Abstract
Purpose
To evaluate optical coherence tomography thickness of the macula in people with diabetes but minimal or no retinopathy and compare these findings with published normative data in the literature from subjects reported to have no retinal disease.
Design
Cross-sectional study.
Methods
Setting: Multicenter, community- and university-based practices.
Study Population: 97 subjects with diabetes with no or minimal diabetic retinopathy and no central retinal thickening on clinical examination and a center point thickness ≤225 microns on a Stratus™ (Carl Zeiss Meditec, Dublin, CA) optical coherence tomography (Stratus OCT™).
Observation Procedures: Electronic-ETDRS best-corrected visual acuity, 7-field stereoscopic color fundus photographs, Stratus OCT™ fast macular scan.
Main Outcome Measures: Central subfield thickness measured on Stratus OCT™.
Results
On average, central subfield thickness was 201 ± 22 microns. Central subfield thickness was significantly greater in retinas from men than retinas from women subjects (mean 209 ± 18 microns versus 194 ± 23 microns, P<0.001). After adjusting for gender, no additional factors were found to be significantly associated with central subfield thickness (P>0.10).
Conclusions
Central subfield thicknesses on Stratus OCT™ in people with diabetes and minimal or no retinopathy are similar to thicknesses reported from a normative database of people without diabetes. Central subfield thickness is greater in men than in women, consistent with many, but not all, previous reports. Studies involving comparisons of retinal thickness to expected norms should consider different mean values for women and men.
Introduction
Before Optical Coherence Tomography (OCT), the standard method for assessing macular thickness in the clinic was stereoscopic biomicroscopy, a subjective process dependent on observer skill, patient cooperation, degree of pupillary dilation, clarity of ocular media and characteristics of the retinal swelling. OCT has added objectivity and sensitivity to the assessment of macular edema previously available from clinical examination and analysis of stereoscopic fundus photographs, assisted clinicians in routine clinical care, and provided data used for outcome assessment in clinical studies.
Quantitative thickness measurements derived from the “fast macular scan” algorithm report retinal thickness at several locations including the center point, central subfield, and 4 inner and 4 outer zones of the macula.1 From these data using an OCT machine (Stratus OCT™, Carl Zeiss Meditec, Dublin, California), an estimate of thickening can be calculated based on a comparison with a normal patient database obtained by Carl Zeiss Meditec (Dublin, California), reported in the StratusOCT™ Software Version 4.0 Brochure and which is the same database (personal communication, J. Lipyanik, Carl Zeiss Meditec, May 31, 2007) as was presented at the 2005 Annual Meeting of the Association for Research in Vision and Ophthalmology. (Fraser-Bell S, et al. IOVS 2005; ARVO E-Abstract 1542) This normative database suggests a difference in thickness by age, gender, ethnicity and refractive error, with thinner values in older individuals, women, African-Americans, and persons with myopia of −5D or more. (Fraser-Bell S, et al. IOVS 2005; ARVO E-Abstract 1542)
Two studies2, 3 have suggested that patients with diabetes mellitus and no retinopathy have retinal thickness values that are similar to values from populations without diabetes and a normal retina. However, these studies used an earlier model of OCT than is commonly available today. Three additional studies provide data on individuals with diabetes, but do not specify results for the subgroup of individuals without diabetic retinopathy.4–6 In addition to the proprietary normative database used for the Stratus OCT™ which reported a difference in thickness by gender (Fraser-Bell S, et al. IOVS 2005; ARVO E-Abstract 1542), several reports in the literature using an older version of OCT also suggest a difference by gender in individuals with no diabetes.2, 3, 7
For persons with diabetes and no retinopathy, there is limited or no published Stratus OCT™ normative data nor assessments of possible interactions by age, gender, ethnicity, or refractive error. Such normative values are desirable to help in the design and interpretation of clinical trials evaluating diabetic macular edema. For example, when setting criteria for central subfield retinal thickening at a specific value to determine eligibility for participation in a study of diabetic macular edema, it is helpful to know whether that value should differ when enrolling women compared with men. To address these topics, we studied Stratus OCT™-measured thickness of the retina in diabetic subjects without retinopathy or with very mild retinopathy (a few microaneurysms and no other retinal abnormalities associated with diabetic retinopathy). We compared these results with published Stratus OCT™ normative data in the literature in healthy subjects (Fraser-Bell S, et al. IOVS 2005; ARVO E-Abstract 1542 and Carl Zeiss Meditec, Inc., Stratus OCT™ Software Version 4.0 Brochure),6, 8, 9 and we reviewed similar comparisons in the literature between healthy subjects and subjects with diabetes but no retinopathy which used earlier OCT models.2, 3, 10, 11 The effect of the presence of very mild retinopathy compared with no retinopathy, duration of diabetes, type of diabetes, and other factors including age, gender, ethnicity, and refractive error on the retinal thickness measurements also was explored.
Methods
This study was conducted by the Diabetic Retinopathy Clinical Research Network (DRCR.net) at 7 clinical sites as part of another study evaluating longitudinal follow up of eyes with subclinical diabetic macular edema. The investigation was funded by the National Eye Institute and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health, U.S. Department of Health and Human Services. The protocol and HIPAA-compliant informed consent forms were approved by multiple institutional review boards. Each subject gave written informed consent for participation in the study. The entire protocol is available on the public site at www.drcr.net by clicking on “Information about the network” and then clicking on “DRCR.net Studies”, under the study title, “Subclinical Diabetic Macular Edema Study.”
To avoid ambiguity with other reports, the following definitions were used:
Center Point – the intersection of the six radial scans of the fast macular thickness protocol of the OCT.
Central Subfield – the circular area of diameter 1 mm centered around the center point.
Central Subfield Mean Thickness – the mean value of the 128 thickness values obtained in the central subfield.
Center Point Thickness – the average of the thickness values for the 6 radial scans at their point of intersection.
Retinal Thickening – the calculated value equal to the thickness minus the population mean for the variable under consideration (either center point thickness or central subfield thickness).
Study Population
Eligible subjects were at least 18 years old with type 1 or type 2 diabetes, no history of renal failure requiring dialysis, no renal or pancreatic transplant, no medical treatment for retinal disorders or any therapy that has been demonstrated to affect retinal edema. An eye was eligible if it met the following criteria: (1) no retinal thickening involving the center point of the macula due to diabetic retinopathy based on clinical examination (2) no diabetic retinopathy (Early Treatment Diabetic Retinopathy Study (ETDRS) level 10) or microaneurysms only (ETDRS level 20) based on Reading Center grading of fundus photos12 (3) best corrected electronic-ETDRS (E-ETDRS) visual acuity13 letter score ≥ 74 (approximately 20/32 or better), and (4) no prior treatment for macular edema.
Study Procedures
Following pupil dilation, OCT fast macular scans were obtained by a certified operator from each eye of the participant using the Stratus OCT™ version 4 system and 6.0 mm scans. Scans were sent to the DRCR.net Reading Center at the University of Wisconsin-Madison where they were visually inspected. Center point thickness was measured manually with calipers at the presumed center of the fovea from printouts of the retina thickness map scans in 16(10%) of the images with at least one of the following findings: (1) a standard deviation of the center point thickness ≥10.5%, (2) inaccurately drawn automated boundary lines, or (3) decentration. For 15 of these 16 images, the automated central subfield thickness measurements were judged by the Reading Center to be inaccurate and center point thickness (manually measured) was used to impute a value for the central subfield (because correlation of the two measures is 0.98 as published previously).14 The equation used for the imputation is: central subfield = 63.6 + 0.84 * center point, based on a regression analysis from a prior study (data not published). The study results were similar when the imputed values were excluded (data not shown).
Diabetic retinopathy grading of ETDRS 7 standard field stereoscopic fundus photography was performed by the DRCR.net Reading Center using the ETDRS protocol. Visual acuity was measured by a certified tester using the E-ETDRS procedure following a standardized refraction.
Statistical Methods
Central subfield thickness was the primary OCT parameter used in the analysis. Three eyes met eligibility criteria but were excluded because they were extreme outliers (central subfields measuring 281, 293, 315). On review of these OCT scans by two of us (NMB, RPD) and their corresponding fundus photographs by one of us (RPD), there was no evidence of any other morphologic abnormality on OCT and no evidence of any retinal disease on fundus photographs except for 1 microaneurysm nasal to the optic nerve in one eye. Central subfield thickness was highly correlated between eyes in subjects with two study eyes. For the 58 subjects with two eyes in the study, the correlation of central subfield thickness between the two eyes was 0.89. The average absolute difference of the central subfield between the two eyes was 8 ± 7 microns with a maximum difference of 29 microns. Therefore, only one eye of each subject was used for all subsequent analyses in this report. The eye to use from subjects with two study eyes was chosen at random.
Mean central subfied thickness values were evaluated according to clinical chacteristics, categorizing continuous parameters according to the following: age and duration of diabetes cutoffs were determined using the median; visual acuity cutoffs group eyes with 20/20 or better vision in one category, and then each 1 line interval below that in a category; HbA1c and refractive error cutoffs were determined using clinically meaningful values. Separate unadjusted least squares regression models were used to evaluate the relationship of clinical characteristics with central subfield thickness. All models used the continuous version of parameters where applicable. Models were repeated, adjusting for factors found to have a relationship with central subfield thickness. The differences between men and women in mean retinal thickness within each macular region were evaluated using t-tests.
All P-values reported are two-tailed. Statistical analyses were performed using SAS software version 9.1.
Results
The study included 97 subjects who had at least one eye meeting study eligibility criteria. Average age was 55 ± 15 years, 48 (49%) were women, and 18 (19%) were not Caucasian. Type 1 diabetes was present in 24 (25%) subjects and type 2 in 73 (75%). The average duration of diabetes was 13 ± 8 years for type 1 subjects and 9 ± 7 years for type 2 subjects. Mean HbA1c was 7.8 ± 1.1% and 7.3 ± 1.4% for type 1 and type 2 subjects respectively. Visual acuity ranged from a letter score (approximate Snellen equivalent) of 93 (20/12) to 74 (20/32), and was >79 (20/25 or better) in 75 eyes (77%). The average spherical equivalent was −0.66 ± 2.33.
Figure 1 displays the mean, standard deviation, and range for the center point, central subfield, 4 inner subfields (within 500 to 1500 microns of the center), 4 outer subfields (within 1500 and 3000 microns of the center), and retinal volume. On average, the central subfield was 201 ± 22 microns. The inner subfields were thicker than the outer subfields, and the nasal subfields were thicker than the temporal subfields. In contrast, the inner superior and inferior subfields were similar to each other, as were the outer superior and inferior subfields. The standard deviation of the thickness was similar in the inner and outer subfields (ranging from 15 to 19) and slightly larger in the central subfield and center point, (22 and 23 microns, respectively). With exclusion of the extreme outliers as described in the methods, the central subfield for the 97 subjects in this study was no greater than 247 microns while the center point thickness was no greater than 225 microns.
Figure 1.
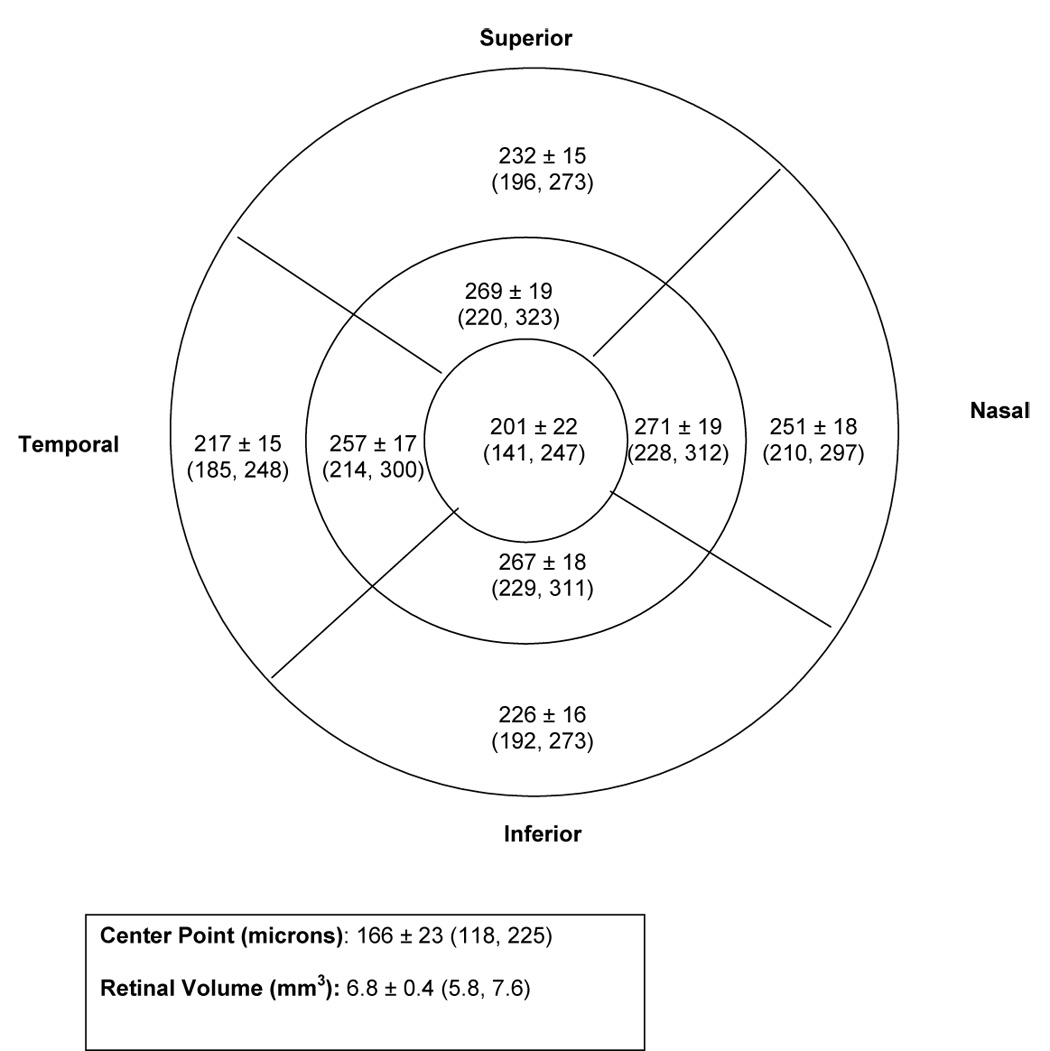
Distribution of Stratus OCT™ retinal thickness in diabetic subjects with minimal or no retinopathy: Mean ± SD (Minimum, Maximum)
Central subfield thickness was significantly greater in retinas from men than retinas from women subjects (P < 0.001, Table 1) with a mean of 209 ± 18 microns in men and 194 ± 23 microns in women. Gender also was associated with level of retinopathy, with 21% having level 20 in women and 41% with this level in men (P=0.05); adjusting for this did not affect the association between gender and central subfield thickness. These gender differences were noted consistently across clinical characteristics (right columns of Table 1). The shift in distribution of the mean central subfield thickness of men compared with women is illustrated in Figure 2. The difference in thickness by gender was not seen or was less pronounced in the inner subfields,outer subfields, and total retinal volume (Table 2). After adjusting for gender, no additional factors were found to be significantly associated with the degree of retinal thickness in the central subfield (P>0.10, Table 1).
Table 1.
Stratus OCT™ Retinal Thickness in the Central Subfield According to Clinical Characteristics in Diabetic Subjects with Minimal or No Retinopathy
| Overall | By Gender |
|||||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | |||||||
| N | Mean ± SD | P-value§ Unadjusted | P-value§ Adjusted for Gender | N | Mean ± SD | N | Mean ± SD | |
| Overall | 97 | 201 ± 22 | ||||||
| Gender | <0.001 | --- | ||||||
| Women | 48 | 194 ± 23 | ||||||
| Men | 49 | 209 ± 18 | ||||||
| Retinopathy Severity | 0.71 | 0.70 | ||||||
| Level 10 (No retinopathy) | 67 | 201 ± 21 | 38 | 196 ± 22 | 29 | 207 ± 20 | ||
| Level 20 (Very mild NPDR) | 30 | 203 ± 23 | 10 | 185 ± 27 | 20 | 211 ± 15 | ||
| Age | 0.63‡ | 0.98‡ | ||||||
| < 55 years | 49 | 202 ± 22 | 26 | 194 ± 23 | 23 | 210 ± 18 | ||
| >= 55 years | 48 | 201 ± 22 | 22 | 194 ± 23 | 26 | 207 ± 18 | ||
| Combination Diabetes Type/Duration | 0.15 | 0.11 | ||||||
| Type 1 - < 10 years | 10 | 212 ± 12 | 6 | 214 ± 11 | 4 | 210 ± 16 | ||
| Type 1 - >= 10 years | 14 | 203 ± 24 | 6 | 190 ± 20 | 8 | 212 ± 22 | ||
| Type 2 - < 10 years | 46 | 197 ± 23 | 24 | 189 ± 24 | 22 | 205 ± 19 | ||
| Type 2 - >= 10 years | 27 | 205 ± 20 | 12 | 195 ± 23 | 15 | 212 ± 14 | ||
| Duration of Diabetes | 0.14 | 0.18 | ||||||
| < 10 years | 56 | 199 ± 22 | 30 | 194 ± 24 | 26 | 205 ± 18 | ||
| >= 10 years | 41 | 204 ± 21 | 18 | 193 ± 21 | 23 | 212 ± 17 | ||
| Diabetes Type | 0.17 | 0.14 | ||||||
| Type 1 | 24 | 207 ± 20 | 12 | 202 ± 20 | 12 | 211 ± 20 | ||
| Type 2 | 73 | 200 ± 22 | 36 | 191 ± 24 | 37 | 208 ± 17 | ||
| HbA1c* | 0.37‡ | 0.23‡ | ||||||
| < 8% | 34 | 210 ± 18 | 13 | 200 ± 16 | 21 | 216 ± 17 | ||
| >= 8% | 13 | 215 ± 15 | 6 | 212 ± 14 | 7 | 218 ± 16 | ||
| Fellow Eye Retinopathy Severity † | 0.27 | 0.33 | ||||||
| Level 10 (No retinopathy) | 67 | 200 ± 22 | 34 | 193 ± 23 | 33 | 206 ± 19 | ||
| Level 20 (Very mild NPDR) | 12 | 205 ± 27 | 5 | 185 ± 29 | 7 | 219 ± 14 | ||
| Worse than level 20 | 9 | 208 ± 23 | 4 | 199 ± 30 | 5 | 214 ± 15 | ||
| Best Corrected Visual Acuity (letter score) | 0.21‡ | 0.24‡ | ||||||
| 20/12 – 20/20 (93-85) | 46 | 203 ± 20 | 20 | 197 ± 22 | 26 | 208 ± 17 | ||
| 20/20 – 20/25 (84-80) | 29 | 203 ± 22 | 18 | 198 ± 20 | 11 | 210 ± 23 | ||
| 20/25 – 20/32 (79-74) | 22 | 196 ± 26 | 10 | 179 ± 27 | 12 | 210 ± 15 | ||
| Refractive Error (Spherical Equivalent) | 0.39‡ | 0.26‡ | ||||||
| ≥ 0.00 D | 51 | 201 ± 21 | 25 | 196 ± 21 | 26 | 205 ± 19 | ||
| < 0.00 D to −3.00 D | 35 | 201 ± 24 | 18 | 189 ± 25 | 17 | 213 ± 15 | ||
| < −3.00 D | 11 | 207 ± 21 | 5 | 204 ± 24 | 6 | 210 ± 19 | ||
1 subject completed the fundus photography 11 weeks after completion of OCT
Not done for 50 subjects
Not done for 8 subjects and nongradable for 1 subject
Continuous measure of characteristic used in calculation of p-value
P-value from least squares regression models
Figure 2.
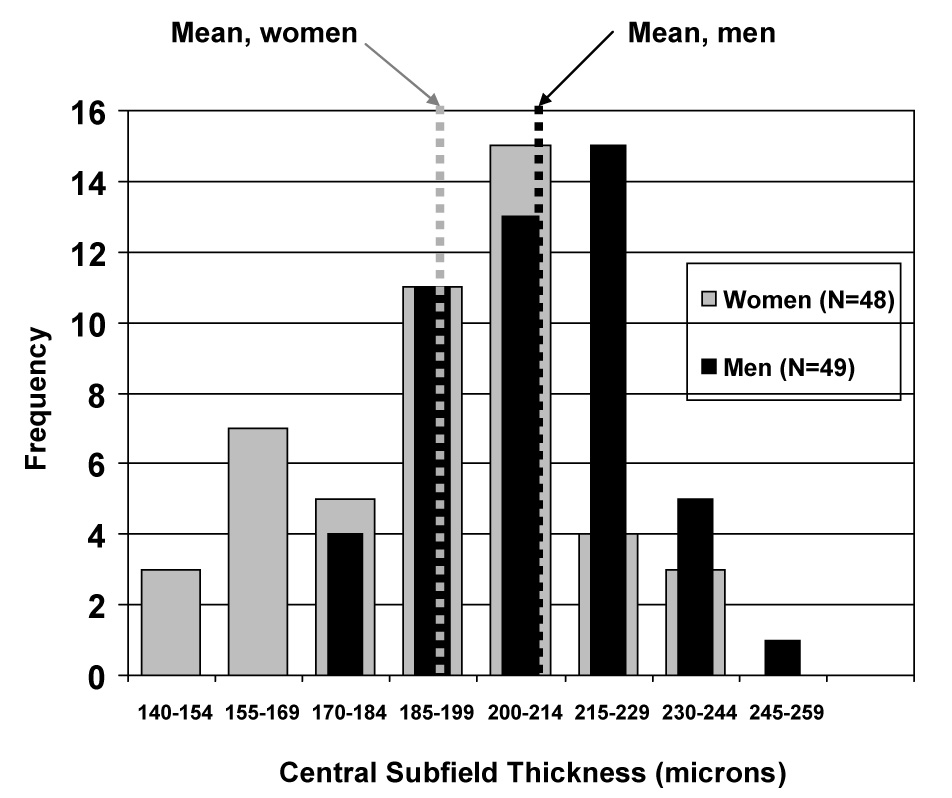
Shift in distribution of the mean central subfield thickness by gender in diabetic subjects with minimal or no retinopathy
Table 2.
Retinal Thickness Measurements using Stratus OCT™ According to Gender in Diabetic Subjects with Minimal or No Retinopathy
| Women N = 48 Mean ± SD | Men N = 49 Mean ± SD | P-value* | |
|---|---|---|---|
| Center Point | 159 ± 22 | 174 ± 21 | <0.001 |
| Central Subfield | 194 ± 23 | 209 ± 18 | <0.001 |
| Inner Zone | |||
| Superior | 268 ± 19 | 270 ± 18 | 0.70 |
| Nasal | 267 ± 20 | 274 ± 16 | 0.10 |
| Inferior | 264 ± 19 | 270 ± 17 | 0.12 |
| Temporal | 255 ± 18 | 260 ± 17 | 0.13 |
| Outer Zone | |||
| Superior | 234 ± 15 | 229 ± 16 | 0.12 |
| Nasal | 252 ± 17 | 250 ± 19 | 0.67 |
| Inferior | 226 ± 15 | 226 ± 17 | 0.86 |
| Temporal | 216 ± 14 | 218 ± 16 | 0.53 |
| Total Retinal Volume | 6.75 ± 0.40 | 6.76 ± 0.42 | 0.91 |
1 subject completed the fundus photography 11 weeks after completion of OCT. 11–12 missing for each of the 8 non-center subfields (due to nongradable).
P-value from t-tests
Discussion
A comparison of relevant databases in the literature describing center point thickness or central subfield thickness in subjects without diabetes or in subjects with diabetes but no retinopathy, summarized in Table 3, shows that Stratus OCT™-measured thickness of the retina in diabetic subjects without retinopathy or with very mild retinopathy in the DRCR Network is similar to Stratus OCT™ data in healthy subjects in the literature6, 8 (Fraser-Bell S, et al. IOVS 2005; ARVO E-Abstract 1542) with respect to the central subfield or center point. These results are consistent with two studies using earlier OCT models.2, 3 This, however, is in contrast to two other studies which did not use an Stratus OCT™ machine which found increased retinal thickness in subjects with diabetes but no retinopathy. Lattanzio’s10 methods differ from the DRCR.net results not only in that an Stratus OCT™ machine was not used but also because the number of women in the group with diabetes but no retinopathy is unknown. It is possible that a greater proportion of men in that study could have resulted in thicker measurements. Using a retinal thickness analyzer, not an OCT, Fritsche’s data11 also suggested that the central subfield was thicker in subjects with diabetes but no retinopathy compared with subjects without diabetes, but was based on only 9 and 10 eyes, respectively.
Table 3.
Relevant Databases in Literature: Mean + SD Retinal Thickness
| Result By Gender (CP=Center Point; CSF=Central Subfield) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference | Machine | Population | Gender, Mean or Median Age | N | Center Point | Central Subfield | Men | Women | P-Value if available |
| Hee2 | OCT (not Stratus OCT™) | Healthy | 45% Men, Age 38 yrs | 73 Eyes (41 Subj) | 152 + 21 | 174 + 18 | CSF = 181 | CSF = 169 | P =.04 (CSF) |
| Diabetic, no DR | 75% Men, Age 55 yrs | 55 Eyes (31 Subj) | 158 + 20 | 179 + 17 | Not Available | ||||
| Lattanzio10 | OCT* | Healthy | 64% Men, Age 52 yrs | 50 Eyes (50 Subj) | 162 + 13 | Not Available | CP Not Correlated | ||
| Diabetic, no DR | Not Available | 46 Eyes (Subj # unk) | 211 + 38 | Not Available | Not Available | ||||
| Fritsche11 | RTA† | Healthy | 50% Men, Age 30 yrs | 10 Eyes (10 Subj) | Not Available | 152 + 15 | CSF = 154 | CSF = 151 | |
| Diabetic, no DR | 56% Men, Age 54 yrs | 9 Eyes (9 Subj) | Not Available | 181 + 26 | CSF = 189 | CSF = 172 | |||
| Massin3 | OCT (not Stratus OCT™) | Healthy | 40% Men, Age 46 yrs | 60 Eyes (30 Subj) | 146 + 20 | 170 + 18 | CSF = 178 CP = 153 | CSF = 165 CP = 140 | P=.0139 (CSF) |
| Diabetic, no DR | Not Available | 30 Eyes (15 Subj) | 144 + 14 | 174 + 19 | Not Available | ||||
| Wong7 | OCT2 | Healthy | 51% Men, Age 41 yrs | 117 Eyes (117 Subj) | Not Available | Not Available | CSF = 203 CP=174 | CSF = 189 CP=168 | P = .001 (CSF) |
| Paunescu8 | Stratus OCT™ | Healthy | 60% Men, Age 31 yrs | 10 Eyes (10 Subj) | Std=178+29‡ High=164+21‡ | Std=206+21‡ High=204+20‡ | Not Available | ||
| Browning6 | Stratus OCT™ | Healthy§ | Age 64 yrs | 52 Eyes (48 Subj) | Not Available | 197 + 31 | Not Available | ||
| Chan9 | Stratus OCT™ | Healthy | 30% Men, Age 43 yrs | 37 Eyes (37 Subj) | Auto=182+23¶ Manual=170+18¶ | 212 + 20 | CSF = 204 | CSF = 207 | |
| Fraser-Bell** | Stratus OCT™ | Healthy | 48% Men, Age 48 yrs | 245 Eyes (245 Subj) | 172 | 203 | CSF = 205 | CSF = 196 | P <.001 (CSF) |
| DRCR.net | Stratus OCT™ | Diabetic, no DR | 51% Men, Age 55 yrs | 97 Eyes (97 Subj) | 166 + 23 | 201 + 22 | CSF = 209 | CSF = 194 | P<.001 |
Not OCT 3. Center point obtained from average of three line scans through presumed foveal center.
Retinal Thickness Analyzer, not an OCT, evaluation of mean foveal thickness.
Results using 6 mm scan (fast macular scans) and 3.5 mm scan (high density).
Article provided data on subjects with diabetes but not specifically subjects with diabetes and no retinopathy.
Automatically generated values and values determined by manually locating the minimum value along each radial scan using the raw data and averaging over those values.
Fraser-Bell S, et al. IOVS 2005; ARVO E-Abstract 1542
As expected anatomically, results from the DRCR Network showed thicker nasal subfields compared with temporal subfields. In contrast, the database from Fraser-Bell et al (Fraser-Bell S, et al. IOVS 2005; ARVO E-Abstract 1542) reported similar thicknesses for the nasal and temporal subfields, both for the inner zone and outer zone (Table 4).
Table 4.
Retinal Thickness Measurements using Stratus OCT™ in Current Study (Diabetic Subjects with Minimal or No Retinopathy) and from Healthy Eyes in Previous Studies
| Eyes from Diabetic Subjects with Minimal or No Retinopathy |
Eyes from Healthy Subjects |
|
|---|---|---|
| Present Study* (N = 97, 51% Men, Mean Age 55 Yrs) |
Stratus OCT™ Brochure (N = 245, 48% Men, Mean Age 48 Yrs) |
|
| Macular Region | Mean (5th and 95th Percentiles) | Mean (5th and 95th Percentiles) |
| Center Point | 166 (128–211) | 172 (135–215) |
| Central Subfield | 201(161–234) | 203 (168–239) |
| Inner Zone | ||
| Superior | 269 (236–301) | 243–296 |
| Nasal | 271 (236–306) | 240–297 |
| Inferior | 267 (233–297) | 246–297 |
| Temporal | 257 (226–285) | 240–294 |
| Outer Zone | ||
| Superior | 232 (208–258) | 207–256 |
| Nasal | 251 (224–281) | 198–274 |
| Inferior | 226 (201–249) | 207–256 |
| Temporal | 217 (190–240) | 199–276 |
1 subject completed the fundus photography 11 weeks after completion of OCT. 11–12 missing for each of the 8 non-center subfields (due to nongradable).
Means not available for all subfields
There were insufficient numbers of subjects in our study who were not Caucasian to permit evaluation of the data by race. However, in an exploration of the potential effect of other factors on OCT-measured central subfield thickness (Table 1), including presence of very mild retinopathy compared with no retinopathy, duration of diabetes, type of diabetes, age and refractive error, only gender was found to have an effect, with mean central subfield thicknesses greater in men than women. These results were similar to Massin,3 Hee2 and Wong 7 who used an OCT that was not an Stratus OCT™, and reported that average central subfield thicknesses were greater in men than women without diabetes, but did not describe whether there were gender differences for subjects with diabetes and no retinopathy. This was confirmed in the normative database presented by Fraser-Bell et al who found a difference in mean central subfield thickness of 9 microns, compared to 15 microns in the current study. (Fraser-Bell S, et al. IOVS 2005; ARVO E-Abstract 1542). It is unknown why this gender difference was not detected in the 37 eyes from subjects without diabetes reported by Chan and colleagues in which the mean central subfield values were similar in men and women.9 Since only about one-third of the subjects in the report by Chan and colleagues were men, one might have expected a thinner mean central subfield thickness compared with other reports.
The consistency of data across studies appears large enough to consider separating norms by gender when designing clinical trials evaluating diabetic macular edema based on OCT and determining upper limits of normal for eligibility and outcomes. For example, consider a study of diabetic macular edema with an eligibility requirement of a central subfield thickness of at least 250 microns. While this value would be more than 2 standard deviations beyond the norm for both men and women, it would be a greater amount of edema compared with norms for women than for men. Such a cutoff would mean that some women with true edema more likely would be excluded from entry than men. Further, if one chooses treatment criteria based on a central subfield thickness of 250 microns or less as a level to withhold additional treatment, one would be more likely to withhold treatment in an eye that might still have true edema in a woman than a man. This 15 micron difference between the means of the two populations (Figure 2) should not be equated with a 15 micron difference in an individual patient between one visit or measurement and a subsequent visit or measurement. A 15 micron difference between measurements in an individual might be considered within the reproducibility of the measurement,15 whereas this difference between the means of two populations represents groups with quite different distributions of thicknesses from which the means are derived (Figure 2).
The Network study has a few potential limitations that should be considered. First, myopic refractive errors that were associated with retinal pathology were to be excluded from this study; reports have indicated that the eyes of such individuals may have altered macular thickness (Fraser-Bell S, et al. IOVS 2005; ARVO E-Abstract 1542). Second, there were few blacks or Latinos enrolled to be able to generalize the results to these groups. Third, extreme outliers were excluded from this study and subjects in this study were culled from practices in which some patients likely were referred to an ophthalmologist specializing in retinal diseases, and thus, this cohort may not be a representative sample of the population of all patients with diabetes and no or very mild retinopathy.
In conclusion, this study showed that Stratus OCT™-measured thickness of the retina in diabetic subjects without retinopathy or with very mild retinopathy was comparable to previously published central subfield Stratus OCT™ data from individuals without diabetes, which is consistent with other reports using earlier OCT models comparing similar populations. Our data suggest that the nasal inner and outer zones are thicker than the temporal inner and outer zones. This is consistent with some published reports6, 9 but in contrast to the normative data for the Stratus OCT™ software. (Fraser-Bell S, et al. IOVS 2005; ARVO E-Abstract 1542) Our study also showed that the average central subfield, but not the inner or outer zones, was thicker in men compared with women, again consistent with previously published reports in individuals without diabetes. The gender differences appear large enough to consider separating norms by gender when designing clinical trials evaluating diabetic macular edema based on OCT and determining upper limits of normal for eligibility and outcomes.
Acknowledgements
A. Funding/Support: Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases EY14231, EY14269, EY14229.
B. Financial Disclosures: For a listing of financial disclosures for all authors as of date of submission go to the journal website (AJO.com).
C. Contributions of Authors: Design of the study (RB, NB, DB, AC, ME, AG, TS); All authors contributed to collection, management, analysis, or interpretation of the data; All authors contributed to preparation, review, or approval of the manuscript.
D. Conformity with Author Information: The protocol and HIPAA-compliant informed consent forms were approved by multiple institutional review boards.
Other Acknowledgements: Steering Committee: Lloyd P. Aiello, Neil M. Bressler, Alexander J. Brucker, Steve Carlton, Emily Y. Chew, Ronald P. Danis, Frederick Ferris, Don S. Fong, Adam R. Glassman, Jeffrey G. Gross, Julia A. Haller, Helen K. Li, Päivi Miskala, Angela K. Price (2005)
The Diabetic Retinopathy Clinical Research Network Clinical Sites that Participated in this Protocol: Sites are listed in order by number of subjects enrolled into the study. The number of subjects enrolled is noted in parenthesis preceded by the site location and the site name. Personnel are listed as (I) for Investigator, (C) for Coordinator, (V) for Visual Acuity Tester, and (P) for Photographer.
Charlotte, NC Charlotte Eye, Ear, Nose and Throat Assoc., PA (39): David Browning (I); Andrew N. Antoszyk (I); Melissa K. Cowen (C,V); Alison H. Stallings (C); Jennifer V. Helms (C,V); Angela K. Price (C,V); Heather L. Murphy (V); Linda M Davis (P); Michael D. McOwen (P); Donna McClain (P); Loraine M. Clark (P); Boston, MA Joslin Diabetes Center (25): George S. Sharuk (I); Deborah K. Schlossman (I); Jennifer K. Sun (I); Timothy J. Murtha (I); Sabera T. Shah (I); Paul G. Arrigg (I); Margaret E Stockman (C,P,V); Ann Kopple (C); Richard M. Calderon (P); Leila Bestourous (V); Ellen L. Casazza (P); Robert W. Cavicchi (P); Baltimore, MD Elman Retina Group, P.A. (22): Michael J. Elman (I); Michelle D. Sloan (C); JoAnn Starr (C,V); Dena Salfer-Firestone (V); Giorya Shabi (P); Terri Cain (P); Peter Sotirakos (P); Lakeland, FL Central Florida Retina Institute (8): Scott M. Friedman (I); Kelly A. Blackmer (C); Steve D. Carlton (C,P); Virginia Gregory (C,P,V); Jolleen S. Key (P,V); Columbia, SC Carolina Retina Center (1): Jeffrey G. Gross (I); Peggy W. Cummings (C); Regina A. Gabriel (V); Randall L. Price (P); Denver, CO Denver Health Medical Center (1): Antonio P. Ciardella (I); Melissa A. Stillberger (C); Janelle Dane Zapata (V); Debbie M. Brown (P); Houston, TX Retina and Vitreous of Texas (1): H. Michael Lambert (I); Arthur W. Willis (I); Susan Busch (C,V); Joseph A. Morales (P)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Institutional affiliations can be found in the acknowledgements, under the listing of clinical sites that participated in this protocol and under the committee listings posted on the online journal website (AJO.com).
References
- 1.Chan A, Duker JS. A standardized method for reporting changes in macular thickening using optical coherence tomography. Arch Ophthalmol. 2005;123:939–943. doi: 10.1001/archopht.123.7.939. [DOI] [PubMed] [Google Scholar]
- 2.Hee M, Puliafito C, Duker J, et al. Topography of diabetic macualr edema with optical coherence tomography. Ophthalmology. 1998;105:360–370. doi: 10.1016/s0161-6420(98)93601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massin P, Erginay A, Haouchine B, Mehidi AB, Paques M, Gaudric A. Retinal thickness in healthy and diabetic subjects measured using optical coherence tomography mapping software. Eur J Ophthalmol. 2002;12:102–128. doi: 10.1177/112067210201200205. [DOI] [PubMed] [Google Scholar]
- 4.Goebel W, Kretzchmar-Gross T. Retinal thickness in diabetic retinopathy. A study using optical coherence tomography (OCT) Retina. 2002;22:759–767. doi: 10.1097/00006982-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Laursen ML, Moeller F, Sander B, Sjoelie AK. Subthreshold micropulse diode laser treatment in diabetic macular edema. Br J Ophthalmol. 2004;88:1173–1179. doi: 10.1136/bjo.2003.040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browning DJ, Fraser CM. Regional patterns of sight-threatening diabetic macular edema. Am J Ophthalmol. 2005;140:117–124. doi: 10.1016/j.ajo.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Wong A, Chan C, Hui S. Relationship of gender, body mass index, and axial length with central retinal thickness using optical coherence tomography. Eye. 2005;19:292–297. doi: 10.1038/sj.eye.6701466. [DOI] [PubMed] [Google Scholar]
- 8.Paunescu LA, Schuman JS, Price LL, et al. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using StratusOCT. Invest Ophthalmol Vis Sci. 2004;45:1716–1724. doi: 10.1167/iovs.03-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan A, Duker JS, Ko TH, Fujimoto JG, Schuman JS. Normal macular thickness measurements in healthy eyes using Stratus optical coherence tomography. Arch Ophthalmol. 2006;124:193–198. doi: 10.1001/archopht.124.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lattanzio R, Branacto R, Pierro L, et al. Macular thickness measured by optical coherence tomography (OCT) in diabetic patients. Eur J Ophthalmol. 2002;12:482–487. doi: 10.1177/112067210201200606. [DOI] [PubMed] [Google Scholar]
- 11.Fritsche P, Van Der Heijde R, Suttorp-Shulten M, Polak B. Retinal thickness analysis(RTA): an objective method to assess and quantify the retinal thickness in healthy controls and in diabetics without diabetic retinopathy. Retina. 2002;22:768–771. doi: 10.1097/00006982-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98:823–833. [PubMed] [Google Scholar]
- 13.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135:194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 14.Diabetic Retinopathy Clinical Research Network. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114:525–536. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diabetic Retinopathy Clinical Research Network. Reproducibility of macular thickness and volume using Zeiss optical coherence tomography in patients with diabetic macular edema. Ophthalmology. 2007;114:1520–1525. doi: 10.1016/j.ophtha.2006.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]


