Abstract
There has been remarkable progress in the last 20 years in understanding mechanisms that underlie the success of axonal regeneration in the peripheral nervous system, and the failure of axonal regeneration in the central nervous system. Following the identification of these underlying mechanisms, several distinct therapeutic approaches have been tested in in vivo models of spinal cord injury to enhance central axonal structural plasticity, including the therapeutic administration of neurotrophic factors. While several tested mechanisms apparently enhance axonal growth, more recent, properly controlled studies indicate that experimental approaches to combine therapies that target distinct neural mechanisms achieve greater axonal growth than therapies applied in isolation. The search for combination therapies that optimize axonal growth after SCI continues.
INTRODUCTION
The adult mammalian spinal cord fails to regenerate, whereas the crushed peripheral nerve often successfully regenerates. Several mechanisms have been identified that contribute to the success of peripheral regeneration, including the following: 1) Nervous system growth factors, or neurotrophic factors, are secreted in appropriate physical and temporal gradients to support axonal regeneration after peripheral nerve injury (Terenghi, 1999; Boyd and Gordon, 2003). Schwann cells produce many of these growth factors in the local injured and regenerating milieu (Bhatheja and Field, 2006). 2) Physical bridges form at sites of peripheral nerve injury that “fill-in” the lesion cavity, establishing a permissive physical matrix to support growth (Chernousov and Carey, 2000; Dubovy, 2004). This matrix includes collagen, fibronectin, laminin, Schwann cells and fibroblasts. 3) A set of genes is activated in the nucleus of the damaged peripheral neuron that supports axonal regeneration, including GPA-43, CAP-23, ß-tubulin, and others (Kury et al., 2001; Navarro et al., 2007). 4) Inhibitors to axonal regeneration have not been detected in the injured peripheral nerve to the extent that they are present in the CNS. In the injured central nervous system (CNS), these supportive responses to injury do not occur (Schwab et al., 2006). Further, molecules of two classes have been identified that actively suppress axonal sprouting and regeneration in the CNS: molecules associated with central myelin, including nogo (Buchli and Schwab, 2005), myelin-associated glycoprotein (MAG), oligodendrocyte-myelin glycoprotein (OMgp), semaphorins, and netrin, and molecules present in the extracellular matrix (ECM), particularly the chondroitin sulfate proteoglycan (CSPG) molecule NG2 (Jones et al., 2002; Silver and Miller, 2004; Fawcett, 2006).
Experimental efforts to enhance regeneration of the spinal cord have individually targeted many of the mechanisms listed in the preceding paragraph. These experimental approaches include placement of molecular, cellular or “synthetic” bridges in the lesion cavity (Lakatos and Franklin, 2002; Novikova et al., 2003); stimulation of the injured spinal cord with growth factors (Tuszynski, 2002); “conditioning” of neurons to activate intrinsic genetic programs and proteins related to an active growth state (Neumann et al., 1999, 2002; Qiu et al., 2002; Rossi et al., 2007); and efforts to neutralize myelin- or ECM-related inhibitors (Silver and Miller, 2004; Fawcett, 2006). In many cases, experiments combined two of these approaches by placing cellular transplants in a lesion cavity while simultaneously, for example, administering neurotrophic factors (Lu et al., 2004) or raising cAMP levels (Nikulina et al., 2004; Pearse et al., 2004). Many of these studies reported enhancement of axonal growth and, in some cases, incremental improvement in functional outcome. However, no properly conducted and controlled study to date has demonstrated highly extensive structural or functional recovery after SCI, and in no case has convincing improvement of plasticity and regeneration been demonstrated in a larger animal model.
Indeed, the challenges presented in attempting to achieve recovery of function after spinal cord injury are substantial. The adult human spinal cord contains in excess of 3 million axons projecting rostrally and caudally. The normal patterning of axonal projections during development is established by a detailed and exquisitely orchestrated set of mechanisms that occur both intrinsically within the neuron, and extrinsically in the environment surrounding the growing axon. Within the neuron, genes are sequentially activated that begin the process of axonal elongation, cytoskeletal stabilization, and receptor expression on the tips of growth cones that sense substrates and diffusible signals in the extracellular environment. In turn, the environment influences the extending axon with diffusible molecules such as netrins, semaphorins, growth factors, and extracellular matrix molecules that contribute to attraction or repelling of the growing axon. This array of intrinsic and extrinsic processes that control axon growth occurs in a precisely timed set of events; perturbation of the timing or single components of axonal elongation in the developing nervous system can lead to mistargeting, axonal withdrawal or neuron death.
Clearly, axonal elongation during development depends on “combinations” of events. Both the developing axon and its environment utilize a number of disparate mechanisms to cooperate in an orchestrated series of events that result in growth that is remarkably rapid, accurate and precise. If substantial axonal growth is to be achieved in the injured adult CNS, it seems reasonable to expect that multiple mechanisms will also need to be addressed. The manipulation of any single or pair of factors may be sufficient to detect axonal growth in a rodent model of SCI; however, the probability of achieving growth of sufficient numbers of axons, and over minimal distances of several centimeters in the larger primate spinal cord, will likely require convergence of treatments targeting multiple cellular and extracellular mechanisms. Simple, one-therapy solutions seem unlikely to address this complex problem.
Effects of Growth Factors and Bridges
Several years ago we and others examined the ability of injured adult spinal cord axons to respond to growth factors (Schnell et al., 1994; Tuszynski et al., 1994, 1996; Xu et al., 1995; Bregman et al., 1997; Grill et al., 1997; Kobayashi et al., 1997; Ye and Houle, 1997; Jackeman et al., 1998; Blesch et al., 1999; Bradbury et al., 1999; Liu et al., 1999; Lu et al., 2001; Ruitenberg et al., 2003). Our group introduced nerve growth factor (NGF) into the intact and partially lesioned spinal cord, using techniques of gene delivery. Suspensions of autologous fibroblasts genetically modified to secrete NGF were injected into either the central gray matter of the non-lesioned thoracic spinal cord, or were embedded into collagen matrices that were then injected into dorsally hemisected spinal cord lesion cavities of adult rats (Tuszynski et al., 1994, 1996;). We found similar patterns of axonal responsiveness to NGF in both the non-lesioned and lesioned spinal cord: dorsal root ganglion (DRG) nociceptive axons and cerulospinal axons densely penetrated NGF-secreting cell grafts placed in the adult spinal cord (Fig. 1). This finding established the fact that adult axons retain sensitivity to growth factors, that patterns of growth factor sensitivity after injury in adulthood recapitulate developmental patterns of growth factor sensitivity, and that axons grow in rather large numbers when presented with a permissive growth environment containing growth factors. In this set of studies, a “bridge” to support axonal penetration was provided by the cellular graft in the lesion cavity. These results generally supported much earlier findings of Aguayo and colleagues indicating that central axons can extend for essentially unlimited distances in the permissive environment of the peripheral nerve “bridge” (Richardson et al., 1982). Indeed, we now know that peripheral nerve bridges contain substantial quantities of NGF, BDNF, neurotrophin-3 (NT-3), ciliary neurotrophic factor (CNTF) and glial cell line-derived neurotrophic factor (GDNF) secreted by Schwann cells, which likely stimulate axon growth.
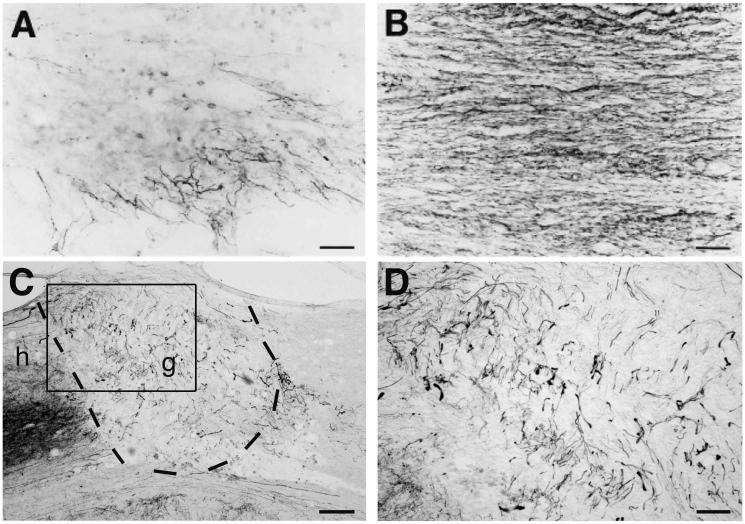
Figure. 1.
Spinal cord axon growth is induced by grafts of NGF- or BDNF-secreting cell grafts. (A) Neurofilament immunolabel shows modest axon penetration into control fibroblast graft, whereas (B) NGF-secreting graft is densely penetrated by axons 3 mo post-injury (see Table 1 for list of responding axons). (C) BDA-labeled reticulospinal axons also extensively penetrate a BDNF-secreting bone marrow stromal cell graft (outlined by dashed lines) placed into site of mid-cervical injury. g, graft; h, host; graft shown 3 mo post-injury. (D) Higher magnification of boxed areas from panel C, demonstrating BDA-labeled reticulospinal axons in BDNF-expressing bone marrow stromal cell graft. Scale bar, A-B, 15 μm; C, 210 μm; D, 80 μm.
Over subsequent years, a number of groups proceeded to define the spectrum of axonal sensitivity to various growth factors in the injured spinal cord (Table 1). Of note, BDNF supports the growth of a number of neuronal populations that modify the activity of spinal cord motor neurons, including cerulospinal, rubrospinal, raphaepsinal and reticulospinal axons (Kobayashi et al., 1997; Ye and Houle, 1997; Jackeman et al., 1998; Menei et al., 1998; Liu et al., 1999; Lu et al., 2001, 2005; Jin et al., 2002; Ruitenberg et al., 2003). Significantly, NT-3 enhances the growth of the most important upper motor neuron population in primates, corticospinal axons (Schnell et al., 1994; Grill et al., 1997; Blits et al., 2003). NT-3 also enhances the growth of ascending dorsal column sensory axons, a model system of great utility in studying both mechanisms and empirical features of axonal elongation in the adult spinal cord (Bradbury et al., 1999; Ramer et al., 2000; Lu et al., 2003; Taylor et al., 2006) (Table 1). These growth factors emerge as the most frequent subjects of therapeutic analysis in models of SCI because of their ability to influence the growth of motor axons, although other growth factors, including insulin-like growth factor -1 (IGF-1), the fibroblast growth factor (FGF) family, and others - remain of considerable potential interest. NGF and GDNF, on the other hand, by promoting growth of nociceptive spinal axons, may risk worsening dysfunctional axonal sprouting after SCI and worsening pain. This point highlights the fact that axonal growth is not a uniformly advantageous phenomenon: aberrant growth of either sensory or motor systems could have deleterious anatomical and functional consequences.
Table 1.
Sensitivity of Spinal Cord Axons to Growth Factors
| Growth Factors | Injured Axons | References |
|---|---|---|
| NGF | nociceptive spinal axons | Tuszynski et al., 1994, 1996 |
| Ramer et al., 2000 | ||
| cerulospinal axons | Tuszynski et al., 1994, 1996 | |
| BDNF | rubrospinal axons | Kobayashi et al., 1997 |
| Ye and Houle, 1997 | ||
| Liu et al., 1999 | ||
| raphespinal axons | Bregman et al., 1997 | |
| Menei et al., 1998 | ||
| coerulospinal axons | Menei et al., 1998 | |
| reticulospinal axons | Ye and Houle, 1997 | |
| Jin et al., 2002 | ||
| vestibulospinal | Jin et al., 2002 | |
| local motor axon | Lu et al., 2001 | |
| local sensory axon (CGRP) | Lu et al., 2001 | |
| NT-3 | corticospinal axons | Schnell et al., 1994 |
| Grill et al., 1997 | ||
| dorsal column sensory axons | Bradbury et al., 1999 | |
| NT-4/5 | local motor axons | Blesch et al., 2004 |
| coerulospinal axons | Blesch et al., 2004 | |
| reticulospinal axons | Blesch et al., 2004 | |
| propriospinal axons | Blesch et al., 2004 | |
| GDNF | local motor axons | Blesch et al., 2003 |
| propriospinal axons | Blesch et al., 2003 | |
| dorsal column sensory axons | Blesch et al., 2003 | |
| nociceptive spinal axons | Ramer et al., 2003 | |
BDNF Effects on Axonal Growth
In a series of studies, several groups reported that BDNF could enhance the growth of supraspinal motor axons into permissive growth milieus placed at sites of SCI. Kobayashi and colleagues reported in 1997 that BDNF promotes the growth of rubrospinal axons into a peripheral nerve graft placed at a site of cervical SCI, and prevents degeneration of the red nucleus cell body in the brainstem (Kobayashi et al., 1997). Further, delayed delivery of BDNF to the red nucleus by one year in this model continued to demonstrate neurotrophic actions, reversing atrophy of neurons in the red nucleus even after this extended time point (Kwon et al., 2002). Liu and colleagues also reported in 1999 that rubrospinal axons regenerated into cellular grafts of autologlous fibroblasts genetically modified to secrete BDNF in a mid-cervical spinal cord lesion site (Liu et al., 1999). They further noted improvement in use of the forepaw on the side affected by the lesion, possibly as a result of local sprouting stimulated by BDNF. Reticulospinal axons, which modulate activity of motor neurons in the spinal cord, also respond to BDNF (Jin et al., 2002) (Fig. 1). Lu and colleagues reported that BDNF did not influence the growth of corticospinal axons in the spinal cord, even though BDNF prevents the death of corticospinal neuronal cell bodies when applied to the cortex, most likely because the BDNF receptor trkB was not trafficked from the cortical soma to the spinal axon (Lu et al., 2001). Others had also previously described protective effects of BDNF on cortical neurons (Giehl and Tetzlaff, 1996). In none of these papers was axonal growth beyond the BDNF cell graft or peripheral nerve bridge demonstrated, however. During development, neurotrophic factors classically promote the growth of their responsive axons along chemotropic gradients, with growth maximal at the point of peak growth factor concentration. This appears to be the case in the adult injured spinal cord also: axons readily penetrate BDNF-secreting grafts, but have not been reliably observed to emerge from the grafts again.
NT-3 Effects on Axonal Growth
A related but slightly distinct phenomenon has been observed in assessing responsiveness of corticospinal axons to NT-3. In 1994, Schnell and colleagues reported that a single injection of NT-3 above a spinal cord lesion site, when combined with administration of the Nogo neutralizing body IN-1, significantly enhanced the distance that a corticospinal axon extends below a mid-thoracic spinal dorsal hemisection lesion site (Schnell et al., 1994). Corticospinal axons observed below the lesion site in this experiment might have originated either from: 1) dorsally lesioned corticospinal axons that regenerated through spared host gray matter underlying the dorsal hemisection lesion, or 2) from ventral corticospinal axons, spared by the dorsal hemisection lesion, that subsequently sprouted below the lesion site in response to IN-1 antibody treatment. The single injection of NT-3 protein increased corticospinal axon sprouting above the lesion, and may have slightly increased the density of axons detected below in the lesion. In 1997, Grill and colleagues implanted autologous fibroblasts genetically modified to secrete NT-3 in a mid-thoracic spinal cord dorsal hemisection lesion site (Grill et al., 1997). Interestingly, corticospinal axons did not penetrate the NT-3 secreting cell graft, but did exhibit enhancing sprouting in host gray matter underlying the graft in the lesion site. Sprouting of CST axons occurred in gray matter above, underlying, and 1 mm caudal to the NT-3 graft/lesion site. Rats treated with NT-3 showed partial recovery of locomotion on a horizontal ladder (Grill et al., 1997). This report confirmed that NT-3 can influence injured adult corticospinal axons, and also revealed that corticospinal axons are uniquely repelled by growth into any substrate other than adult gray matter. To the present day, 10 years later, we and to our knowledge others have not clearly succeeded in promoting growth of injured adult corticospinal axons into a substrate placed into the site of an adult spinal cord lesion.
Sensory axons also respond to NT-3 (Bradbury et al., 1999; Ramer et al., 2000; Lu et al., 2003; Taylor et al., 2006). Unlike corticospinal tract axons, however, dorsal column sensory axons successfully penetrate a cell graft placed in a spinal cord lesion site, and their growth into a graft is significantly enhanced by NT-3 (Lu et al., 2003). Like BDNF-responsive descending motor axons, however, ascending dorsal column sensory axons do not emerge from grafts placed in lesion sites to bridge beyond the lesion cavity.
In summary, studies over the past decade clearly demonstrated that injured adult axons retain sensitivity to various growth factors. While mechanistically informative, these findings were of little practical value: axons must grow beyond the lesion site to have a potential for facilitating functional recovery. The observation that corticospinal axons sprout through host gray matter surrounding a dorsal hemisection lesion site was also informative but of limited value in a practical sense: gray matter is usually entirely destroyed at sites of SCI in humans, due its greater vulnerability to ischemia, inflammation and secondary injury. A next step was required.
Combinatorial Therapies Support Axonal Bridging Beyond Spinal Cord Lesion Sites
In 2004, several groups published findings that combinatorial therapies exert effects in models of SCI that are not fully observed when fewer treatments are applied (Lu et al., 2004; Nikulina et al., 2004; Pearse et al., 2004). Prior to this time, most working in the field expected that treatment combinations would be more potent than individual, incremental approaches to enhancing axonal plasticity and regeneration, but little rigorous and objective data existed to support this belief.
Testing the hypothesis that combinatorial therapies exert greater effects on regeneration than single therapies is not a simple matter. One must control each variable, and if there are more than two combinations to the therapy, then each potential combination must be tested. If there are two therapies, then 3 groups must be tested. If three combinations are tested, then there must be 7 groups. And if 4 potential combinations are tested, then 15 groups must be tested (see Table 2). In our laboratory’s models of dorsal column transection, we typically require a sample size of 10-12 animals per group to detect an effect that by convention is statistically significant at the p<0.05 level. Thus, testing 3 combinations requires approximately 70-80 rats, and testing 4 combinations requires 150-170 rats. These experiments rapidly become impractical for laboratories with limited personnel, time and financial support.
Table 2.
Controls for Combinatorial Growth Factor/cAMP Therapy
| A. Groups to be assigned: | |
|---|---|
| MSC | Marrow stromal cell |
| NT-3 | NT-3 in lesion site |
| NT-3 Beyond | NT-3 beyond lesion site |
| cAMP | cAMP injection into DRG |
| B. Hypothetical experimental groups for full control: |
|---|
| 1) MSC |
| 2) MSC + NT-3 |
| 3) MSC + NT-3 + NT-3 Beyond |
| 4) MSC + NT-3 + NT-3 Beyond + cAMP |
| 5) MSC + cAMP |
| 6) MSC + NT-3 Beyond |
| 7) MSC + NT-3 + cAMP |
| 8) MSC + NT-3 Beyond + cAMP |
| 9) NT-3 |
| 10) NT-3 + NT-3 Beyond |
| 11) NT-3 + NT-3 Beyond + cAMP |
| 12) NT-3 + cAMP |
| 13) NT-3 Beyond |
| 14) NT-3 Beyond + cAMP |
| 15) cAMP |
| 16) No MSC, no NT-3, no NT-3 beyond, and no cAMP |
Note: Underlined groups were included in final experiments.
Controls for NT-3 or cAMP injections consisted of vehicle solution injections into the respective target sites (see Lu et al., J Neurosci 2004; 24:6402-6409).
Nonetheless, as an initial rigorous test of the value of combinatorial therapies, many combinations were subjected to experimental control. We tested the hypothesis that to achieve sensory axonal bridging beyond a C4 spinal cord dorsal column lesion site, the following combinatorial therapies would be required:
1) A cell bridge in the lesion site
In this experiment, we used syngenic bone marrow stromal cells, which establish a “passive” cellular matrix within the lesion site, without producing growth factors or migrating from the lesion site after injection (Lu et al., 2005). These cells integrate well into a lesion site and reestablish cellular connectivity between the proximal and distal ends of the lesion cavity.
2) A growth factor within the bridge in the lesion site
We injected recombinant human NT-3 protein (600 ng) once into the marrow stromal cell suspension in the lesion site at the time of lesion and grafting to provide a trophic stimulus for attracting injured dorsal column sensory axons into the lesion site. Decline in the level of NT-3 protein over time (based on utilization and diffusion) would eliminate it as a sustained trophic stimulus that would otherwise risk retaining axons in the lesion site.
3) A growth factor beyond the bridge in the lesion site
To test the hypothesis that a gradient of NT-3 beyond a lesion site would elicit sensory axonal bridging not only into, but beyond, the lesion site, one week after the initial lesion, 1000 ng NT-3 was injected into the dorsal column white matter 1.5 mm rostral to the lesion site, to act as a delayed chemotropic stimulus to attract regenerating axons out of the cell graft in the lesion site.
4) A conditioning stimulus to the injured neuron, to “prime” the growth state
Both remote and more recent studies demonstrate that a conditioning (crush) lesion to the peripheral branch of a dorsal root ganglion neuron enhances the sprouting of its central axon after a spinal cord lesion (McQuarrie et al., 1977; Neumann et al., 1999). In contrast, a conditioning lesion of the central branch of a dorsal root ganglion neuron does not enhance growth after either a subsequent peripheral or central lesion. Thus, the peripheral crush “primes” the neuron to enter a growth state. This phenomenon is in part cAMP dependent, and can be replicated by injection of cAMP into the dorsal root ganglion (Neumann et al., 2002; Qiu et al., 2002). The mechanism of central growth activation may be related to upregulation of regeneration-associated genes such as GAP-43, C-jun and ß-tubulin, and reduced sensitivity of the injured axon to myelin-associated inhibitors of growth. In our combinatorial study, we tested the hypothesis that the addition of a cAMP injection into the dorsal root ganglion, NT-3 gradients within and beyond a C4 dorsal column lesion cavity, and a cell bridge in the lesion cavity, would support axonal bridging (if a simpler combination did not).
This ambitious experiment manipulated four variables: 1) cell bridge, 2) growth factor within the lesion site, 3) growth factor beyond the lesion site, and 4) cAMP into the dorsal root ganglion cell body. Thus, 16 experimental groups would be required to fully control the experiment and determine the necessity of each component of the combination, as shown in Table 2. We reduced this to a more manageable number based on observations from previous studies over which there would be little disagreement. First, axons cannot extend into the lesion cavity in the absence of a bridging substrate or, in this experiment, the marrow stromal cell graft. In previous experiments in our laboratory and that of all others that we are aware of, working in rat models, axons will not bridge beyond a transection lesion in the absence of a physical substrate for axonal attachment in the lesion cavity. Thus, this consideration allowed exclusion of all groups that did not contain a MSC graft, groups 9-15 in Table 2. We retained group 16, a no-treatment lesion control, to provide a baseline for injury severity in the absence of treatment. We were left with 9 groups to test.
Of these 9 remaining experimental groups, we eliminated another 3 groups as potentially non-essential control conditions. Group 6 from Table 2 would receive a graft and only NT-3 beyond the lesion site. If group 3 (graft + NT-3 in the graft + NT-3 beyond the graft) did not show bridging axons, then group 3 would not either. Indeed, this proved to be the case at the conclusion of the experiment, and group 6 was not needed as a control. Group 7 from Table 2 would receive a graft, NT-3 in the lesion site, and cAMP into the DRG: we hypothesized that in the absence of a neurotrophic gradient beyond the graft, axons would not extend for considerable distances beyond the graft. Group 8 from Table 2 would receive a graft, NT-3 beyond the lesion site and cAMP into the DRG: because the distal NT-3 injection is performed one week after the original lesion, we reasoned it unlikely that axons would extend into the lesion site in sufficient numbers in this group to represent a quantitative “pool” for potential emergence from the graft. While findings of the experiment suggested that the exclusion of the latter two control groups (7 and 8) were likely justified as results below indicate, rigorous proof would require completion of experiments in these groups.
Thus, the experimental design ultimately examined five groups of animals, and 12 animals per group, for a total of 60 subjects, plus lesioned, non-grafted controls. At the conclusion of the experiment, we found that axons bridged beyond the lesion cavity only in the full treatment group that received a cell bridge, NT-3 within and beyond the lesion site, and cAMP priming of the injured neuron (Fig. 2). None of the remaining groups exhibited bridging axons, although in some groups (bridge + cAMP; bridge + NT-3 within and beyond the lesion site) axons extended to the interface of the graft with the rostral host spinal cord.
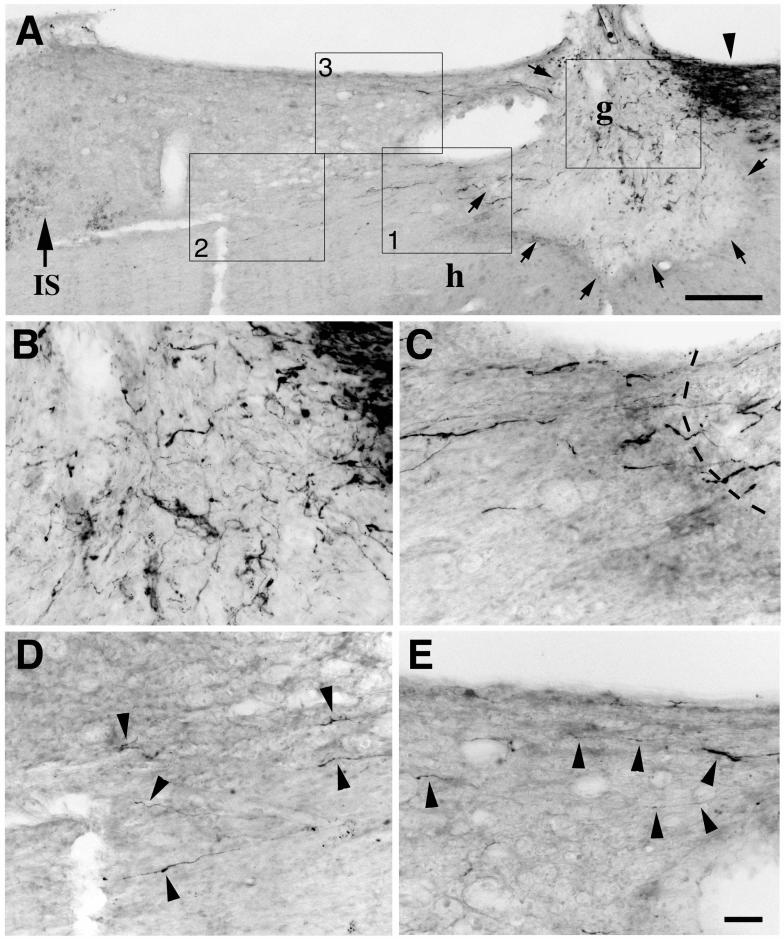
Figure 2.
Sensory axons regenerate beyond spinal cord lesion sites after combined administration of intraganglionic cAMP and axonal application of NT-3. (A) Lower magnification view of sagittal section of spinal cord illustrating CTB-labeled dorsal column sensory axons approaching lesion site (arrowhead on upper right), MSC graft in lesion cavity (g; arrows indicate host/graft interface), and region rostral to lesion site (left side of figure). Large arrow and IS indicate rostral Injection Site of NT-3. (B) Boxed area of graft: CTB-labeled sensory axons penetrate graft in lesion site. (C) Higher magnification of box 1 from panel A, demonstrating crossing of CTB-labeled axons from graft into host white matter beyond the graft. Dashed lines indicate host/graft interface. This crossing occurs at a point well away from dorsal or ventral lesion regions, reducing likelihood that axons were spared by lesion. Lesion completeness was confirmed by failure to observe CTB-labeled sensory axons in medulla in lesioned subjects. (D) Higher magnification of box 2 from panel A, demonstrating several varicose CTB-labeled axons extending 0.5 - 0.7 mm beyond lesion site (arrowheads). (E) Higher magnification of box 3 from panel A, showing additional axons extending under the dorsal aspect of the spinal cord beyond the lesion site. Scale bars = 280 μm (A), 44 μm (B-E). (From Lu et al., J Neurosci 2004; 24:6406).
The risk of misinterpreting spared axons for regenerating axons looms large in all spinal cord studies. In this experiment, we confirmed that lesions were complete by sectioning the nucleus gracilis in every animal: no spared axons reaching the nucleus were observed. Further, axons observed to extend beyond the lesion site in this experiment emerged directly across the graft-host interface (labeled clearly with the reporter gene green fluorescent protein, GFP), and frequently from the center of the graft rather than only the most dorsal or ventral aspects of the lesion site, where spared axons are most likely to be located. Finally, axons extending beyond the lesion site had an irregular and often circuitous course, unlike linear intact axons. For these reasons, it appeared that axons truly bridged beyond the lesion cavity (Fig. 2).
Two other reports published in 2004 suggested further that combination therapies can generate more extensive anatomical or behavioral plasticity than individual therapies (Pearse et al., 2004; Nikulina et al., 2004). Interestingly, both of these studies, as well as our own, included cAMP augmentation of the neuronal growth state as a component of a combination therapy to improve outcomes in models of SCI. Augmentation of the neuronal growth state remains a major focus of several current efforts in SCI research, including attempts discover novel genes related to enhanced central plasticity and regeneration (Costigan et al., 2002).
CONCLUSION
Reports continue to emerge supporting the concept that combination therapies elicit significantly greater degrees of axon growth than individual therapies (Houle et al., 2006; Tan et al., 2006; Massey et al., 2007). Combinations of bridges, growth factors, degrading enzymes of the extracellular matrix, and myelin neutralization will require systematic but focused examination to design a combinatorial approach most meritorious of testing in larger animal models. The practical hurdles to this approach are not unsubstantial, but must be overcome to generate approaches that may ultimately benefit the human condition.
ACKNOWLEDGEMENTS
Supported by the NIH (NS09881, NS42291), the Veterans Administration, the Canadian Spinal Research Organization, and the Adelson Medical Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bhatheja K, Field J. Schwann cells: origins and role in axonal maintenance and regeneration. Int J Biochem Cell Biol. 2006;38:1995–1999. doi: 10.1016/j.biocel.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Blesch A, Uy HS, Grill RJ, Cheng JG, Patterson PH, Tuszynski MH. Leukemia inhibitory factor augments neurotrophin expression and corticospinal axon growth after adult CNS injury. J Neurosci. 1999;19:3556–3566. doi: 10.1523/JNEUROSCI.19-09-03556.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blits B, Oudega M, Boer GJ, Bunge M, Verhaagen J. Adeno-associated viral vector-mediated neurotrophin gene transfer in the injured adult rat spinal cord improves hindlimb function. Neuroscience. 118:271–281. doi: 10.1016/s0306-4522(02)00970-3. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27:277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Khemani S, Von R, King, Priestley JV, McMahon SB. NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur J Neurosci. 1999;11:3873–3883. doi: 10.1046/j.1460-9568.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- Bregman BS, McAtee M, Dai HN, Kuhn PL. Neurotrophic factors increase axonal growth after spinal cord injury and transplantation in the adult rat. Exp Neurol. 1997;148:475–494. doi: 10.1006/exnr.1997.6705. [DOI] [PubMed] [Google Scholar]
- Buchli AD, Schwab ME. Inhibition of Nogo: a key strategy to increase regeneration, plasticity and functional recovery of the lesioned central nervous system. Ann Med. 2005;37:556–567. doi: 10.1080/07853890500407520. [DOI] [PubMed] [Google Scholar]
- Chernousov MA, Carey DJ. Schwann cell extracellular matrix molecules and their receptors. Histol Histopathol. 2000;15:593–601. doi: 10.14670/HH-15.593. [DOI] [PubMed] [Google Scholar]
- Costigan M, Befort K, Karchewski L, Griffin RS, D’Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovy P. Schwann cells and endoneurial extracellular matrix molecules as potential cues for sorting of regenerated axons: a review. Anat Sci Int. 2004;79:198–208. doi: 10.1111/j.1447-073x.2004.00090.x. [DOI] [PubMed] [Google Scholar]
- Fawcett JW. Overcoming inhibition in the damaged spinal cord. J Neurotrauma. 2006;23:371–383. doi: 10.1089/neu.2006.23.371. [DOI] [PubMed] [Google Scholar]
- Giehl KM, Tetzlaff W. BDNF and NT-3, but not NGF, prevent axotomy-induced death of rat corticospinal neurons in vivo. Eur J Neurosci. 1996;8:1167–1175. doi: 10.1111/j.1460-9568.1996.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Grill R, Murai K, Blesch A, Gage FH, Tuszynski MH. Cellular delivery of neurotrophin-3 promotes corticospinal axonal growth and partial functional recovery after spinal cord injury. J Neurosci. 1997;17:5560–5572. doi: 10.1523/JNEUROSCI.17-14-05560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakeman LB, Wei P, Guan Z, Stokes BT. Brain-derived neurotrophic factor stimulates hindlimb stepping and sprouting of cholinergic fibers after spinal cord injury. Exp Neurol. 1998;154:170–184. doi: 10.1006/exnr.1998.6924. [DOI] [PubMed] [Google Scholar]
- Jin Y, Fischer I, Tessler A, Houle JD. Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury. Exp Neurol. 2002;177:265–275. doi: 10.1006/exnr.2002.7980. [DOI] [PubMed] [Google Scholar]
- Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi NR, Fan DP, Giehl KM, Bedard AM, Wiegand SJ, Tetzlaff W. BDNF and NT-4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate GAP-43 and Talpha1-tubulin mRNA expression, and promote axonal regeneration. J Neurosci. 1997;17:9583–9595. doi: 10.1523/JNEUROSCI.17-24-09583.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kury P, Stoll G, Muller HW. Molecular mechanisms of cellular interactions in peripheral nerve regeneration. Curr Opin Neurol. 2001;14:635–639. doi: 10.1097/00019052-200110000-00013. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Liu J, Messerer C, Kobayashi NR, McGraw J, Oschipok L, Tetzlaff W. Survival and regeneration of rubrospinal neurons 1 year after spinal cord injury. Proc Natl Acad Sci U S A. 2002;99:3246–3251. doi: 10.1073/pnas.052308899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos A, Franklin RJ. Transplant mediated repair of the central nervous system: an imminent solution? Curr Opin Neurol. 2002;15:701–705. doi: 10.1097/01.wco.0000044766.39452.f6. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kim D, Himes BT, Chow SY, Schallert T, Murray M, Tessler A, Fischer I. Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J Neurosci. 1999;19:4370–4387. doi: 10.1523/JNEUROSCI.19-11-04370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Blesch A, Tuszynski MH. Neurotrophism without neurotropism: BDNF promotes survival but not growth of lesioned corticospinal neurons. J Comp Neurol. 2001;436:456–470. doi: 10.1002/cne.1080. [DOI] [PubMed] [Google Scholar]
- Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Lu P, Jones LL, Tuszynski MH. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp Neurol. 2005;191:344–360. doi: 10.1016/j.expneurol.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Lu P, Yang H, Jones LL, Filbin MT, Tuszynski MH. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neurosci. 2004;24:6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey JM, Amps J, Viapiano MS, Matthews RT, Wagoner MR, Whitaker CM, Alilain W, Yonkof AL, Khalyfa A, Cooper NG, Silver J, Onifer SM. Increased chondroitin sulfate proteoglycan expression in denervated brainstem targets following spinal cord injury creates a barrier to axonal regeneration overcome by chondroitinase ABC and neurotrophin-3. Exp Neurol. 2008;209:426–475. doi: 10.1016/j.expneurol.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuarrie IG, Graftstein B, Gershon MD. Axonal regeneration in the rat sciatic nerve: effect of a conditioning lesion and of dbcAMP. Brain Res. 132:443–453. doi: 10.1016/0006-8993(77)90193-7. [DOI] [PubMed] [Google Scholar]
- Menei P, Montero-Menei C, Whittemore SR, Bunge RP, Bunge MB. Schwann cells genetically modified to secrete human BDNF promote enhanced axonal regrowth across transected adult rat spinal cord. Eur J Neurosci. 1998;10:607–621. doi: 10.1046/j.1460-9568.1998.00071.x. [DOI] [PubMed] [Google Scholar]
- Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikova LN, Novikov LN, Kellerth JO. Biopolymers and biodegradable smart implants for tissue regeneration after spinal cord injury. Curr Opin Neurol. 2003;16:711–715. doi: 10.1097/01.wco.0000102620.38669.3e. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Bradbury EJ, Michael GJ, Lever IJ, McMahon SB. Glial cell line-derived neurotrophic factor increases calcitonin gene-related peptide immunoreactivity in sensory and motoneurons in vivo. Eur J Neurosci. 2003;18:2713–2721. doi: 10.1111/j.1460-9568.2003.03012.x. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403:312–316. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- Rossi F, Gianola S, Corvetti L. Regulation of intrinsic neuronal properties for axon growth and regeneration. Prog Neurobiol. 2007;81:1–28. doi: 10.1016/j.pneurobio.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Richardson PM, McGuiness UM, Aguayo AJ. Axons from CNS neurons regenerate into PNS grafts. Nature. 284:264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- Ruitenberg MJ, Plant GW, Hamers FP, Wortel J, Blits B, Dijkhuizen PA, Gispen WH, Boer GJ, Verhaagen J. Ex vivo adenoviral vector-mediated neurotrophin gene transfer to olfactory ensheathing glia: effects on rubrospinal tract regeneration, lesion size, and functional recovery after implantation in the injured rat spinal cord. J Neurosci. 2003;23:7045–7058. doi: 10.1523/JNEUROSCI.23-18-07045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Brechtel K, Mueller CA, Failli V, Kaps HP, Tuli SK, Schluesener HJ. Experimental strategies to promote spinal cord regeneration--an integrative perspective. Prog Neurobiol. 2006;78:91–116. doi: 10.1016/j.pneurobio.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Tan AM, Colletti M, Rorai AT, Skene JH, Levine JM. Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J Neurosci. 2006;26:4729–4739. doi: 10.1523/JNEUROSCI.3900-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26:9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J Anat. 1999;194(Pt 1):1–14. doi: 10.1046/j.1469-7580.1999.19410001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski MH. Growth-factor gene therapy for neurodegenerative disorders. Lancet Neurol. 2002;1:51–57. doi: 10.1016/s1474-4422(02)00006-6. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Gabriel K, Gage FH, Suhr S, Meyer S, Rosetti A. Nerve growth factor delivery by gene transfer induces differential outgrowth of sensory, motor, and noradrenergic neurites after adult spinal cord injury. Exp Neurol. 1996;137:157–173. doi: 10.1006/exnr.1996.0016. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Peterson DA, Ray J, Baird A, Nakahara Y, Gage FH. Fibroblasts genetically modified to produce nerve growth factor induce robust neuritic ingrowth after grafting to the spinal cord. Exp Neurol. 1994;126:1–14. doi: 10.1006/exnr.1994.1037. [DOI] [PubMed] [Google Scholar]
- Xu XM, Guenard V, Kleitman N, Aebischer P, Bunge MB. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp Neurol. 1995;134:261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- Ye JH, Houle JD. Treatment of the chronically injured spinal cord with neurotrophic factors can promote axonal regeneration from supraspinal neurons. Exp Neurol. 1997;143:70–81. doi: 10.1006/exnr.1996.6353. [DOI] [PubMed] [Google Scholar]
- Blesch A, Tuszynski MH. Cellular GDNF delivery promotes growth of motor and dorsal column sensory axons after partial and complete spinal cord transections and induces remyelination. J Comp Neurol. 2003;467:403–417. doi: 10.1002/cne.10934. [DOI] [PubMed] [Google Scholar]
- Blesch A, Yang H, Weidner N, Hoang A, Otero D. Axonal responses to cellularly delivered NT-4/5 after spinal cord injury. Mol Cell Neurosci. 2004;27:190–201. doi: 10.1016/j.mcn.2004.06.007. [DOI] [PubMed] [Google Scholar]