured in response to increasing doses of either ACh, an endothelium-dependent vasodilator, or nitroprusside, an endothelium-independent vasodilator. Relaxation to both agonists was similar in aorta from L/+ and +/+ mice (Figure S1, http://hyper.ahajournals.org). The contractile response to PGF2α, 5-HT, and ET-1 was increased in aorta from L/+ mice versus controls (Figure S2, http://hyper.ahajournals.org). The response to the first two agonists in L/+ mice was greater in males than females, whereas ET-1 mediated contraction in L/+ mice was observed primarily in females (Figure S3, http://hyper.ahajournals.org). There was no difference in the contractile response to KCl (Figure S2, http://hyper.ahajournals.org) indicating the increase in aortic contraction was selective.
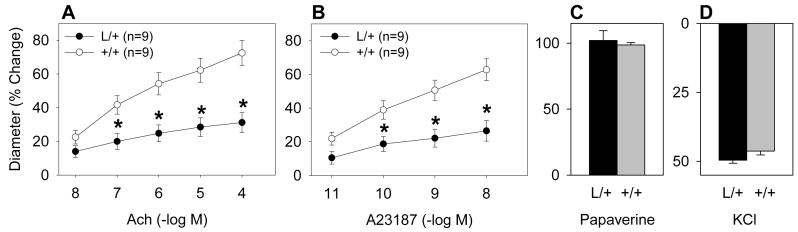
To examine the role of PPARγ in resistance vessels supplying a vital organ, we tested responses in a cerebral artery and in cerebral arterioles. Unlike the aorta, dilation of the basilar artery in response to the endothelial-dependent agonists ACh and A23187 was markedly impaired in L/+ mice (Figure 1A and B). Maximal vasodilation to papaverine was similar in the L/+ and +/+ mice (Figure 1C), indicating that the reduced responses to ACh and A23187 were selective. There was no difference in the contraction caused by KCl (Figure 1D) or U46619 (data not shown).
Figure 1. Impaired Endothelial Function in Basilar Artery from P465L Knockin Mice.
Dilation of the basilar artery from L/+ and +/+ mice in response to ACh (A), A23187 (B), or 100 mmol/L papaverine (C). The constrictor response to KCl (50 mmole/L) was measured (D). *, P<0.01. The P value comparing the dose response curves is indicated.
Dilation of the cerebral arterioles (∼30 μm in diameter) to ACh was markedly reduced in L/+ mice compared to controls (Figure 2A). On the contrary, the vasodilator response to submaximal concentrations of nitroprusside and papaverine were similar in both groups of mice (Figure 2B and C). We measured superoxide levels using dihydroethidine (DHE) staining and observed a nearly a 2-fold increase in fluorescence in arterioles from L/+ mice (Figure 3). Consistent with increased oxidative stress, the impairment of ACh-mediated vasodilation from L/+ mice was reversed by Tempol, a scavenger of superoxide (Figure 4). Tempol alone had no effect on baseline diameter of cerebral arterioles (31±1 vs 32±1 μm).
Figure 2. Impaired Endothelial Function in the Cerebral Circulation In Vivo.
Dilation of cerebral arterioles from L/+ and +/+ mice measured in response to ACh (A),nitroprusside (B), or papaverine (C). *, P<0.05.
Figure 3. Increased Superoxide in Cerebral Arterioles.
Representative confocal fluorescent sections and relative fluorescence in cerebral arterioles of wild-type mice (+/+, left) and P465L mice (L/+, center) incubated with hydroethidine (2 μmol/L for 30 minutes) for detection of O2−. Values are means ±SEM in 37 vessels from 10 +/+ mice and 39 vessels from 10 L/+ mice. *P<0.05 vs wild-type mice. Scale bar = 20 μm.
Figure 4. Tempol Reverses Endothelial Dysfunction in the Cerebral Circulation In Vivo.
Dilation of cerebral arterioles from untreated +/+, untreated L/+ and Tempol-treated L/+ mice measured in response to ACh. *, P<0.05.
Before deactivation with EDTA, diameter of cerebral arterioles was not different in L/+ mice from that in control mice (Table S3, http://hyper.ahajournals.org). During maximal dilation with EDTA, external and internal diameter was less in cerebral arterioles in L/+ mice at all levels of arteriolar pressure (Figure 5A and D). Wall thickness and cross-sectional area of the vessel wall was greater in L/+ mice compared to controls (Figure 5B and C). The stress-strain curve in cerebral arterioles in L/+ mice was shifted to the right of the curve in wild-type mice (Figure S4, http://hyper.ahajournals.org). Thus, cerebral arterioles in L/+ mice underwent hypertrophy with an increase in distensibility and a reduction in external diameter. The changes we observed occurred in mice exhibiting only a small average (7 mmHg) increase in systolic blood pressure, consistent with previous work 12. When separated by gender, male mice exhibited a 10 mmHg increase in blood pressure whereas blood pressure in female mice was unchanged (Table S3, http://hyper.ahajournals.org). Despite the difference in blood pressure by gender, there was an equivalent reduction in external diameter and increase in cross-sectional area in both males and females. Therefore it is unlikely that the structural changes observed are due to the small increase in systemic arterial pressure.
Figure 5. Structural and Mechanical Changes in Cerebral Arterioles in P465L mice.
External and internal diameter of cerebral arterioles after maximal dilation (A), wall thickness (B) cross-sectional area (CSA) of the vessel wall in arterioles (C), pressure-internal diameter relationships in arterioles during maximal dilation (D), in wild-type (+/+) and L/+ mice.
Discussion
We addressed the hypothesis that PPARγ maintains normal vascular structure and function. We reasoned that if PPARγ directly plays a protective role in the vasculature, a dominant negative mutation causing impaired PPARγ activity should result in impaired vascular function and altered vascular growth. Moreover, it allowed us to examine the importance of PPARγ using a genetic approach without the need for synthetic agonists which may have non-specific PPARγ-independent effects [reviewed in 11].
There are a number of key findings from our work. First, there was no evidence for impaired endothelial function in the aorta as measured by the response to ACh. However, an increase in vasoconstrictor responses was observed to several receptor-mediated agonists but not to KCl. Second, we showed that interference with PPARγ signaling decreased responses to endothelial-dependent vasodilators in the cerebral vasculature both in vitro and in vivo. That the response to the endothelial-independent agonists were normal suggests the dysfunction lies primarily in the endothelium and not smooth muscle. Third, the mechanism causing endothelial dysfunction in cerebral arterioles in response to interference with PPARγ activity involved oxidative stress since superoxide was increased and Tempol restored responses to ACh. Fourth, interference with PPARγ activity resulted in hypertrophy (an increase in vessel cross sectional area) and inward remodeling in cerebral arterioles. Collectively, these data suggest that PPARγ plays a pivotal role in regulating vascular structure and function. The impact of PPARγ was prominent in the cerebral circulation but the data also imply that PPARγ may play a greater role in resistance vessels than in larger conduit vessels.
We previously reported that rosiglitazone, a high affinity synthetic PPARγ agonist improved endothelial function in a model of non-diabetic hypertension 5. Consequently, we were surprised that the dominant negative mutation in PPARγ did not cause obvious impairment of endothelial function in the aorta although contractility was modestly augmented for selected agonists. Interestingly, heterozygous endothelial NO synthase (eNOS) deficient mice respond normally to ACh but their vasoconstrictor response to 5-HT is augmented 18. The increased contraction to 5-HT, and perhaps PGF2α, is particularly interesting because vasoconstriction to 5-HT is normally inhibited by eNOS 19. Thus under baseline conditions in P465L mice, increased contractility to 5-HT, PGF2α and ET-1 may be due to a decrease in basal NO.
Unlike the aorta, we observed marked impairment in endothelial function in the cerebral circulation. Several possibilities may explain this striking difference in vascular phenotype. First, it is possible that the impact of PPARγ on endothelial function is greater in resistance vessels than in larger vessels such as aorta. Second, there may be regional differences in the functional importance of PPARγ. For example, endothelium of the brain constitutes the blood-brain barrier which has unique biochemical and metabolic characteristics. Third, levels of expression of PPARγ may be higher in cerebral blood vessels than in conduit vessels or in other vascular beds such that interference with normal PPARγ activity has a greater functional impact in cerebral circulation. Fourth, differences in pro- and anti-oxidant mechanisms in the vessel wall and the impact of PPARγ on these mechanisms may differ in cerebral blood vessels.
Our observations of increased superoxide in cerebral arterioles and that Tempol restores responses to ACh suggests that inhibition of oxidative stress is one of the key mechanisms by which PPARγ exerts protective effects on endothelial function. One source of oxygen-derived free radicals in the vasculature is NAD(P)H oxidase. PPARγ inhibits NAD(P)H oxidase activity but also induces expression of CuZn-SOD, critical in the scavenging of superoxide 10, 20. These results suggest that PPARγ expression in the vascular wall may play a physiologic role as a regulator of genes involved in oxidative stress, even though the details of these actions remain to be completely defined.
Our data are very consistent with vascular protective effects predicted for PPARγ based on the protective effects of PPARγ agonists in humans and animal models. However, the strength of our data lies in making a genetic connection between PPARγ and vascular structure and function independent of off-target effects of PPARγ agonists. Indeed, non-specific effects of PPARγ agonists have been reported [reviewed in 11].
One of the most interesting phenotypes we observed in P465L mice relates to altered structure of the vasculature. There are multiple determinants of vascular hypertrophy including blood pressure, the renin-angiotensin system, and reactive oxygen species. Tsai et al. previously reported that L/+ mice exhibit an small increase in blood pressure 12, a finding replicated in the male L/+ mice examined herein. That cerebral arteriolar pressure does not differ between L/+ and +/+ mice, and the systemic arterial pressure of female L/+ mice was not different from control mice despite a similar change in hypertrophy and remodeling, strongly suggests that these structural and functional changes occur independent of blood pressure.
Hypertrophy of cerebral arterioles has been observed previously in several models including mice with angiotensin-II-dependent hypertension 21, 22. In the present study, we found that cerebral arterioles in P465L mice had increased cross-sectional area compared to controls. These findings are consistent with previous studies in vascular muscle in culture which suggested that pharmacological activation of PPARγ reduces vascular hypertrophy, possibly through effects on the renin-angiotensin system 23. In vivo, synthetic activators of PPARγ protect against abnormal vascular growth during angiotensin II-dependent hypertension 24. In contrast to hypertrophy, much less is known regarding determinants of vascular remodeling. We define inward vascular remodeling as a reduction in vessel diameter that cannot be attributed to altered distensibility of the vessel wall. Although vascular hypertrophy is present in diverse models 16, 21, 22, inward vascular remodeling was only observed in mice with angiotensin II-dependent hypertension 22. Based on these findings, we have proposed that the renin-angiotensin system may be a key determinant of vascular remodeling 22. Similar to what is observed with angiotensin II-dependent hypertension, hypertrophy and inward vascular remodeling were seen in mice expressing a dominant negative form of PPARγ. In this regard, it is noteworthy that angiotensin II produces such vascular changes and the renin-angiotensin system is a target of PPARγ.
Perspectives
Our data provide compelling genetic evidence that PPARγ plays an important role in protecting the vasculature. Interference with PPARγ results in hypertrophy and inward remodeling of resistance vessels and impairment of endothelial function in the cerebral circulation. The model employed herein is one of global interference with PPARγ function. Parallel efforts are beginning to define the role of PPARγ in specific cell types. For example, endothelial-specific knockout of PPARγ using cre-loxP caused an increase in arterial pressure after feeding a high fat diet 25. Similarly, strong interference with PPARγ function specifically in vascular muscle caused vascular dysfunction and hypertension 26.
Supplementary Material
Acknowledgments
P465L mice were maintained at the University of Iowa Transgenic Animal Facility supported by the Carver College of Medicine. We would like to thank Drs. Sean Didion and Michael Ryan for their advice during the inception of this project. We gratefully acknowledge the generous research support of the Roy J. Carver Trust.
Sources of Funding
This work was supported by grants from the National Institutes of Health (HL48058, HL61446, HL55006, HL38901, HL62984, NS24621, HL22149, HL42630, HL67320, and GM08629) and the AHA (0575092N, 0415460Z).
Footnotes
Conflict of Interest
No conflict of interest exists with an author on this paper.
Disclosures
NIH funding to Sigmund, Faraci, Baumbach, Halabi and Maeda AHA Funding to Faraci, Beyer
References
- 1.Schiffrin EL, Amiri F, Benkirane K, Iglarz M, Diep QN. Peroxisome proliferator-activated receptors: vascular and cardiac effects in hypertension. Hypertension. 2003;42:664–668. doi: 10.1161/01.HYP.0000084370.74777.B6. [DOI] [PubMed] [Google Scholar]
- 2.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK, O'Rahilly S. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 3.Nolan JJ, Ludvik B, Beerdsen P, Joyce M, Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N Engl J Med. 1994;331:1188–1193. doi: 10.1056/NEJM199411033311803. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan TA, Meehan WP, Jeng YY, Yang D, Chan TM, Nadler JL, Scott S, Rude RK, Hsueh WA. Blood pressure lowering by pioglitazone. Evidence for a direct vascular effect. J Clin Invest. 1995;96:354–360. doi: 10.1172/JCI118041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. PPARγ agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension. 2004;43:661–666. doi: 10.1161/01.HYP.0000116303.71408.c2. [DOI] [PubMed] [Google Scholar]
- 6.Marx N, Bourcier T, Sukhova GK, Libby P, Plutzky J. PPARgamma activation in human endothelial cells increases plasminogen activator inhibitor type-1 expression: PPARγ as a potential mediator in vascular disease. Arterioscler Thromb Vasc Biol. 1999;19:546–551. doi: 10.1161/01.atv.19.3.546. [DOI] [PubMed] [Google Scholar]
- 7.Iijima K, Yoshizumi M, Ako J, Eto M, Kim S, Hashimoto M, Sugimoto N, Liang YQ, Sudoh N, Toba K, Ouchi Y. Expression of peroxisome proliferator-activated receptor gamma (PPARγ) in rat aortic smooth muscle cells. Biochem Biophys Res Commun. 1998;247:353–356. doi: 10.1006/bbrc.1998.8794. [DOI] [PubMed] [Google Scholar]
- 8.Satoh H, Tsukamoto K, Hashimoto Y, Hashimoto N, Togo M, Hara M, Maekawa H, Isoo N, Kimura S, Watanabe T. Thiazolidinediones suppress endothelin-1 secretion from bovine vascular endothelial cells: a new possible role of PPARγ on vascular endothelial function. Biochem Biophys Res Commun. 1999;254:757–763. doi: 10.1006/bbrc.1998.0126. [DOI] [PubMed] [Google Scholar]
- 9.Fukunaga Y, Itoh H, Doi K, Tanaka T, Yamashita J, Chun TH, Inoue M, Masatsugu K, Sawada N, Saito T, Hosoda K, Kook H, Ueda M, Nakao K. Thiazolidinediones, peroxisome proliferator-activated receptor gamma agonists, regulate endothelial cell growth and secretion of vasoactive peptides. Atherosclerosis. 2001;158:113–119. doi: 10.1016/s0021-9150(01)00430-0. [DOI] [PubMed] [Google Scholar]
- 10.Inoue I, Goto S, Matsunaga T, Nakajima T, Awata T, Hokari S, Komoda T, Katayama S. The ligands/activators for peroxisome proliferator-activated receptor alpha (PPARalpha) and PPARγ increase Cu2+,Zn2+-superoxide dismutase and decrease p22phox message expressions in primary endothelial cells. Metabolism. 2001;50:3–11. doi: 10.1053/meta.2001.19415. [DOI] [PubMed] [Google Scholar]
- 11.Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V, Dello RC. Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochem Pharmacol. 2005;70:177–188. doi: 10.1016/j.bcp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Tsai YS, Kim HJ, Takahashi N, Kim HS, Hagaman JR, Kim JK, Maeda N. Hypertension and abnormal fat distribution but not insulin resistance in mice with P465L PPARγ. J Clin Invest. 2004;114:240–249. doi: 10.1172/JCI20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res. 2002;91:938–944. doi: 10.1161/01.res.0000043280.65241.04. [DOI] [PubMed] [Google Scholar]
- 14.Faraci FM, Modrick ML, Lynch CM, Didion LA, Fegan PE, Didion SP. Selective cerebral vascular dysfunction in Mn-SOD-deficient mice. J Appl Physiol. 2006;100:2089–2093. doi: 10.1152/japplphysiol.00939.2005. [DOI] [PubMed] [Google Scholar]
- 15.Didion SP, Lynch CM, Baumbach GL, Faraci FM. Impaired endothelium-dependent responses and enhanced influence of Rho-kinase in cerebral arterioles in type II diabetes. Stroke. 2005;36:342–347. doi: 10.1161/01.STR.0000152952.42730.92. [DOI] [PubMed] [Google Scholar]
- 16.Baumbach GL, Didion SP, Faraci FM. Hypertrophy of cerebral arterioles in mice deficient in expression of the gene for CuZn superoxide dismutase. Stroke. 2006;37:1850–1855. doi: 10.1161/01.STR.0000227236.84546.5a. [DOI] [PubMed] [Google Scholar]
- 17.Baumbach GL, Sigmund CD, Bottiglieri T, Lentz SR. Structure of cerebral arterioles in cystathionine beta-synthase-deficient mice. Circ Res. 2002;91:931–937. doi: 10.1161/01.res.0000041408.64867.1d. [DOI] [PubMed] [Google Scholar]
- 18.Lamping K, Faraci F. Enhanced vasoconstrictor responses in eNOS deficient mice. Nitric Oxide. 2003;8:207–213. doi: 10.1016/s1089-8603(03)00028-4. [DOI] [PubMed] [Google Scholar]
- 19.Lamping KG, Faraci FM. Role of sex differences and effects of endothelial NO synthase deficiency in responses of carotid arteries to serotonin. Arterioscler Thromb Vasc Biol. 2001;21:523–528. doi: 10.1161/01.atv.21.4.523. [DOI] [PubMed] [Google Scholar]
- 20.Von Knethen A, Brune B. Activation of peroxisome proliferator-activated receptor gamma by nitric oxide in monocytes/macrophages down-regulates p47phox and attenuates the respiratory burst. J Immunol. 2002;169:2619–2626. doi: 10.4049/jimmunol.169.5.2619. [DOI] [PubMed] [Google Scholar]
- 21.Baumbach GL, Sigmund CD, Faraci FM. Structure of cerebral arterioles in mice deficient in expression of the gene for endothelial nitric oxide synthase. Circ Res. 2004;95:822–829. doi: 10.1161/01.RES.0000146279.11923.14. [DOI] [PubMed] [Google Scholar]
- 22.Baumbach GL, Sigmund CD, Faraci FM. Cerebral arteriolar structure in mice overexpressing human renin and angiotensinogen. Hypertension. 2003;41:50–55. doi: 10.1161/01.hyp.0000042427.05390.5c. [DOI] [PubMed] [Google Scholar]
- 23.Sugawara A, Takeuchi K, Uruno A, Ikeda Y, Arima S, Kudo M, Sato K, Taniyama Y, Ito S. Transcriptional suppression of type 1 angiotensin II receptor gene expression by peroxisome proliferator-activated receptor-gamma in vascular smooth muscle cells. Endocrinology. 2001;142:3125–3134. doi: 10.1210/endo.142.7.8272. [DOI] [PubMed] [Google Scholar]
- 24.Diep QN, El Mabrouk M, Cohn JS, Endemann D, Amiri F, Virdis A, Neves MF, Schiffrin EL. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: role of peroxisome proliferator-activated receptor-gamma. Circulation. 2002;105:2296–2302. doi: 10.1161/01.cir.0000016049.86468.23. [DOI] [PubMed] [Google Scholar]
- 25.Nicol CJ, Adachi M, Akiyama TE, Gonzalez FJ. PPARγ in endothelial cells influences high fat diet-induced hypertension. Am J Hypertens. 2005;18:549–556. doi: 10.1016/j.amjhyper.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 26.Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM, Sigmund CD. Interference with PPARγ Function in Smooth Muscle Causes Vascular Dysfunction and Hypertension. Cell Metabolism. 2008 doi: 10.1016/j.cmet.2007.12.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.