Abstract
Androgens act on the CNS to affect motor function through interaction with a widespread distribution of intracellular androgen receptors (AR). This review highlights our work on androgens and process outgrowth in motoneurons, both in vitro and in vivo. The actions of androgens on motoneurons involve the generation of novel neuronal interactions that are mediated by the induction of androgen-dependent neurite or axonal outgrowth. Here, we summarize the experimental evidence for the androgenic regulation of the extension and regeneration of motoneuron neurites in vitro using cultured immortalized motoneurons, and axons in vivo using the hamster facial nerve crush paradigm. We place particular emphasis on the relevance of these effects to SBMA and peripheral nerve injuries.
Keywords: androgens, motoneurons, peripheral nerve injury, spinal cord injury, motoneuron disease, gonadal steroids
1. Introduction
One of the major targets of the androgenic steroids is the nervous system, where they play a part in regulating sexual, reproductive, and aggressive behaviors (Blaustein and Erskine, 2002; Hull et al., 2002; Simon, 2002). Androgens exert long-term organizational effects during developmental periods, and also exert transient activational effects, including during regeneration events. Many of the organization and activational effects of androgens in the nervous system involve the establishment of appropriate neuronal connectivity through regulated dendritic and axonal outgrowth.
Androgenic steroids activate an intracellular receptor known as the androgen receptor (AR). Although recent evidence suggests that AR may have nongenomic effects in the nervous system, and that androgens may act independently of the AR (Foradori et al., 2007; Losel et al., 2003; Sarkey et al., this issue), the classical view is that most of the actions of androgens are mediated by AR acting at the genomic level as a transcription factor. In fact, many of the nuclei in the nervous system that are implicated in sex differences in steroid-dependent functions exhibit a sexual dimorphism in AR expression (Cooke et al., 1998).
Lower motoneurons in the spinal cord and brainstem are particularly rich with AR (Matsumoto, 1997; Yu and McGinnis, 2001; Tetzlaff et al., 2007a), and the growth of their elaborate dendritic arbors and extremely long axons is regulated by androgens, in both development and regeneration. In this review, we will summarize the experimental evidence for the androgenic regulation of the extension and regeneration of motoneuron neurites in vitro and axons in vivo, with particular emphasis on the relevance to Kennedy's Disease and peripheral nerve injuries.
2. Androgen regulation of neurite extension in vitro
The lower motoneurons of the brainstem and the anterior (ventral) horns of the spinal cord are among the primary targets of androgens in the nervous system (for reviews, see Matsumoto, 1997; and Poletti, 2004), and these motoneurons express very high levels of AR protein (Matsumoto, 1997; Yu and McGinnis, 2001; Tetzlaff et al., 2007a). Clinically, these neurons are lost during the progression of Kennedy Disease (or Spinal and Bulbar Muscular Atrophy, SBMA; Kennedy et al., 1968), an X-linked condition caused by a toxic mutation of the AR gene that leads to an abnormally long polyglutamine tract (see below; La Spada et al., 1991). These neurons are also lost or damaged in several other neurodegenerative diseases (notably, ALS) and by traumatic injuries to the central nervous system or the nerves. Interestingly, the motoneurons located in Onuf's nucleus are spared in ALS, and may also be spared in SBMA. This is of particular interest because the motoneurons in the rodent homologue of Onuf's express androgen receptors and coactivators at greater levels than do other motoneurons (Breedlove and Arnold, 1983c; Jordan et al., 1997; O'Bryant and Jordan, 2005; Ranson et al., 2003). Therefore, understanding the effects of androgens in these cells, with both normal and abnormal AR, is of great importance, and several in vitro and in vivo models have been used in this effort. In this section, we focus on cell culture models.
2.1 Cultured neurons and neuron-like cells
Several groups have utilized neuronal cell culture to analyze the molecular mechanisms that underly androgen-driven neuronal differentiation, to study the effects of androgens on neurite dynamics in both physiological and pathological conditions [injury, motoneuron diseases such as SBMA or amyotrophic lateral sclerosis (ALS), etc.], and to examine the effects of androgens on neurite elongation and branching. For example, Butler and colleagues (2001) have used proliferating human neuroblastoma cells (SH-SY5Y, a line of neuron-like cells) to study androgen effects on tubulin metabolism. They demonstrated that testosterone directly upregulates both α- and β-tubulin, but not the microtubule-associated proteins tau or MAP2b. The increase in tubulin levels involved both the ubiquitous βII-tubulin and neuron-specific βIII-tubulin isoforms. Furthermore, this effect was counteracted by the antiandrogens cyproterone acetate and flutamide, suggesting that it was AR-dependent.
Other neuron-like cell lines, such as GT1 cells, have been used to analyze the effects of androgens on physiological processes such as control of the hypothalamic-pituitary-gonadal axis and regulation of GnRH production and secretion (Belsham et al., 1998; Poletti et al., 1994, 2001). While cell lines such as SH-SY5Y and GT1 cells may display a general neuron-like phenotype, they are not ideal for the in vitro study of the effects of androgens on the survival, differentiation, and neurite dynamics of motoneurons, specifically. For that, we turn to cultured motoneurons and motoneuron-like cells.
2.2 Cultured motoneuron-like cells
One of the first cell culture models based on motoneurons and used for studying androgen effects was developed and described by Brooks and colleagues (1998). They utilized a line of cells derived from the embryonic mouse spinal cord and hybridized with neuroblastoma cells (motoneuron-neuroblastoma hybrid cells, or MN hybrid cells; Salazar-Grueso et al., 1991), and stably transfected it with human AR. These cells express markers typical of anterior horn motoneurons, and are now widely used as an in vitro model to study motoneuron function. Notably, motoneurons in vivo express AR normally (Matsumoto, 1997; Yu and McGinnis, 2001), while MN hybrid cells only express AR when transfected. This important feature allows the cell line to be used to differentiate between AR-dependent and AR-independent androgen effects in motoneurons (for an example, see the description of neuritin expression in these cells in Section 3.4).
Androgen treatment increases in a dose-dependent manner the number of hybrid cells showing a motoneuronal phenotype by promoting their differentiation. The morphological modifications induced by androgens in these cells include the development of larger cell bodies and broader neurites. Additionally, androgens are able to increase the survival of MN hybrid cells under low-serum conditions. The effects of androgens on the survival and morphological differentiation of MN hybrid cells are detectable only in the AR-transfected cells, not in the AR-free control cells, suggesting that the effects must be directly linked to AR activation (Brooks et al., 1998; also see Figure 1). Although Hauser and Toran-Allerand (1989) had already shown that androgens can increase motoneuron survival in organotypic spinal cord explants independent of the target muscles, this first report on isolated motoneuron-like cells demonstrated that motoneurons themselves can be the direct targets of the trophic actions of androgens. Androgen enhanced neurite elongation and cell survival in the absence of afferent input, surrounding central glial cells, the peripheral axon-associated Schwann cells, or target muscle cells [which are also particularly sensitive to androgens and express high levels of AR (Herbst and Bhasin, 2004)]. Indeed, in vitro data from these cultured cells may allow to us to discriminate between cell-autonomous, AR-mediated effects and those mediated by neighboring cells.
Figure 1.
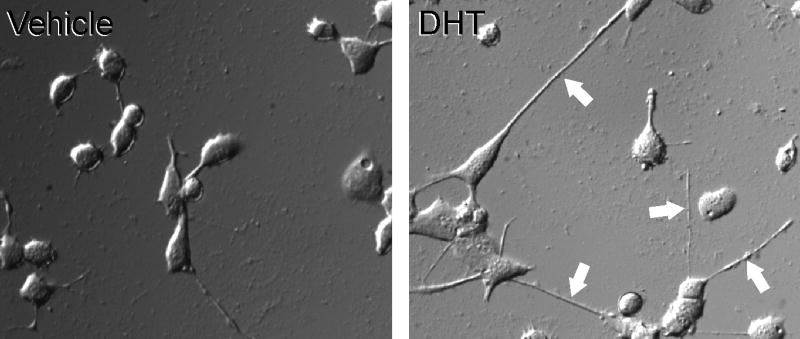
Differential interference contrast photomicrograph of MN hybrid cells transfected with AR and treated for 48 hours with either 100 nM DHT or vehicle control. Note that DHT-treated cells have longer neurites (arrows). (After DeLucia et al., 2007.)
In vivo, afferents, central glial cells, Schwann cells, and target muscle cells may all play important trophic roles in enhancing the survival and differentiation of motoneurons. In some cases, these cells may play a role by producing various neurotrophic factors (or their receptors), which may also be regulated by androgens. For example, skeletal muscle satellite cells produce brain derived neurotrophic factor (BDNF; Mousavi and Jasmin, 2006), and levels of both BDNF and its receptor TrkB are controlled by androgens in at least some motoneurons (Osborne et al., 2007; Ottem et al., 2007). It is thought that BDNF produced by the muscle acts as a retrogradely transported trophic factor to support motoneurons throughout their lifespan. In vivo, BDNF can induce axon outgrowth in injured motoneurons (Kishino et al., 1997), and it interacts with androgen to maintain normal motoneuron dendritic length (Yang et al. 2004). Furthermore, androgens control the levels BDNF protein in motoneuron dendrites and in their glutamatergic afferents, many of which express TrkB (Ottem, et al., 2007), suggesting that androgen-mediated BDNF signaling may support excitatory inputs to motoneurons. Moreover, BDNF regulates the expression of AR in the motoneuron itself (Al-Shamma and Arnold, 1997; Yang and Arnold, 2000), further increasing the difficulty of disentangling cell autonomous and non-cell autonomous androgen effects on motoneurons in vivo. In contrast, the isolated nature of cultured cells allows us to address some of these issues directly.
2.3 NSC34/mAR cells
In order to identify androgen-regulated genes that may mediate neurite outgrowth in motoneurons via interaction with structural proteins, we have produced an in vitro model of highly differentiated motoneuron-like cells. The model is based on the NSC34 line of immortalized mouse motoneurons (Cashman et al., 1992; Durham et al., 1992). NSC34 cells are a hybrid of motoneuron-enriched embryonic mouse spinal cord cells and mouse neuroblastoma cells. NSC34 cells are highly differentiated and display a multipolar motoneuron-like shape with very long neurites. Moreover, NSC34 cells produce, store, and release acetylcholine, and can generate action potentials. NSC34 cells have been fully characterized for their motoneuronal phenotype (Cashman et al., 1992; Durham et al., 1992) and are widely used to study motoneuron development and differentiation.
We have stably transfected NSC34 cells with mouse AR (NSC34/mAR). As with MN cells, the fact that NSC34 cells only express AR when transfected allows us to use these cells to discriminate between AR-dependent and AR-independent androgen effects. In this model, androgens enhance motoneuron differentiation and neurite outgrowth via activation of the AR (Marron et al., 2005). In particular, while untreated cells are characterized by short cell processes, androgen-treated NSC34/mAR cells have a much higher number of cell processes and display a marked motoneuron-like morphology. Androgen-induced neurites are quite long, with a mean length ranging from five to ten times the soma size. Interestingly, these neurites are also immunoreactive for SMI312 (Marron et al., 2005), a cocktail of monoclonal IgG1 antibodies that is specific for phosphorylated medium- and high-molecular weight neurofilaments, selected to provide a marker for axons (Sternberger et al., 1982). Thus, androgen treatment results in the differentiation of axon-like cell processes, which normally do not develop easily in neuron cultures. Moreover, androgens promote the survival and differentiation of NSC34/mAR cells even when the cells express a toxic AR mutant (ARpolyQ; see Section 2.4 below).
Testosterone is converted to androgenic metabolite dihydrotestosterone (DHT) by the enzyme 5α-reductase, which is expressed at very high levels in the anterior horn of the spinal cord (Poletti, 2004; Pozzi et al., 2003), thereby ensuring that motoneurons are exposed to both testosterone and DHT. We have used NSC34/mAR cells to examine the differential effects of these two androgens in promoting motoneuron differentiation (Marron et al., 2005). Cells were treated for 48 hours with either testosterone or DHT, and axon outgrowth was measured by counting the number of neurites positive for SMI312 (considered a specific marker for axons; Sternberger et al., 1982; see above). Both testosterone and DHT increased the number of axon-like processes in NSC34/mAR cells, but DHT treatment led to significantly more axon outgrowth than did testosterone. Critically, the effects of testosterone were completely counteracted by the addition of finasteride, a selective inhibitor of 5α-reductase. Thus, androgen-dependent axon outgrowth in motoneurons may be driven primarily by DHT, rather than testosterone.
The androgen-dependent differentiation of NSC34/mAR cells into a motoneuron-like phenotype depends at least in part on AR-mediated changes in gene transcription. We have used differential display PCR (DD-PCR), confirmed with RT-PCR, to identify several genes that are either activated or repressed by androgen treatment (Marron et al., 2005). One of the primary genes upregulated by androgen is neuritin, also known as cpg15 (for candidate plasticity-related gene 15; Naeve et al., 1997; Nedivi et al., 1993). Neuritin is a small, highly conserved protein linked to the extracellular membrane through a glycosylphosphatidylinositol (GPI) anchor, and is known to be associated with neurite elongation in several areas of the nervous system.
Neuritin expression peaks during neuronal development (Cantallops et al., 2000; Corriveau et al., 1999; Nedivi et al., 1996), and is particularly elevated in embryonic proliferative zones (Putz et al., 2005). Moreover, recombinant neuritin promotes neurite outgrowth and arborization in primary cultures of embryonic hippocampal and cortical cells (Naeve et al., 1997). Neuritin expression is also associated with dramatic growth of dendritic arbors (Nedivi et al., 1998), coordinated development of apposing dendritic and axonal arbors, and synaptic maturation (Putz et al., 2005). Neuritin expression is highly upregulated by neuronal activity and the activity-induced neurotrophins BDNF and NT-3 (Naeve et al., 1997), and in cortical neurons during post-stroke reorganization (Han et al., 2007). Furthermore, neuritin is a key player in the NGF-directed neuronal differentiation of PC12 cells, where its upregulation is a critical step in neurite outgrowth and the differentiation of axon-like processes (Cappelletti et al., 2007). It has therefore been hypothesized that neuritin acts as a downstream effector in promoting neurite outgrowth (Cantallops et al., 2000; Lee and Nedivi, 2002; Nedivi et al., 1998).
Interestingly, neuritin is highly expressed in developing motoneurons, where it enhances the development of axonal arbors by promoting branching and neuromuscular synaptogenesis (Javaherian and Cline, 2005). Neuritin expression is also affected by motoneuron injury. For example neuritin is transiently downregulated in the injured spinal cord (Di Giovanni et al., 2005), and can be upregulated in the brainstem following peripheral motor axon injury (see Section 3.4). Moreover, an in silico analysis performed using MatInspector (www.genomatix.de) identified seven putative androgen response elements (AREs) in the promoter region of the neuritin gene, suggesting that neuritin expression may be under the direct control of steroid-bound AR. We have used NSC34/mAR cells to study the interaction between androgens and neuritin in promoting neurite outgrowth in motoneurons (Marron et al., 2005). NSC34/mAR cells express neuritin at basal levels even in the absence of androgens. However, the addition of DHT to the culture medium increases the expression of neuritin up to two-fold in a dose-dependent manner, and this effect can be completely blocked by the AR antagonist bicalutamide (Casodex). This suggests the hypothesis that androgens may enhance neurite extension in these cells by upregulating neuritin expression. We tested this hypothesis using RNA interference. Silencing neuritin mRNA expression with a short hairpin RNA (shRNA) completely abolished the effect of androgens on neurite outgrowth. Furthermore, neuritin overexpression enhanced neurite outgrowth, even in the absence of androgens. Taken together, these data provide strong evidence for the role of neuritin as a downstream effector molecule mediating the trophic effects of androgens on neurite outgrowth in motoneurons (Marron et al., 2005). We have also used MN hybrid cells to confirm that androgens regulate neuritin expression in cultured motoneurons (see section 3.4).
2.4 Models of SBMA
The effects of androgens on neurite outgrowth and neuritin expression may be particularly relevant in the pathogenesis of SBMA. Typical features of SBMA are the death of motoneurons in the spinal cord and brainstem, and associated muscular atrophy (Kennedy et al., 1968; Stefanis et al., 1968, 1975). The disease affects only men, and the age of onset is usually between 30 and 50 years. Clinical symptoms include muscle cramps and fasciculations, and progressive weakness and wasting of the muscles of the limbs and face. In many patients, a partial loss of androgen sensitivity is present and can lead to gynecomastia, infertility, and testicular atrophy. SBMA is caused by an abnormal elongation of the polyglutamine (polyQ) tract in the N-terminal transactivation domain of the AR, which confers a toxic gain-of-function to the mutant AR (Brooks et al., 1998; Poletti, 2004). However, the elongated polyQ tract also reduces the transcriptional competence of AR (Abdullah et al., 1998; Kazemi-Esfarjani et al., 1995; Mhatre et al., 1993). It has therefore also been proposed that a loss-of-function of the receptor may be involved in SBMA pathogenesis, and mice expressing only the mutant SBMA AR that does not carry out the normal functions of AR display a more rapid neurodegeneration (Thomas et al., 2006).
It is possible that SBMA may interfere with neuritin-mediated androgenic control of neurite outgrowth in motoneurons, thereby preventing them from maintaining their normal patterns of connectivity. In immortalized motoneurons expressing mutant SBMA AR, neurites are short and dystrophic and display abnormal branching patterns (Poletti, 1999); these alterations in morphology apparently correlate with toxicity induced by the mutant AR, raising the possibility that the motoneuron pathogenesis may be initiated in the dendrites and axons (Avila et al., 2003; Pozzi et al., 2003; Szebenyi et al., 2003). In this view, the loss of connectivity with afferents or with the target musculature may be responsible for the eventual death of the motoneuron.
A common feature of polyglutamine diseases, such as Huntington's disease and SBMA, is the formation of intracellular aggregates of the mutant protein. Aggregates found in cellular processes, rather than nuclei or perikarya, are referred to as “neuropil aggregates”. In NSC34/mAR cells expressing mutant SBMA AR, androgen treatment induces the formation of prominent neuropil aggregates in processes that stain positively for axonal markers (Piccioni et al., 2002). It is unclear whether these neuropil aggregates are formed in loco or in the cell body and then transported into the processes (DonCarlos et al., 2003; Pozzi et al., 2003). Neuropil aggregates could alter motoneuron function by blocking the transport of organelles, vesicles, and polyribosomes along the axons and dendrites (Nagai et al., 1999; Poletti, 1999). In fact, in NSC34/mAR cells expressing mutant SBMA AR, neuropil aggregates induced by testosterone impair kinesin-mediated transport and lead to an accumulation of kinesin protein, filamentous actin, and mitochondria in the cell processes (Piccioni et al., 2002; Poletti, 2004). This process leads to altered cellular morphology and may induce axonal strangulation or reduce the bioavailability of components essential for synaptic functions, resulting in neuronal dysfunction (Poletti, 2004).
Thus, the SBMA mutation may lead to a two-fold problem for motoneurons. First, the gain-of-function would lead to aggregates in the cellular processes, which alter trafficking and connectivity. In this view, it is possible that the selective degeneration of motoneurons in SBMA is related to the fact that motoneurons have incredibly long axons and dendrites, making them particularly susceptible to interruptions in axonal or dendritic trafficking (Ellerby et al., 1999). Second, the loss-of-function conferred by the SBMA AR mutation would interfere with the normal role of androgens in upregulating neuritin, which might otherwise ameliorate the effects of the neuropil aggregates and help to maintain or recover normal axonal and dendritic functionality.
3. Androgen regulation of axonal regeneration in vivo
In mammals, axons in the injured central nervous system generally do not regenerate well. In contrast, axons in the peripheral nervous system are known for their remarkable regenerative capacity. As long as the connective tissues of the nerve sheath are left relatively intact, most peripheral axons readily regrow and reinnervate their original targets with a high degree of specificity. This is true of both cranial and spinal motoneurons. However, the regenerative capacity of peripheral motoneuron axons is not limitless. Both severity of the nerve injury and proximity of the injury to the cell body can lead to relatively poor nerve regeneration (Sunderland, 1981). It is therefore important to uncover the factors that determine the regenerative ability of injured neurons, and begin to understand how to enhance these abilities. In fact, a significant amount of research has been done on these topics (Makwana and Raivich, 2005; Moran and Graeber, 2004). This portion of the review will focus on one of the most promising areas of this research, androgen enhancement of nerve regeneration, with particular attention paid to the hamster facial nerve injury model.
3.1 The facial nerve injury paradigm
One of the most useful in vivo models of androgen regulation of axonal regeneration is the rodent facial nerve axotomy model (Jones et al., 2001; Moran and Graeber, 2004). Facial nerve injury at its exit from the skull through the stylomastoid foramen leaves the blood-brain barrier intact and does not directly damage the cell bodies within the brainstem. Furthermore, the uninjured side can be used as an internal control for each animal in the experiment. The facial nerve (cranial nerve VII) innervates most of the muscles of the face and ears. The cell bodies that give rise to the axons in the facial nerve are located in the facial motor nucleus, in the ventral portion of the brainstem just caudal to the pontine band and rostral to the trapezoid body. The facial nerve exits the skull at the stylomastoid foramen before branching into several trunks. In most rodents, the nerve is easily accessible at this point. In addition to being surrounded by endoneurium, the axons here are afforded protection against traction deformation by layers of perineurium and protection against compression injury by a sheath of epineurium (Sunderland, 1981).
Facial nerve damage can vary in severity. The typical injury in an experimental study of nerve regeneration consists of a crush axotomy. Briefly, the nerve is exposed at its exit from the stylomastoid foramen, and is subjected to two 30-second crushes with fine forceps. In rodents, complete recovery of gross motor movements is nearly always observed within three weeks. In adult hamsters specifically, almost all facial motoneurons survive facial nerve crush (Lavelle and Lavelle, 1984); this fact makes hamster facial nerve crush a particularly informative model for axon regeneration studies because it is not confounded with issues related to cell death or survival.
3.2 Androgen-enhanced regeneration of the hamster facial nerve
The first indication that androgens might regulate axonal regeneration in the facial nerve was in a series of experiments published in 1989 (Kujawa et al., 1989). In this paper, the authors reported experiments in which adult male Syrian hamsters were subjected to unilateral facial nerve crush, then treated with various regimens of an ester of testosterone—testosterone propionate (TP)—and evaluated for the length of time until recovery of facial motor function. In the first experiment, TP was injected subcutaneously every other day in a sesame oil vehicle (5 mg/ml, 0.1 ml per injection) following the crush injury. In the second experiment, TP was delivered daily and at a higher dose. In the third experiment, TP was delivered by either subcutaneous injection or Silastic implants (see Smith et al., 1977, for technical details on Silastic implants). Additionally, in order to determine the effects of endogenous testosterone, all animals were castrated in the third experiment. The results demonstrated that testosterone exerts a dramatic effect on the speed of functional recovery, with every testosterone-treated group experiencing an acceleration of recovery. Furthermore, the experiments revealed a dose-response relationship, with higher doses and more frequent treatments producing better results; a continuous dose achieved by Silastic implants yielded the best results. Finally, the castrated animals were no worse off than the gonadally intact, untreated animals, suggesting that normal endogenous levels of testosterone have little to no effect on functional recovery rates.
The initial finding that testosterone accelerates functional recovery following facial nerve injury led to the hypothesis that testosterone might exert its beneficial effect by increasing the temporal rate of regeneration of the injured axons. To test this hypothesis, Kujawa and colleagues (1991) injected tritiated amino acids into the facial motor nuclei of male and female hamsters with facial nerve crush injuries. Because these radiolabeled amino acids were incorporated into protein and transported to the growing tips of the axons by fast axonal transport, it was then possible to measure the distance traveled by the fastest growing axons within the facial nerve. Testosterone administration led to a 26-30% increase in the rate of regeneration in males (Figure 3); this increase was apparent regardless of whether or not the animals had been previously castrated, indicating that endogenous levels of testosterone in males had little effect on this process. Interestingly, baseline axonal regeneration rates were higher in females than in males. Testosterone also increased axonal regeneration rates in females, but to a lesser degree than in males. There is a corresponding sex difference in speed of functional recovery following facial nerve crush, with females recovering before males (Jones, 1993); and testosterone treatment speeds functional recovery in males, but has no significant effect on speed of recovery in females (Jones, 1993).
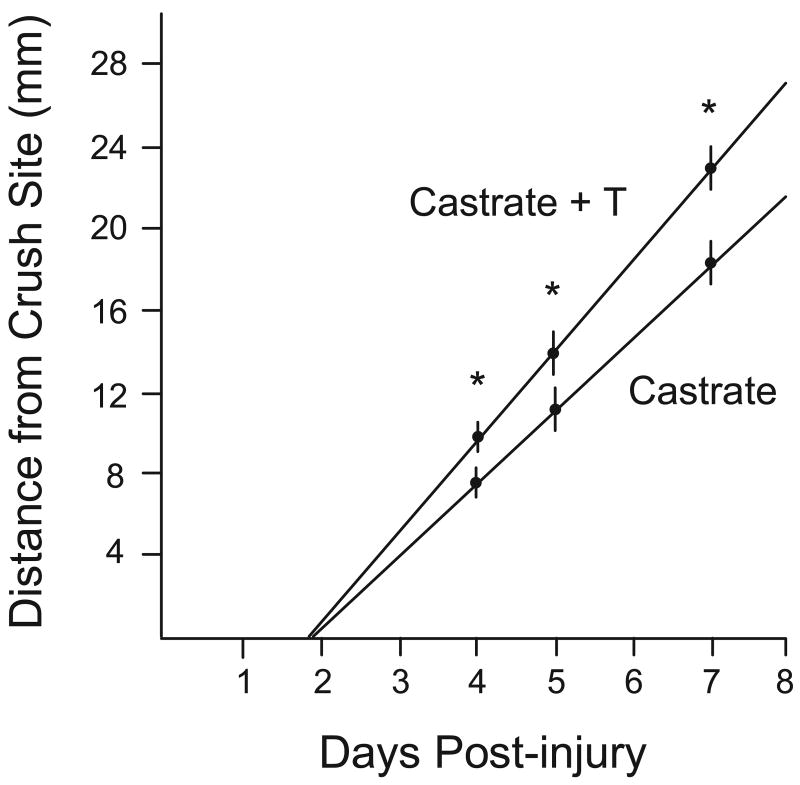
Figure 3.
Outgrowth distances from the point of nerve crush to the leading edge of the growing axons at 4 days, 5 days, and 7 days following injury in castrated male hamsters and castrated male hamsters given systemic testosterone treatment. Circles represent means and vertical lines represent SEMs. * = p < .05. Diagonal lines represent extrapolation of the data. Note that the data extrapolate to approximately 2 days post-injury for the zero outgrowth distance. This time point corresponds both to the androgen-induced spike in neuritin mRNA levels (see Section 3.4) and to the earliest time point at which androgen treatment augments tubulin upregulation following axotomy (Jones and Oblinger, 1994; Jones et al., 1999b). (After Kujawa et al., 1991.)
This finding of a sex difference in testosterone-enhanced axonal regeneration suggests that the facial motoneurons of male and female hamsters may be differentially responsive to androgens, perhaps due in part to an inherent sex difference in basal AR levels. This hypothesis is strengthened by the fact that administration of flutamide, a potent AR blocker, completely abolishes the ability of testosterone to enhance facial nerve regeneration rates, indicating that the effect of testosterone is indeed AR dependent (Kujawa et al., 1995). Additionally, DHT, which is a more potent ligand for AR than testosterone, induces a greater accelerative effect than testosterone (Tanzer and Jones, 1997). Drengler and colleagues (1996) provided evidence for a sex difference in AR levels by demonstrating that female hamsters express AR mRNA in their facial motoneuron somata at only about half the levels that male hamsters do. It is unknown whether this difference in message is translated into a difference in protein, but if so, this could account for the reduced effect of testosterone on axonal regeneration rates in females. There is a known sex difference in AR protein in rat facial motoneuron somata, with males having more (Yu and McGinnis, 2001). Interestingly, while testosterone treatment of castrated males increases AR mRNA in facial motoneuron somata, this effect is completely blocked by axotomy (Drengler et al., 1997; Larkowski et al., 2000). Axotomy-induced downregulation of AR has also been found in spinal motoneurons (Lubischer and Arnold, 1995; Yang and Arnold, 2000). These findings suggest that the regulation of AR levels in motoneuron somata depends at least in part on a peripherally-derived factor. More importantly, however, they suggest that the accelerative effects of testosterone on axonal regeneration do not depend on increased transcription of AR message in the regenerating motoneuron.
Androgen enhancement of facial nerve regeneration has important temporal requirements. An early study of the effects of testosterone on the recovery of facial motor function following crush injury suggests that the first week following injury represents a “critical period” (Kujawa and Jones, 1990). If TP treatment is delayed until 6 days after the injury, it has no accelerative effect. However, if TP treatment is given immediately, it is beneficial even if withdrawn after 7 days, well before full behavioral recovery. In fact, the effective temporal window of testosterone is much shorter than this (Tanzer and Jones, 2004). Administration of testosterone for only 6 hours post-injury has the same effectiveness on both functional recovery and axonal regeneration rates as does continuous administration of testosterone throughout the full recovery phase. Moreover, delaying administration of testosterone until 6 hours after injury completely eliminates its effectiveness, even if it is administered continuously from that point forward.
3.3 Other models
While the hamster facial motor nerve injury model has been the most fruitful model of peripheral nerve regeneration, it is not the only model. Androgen treatment also accelerates functional recovery from facial nerve crush in mice (Tetzlaff et al., 2006). Early studies by Yu and colleagues demonstrated a sex difference in the rates of axonal regeneration (males regenerating faster) following axotomy of the hypoglossal nerve in rats (Yu, 1982), and that androgen treatment enhances axonal regeneration rates in both male (Yu, 1982; Yu and Yu, 1983) and female (Yu and Srinivasan, 1981) rats. Facial and hypoglossal motoneurons are both cranial motoneurons, but androgens also have beneficial effects for spinal motoneurons. For example, testosterone has been shown to accelerate functional recovery from hind limb paralysis following sciatic nerve crush in rats (Brown et al., 1999), and to enhance sciatic axon regeneration rates (Kujawa et al, 1993). Interestingly, the sciatic nerve is known to express AR (Magnaghi et al., 1999), apparently in the endoneurium (Jordan et al., 2002), raising the possibility that androgens exert some of their beneficial effects by acting in the periphery.
3.4 Cellular and molecular mediators
To paraphrase the poet John Donne, “no neuron is an island, entire of itself”. Indeed, one of the essential features of motoneurons is their connectivity with, and dependence on, other neurons, glia, and their target muscles. It is therefore important to examine the effects of androgens on glial activity and neuronal connectivity in motor pools following axotomy. In the case of the facial motor nucleus, axotomy is known to result in a process known as synaptic stripping, in which the synaptic input to the injured motoneurons is dramatically reduced, and neuron-neuron contacts are replaced by glia-neuron contacts (Jones et al., 1997b). This is accompanied by an upregulation in the facial motor nucleus of the glial marker GFAP at both the mRNA (Jones et al., 1997c) and protein (Coers et al., 2002) levels. However, androgen treatment attenuates the process of synaptic stripping, preserving central input to the motoneurons (Jones et al., 1997b), as well as attenuating the axotomy-induced upregulation of GFAP (Coers et al., 2002; Jones et al., 1997c; for a more in-depth review of glial mediation of androgen effects in nerve regeneration, see Jones et al., 1999a). Similar effects have also been observed in the rat (see Moran and Graeber, 2004, for a review). Finally, there is some evidence that androgens may work at the muscle in promoting axon regeneration (Yu and Cao, 1991), though this has not been tested in hamster facial motoneurons.
Of course, androgen treatment following axotomy also alters some processes that are thought to be cell-autonomous. In order to mount an effective regeneration program, the injured motoneuron must rapidly increase synthesis of regeneration-associated proteins, driven by increased levels of ribosomal RNA (rRNA). However, the neuron must also deal simultaneously with the stress of the injury itself. We have hypothesized that androgen treatment alleviates initial motoneuron stress, allowing the cell to shunt its metabolic energy toward reparative processes (Jones et al., 2000). One of the primary features of the stress response is an increase in heat shock protein (HSP) levels. HSPs normally complex with, and are therefore sequestered by, unbound AR in the cytoplasm. Treatment with androgen liberates these stored HSPs, obviating the need for the cell to produce more for at least 10 hours following axotomy (Tetzlaff et al., 2007b). This delay in the stress response is accompanied by an enhanced early regeneration response. In untreated hamsters, facial nerve axotomy causes an upregulation of rRNA by 24 hours following injury; however, androgen treatment results in rRNA upregulation as early as 6 hours following injury (Kinderman and Jones, 1993), the same point in time after which delayed androgen treatment is no longer effective in accelerating axonal regeneration. Thus, it is likely that the effect of androgen in creating an enhanced early cellular regeneration response underlies its ability to enhance axonal regeneration rates following axotomy.
It has been noted that some regeneration-associated proteins are expressed in the peripheral but not central nervous system, and that these differences may be related to the well known advantage of the peripheral nervous system in regenerative potential (Sanes and Jessell, 2000). One such protein is GAP-43, which is a cytoskeletal protein associated with the membranes of axons but not dendrites. GAP-43 is expressed at high levels in both central and peripheral nervous tissues during development, but only in the peripheral nervous system during adulthood. Furthermore, GAP-43 is upregulated by axotomy in the peripheral nervous system, but this response is muted in the central nervous system (Tetzlaff et al., 1991). Injury of the facial motor nerve increases GAP-43 mRNA and protein in the facial nucleus, and androgen treatment augments this response (Coers et al., 2002; Jones et al., 1997a).
Other cytoskeletal proteins are also involved in androgen enhanced nerve regeneration. The primary cytoskeletal structures in axons are microtubules, which are composed of tubulin proteins. Tubulin mRNAs are upregulated in facial motoneurons as the axons regenerate following axotomy (Jones and Oblinger, 1994; Jones et al., 1999b; Tetzlaff et al., 1991); and androgen treatment, which enhances axon regeneration rates, causes an additional upregulation of tubulin mRNAs above axotomy alone (Jones and Oblinger, 1994; Jones et al., 1999b). Importantly, tubulin mRNAs are upregulated by androgen treatment selectively. While αI-tubulin, βII-tubulin, and βIII-tubulin are all upregulated by axotomy, only βII-tubulin has been shown to be under the control of androgen in this model (Jones and Oblinger, 1994; Jones et al., 1999b). Tubulin genes are also regulated by androgen in axotomized sciatic motoneurons (Brown et al., 2001) and rubrospinal motoneurons (Storer et al., 2002; DeLucia et al., 2007), and in the spinal nucleus of the bulbocavernosus (SNB; Matsumoto et al., 1993, 1994), a group of motoneurons whose dendritic extension and retraction is under the control of androgens (Fargo and Sengelaub, 2007b; Kurz et al., 1986), raising the possibility that this may be a general phenomenon in motoneurons.
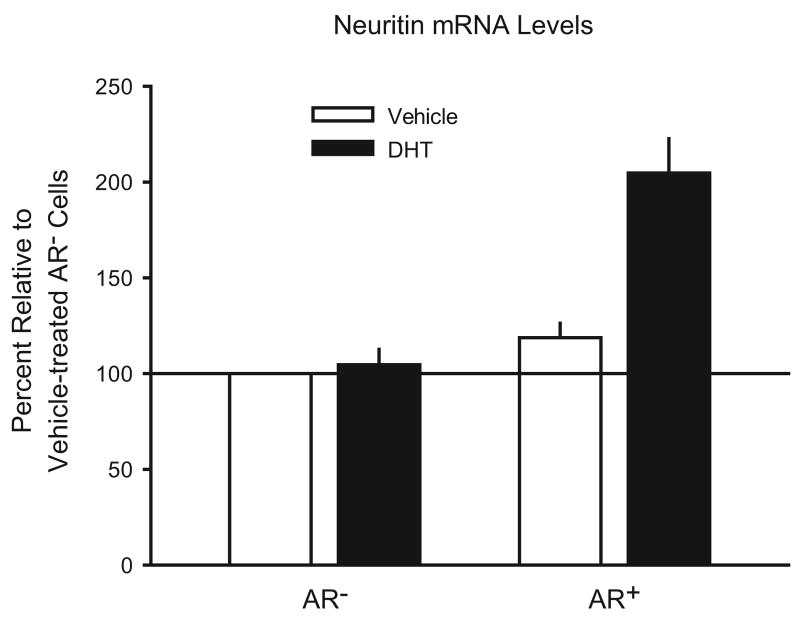
Androgenic enhancement of axon regeneration may also be under the control of neuritin. As discussed in Section 2, neuritin is a critical downstream mediator of the ability of androgens to increase neurite outgrowth in cultured NSC34/mAR cells (Marron et al. 2005). We have confirmed this finding in MN hybrid cells. We treated MN cells, either expressing AR (AR+) or not expressing AR (AR−), with either 100 nM DHT or vehicle control for 48 hours. For each group, three samples of approximately 5 × 105 cells each were harvested from separate wells. Total mRNA was extracted by guanidinium-thiocyanate extraction (Chomczynski and Sacchi, 1987), and neuritin mRNA levels were assessed by reverse transcription and real-time PCR. GAPDH served as the reference gene. PCRs were run in triplicate for each sample, with the average result serving as the sample score. Neuritin mRNA levels were determined with the ΔΔCT method, using vehicle-treated AR− cells as the control. DHT caused neuritin mRNA levels to double in AR+ MN cells [see Figure 4; F(2, 6) = 14.96, p < .01; post hoc analysis, Fisher's LSD, p < .01, compared to vehicle-treated AR+ cells]. However, DHT had no effect on neuritin mRNA levels in AR− MN cells, again indicating that the effect of androgen on neuritin mRNA expression is AR-dependent.
Figure 4.
Neuritin mRNA levels in AR− and AR+ cultured MN cells, treated with either 100 nM DHT (filled bars) or vehicle only (open bars). DHT treatment doubled neuritin mRNA levels, but only in AR+ cells. Bars heights represent means (± SEM).
We have also examined the in vivo expression of neuritin mRNA in the hamster facial motor nucleus following facial nerve injury and testosterone treatment. Facial motoneurons were axotomized by severing the right facial nerve at its exit from the stylomastoid foramen in adult, male hamsters. At the same time, some of the animals were given subcutaneous, interscapular implants of Silastic capsules containing testosterone propionate (3.18 mm OD, 1.57 mm ID, 10 mm long). Postoperative survival times were 6 hours, or 1, 2, 4, 6, or 7 days. Based on initial results, another group of animals was axotomized and treated with testosterone as described, but also given daily subcutaneous injections of flutamide (15 mg in 0.2 ml of a 50:50 v/v solution of ethanol and propylene glycol); this group had a postoperative survival time of 2 days. Tissue punches containing the facial nucleus were harvested from both sides of the brain stem according to the method of Yu (1989). Tissue punches were homogenized with Lysing Matrix D, and mRNA was extracted and quantified as described in the preceding paragraph, with the left side serving as an internal control for each animal (n = 3-6 animals per group).
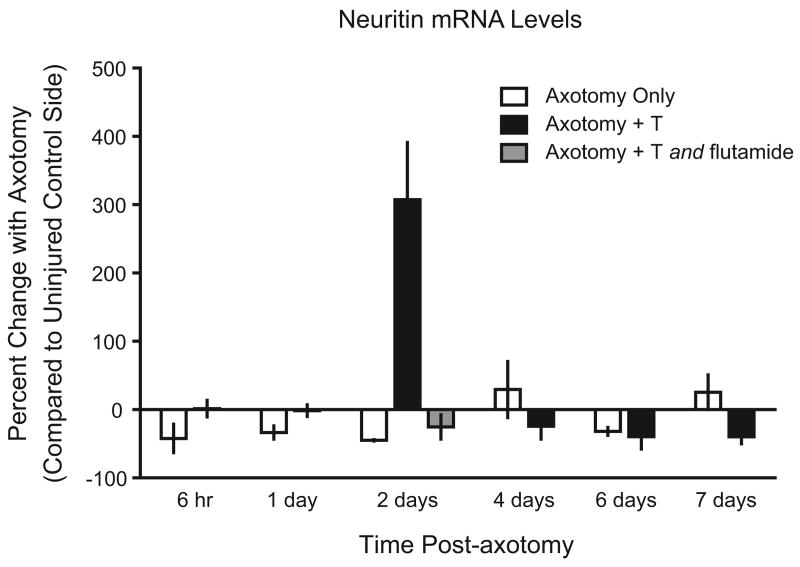
As illustrated in Figure 5, a two-way ANOVA revealed a main effect of time point [F(5, 28) = 7.67, p < .001], a main effect of testosterone treatment [F(1, 28) = 8.17, p < .01], and an interaction between time point and testosterone treatment [F(5, 28) = 12.64, p < .001]. Post hoc analysis indicated that neuritin mRNA levels were significantly higher in testosterone-treated animals two days post-axotomy than in any other group (Fisher's LSD, ps < .001), increasing by approximately 300% above the uninjured control side. In contrast, no such increase occurred in untreated animals (Tetzlaff et al., 2006). Because testosterone treatment increases the rate of axon regeneration in injured hamster facial motoneurons (Kujawa et al., 1991), these data demonstrate a relationship between neuritin expression and androgen-enhanced axon regeneration in vivo. This relationship is further supported by the fact that blocking AR with flutamide, which is known to block the effects of testosterone on axon regeneration rates (Kujawa et al., 1995), completely abolished the effect of testosterone on neuritin mRNA expression in the injured hamster facial nucleus.
Figure 5.
Neuritin mRNA levels in axotomized hamsters (open bars) and axotomized hamsters treated with testosterone (black bars) at 6 hours and 1, 2, 4, 6, and 7 days post-axotomy. Also shown are axotomized hamsters treated with both testosterone and flutamide for 2 days following axotomy (gray bar). Neuritin mRNA levels are displayed as the percent difference between the axotomized side and the control side within each animal. Testosterone caused a dramatic increase in neuritin mRNA levels 2 days post-axotomy; however, this increase was completely prevented by flutamide treatment. Bars heights represent means (± SEM).
The time course of androgenic regulation of neuritin expression in the hamster facial motor nucleus, with the most pronounced effect of testosterone occurring at two days post-injury, is particularly interesting for two reasons. First, extrapolation of hamster facial motoneuron axon regeneration data indicates that axon sprouting occurs at about two days post-injury (see Figure 3; Kujawa et al., 1991). Second, androgen treatment enhances axotomy-induced upregulation of βII-tubulin expression between two days and seven days post-injury (Jones and Oblinger, 1994; Jones et al., 1999b). Considered together with the fact that neuritin is an essential mediator of androgen-induced axon outgrowth in NSC34/mAR cells (Marron et al., 2005), these data suggest the hypothesis that neuritin may be involved in coordinating androgen-enhanced axon regeneration in the injured facial nerve.
4. Future directions
It is now possible to begin to make some connections between what in vitro and in vivo studies tell us about the effects of androgens on the growth of motoneuron processes. For example, it has long been established that androgens can increase soma size and dendritic arborization in spinal motoneurons (Breedlove and Arnold, 1981; Forger and Breedlove, 1987; Kurz et al., 1986). The fact that androgens increase cell size and process outgrowth in MN hybrid cells, but only in those expressing AR, provides evidence that androgens are capable of acting directly on motoneurons to affect these properties in a cell autonomous fashion in vitro (Brooks et al., 1998). In accordance, a series of experiments using rats with mosaic androgen insensitivity (in which a portion of motoneurons do not express functional AR) has demonstrated that androgen can regulate motoneurons in a cell autonomous manner in vivo as well (Monks et al., 1999; Monks and Watson, 2001; Watson et al., 2001).
Similarly, androgens promote the survival of AR-expressing MN hybrid cells in low-serum conditions in a cell autonomous manner in vitro (Brooks et al., 1998). In vivo, androgens also promote the survival of motoneurons in both the spinal cord (SNB motoneurons; Breedlove and Arnold, 1983a,b; Nordeen et al., 1985) and brainstem (facial motoneurons; Huppenbauer et al., 2005; Tetzlaff et al., 2006). While the androgen-dependent survival of SNB motoneurons during development is traditionally thought of as being mediated by their target muscles (Kurz et al., 1992; Johansen et al., 2004), androgens are capable of preventing the death of facial motoneurons that have been disconnected from their target muscles by axotomy (Huppenbauer et al., 2005; Tetzlaff et al., 2006).
Furthermore, in vitro studies of NSC34/mAR cells have indicated that androgens can act directly on motoneurons to drive process outgrowth, and that this effect is AR-mediated (Marron et al., 2005). In vivo studies have shown that androgens are also responsible for process outgrowth in motoneurons, for example maintaining appropriate adult dendritic length (Kurz et al., 1986; Sasaki and Arnold, 1991; Rand and Breedlove, 1995, Fargo and Sengelaub, 2007a,b) and enhancing axon regeneration after peripheral nerve axotomy (Kujawa et al., 1991). Additionally, AR is present in both motoneurons and muscles in vivo (Matsumoto, 1997; Yu and McGinnis, 2001; Tetzlaff et al., 2007a; Monks et al., 2004), and blocking androgen receptors with flutamide or hydroxyflutamide abolishes the ability of androgens to maintain dendritic length (Rand and Breedlove, 1995) and enhance axon regeneration (Yu and Cao, 1991; Kujawa et al., 1995). However, one possible difference between in vitro and in vivo studies of androgen regulation of process outgrowth in motoneurons relates to the question of whether androgens act directly on motoneurons to affect changes in cellular morphology. While in vitro studies of motoneuron-like cells indicate that androgens can drive cell-autonomous increases in process outgrowth (Brooks et al., 1998; Marron et al., 2005), there is evidence that androgenic control of the maintenance of dendritic length and axon regeneration in vivo may be mediated by the target muscles (Rand and Breedlove, 1995; Yu and Cao, 1991), or other central or peripheral factors, as discussed above.
Another connection between in vitro and in vivo studies on androgen regulation of process outgrowth in motoneurons lies in the fact that both lines of inquiry point to DHT as an important steroid in these effects. For example, both testosterone and DHT increase the growth of axon-like processes in NSC34/mAR cells in vitro, but DHT has a larger effect (Marron et al., 2005). In vivo, DHT produces a greater acceleration of motoneuron axon regeneration than does testosterone (Tanzer and Jones, 1997), and DHT is at least as effective as testosterone in maintaining dendritic length (Fargo and Sengelaub, 2007a). Finally, both in vitro and in vivo studies point to the importance of cytoskeletal proteins in mediating androgenic control of process outgrowth. Testosterone upregulates α-tubulin, βII-tubulin, and βIII-tubulin in cultured SH-SY5Y cells in an AR-dependent manner (Butler et al., 2001). In vivo, βII-tubulin expression is upregulated during androgen-enhanced nerve regeneration in axotomized facial (Jones and Oblinger, 1994; Jones et al., 1999b) and sciatic (Brown et al., 1999, 2001) motoneurons, and androgens regulate levels of both β-actin and β-tubulin in SNB motoneurons (Matsumoto et al., 1992, 1993, 1994).
Androgens enhance neurite extension in vitro and axonal regeneration in vivo by a variety of mechanisms. While progress has been made in illuminating the cellular and molecular events involved in these effects, much more work is needed. Further elucidation of the mechanisms by which androgens promote axonal elongation will contribute to understanding aspects of several key neuropathological situations. For example, the androgen-upregulated protein neuritin could be an important mediator of enhanced recovery from traumatic nervous system injuries, as well as playing a role in attenuating the effects of neurodegenerative diseases such as SBMA and ALS. We have begun exploring the possibilities of using androgens as an adjuvant therapy in several experimental paradigms with translational implications. We have combined testosterone treatment with other therapies, including grafting of peripheral nerves into the injured spinal cord, to enhance the expression of cytoskeletal genes in injured rubrospinal motoneurons (Storer et al., 2002; DeLucia et al., In press). We have also shown that testosterone treatment enhances the ability of electrical stimulation to increase peripheral axon regeneration rates (Lal et al., 2007; Hetzler et al., 2007), and are applying that to clinical situations in which the facial nerve is damaged following acoustic neuroma formation. These studies provide the foundation for future work that will further our understanding of the neurotherapeutic roles of androgens in neural repair, with the goal of finding novel strategies for enhancing recovery from injury and overcoming the debilitating effects of neurodegenerative diseases.
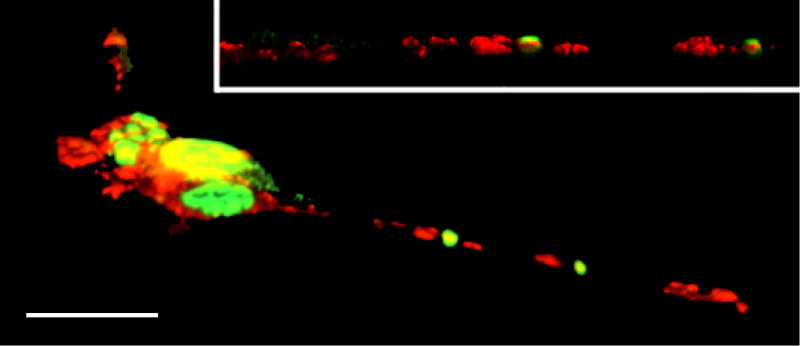
Figure 2.
Confocal microscopy image of NSC34/mAR cells expressing the mutant SBMA AR and treated with testosterone. Several SBMA AR neuropil aggregates have formed (green), along with an accumulation of the motor protein kinesin (red). Scale bar = 20 μm. Inset: closer view of a neurite. (After Piccioni et al, 2002.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdullah AAR, Trifiro MA, Panetraymond V, Alvarado C, Detourreil S, Frankel D, Schipper HM, Pinsky L. Spinobulbar muscular atrophy - polyglutamine-expanded androgen receptor is proteolytically resistant in vitro and processed abnormally in transfected cells. Hum Mol Genet. 1998;7:379–384. doi: 10.1093/hmg/7.3.379. [DOI] [PubMed] [Google Scholar]
- Al-Shamma HA, Arnold AP. Brain-derived neurotrophic factor regulates expression of androgen receptors in perineal motoneurons. Proc Natl Acad Sci U S A. 1997;94:1521–1526. doi: 10.1073/pnas.94.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila DM, Allman DR, Gallo JM, McPhaul MY. Androgen receptors containing expanded polyglutamine tracts exhibit progressive toxicity when stably expressed in the neuroblastoma cell line, SH-SY 5Y. Exp Biol Med. 2003;228:982–990. doi: 10.1177/153537020322800815. [DOI] [PubMed] [Google Scholar]
- Bardo S, Cavazzini MG, Emptage N. The role of the endoplasmic reticulum Ca2+ store in the plasticity of central neurons. Trends Pharmacol Sci. 2006;27:78–84. doi: 10.1016/j.tips.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Belsham DD, Evangelou A, Roy D, Vinh Le D, Brown TJ. Regulation of gonadotropin-releasing hormone (GnRH) gene expression by 5alpha-dihydrotestosterone in GnRH-secreting GT1-7 hypothalamic neurons. Endocrinology. 1998;139:1108–1114. doi: 10.1210/endo.139.3.5846. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Erskine MS. Feminine sexual behavior: Cellular integration of hormonal and afferent information in the rodent brain. In: Pfaff DW, Arnold A, Etgen A, Fahrbach S, Rubin R, editors. Hormones, Brain and Behavior. Vol. 1. Academic Press; New York: 2002. pp. 139–214. [Google Scholar]
- Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225:297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- Breedolve SM, Arnold AP. Hormonal control of a developing neuromuscular system. I. Complete Demasculinization of the male rat spinal nucleus of the bulbocavernosus using the anti-androgen flutamide. J Neurosci. 1983a;3:417–423. doi: 10.1523/JNEUROSCI.03-02-00417.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedolve SM, Arnold AP. Hormonal control of a developing neuromuscular system. II. Sensitive periods for the androgen-induced masculinization of the rat spinal nucleus of the bulbocavernosus. J Neurosci. 1983b;3:424–432. doi: 10.1523/JNEUROSCI.03-02-00424.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Sex differences in the pattern of steroid accumulation by motoneurons in the rat lumbar spinal cord. J Comp Neurol. 1983c;215:211–216. doi: 10.1002/cne.902150208. [DOI] [PubMed] [Google Scholar]
- Brooks BP, Merry DE, Paulson HL, Lieberman AP, Kolson DL, Fischbeck KH. A cell culture model for androgen effects in motor neurons. J Neurochem. 1998;70:1054–1060. doi: 10.1046/j.1471-4159.1998.70031054.x. [DOI] [PubMed] [Google Scholar]
- Brown TJ, Kahn T, Jones KJ. Androgen induced acceleration of functional recovery after rat sciatic nerve injury. Restor Neurol Neurosci. 1999;15:289–295. [PubMed] [Google Scholar]
- Brown TJ, Storer P, Oblinger M, Jones KJ. Androgenic enhancement of βII-tubulin mRNA in spinal motoneurons following sciatic nerve injury. Restor Neurol Neurosci. 2001;18:191–198. [PubMed] [Google Scholar]
- Butler R, Leigh PN, Gallo JM. Androgen-induced up-regulation of tubulin isoforms in neuroblastoma cells. J Neurochem. 2001;78:854–861. doi: 10.1046/j.1471-4159.2001.00475.x. [DOI] [PubMed] [Google Scholar]
- Cantallops I, Haas K, Cline HT. Postsynaptic CPG15 promotes synaptic maturation and presynaptic axon arbor elaboration in vivo. Nat Neurosci. 2000;3:1004–1011. doi: 10.1038/79823. [DOI] [PubMed] [Google Scholar]
- Cappelletti G, Galbiati M, Ronchi C, Maggioni MG, Onesto E, Poletti A. Neuritin (cpg15) enhances the differentiating effect of NGF on neuronal PC12 cells. J Neurosci Res. 2007;85:2702–2713. doi: 10.1002/jnr.21235. [DOI] [PubMed] [Google Scholar]
- Cashman NR, Durham HD, Blusztajn JK, Oda K, Tabira T, Shaw IT, Dahrouge S, Antel JP. Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dyn. 1992;194:209–221. doi: 10.1002/aja.1001940306. [DOI] [PubMed] [Google Scholar]
- Celotti F, Negri-Cesi P, Poletti A. Steroid metabolism in the mammalian brain: 5alpha-reduction and aromatization. Brain Res Bull. 1997;44:365–375. doi: 10.1016/s0361-9230(97)00216-5. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coers S, Tanzer L, Jones KJ. Testosterone treatment attenuates the effects of facial nerve transection on glial fibrillary acidic protein (GFAP) levels in the hamster facial motor nucleus. Metab Brain Dis. 2002;17:55–63. doi: 10.1023/a:1015415226799. [DOI] [PubMed] [Google Scholar]
- Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: Principles and mechanisms. Front Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Shatz CJ, Nedivi E. Dynamic regulation of cpg15 during activity-dependent synaptic development in the mammalian visual system. J Neurosci. 1999;19:7999–8008. doi: 10.1523/JNEUROSCI.19-18-07999.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucia TA, Alexander T, Fargo KN, Jones KJ. Effects of single versus combinatorial treatment strategies on beta II-tubulin gene expression in axotomized hamster rubrospinal motoneurons. Restor Neurol Neurosci. In press. [PubMed] [Google Scholar]
- Di Giovanni S, Faden AI, Yakovlev A, Duke-Cohan JS, Finn T, Thouin M, Knoblach S, De Biase A, Bregman BS, Hoffman EP. Neuronal plasticity after spinal cord injury: identification of a gene cluster driving neurite outgrowth. FASEB J. 2005;19:153–154. doi: 10.1096/fj.04-2694fje. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, Garcia-Ovejero D, Sarky S, Garcia-Segura LM, Azcoitia I. Androgen receptor immunoreactivity in forebrain axons and dendrites in the rat. Endocrinology. 2003;144:3632–3638. doi: 10.1210/en.2002-0105. [DOI] [PubMed] [Google Scholar]
- Drengler SM, Handa RJ, Jones KJ. Sex differences in androgen receptor mRNA levels and regulation in hamster facial motoneurons. Mol Brain Res. 1996;35:131–138. doi: 10.1016/0169-328x(95)00197-z. [DOI] [PubMed] [Google Scholar]
- Drengler SM, Handa RJ, Jones KJ. Effects of axotomy and testosterone on androgen receptor mRNA expression in hamster facial motoneurons. Exp Neurol. 1997;146:374–379. doi: 10.1006/exnr.1997.6537. [DOI] [PubMed] [Google Scholar]
- Durham HD, Dahrouge S, Cashman NR. Evaluation of the spinal cord neuron X neuroblastoma hybrid cell line NSC-34 as a model for neurotoxicity testing. Neurotoxicology. 1992;14:387–395. [PubMed] [Google Scholar]
- Ellerby LM, Hackam AS, Propp SS, Ellerby HM, Rabizadeh S, Cashman NR, Trifiro MA, Pinsky L, Wellington CL, Salvesen GS, Hayden MR, Bredesen DE. Kennedy's disease: caspase cleavage of the androgen receptor is a crucial event in cytotoxicity. J Neurochem. 1999;72:185–195. doi: 10.1046/j.1471-4159.1999.0720185.x. [DOI] [PubMed] [Google Scholar]
- Estrada M, Uhlen P, Ehrlich BE. Ca2+ oscillations induced by testosterone enhance neurite outgrowth. J Cell Sci. 2006;119:733–743. doi: 10.1242/jcs.02775. [DOI] [PubMed] [Google Scholar]
- Fargo KN, Sengelaub DR. Androgenic, but not estrogenic, protection of motoneurons from somal and dendritic atrophy induced by the death of neighboring motoneurons. Dev Neurobiol. 2007a;67:1094–1106. doi: 10.1002/dneu.20454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargo KN, Sengelaub DR. Exogenous testosterone reverses age-related atrophy in a spinal neuromuscular system. Horm Behav. 2007b;51:20–30. doi: 10.1016/j.yhbeh.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, Breedlove SM. Seasonal variation in mammalian striated muscle mass and motoneuron morphology. J Neurobiol. 1987;18:155–165. doi: 10.1002/neu.480180204. [DOI] [PubMed] [Google Scholar]
- Han Y, Chen X, Shi F, Li S, Huang J, Xie M, Hu L, Hoidal JR, Xu P. CPG15, a new factor upregulated after ischemic brain injury, contributes to neuronal network re-establishment after glutamate-induced injury. J Neurotrauma. 2007;24:722–731. doi: 10.1089/neu.2006.0174. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Toran-Allerand CD. Androgen increases the number of cells in fetal mouse spinal cord cultures: implications for motoneuron survival. Brain Res. 1989;485:157–164. doi: 10.1016/0006-8993(89)90677-x. [DOI] [PubMed] [Google Scholar]
- Herbst KL, Bhasin S. Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7:271–277. doi: 10.1097/00075197-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Hetzler LET, Sharma N, Wurster R, Leonetti JP, Marzo SJ, Jones K, Foecking E. Accelerated rat facial nerve recovery: Testosterone plus electrostimulation. Official Program of the American Academy of Otolaryngology-Head and Neck Surgery Foundation 2007 [Google Scholar]
- Hull EM, Meisel RL, Sachs BD. Male sexual behavior. In: Pfaff DW, Arnold A, Etgen A, Fahrbach S, Rubin R, editors. Hormones, Brain and Behavior. Vol. 1. Academic Press; New York: 2002. pp. 1–138. [Google Scholar]
- Huppenbauer CB, Tanzer L, DonCarlos LL, Jones KJ. Gonadal steroid attenuation of developing hamster facial motoneuron loss by axotomy: equal efficacy of testosterone, dihydrotestosterone, and 17-beta estradiol. J Neurosci. 2005;25:4004–4013. doi: 10.1523/JNEUROSCI.5279-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques-Fricke BT, Seow Y, Gottlieb PA, Sachs F, Gomez TM. Ca2+ influx through mechanosensitive channels inhibits neurite outgrowth in opposition to other influx pathways and release from intracellular stores. J Neurosci. 2006;26:5656–5664. doi: 10.1523/JNEUROSCI.0675-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaherian A, Cline HT. Coordinated motor neuron axon growth and neuromuscular synaptogenesis are promoted by CPG15 in vivo. Neuron. 2005;45:505–512. doi: 10.1016/j.neuron.2004.12.051. [DOI] [PubMed] [Google Scholar]
- Johansen JA, Jordan CL, Breedlove SM. Steroid hormone masculinization of neural structure in rats: a tale of two nuclei. Physiol Behav. 2004;83:271–277. doi: 10.1016/j.physbeh.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Jones KJ. Recovery from facial paralysis following crush injury of the facial nerve in hamsters: differential effects of gender and androgen exposure. Exp Neurol. 1993;121:133–138. doi: 10.1006/exnr.1993.1079. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Alexander TD, Brown TJ, Tanzer L. Gonadal steroid enhancement of facial nerve regeneration: Role of heat shock protein 70. J Neurocytol. 2000;29:341–349. doi: 10.1023/a:1007157105835. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Brown TJ, Damaser M. Neuroprotective effects of gonadal steroids on regenerating peripheral motoneurons. Brain Res Brain Res Rev. 2001;37:372–382. doi: 10.1016/s0165-0173(01)00107-2. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Coers S, Storer PD, Tanzer L, Kinderman NB. Androgenic regulation of the central glia response following nerve damage. J Neurobiol. 1999a;40:560–573. doi: 10.1002/(sici)1097-4695(19990915)40:4<560::aid-neu11>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Drengler SM, Oblinger MM. Gonadal steroid regulation of growth-associated protein GAP-43 mRNA expression in axotomized hamster facial motor neurons. Neurochem Res. 1997a;22:1367–1374. doi: 10.1023/a:1022071123255. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Durica TE, Jacob SK. Gonadal steroid preservation of central synaptic input to hamster facial motoneurons following peripheral axotomy. J Neurocytol. 1997b;26:257–266. doi: 10.1023/a:1018596316465. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Kinderman NB, Oblinger MM. Alterations in glial fibrillary acidic protein (GFAP) mRNA levels in the hamster facial motor nucleus: effects of axotomy and testosterone. Neurochem Res. 1997c;22:1359–1366. doi: 10.1023/a:1022019106417. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Oblinger MM. Androgenic regulation of tubulin gene expression in axotomized hamster facial motoneurons. J Neurosci. 1994;14:3620–3627. doi: 10.1523/JNEUROSCI.14-06-03620.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KJ, Storer PD, Drengler SM, Oblinger MM. Differential regulation of cytoskeletal gene expression in hamster facial motoneurons: effects of axotomy and testosterone treatment. J Neurosci Res. 1999b;57:817–823. [PubMed] [Google Scholar]
- Jordan CL, Padgett B, Hershey J, Prins G, Arnold A. Ontogeny of androgen receptor immunoreactivity in lumbar motoneurons and in the sexually dimorphic levator ani muscle of male rats. J Comp Neurol. 1997;379:88–98. [PubMed] [Google Scholar]
- Jordan CL, Price RH, Jr, Handa RJ. Androgen receptor messenger RNA and protein in adult rat sciatic nerve: implications for site of androgen action. J Neurosci Res. 2002;69:509–518. doi: 10.1002/jnr.10324. [DOI] [PubMed] [Google Scholar]
- Kazemi-Esfarjani P, Trifiro MA, Pinsky L. Evidence for a repressive function of the long polyglutamine tract in the human androgen receptor: possible pathogenetic relevance for the (CAG)n-expanded neuronopathies. Hum Mol Genet. 1995;4:523–527. doi: 10.1093/hmg/4.4.523. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Alter M, Sung JH. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology. 1968;18:671–680. doi: 10.1212/wnl.18.7.671. [DOI] [PubMed] [Google Scholar]
- Kinderman NB, Jones KJ. Testosterone enhancement of the nerve cell body response to injury: evidence using in situ hybridization and ribosomal DNA probes. J Neurosci. 1993;13:1523–1532. doi: 10.1523/JNEUROSCI.13-04-01523.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishino A, Ishige Y, Tatsuno T, Nakayama C, Noguchi H. BDNF prevents and reverses adult rat motor neuron degeneration and induces axonal outgrowth. Exp Neurol. 1997;144:273–286. doi: 10.1006/exnr.1996.6367. [DOI] [PubMed] [Google Scholar]
- Kujawa KA, Emeric E, Jones KJ. Testosterone differentially regulates the regenerative properties of injured hamster facial motoneurons. J Neurosci. 1991;11:3898–3906. doi: 10.1523/JNEUROSCI.11-12-03898.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa KA, Jacob JM, Jones KJ. Testosterone regulation of the regenerative properties of injured rat sciatic motor neurons. J Neurosci Res. 1993;35:268–273. doi: 10.1002/jnr.490350306. [DOI] [PubMed] [Google Scholar]
- Kujawa KA, Jones KJ. Testosterone-induced acceleration of recovery from facial paralysis in male hamsters: temporal requirements of hormone exposure. Physiol Behav. 1990;48:765–768. doi: 10.1016/0031-9384(90)90223-q. [DOI] [PubMed] [Google Scholar]
- Kujawa KA, Kinderman NB, Jones KJ. Testosterone-induced acceleration of recovery from facial paralysis following crush axotomy of the facial nerve in male hamsters. Exp Neurol. 1989;105:80–85. doi: 10.1016/0014-4886(89)90174-x. [DOI] [PubMed] [Google Scholar]
- Kujawa KA, Tanzer L, Jones KJ. Inhibition of the accelerative effects of testosterone on hamster facial nerve regeneration by the antiandrogen flutamide. Exp Neurol. 1995;133:138–143. doi: 10.1006/exnr.1995.1016. [DOI] [PubMed] [Google Scholar]
- Kurz EM, Cover AR, Sengelaub DR. Testosterone fails to save androgen-sensitive rat motoneurons following early target removal. Dev Brain Res. 1992;70:181–189. doi: 10.1016/0165-3806(92)90196-4. [DOI] [PubMed] [Google Scholar]
- Kurz EM, Sengelaub DR, Arnold AP. Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science. 1986;232:395–398. doi: 10.1126/science.3961488. [DOI] [PubMed] [Google Scholar]
- Lal D, Sharma N, Kerns SC, Wurster R, Leonetti JP, Marzo SJ, Jones K, Foecking E. Electrical stimulation facilitates rat facial nerve recovery. Official Program of the American Academy of Otolaryngology-Head and Neck Surgery Foundation. 2007 doi: 10.1016/j.otohns.2008.04.030. [DOI] [PubMed] [Google Scholar]
- La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352:77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Larkowski TD, Drengler SM, Tanzer L, Jones KJ. Androgen receptor mRNA regulation in adult male and female hamster facial motoneurons: Effects of axotomy and exogenous androgens. J Neurobiol. 2000;45(4):207–214. doi: 10.1002/1097-4695(200012)45:4<207::aid-neu2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Lavelle A, Lavelle F. Neuron reaction to injury during development. In: Almli CR, editor. Early Brain Damage: Neurobiology, and Behavior. Academic Press; New York: 1984. pp. 3–16. [Google Scholar]
- Lee WC, Nedivi E. Extended plasticity of visual cortex in dark-reared animals may result from prolonged expression of cpg15-like genes. J Neurosci. 2002;22:1807–1815. doi: 10.1523/JNEUROSCI.22-05-01807.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losel RM, Falkenstein E, Feuring M, Schultz A, Tillmann HC, Rossol-Haseroth K, Wehling M. Nongenomic steroid action: Controversies, questions, and answers. Physiol Rev. 2003;83:965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- Lubischer JL, Arnold AP. Axotomy transiently down-regulates androgen receptors in motoneurons of the spinal nucleus of the bulbocavernosus. Brain Res. 1995;694:61–68. doi: 10.1016/0006-8993(95)00766-j. [DOI] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Handa RJ. Dihydrotestosterone may inhibit hypothalamo-pituitary-adrenal activity by acting through estrogen receptor in the male mouse. Neurosci Lett. 2004;365:43–47. doi: 10.1016/j.neulet.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Magnaghi V, Cavarretta I, Zucchi I, Susani L, Rupprecht R, Hermann B, Martini L, Melcangi RC. Po gene expression is modulated by androgens in the sciatic nerve of adult male rats. Brain Res Mol Brain Res. 1999;70:36–44. doi: 10.1016/s0169-328x(99)00124-2. [DOI] [PubMed] [Google Scholar]
- Makwana M, Raivich G. Molecular mechanisms in successful peripheral regeneration. FEBS J. 2005;272:2628–2638. doi: 10.1111/j.1742-4658.2005.04699.x. [DOI] [PubMed] [Google Scholar]
- Marron TU, Guerini V, Rusmini P, Sau D, Brevini TA, Martini L, Poletti A. Androgen-induced neurite outgrowth is mediated by neuritin in motor neurones. J Neurochem. 2005;92:10–20. doi: 10.1111/j.1471-4159.2004.02836.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto A. Hormonally induced neuronal plasticity in the adult motoneurons. Brain Res Bull. 1997;44:539–547. doi: 10.1016/s0361-9230(97)00240-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Hyodo S. Androgenic regulation of expression of β-tubulin messenger ribonucleic acid in motoneurons of the spinal nucleus of the bulbocavernosus. J Neuroendocrinol. 1993;5:357–363. doi: 10.1111/j.1365-2826.1993.tb00495.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Urano A, Hyodo S. Effect of androgen on the expression of gap junction and β-actin mRNAs in adult rat motoneurons. Neurosci Res. 1992;14:133–144. doi: 10.1016/0168-0102(92)90089-u. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Urano A, Hyodo S. Androgen regulates gene expression of cytoskeletal proteins in adult rat motoneurons. Horm Behav. 1994;28:357–366. doi: 10.1006/hbeh.1994.1032. [DOI] [PubMed] [Google Scholar]
- Mhatre AN, Trifiro MA, Kaufman M, Kazemi-Esfarjani P, Figlewicz D, Rouleau G, Pinsky L. Reduced transcriptional regulatory competence of the androgen receptor in X-linked spinal and bulbar muscular atrophy. Nat Genet. 1993;5:184–188. doi: 10.1038/ng1093-184. [DOI] [PubMed] [Google Scholar]
- Monks DA, O'Bryant EL, Jordan CL. Androgen receptor immunoreactivity in skeletal muscle: enrichment at the neuromuscular junction. J Comp Neurol. 2004;473:59–72. doi: 10.1002/cne.20088. [DOI] [PubMed] [Google Scholar]
- Monks DA, Vanston CM, Watson NV. Direct androgenic regulation of calcitonin gene-related peptide expression in motoneurons of rats with mosaic androgen insensitivity. J Neurosci. 1999;19:5597–5601. doi: 10.1523/JNEUROSCI.19-13-05597.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks DA, Watson NV. N-cadherin expression in motoneurons is directly regulated by androgens: a genetic mosaic analysis in rats. Brain Res. 2001;895:73–79. doi: 10.1016/s0006-8993(01)02031-5. [DOI] [PubMed] [Google Scholar]
- Moran LB, Graeber MB. The facial nerve axotomy model. Brain Res Brain Res Rev. 2004;44:154–178. doi: 10.1016/j.brainresrev.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Mousavi K, Jasmin BJ. BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. J Neurosci. 2006;26:5739–5749. doi: 10.1523/JNEUROSCI.5398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeve GS, Ramakrishnan M, Kramer R, Hevroni D, Citri Y, Theill LE. Neuritin: a gene induced by neural activity and neurotrophins that promotes neuritogenesis. Proc Natl Acad Sci U S A. 1997;94:2648–2653. doi: 10.1073/pnas.94.6.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Onodera O, Strittmatter WJ, Burke JR. Polyglutamine domain proteins with expanded repeats bind neurofilament, altering the neurofilament network. Ann N Y Acad Sci. 1999;893:192–202. doi: 10.1111/j.1749-6632.1999.tb07826.x. [DOI] [PubMed] [Google Scholar]
- Nedivi E, Fieldust S, Theill LE, Hevron D. A set of genes expressed in response to light in the adult cerebral cortex and regulated during development. Proc Natl Acad Sci U S A. 1996;93:2048–2053. doi: 10.1073/pnas.93.5.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedivi E, Hevroni D, Naot D, Israeli D, Citri Y. Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature. 1993;363:718–722. doi: 10.1038/363718a0. [DOI] [PubMed] [Google Scholar]
- Nedivi E, Wu GY, Cline HT. Promotion of dendritic growth by CPG15, an activity-induced signaling molecule. Science. 1998;281:1863–1866. doi: 10.1126/science.281.5384.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri-Cesi P, Colciago A, Poletti A, Motta M. 5alpha-reductase isozymes and aromatase are differentially expressed and active in the androgen-independent human prostate cancer cell lines DU145 and PC3. Prostate. 1999;41:224–232. doi: 10.1002/(sici)1097-0045(19991201)41:4<224::aid-pros2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Negri-Cesi P, Poletti A, Celotti F. Metabolism of steroids in the brain: a new insight into the role of 5alpha-reductase and aromatase in brain differentiation and functions. J Steroid Biochem Mol Biol. 1996;58:455–466. doi: 10.1016/0960-0760(96)00083-0. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Sengelaub DR, Arnold AP. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985;229:671–673. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- O'Bryant EL, Jordan CL. Expression of nuclear receptor coactivators in androgen-responsive and -unresponsive motoneurons. Horm Behav. 2005;47:29–38. doi: 10.1016/j.yhbeh.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Osborne MC, Verhovshek T, Sengelaub DR. Androgen regulates trkB immunolabeling in spinal motoneurons. J Neurosci Res. 2007;85:303–309. doi: 10.1002/jnr.21122. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Beck LA, Jordan CL, Breedlove SM. Androgen-dependent regulation of brain-derived neurotrophic factor and tyrosine kinase B in the sexually dimorphic spinal nucleus of the bulbocavernosus. Endocrinology. 2007;148:3655–3665. doi: 10.1210/en.2007-0308. [DOI] [PubMed] [Google Scholar]
- Pak TR, Chung WC, Lund TD, Hinds LR, Clay CM, Handa RJ. The androgen metabolite, 5alpha-androstane-3beta, 17beta-diol, is a potent modulator of estrogen receptor-beta1-mediated gene transcription in neuronal cells. Endocrinology. 2005;146:147–155. doi: 10.1210/en.2004-0871. [DOI] [PubMed] [Google Scholar]
- Piccioni F, Pinton P, Simeoni S, Pozzi P, Fascio U, Vismara G, Martini L, Rizzuto R, Poletti A. Androgen receptor with elongated polyglutamine tract forms aggregates that alter axonal trafficking and mitochondrial distribution in motor neuronal processes. FASEB J. 2002;160:1418–1420. doi: 10.1096/fj.01-1035fje. [DOI] [PubMed] [Google Scholar]
- Poletti A. CAG expansion in androgen receptor gene and neuronal cell death. Recent Res Devel Neurochem. 1999;2:507–515. [Google Scholar]
- Poletti A. The polyglutamine tract of androgen receptor: from functions to dysfunctions in motor neurons. Front Neuroendocrinol. 2004;25:1–26. doi: 10.1016/j.yfrne.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Poletti A, Martini L. Androgen-activating enzymes in the central nervous system. J Steroid Biochem Mol Biol. 1999;69:117–122. doi: 10.1016/s0960-0760(98)00150-2. [DOI] [PubMed] [Google Scholar]
- Poletti A, Melcangi RC, Negri-Cesi P, Maggi R, Martini L. Steroid binding and metabolism in the luteinizing hormone-releasing hormone-producing neuronal cell line GT1-1. Endocrinology. 1994;135:2623–2638. doi: 10.1210/endo.135.6.7988451. [DOI] [PubMed] [Google Scholar]
- Poletti A, Negri-Cesi P, Colciago A, Celotti F, Martini L. The 5alpha-reductase isozymes in the central nervous system. Steroids. 1998;63:246–251. doi: 10.1016/s0039-128x(98)00018-x. [DOI] [PubMed] [Google Scholar]
- Poletti A, Negri-Cesi P, Martini L. Reflections on the diseases linked to mutations of the androgen receptor. Endocrine. 2005;28:243–262. doi: 10.1385/ENDO:28:3:243. [DOI] [PubMed] [Google Scholar]
- Poletti A, Negri-Cesi P, Melcangi RC, Colciago A, Martini L, Celotti F. Expression of androgen-activating enzymes in cultured cells of developing rat brain. J Neurochem. 1997;68:1298–1303. doi: 10.1046/j.1471-4159.1997.68031298.x. [DOI] [PubMed] [Google Scholar]
- Poletti A, Rampoldi A, Piccioni F, Volpi S, Simeoni S, Zanisi M, Martini L. 5alpha-reductase type 2 and androgen receptor expression in gonadotropin releasing hormone GT1-1 cells. J Neuroendocrinol. 2001;13:353–357. doi: 10.1046/j.1365-2826.2001.00635.x. [DOI] [PubMed] [Google Scholar]
- Pozzi P, Bendotti C, Simeoni S, Piccioni F, Guerini V, Marron TU, Martini L, Poletti A. Androgen 5-alpha-reductase type 2 is highly expressed and active in rat spinal cord motor neurones. J Neuroendocrinol. 2003;15:882–887. doi: 10.1046/j.1365-2826.2003.01074.x. [DOI] [PubMed] [Google Scholar]
- Putz U, Harwell C, Nedivi E. Soluble CPG15 expressed during early development rescues cortical progenitors from apoptosis. Nat Neurosci. 2005;8:322–331. doi: 10.1038/nn1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand MN, Breedlove SM. Androgen alters the dendritic arbors of SNB motoneurons by acting upon their target muscles. J Neurosci. 1995;15:4408–4416. doi: 10.1523/JNEUROSCI.15-06-04408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson RN, Santer RM, Watson AH. SRC-1 localisation in lumbosacral spinal cord of male and female Wistar rats. Neuroreport. 2003;14:1821–1824. doi: 10.1097/00001756-200310060-00012. [DOI] [PubMed] [Google Scholar]
- Salazar-Grueso EF, Kim S, Kim H. Embryonic mouse spinal cord motor neuron hybrid cells. Neuroreport. 1991;2:505–508. doi: 10.1097/00001756-199109000-00002. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Jessell TM. The formation and regeneration of synapses. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. McGraw-Hill; New York: 2000. pp. 1087–1114. [Google Scholar]
- Sasaki M, Arnold AP. Androgenic regulation of dendritic trees of motoneurons in the spinal nucleus of the bulbocavernosus: reconstruction after intracellular iontophoresis of horseradish peroxidase. J Comp Neurol. 1991;308:11–27. doi: 10.1002/cne.903080103. [DOI] [PubMed] [Google Scholar]
- Simon NG. Hormonal processes in the development and expression of aggressive behavior. In: Pfaff DW, Arnold A, Etgen A, Fahrbach S, Rubin R, editors. Hormones, Brain and Behavior. Vol. 1. Academic Press; New York: 2002. pp. 339–92. [Google Scholar]
- Smith ER, Damassa DA, Davidson JM. Hormone Administration: Peripheral and Intracranial Implants. In: Meyer RD, editor. Methods in Psychobiology. Vol. 3. Academic Press; New York: 1977. pp. 259–279. [Google Scholar]
- Stefanis C, Papapetropoulos T, Scarpalezos S. A peculiar form of X-linked hereditary degenerative motor neuron disease. Presented in the Hellenic Association of Neurology and Psychiatry 26 november 1968.1968. [Google Scholar]
- Stefanis C, Papapetropoulos T, Scarpalezos S, Lygidakis G, Panayiotopoulos CP. X-linked spinal and bulbar muscular atrophy of late onset. A separate type of motor neuron disease? J Neurol Sci. 1975;24:493–403. doi: 10.1016/0022-510x(75)90173-2. [DOI] [PubMed] [Google Scholar]
- Sternberger LA, Harwell LW, Sternberger NH. Neurotypy: regional individuality in rat brain detected by immunocytochemistry with monoclonal antibodies. Proc Natl Acad Sci U S A. 1982;79:1326–1330. doi: 10.1073/pnas.79.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer PD, Houle JD, Oblinger M, Jones KJ. Combination of gonadal steroid treatment and peripheral nerve grafting results in a peripheral motoneuron-like pattern of BII-tubulin mRNA expression in axotomized hamster rubrospinal motoneurons. J Comp Neurol. 2002;449:364–373. doi: 10.1002/cne.10304. [DOI] [PubMed] [Google Scholar]
- Sunderland S. Cranial nerve injury. Structural and pathophysiological considerations and a classification of nerve injury. In: Samii M, Jannetta PJ, editors. The Cranial Nerves. Springer-Verlag; Berlin: 1981. pp. 16–23. [Google Scholar]
- Szebenyi G, Morfini GA, Babcock A, Gould M, Selkoe K, Stenoien DL, Young M, Faber PW, MacDonald ME, McPhaul MJ, Brady ST. Neuropathogenic forms of huntingtin and androgen receptor inhibit fast axonal transport. Neuron. 2003;40:41–52. doi: 10.1016/s0896-6273(03)00569-5. [DOI] [PubMed] [Google Scholar]
- Tanzer L, Jones KJ. Gonadal steroid regulation of hamster facial nerve regeneration: effects of dihydrotestosterone and estradiol. Exp Neurol. 1997;146:258–264. doi: 10.1006/exnr.1997.6529. [DOI] [PubMed] [Google Scholar]
- Tanzer L, Jones KJ. Neurotherapeutic action of testosterone on hamster facial nerve regeneration: temporal window of effects. Horm Behav. 2004;45:339–344. doi: 10.1016/j.yhbeh.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Tetzlaff J, Tanzer L, Jones KJ. Cellular localization of androgen and estrogen receptors in mouse-derived motoneuron hybrid cells and mouse facial motoneurons. Dev Neurobiol. 2007a;67:1362–1370. doi: 10.1002/dneu.20505. [DOI] [PubMed] [Google Scholar]
- Tetzlaff J, Tanzer L, Jones KJ. Exogenous androgen treatment delays the stress response following hamster facial nerve injury. J Neuroendocrinol. 2007b;19:383–389. doi: 10.1111/j.1365-2826.2007.01538.x. [DOI] [PubMed] [Google Scholar]
- Tetzlaff JE, Huppenbauer CB, Tanzer L, Alexander TD, Jones KJ. Motoneuron injury and repair: New perspectives on gonadal steroids as neurotherapeutics. J Mol Neurosci. 2006;28:53–64. doi: 10.1385/jmn:28:1:53. [DOI] [PubMed] [Google Scholar]
- Tetzlaff W, Alexander SW, Miller FD, Bisby MA. Response of facial and rubrospinal neurons to axotomy: changes in mRNA expression for cytoskeletal proteins and GAP-43. J Neurosci. 1991;11:2528–2544. doi: 10.1523/JNEUROSCI.11-08-02528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PS, Jr, Fraley GS, Damien V, Woodke LB, Zapata F, Sopher BL, Plymate SR, La Spada AR. Loss of endogenous androgen receptor protein accelerates motor neuron degeneration and accentuates androgen insensitivity in a mouse model of X-linked spinal and bulbar muscular atrophy. Hum Mol Genet. 2006;15:2225–2238. doi: 10.1093/hmg/ddl148. [DOI] [PubMed] [Google Scholar]
- Watson NV, Freeman LM, Breedlove SM. Neuronal size in the spinal nucleus of the bulbocavernosus: direct modulation by androgen in rats with mosaic androgen insensitivity. J Neurosci. 2001;21:1062–1066. doi: 10.1523/JNEUROSCI.21-03-01062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LY, Arnold AP. Interaction of BDNF and testosterone in the regulation of adult perineal motoneurons. J Neurobiol. 2000;44:308–319. doi: 10.1002/1097-4695(20000905)44:3<308::aid-neu2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Yang LY, Verhovshek T, Sengelaub DR. Brain-derived neurotrophic factor and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology. 2004;145:161–168. doi: 10.1210/en.2003-0853. [DOI] [PubMed] [Google Scholar]
- Yu WH. Sex difference in the regeneration of the hypoglossal nerve in rats. Brain Res. 1982;238:404–406. doi: 10.1016/0006-8993(82)90114-7. [DOI] [PubMed] [Google Scholar]
- Yu WHA. Dissection of motor nuclei of trigeminal, facial, and hypoglossal nerves from fresh rat brain. In: Shahar A, de Vellis J, Vernadakis A, Haber B, editors. A Dissection and Tissue Culture Manual of the Nervous System. Alan R. Liss; New York: 1989. pp. 30–39. [Google Scholar]
- Yu WH, Cao CG. Muscle as the primary site for testosterone to promote survival of axotomized motoneurons. Neuroreport. 1991;2:258–260. doi: 10.1097/00001756-199105000-00011. [DOI] [PubMed] [Google Scholar]
- Yu WH, McGinnis MY. Androgen receptors in cranial nerve motor nuclei of male and female rats. J Neurobiol. 2001;46:1–10. doi: 10.1002/1097-4695(200101)46:1<1::aid-neu1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Yu WH, Srinivasan R. Effect of testosterone and 5 alpha-dihydrotestosterone on regeneration of the hypoglossal nerve in rats. Exp Neurol. 1981;71:431–435. doi: 10.1016/0014-4886(81)90101-1. [DOI] [PubMed] [Google Scholar]
- Yu WH, Yu MC. Acceleration of the regeneration of the crushed hypoglossal nerve by testosterone. Exp Neurol. 1983;80:349–360. doi: 10.1016/0014-4886(83)90288-1. [DOI] [PubMed] [Google Scholar]