Abstract
Examination of factors regulating oocyte chromatin remodeling is crucial to circumvent embryonic aneuploidy and resulting defects. Aurora kinases (AURK) are involved in regulation of chromatin remodeling, however, little attention has been paid to AURKs in regard to oocyte maturation. Meiotically incompetent mouse oocytes contain transcripts for all three Aurk isoforms: A, B and C. Upon achieving meiotic competence, oocytes showed significant increases in transcript levels of all three Aurk isoforms and transcript levels remained unchanged as oocytes progressed through meiosis, with AurkA being the predominant isoform. Inhibition of oocyte AURKs during the prophase–metaphase I (MI) transition via inhibitor ZM447439 (ZM) had no effect on germinal vesicle breakdown. However, meiotic spindles were malformed, and microtubule organizing centers and chromatin were scattered. Chromosomal spreads of MI oocytes indicated AURK inhibition resulted in abnormal chromosome condensation. Furthermore, inhibition of AURK during prophase I–MII prevented completion of MII and extrusion of the polar body. Inhibition of AURKs during the MI–MII transition resulted in significantly fewer cells progressing to MII and induced aberrant chromatin remodeling. Further analysis indicated that inhibition of AURKs resulted in absence of histone-H3 phosphorylation at serine 10 and 28. These data suggest a ZM-sensitive AURK may be an oocyte histone-H3 kinase capable of regulating chromatin remodeling throughout oocyte meiosis, both pre- and post-MI.
Keywords: chromosomal disorders, meiosis, oocyte, signal transduction, kinase
Introduction
In vitro oocyte maturation offers immense potential for treatment of infertility, however, current systems are relatively inefficient (Tan et al., 2007). Additionally, mammalian oocytes are notorious for high rates of chromosomal abnormalities (Hassold and Hunt, 2001), resulting in subsequent embryonic aneuploidy, infertility and congenital defects. Unfortunately, components of successful oocyte maturation and regulation of these events remains enigmatic. Therefore, understanding regulatory mechanisms involved in oocyte meiotic maturation, especially those controlling chromatin remodeling, is imperative to establish therapies to improve current assisted reproductive technologies and circumvent oocyte-derived infertility and aneuploidy-induced congenital defects.
Remodeling of chromosomes during oocyte meiosis begins when homologues initially pair and condense via actions of the synaptonemal complex during initiation of prophase I, accompanied by homologous recombination and crossing-over events (Vallente et al., 2006). Chromatin subsequently decondenses as oocytes enter a phase of quiescence prior to completing prophase I. In response to the pre-ovulatory gonadotropin surge, follicle-enclosed oocytes resume meiosis and homologues condense in preparation for a reductional division (Mehlmann, 2005). A bipolar meiotic spindle forms, consisting of polymerized microtubules, and attaches to homologues at their centromeres. Subsequently, physical contact between homologous pairs at chiasmata counteract forces pulling apart homologues, resulting in alignment of chromosomes along the metaphase plate, signaling completion of metaphase I (MI). The meiotic spindle then facilitates separation and segregation as homologues are pulled toward opposite spindle poles at the beginning of anaphase. Oocytes progress through telophase, resulting in disproportionate cytokinesis and extrusion of the first polar body signaling completion of meiosis I (Wang and Sun, 2006). Subsequently, oocytes forego DNA replication and re-arrest at MII.
Reversible phosphorylation, controlled via actions of protein kinases and phosphatases, is an extremely important regulator of oocyte meiosis, and well-suited to the rapid changes required during the cell cycle (see reviews, Swain, 2007; Swain and Smith, 2007). Extensive research has been conducted on the role of kinases such as cyclin-dependant kinase (CDK1) and mitogen-activated protein kinase (MAPK) in mammalian oocytes (Motlik et al., 1998; Abrieu et al., 2001). Additionally, roles for protein phosphatases (PPP) in control of oocyte germinal vesicle breakdown (GVBD) (Alexandre et al., 1991; Gavin et al., 1991, 1994; Swain et al., 2003), spindle dynamics (Alexandre et al., 1991; Lu et al., 2002) and chromatin remodeling have been identified (Mailhes et al., 2003; Swain et al., 2007). However, relatively little attention has been paid to aurora kinases (AURK) in regard to mammalian oocyte maturation.
AURKs are a family of serine/threonine protein kinases responsible for regulating several mitotic cell cycle events important to maintaining proper cellular ploidy, including chromosome condensation, spindle dynamics and cytokinesis (see review, Carmena and Earnshaw, 2003). Thus, considering high rates of aneuploidy associated with oocyte meiosis, AURKs may play an instrumental role in the female gamete was well. Based primarily on research from lower eukaryotes and mitotically dividing mammalian cells, three AURKs exist categorized by sequence differences and subcellular localization; AURKA, AURKB and AURKC (Nigg, 2001). Aurora-A has been identified in mammalian oocytes localized to nuclei in GV-intact oocytes; to the spindle, spindle poles and condensing chromatin during MI; and to spindle poles at MII (Yao and Sun, 2005). Neutralization of oocyte AURKA activity delays GVBD and distorts MI spindle organization (Yao et al., 2004). Aurora-B kinase is known as the equatorial kinase and localizes to condensed chromatin during mitosis and meiosis in lower eukaryotes, where it is thought to regulate chromosome condensation (Hsu et al., 2000; Murnion et al., 2001) and homologue separation (Kaitna et al., 2002; Rogers et al., 2002). Aurora-C kinase is the least studied AURK isoform, previously reported to be testes-specific with localization to centrosomes (Tseng et al., 1998; Kimura et al., 1999; Hu et al., 2000); though transcript for AurkC has now been identified in human cumulus–oocyte complexes (Assou et al., 2006) and reports indicate that it binds to chromosomal passenger proteins during mitosis (Li et al., 2004; Sasai et al., 2004; Yan et al., 2005).
Objectives of this study were to determine which Aurk isoform transcripts are present in oocytes; examine functional roles of AURK activity during various meiotic stages of mouse oocyte maturation, and identify AURK phosphoprotein substrates and targets of actions, focusing primarily on regulation of chromatin remodeling during the first meiosis.
Materials and Methods
All procedures described within were reviewed and approved by The University Committee on Use and Care of Animals at the University of Michigan and were performed in accordance with the Guiding Principles for the Care and Use of Laboratory Animals.
Mouse stimulation and oocyte collection
Meiotically incompetent GV-intact oocytes were collected from 11-day-old female CF1 mice (Harlan, Indianapolis, IN). Meiotically competent GV-intact oocytes were collected from 20–23-day-old CF1 female mice, 42–44 h following injection with 10 IU eCG (Sigma, St Louis, MO). Oocytes were isolated by manual rupturing of antral ovarian follicles in Hepes-buffered human tubal fluid medium (HTFH; Irvine Scientific, Santa Ana, CA) supplemented with 0.3% w/v polyvinylpyrrolidone (Sigma).
RNA isolation, reverse transcription and real-time PCR
Oocyte total RNA was extracted from 50 oocytes at each development stage using Picopure RNA isolation kit (Arcturus Bioscience, Mountain View, CA) following manufacturer’s instructions. Oocyte cDNA was synthesized using 125 pmol random hexamer, 500 µM dNTP, 20 U RNase inhibitor and 62.5 U MultiScribeTM reverse transcriptase (ABI systems) in a final volume 50 µl. Primers for mouse AurkA, AurkB and AurkC were designed with no sequence overlap between isoforms (AurkA—forward primer: 5′ cactagcaaagagccaacca 3′, reverse primer: 5′ ggtggcttcaatagggtgtt 3′; AurkB—forward primer: 5′ cctgacctactgccacaaga 3′, reverse primer: 5′ gccaaagtctgcaatcttca 3′; AurkC—forward primer: 5′ ctgccatgagaagaaggtga 3′, reverse primer: 5′ gtccagagtcccacacattg 3′). Real-time PCR was performed on Applied Biosystems 7300 Real-Time PCR system. Each PCR was performed with 1.5 oocyte equivalents of cDNA added to SYBR Green PCR Master Mix (Applied BioSystems, Foster City, CA). In addition, control reactions were conducted consisting of no template with primers and master mix. Real-time PCR reactions were carried out for 40 cycles (95°C for 15 s, 60°C for 1 min) after initial 10 min incubation at 95°C. Following PCR, products were isolated and run on a 2% agarose gel for 60 min at 100 V to verify size of the amplified product. Additionally, DNA was isolated from gels using QIAquick Gel Extraction Kit (Qiagen, Chatsworth, CA) and subjected to DNA sequencing to verify identity of the product. Standard curve method was used to compare relative abundance of a single Aurk isoform between oocyte meiotic stages using normalization of β-actin levels. Data were collected over three replicates, with triplicate samples for each isoform and fold increases were based on meiotically incompetent GV-intact oocytes levels, which were normalized to 1. Statistical significance was determined using unpaired Student’s t-test, P < 0.05). To examine relative abundance of all three isoforms within a single time point, we ensured primer efficiency of all samples were within a 5% range of an internal β-actin control. We then analyzed data using comparative Ct method.
Oocyte culture and AURK inhibition
Aurora kinase inhibitor ZM447439 (ZM, Astra Zeneca, Wilmington, DE) was dissolved in dimethylsulphoxide (DMSO) to obtain a 10 mM stock. Stock solution was dissolved in HTF to obtain final concentrations of 0.625, 1.25, 2.5, 5, 10 and 20 µM. Control treatments contained DMSO.
To assess effects of AURK inhibition on oocyte maturation, meiotically competent GV-intact oocytes (prophase I) were placed into culture in presence or absence of varying doses of ZM. Oocytes were assessed for GVBD and MII development at 2 and 16 h, respectively. Experiments were performed in triplicate and statistical differences in development were assessed using chi-square analysis with differences considered significant if P < 0.05.
To determine effects of AURK on chromosome condensation and spindle formation during the prophase I to MI transition, prophase I oocytes were matured in vitro to MI (7 h) in the presence or absence of ZM (10 µM) then subjected to immunocytochemistry (ICC) or processed for chromosome spreading. Prophase-I oocytes were also cultured to a time point consistent with MII to assess spindle and chromatin characteristics following extended AURK inhibition.
To assess effects of AURK inhibition on oocyte meiosis during the MI–MII transition, oocytes were matured for 7 or 9 h in the absence of any chemical manipulation to allow normal spindle formation and chromatin remodeling. Oocytes were then cultured to MII (an additional 9 or 7 h) in presence or absence of 10 µM ZM, followed by assessment of chromatin positioning and spindle configuration. All experiments were performed in triplicate and nonparametric parameters were analyzed for significant differences by Chi-square.
Finally, to begin to determine substrates for oocyte AURK, histone-H3 phosphorylation at ser10 and ser28 was assessed following AURK inhibition at various time points utilizing ICC and western blot analysis.
Immunocytochemistry
To examine effects of AURK inhibition on spindle formation and metaphase chromosome positioning, MI and MII oocytes were attached to poly-lysine coated coverslips, and fixed in 2% (w/v) paraformaldehyde with 0.05% (v/v) Triton X-100 in phosphate-buffered saline (PBS) (pH = 7.3) for 30 min. Oocytes were then blocked overnight with 2% (w/v) bovine serum albumin, 0.1 M glycine and 5% (w/v) dry milk in PBS at 4°C. Oocytes were incubated with β-tubulin antibody (Sigma, 1:200) and pericentrin antibody (Abcam, 1:1000). Negative controls included non-immune mouse serum in place of primary antibody. After three 5 min washes with blocking solution, samples were reacted with the appropriate Alexa 568 and 488 conjugated secondary antibodies (Molecular Probes) at a 1:750 dilution for 1 h at 37°C. Following washing, slides were incubated with Hoescht 33 342 (1 µg/ml) in PBS for 20 min at 37°C. Coverslips were then mounted on glass slides with 90% glycerol in PBS for fluorescence microscopic visualization under ×1000 on a confocal microscope.
Chromosomal spreading and analysis
Following culture to MI in ZM (10 µM), oocytes were collected and prepared for chromosomal spreading (Hodges and Hunt, 2002). Briefly, zona pellucida were removed by exposure to 1% pronase in HTFH. Zona-free oocytes were then washed and fixed by carefully placing them onto a microscope slide dipped in a solution of 1% paraformaldehyde in distilled water (pH 9.2) containing 0.15% Triton X and 3 mM dithiothreitol. Slides were then placed into a humidified chamber overnight before being subjected to triplicate 5 min washes in PBS and air-dried at room temperature. To analyze chromosomal condensation, slides were placed into a 1% solution of Hoescht 33 342 in PBS for 10 min and subjected to three more washes in PBS. Glycerol mounting solution and a coverslip were added and slides were sealed. Chromosomal spreads were analyzed blind to treatment at ×1000 on a Leica DMR microscope. Statistical differences between treatment groups were analyzed using chi-square analysis.
Electrophoresis and western blot analysis
To assess effects of AURK inhibition on oocyte histone-H3 phosphorylation, groups of oocytes (n = 100) were prepared for western blot analysis. Oocytes were placed in 2× sodium dodecyl sulphate (SDS)–polyacrylamide gel electrophoresis (PAGE) sample loading buffer [80 mM Tris–HCl (pH = 6.8), 20% glycerol, 4% SDS, 4% β-mercaptoethanol, 0.04% bromophenol blue], vortexed and placed on ice for 15 min. Following sonication on ice for 10 s, samples were denatured at 90°C for 10 min and loaded for electrophoresis. Total protein from equal numbers of mouse oocytes was loaded in each lane and separated by one-dimensional SDS–PAGE. Resolving gels were cast using 12% acrylamide; stacking gels contained 5% acrylamide. HeLa cell histone lysate was used as a positive control for recognizing phospho-ser10. Gels were equilibrated and transferred to Hybond-P PVDF transfer membrane (Amersham Life Science, Little Chalfont Buckinghamshire, UK) by Semi-Dry Electrophoretic Transfer Cell (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instructions. Blots were blocked in 5% nonfat milk in Tris-buffered saline (TBS)+0.5% Tween (TBST) at room temperature for 1 h and incubated with the appropriate primary antibody diluted in TBST + 5% nonfat milk overnight at 4°C with agitation. Antibodies included anti-phospho-ser10-histone H3 (1:1000, Upstate) and anti-phospho-ser28-histone-H3 antibody (1:500, Upstate). After complete washing in TBST, blots were incubated with the appropriate horse-radish peroxidase-conjugated IgG secondary antibody (diluted 1:2000) at room temperature for 1 h, washed in TBST and developed with ECL Plus reagents (Amersham Life Sciences) according to the manufacturer’s instructions. To verify equal protein loading of lanes to allow densitometric analysis blots were stripped for 30 min in a 50°C water bath with agitation in a stripping buffer (62.5 mM Tris–HCl, pH 6.7, 100 mM β-mercaptoethanol and 2% SDS). Completely stripped blots were blocked in 5% nonfat milk in TBST for 1 h at room temperature, then incubated with histone-H3 antibody (diluted 1:1000, Chemicon) overnight at 4°C with agitation and processed further as described above. Band densities were assessed using NIH imaging software, Image J.
Results
AURK isoforms in mouse oocytes
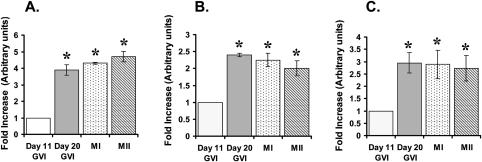
To determine specific AURK isoforms present in mouse oocytes, isoform-specific primers were designed for AurkA, AurkB and AurkC. Transcripts were amplified utilizing real-time PCR for all three isoforms in meiotically incompetent GV-intact oocytes. Significant increases in transcript levels for all three Aurk isoforms were identified in meiotically competent GV-intact and meiotically maturing oocytes, compared with incompetent oocytes (Fig. 1). No differences in transcript levels were identified for any isoform between meiotically competent GV-intact, GVBD, MI or MII oocytes. Additionally, AurkA appeared to be the predominant isoform transcript, displaying an approximate 12-fold increase compared with AurkB and AurkC, which displayed comparable levels, in meiotically competent GV-intact oocytes (data not shown). Single bands were present on agarose gels for each isoform, corresponding to predicted sizes (AurkA—90 bp, AurkB—100 bp, AurkC—145 bp). Sequencing verified that amplified gene products shared 100% homology with mouse Aurk isoforms (data not shown).
Figure 1:
Graphical representation demonstrating fold-increases of aurora kinase (Aurk) isoform levels between various oocyte types obtained from real-time PCR.
Day 11 germinal vesicle intact (GVI) oocytes were used at controls and normalized to 1. (A) AurkA, (B) AurkB and (C) AurkC. Presence of an asterick represents statistical significance compared with groups without an asterick, P < 0.05.
AURKs and oocyte meiotic progression
To determine the influence of AURK on oocyte meiotic progression, meiotically competent GV-intact oocytes (prophase I) were matured in varying doses of the AURK inhibitor ZM and development was checked at 2 and 16 h. Though a significant reduction was observed with 2.5 μM, Inhibition of AURK had no effect on oocyte GVBD at 2 h at any other dose examined compared with control treatments (Table I). Furthermore, no differences were visually apparent at the light microscope level between ZM-treated and control oocytes at a time point consistent with MI development (7 h; Fig. 2, inset). However, concentrations of ZM at 2.5, 5, 10 and 20 µM prevented all oocytes from progressing to MII at 16 h (Fig. 3). Concentrations of 1.25 and 0.675 µM allowed a small portion of oocytes to extrude the first polar body at 16 h, which was significantly less than the percentage of MII oocytes obtained from control treatments, P < 0.01 (Table I). Additionally, culturing GV-intact oocytes for 7 h to MI in presence of 5 or 10 µM ZM, followed by thorough washing and 9 h of culture in the absence of the AURK inhibitor, indicated that, although ZM may be washed out from blocking the ATP binding pockets, defects caused by ZM treatment were not reversible, as oocytes were unable to complete meiosis and extrude the first polar body (data not shown). Based on these data, a dose of 10 µM ZM was selected for future experiments to ensure all AURK isoforms were inhibited. This dose has been used in other studies on mammalian oocytes (Jelinkova and Kulbeka, 2006), and is lower than the 20 µM used in other studies (Gadea and Ruderman, 2005).
Table I.
Development of mouse oocytes following treatment in varying doses of Aurora kinase inhibitor ZM447439 (ZM).
| ZM concentration (µM) | 2 h GVBD | 16 h MII |
|---|---|---|
| 0 | 115/121 (95%)a | 38/52 (73%)a |
| 0.625 | 29/30 (97%) | 8/30 (27%)b |
| 1.25 | 30/35 (86%) | 8/26 (31%)b |
| 2.5 | 84/99 (85%)b | 0/54 (0%)c |
| 5 | 59/66 (89%) | 0/28 (0%)c |
| 10 | 55/64 (86%) | 0/28 (0%)c |
| 20 | 55/64 (85%) | 0/28 (0%)c |
Significant differences in development between treatments within a time point are indicated by different superscripts, P < 0.01.
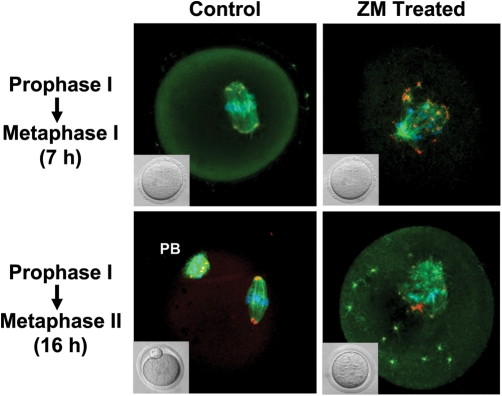
Figure 2:
Representative micrographs demonstrating effect of aurora kinase (AURK) inhibition on oocyte meiotic spindle formation.
Inhibition of mouse oocyte AURKs with 10 µM ZM447439 (ZM) for 7 h had no observable effect on oocyte development at the light microscope level (inset images). However, immunocytochemical examination demonstrated that morphology of the meiotic spindle of ZM-treated oocytes appeared abnormal, with polymerized microtubules (β-tubulin: green), microtubule organizing centers MTOCS (pericentrin: red/orange) and chromatin (blue) was scattered around the metaphase plate, compared with vehicle treated controls. Furthermore, 16 h treatment of oocytes with ZM resulted in arrest of oocytes at a MI-like stage (inset). Immunocytochemical examination demonstrated aberrant polymerized microtubules, MTOCs and condensed chromatin (blue) and MTOCs (red/orange). Polar body of control MII oocytes is indicated by PB.
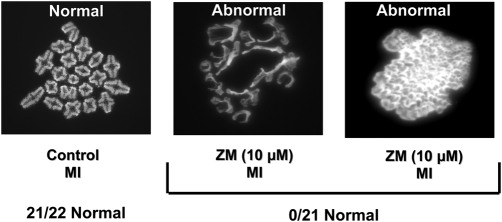
Figure 3:
Representative micrographs of oocyte chromosome spreads demonstrating negative impact of AURK inhibition on oocyte chromatin remodeling.
Treatment of oocytes with ZM447439 (ZM-10 µM) for 7 h resulted in metaphase I oocytes (MI) with significantly greater defects in bivalent formation, compared with controls (P < 0.0001).
AURKs and oocyte spindle morphology and chromatin remodeling
Prophase–MI transition
To begin to determine a temporal window when AURK inhibition may be conveying observed phenotypes, experiments examined the effects of AURK inhibition during the prophase I–MI transition. Aberrant spindle morphology and improper positioning of chromatin were observed in oocytes cultured for 7 h in presence of 10 µM ZM (19% normal, n = 32) compared with controls (91% normal, n = 35; P < 0.0001; Fig. 2). Similar patterns were also obtained from treatments containing 2.5, 5 and 20 µM ZM (data not shown).
To examine effects of AURK inhibition on oocyte chromosome condensation in greater detail, chromosomal spreads of MI oocytes were examined following 7 h of ZM (10 µM) treatment from prophase I. Inhibition of AURKs resulted in oocytes with a significant reduction in normal chromosome condensation (0%, n = 21) compared with control treatments (95%, n = 22), as evidenced by the inability to resolve bivalents, P < 0.001 (Fig. 3).
Prophase I–MII transition
Culture of oocytes for 16 h in 10 µM ZM during the prophase I–MII transition indicated that arrest of oocytes was not due to inability of microtubule polymerization as β-tubulin staining indicated microtubules polymerized around chromatin (Fig. 2). Additionally, pericentrin staining indicated apparent microtubule organizing centers (MTOC) assembly. However, spindle formation and MTOC localization were disrupted compared with untreated controls. Furthermore, chromatin was scattered throughout the meiotic spindle, with the pattern of normal chromatin remodeling significantly reduced following ZM treatment (0%, n = 44) compared with controls (81%, n = 32), P < 0.001.
MI–MII transition
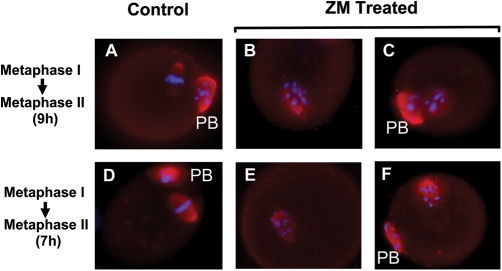
To determine effect of AURK inhibition following normal chromatin remodeling and spindle formation, oocytes were cultured for 7 or 9 h in the absence of ZM (cells typically at MI or AI, respectively). Subsequently, these oocytes were cultured to a time point consistent with MII (an additional 9 or 7 h) in presence or absence of ZM. Treatment of oocytes for 9 h with ZM resulted in significantly less cells progressing to MII (53%, n = 117) compared with 80% of control oocytes (n = 121), P < 0.05. Treatments of oocytes for 7 h with ZM also resulted in significantly fewer cells progressing to MII (58%, n = 88), compared with controls (73%, n = 78; P < 0.05). All MII oocytes obtained following ZM treatment displayed scattered chromatin around the metaphase plate and slightly irregular shaped spindles (Fig. 4).
Figure 4:
Representative micrographs of oocytes cultured to a time point consistent with MII in presence or absence of 10 µM AURK inhibitor ZM447439 during the MI-MII transition.
Control MI oocytes developed normally to MII, extruding the first polar body and displaying condensed chromatin on the metaphase plate (blue) within the meiotic spindle with normally condensed β-tubulin (red) (A). However, following AURK inhibition, a portion of oocytes arrested prior to MII, displaying scattered chromatin within a malformed meiotic spindle (B). Those oocytes that did complete MII under AURK inhibition displayed disorganized chromatin within the meiotic spindle (C). Similar patterns of chromatin disorganization and inability to complete cytokinesis and progress to MII were also observed following culture of oocytes to MII (7 h) following AURK inhibition from a time point where cells are beginning to enter anaphase (E and F) compared with controls (D).
AURKs and oocyte histone phosphorylation
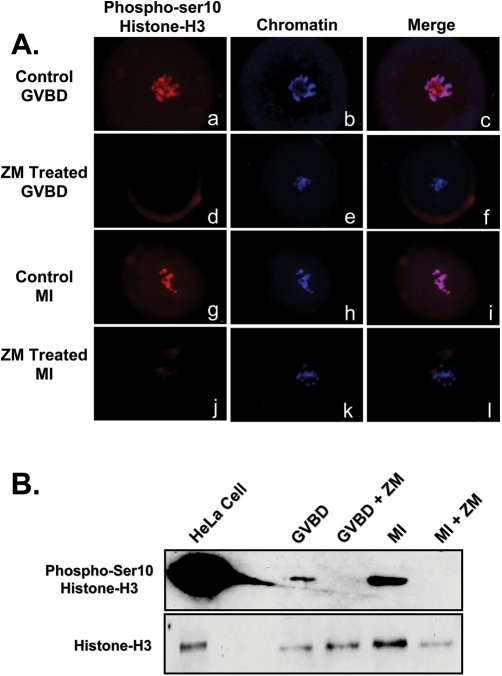
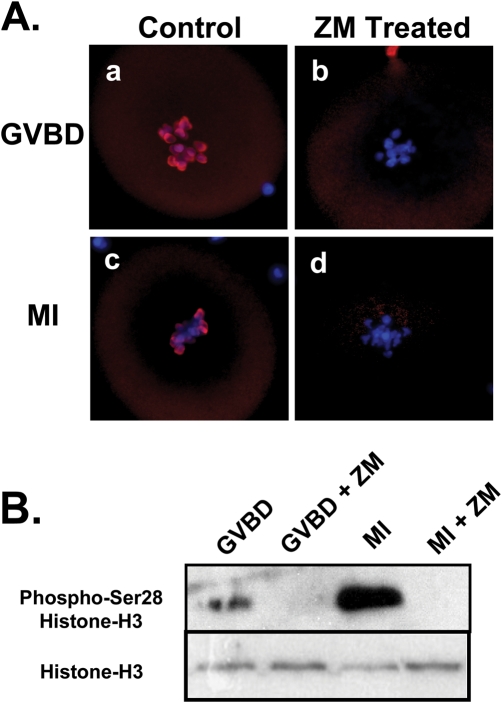
Because of reported roles for histone-H3 phosphorylation in chromatin remodeling, phosphorylation of histone-H3 following AURK inhibition was examined as a possible cause of aberrant condensation during the prophase I to MI transition. Western blot analysis and ICC utilizing phospho-ser10 histone-H3 antibody was performed. Inhibition of AURK for 2 or 7 h with 10 µM ZM resulted in a total lack of histone-H3 ser10 phosphorylation (Fig. 5A and B). Treatment of oocytes with ZM also completely inhibited phosphorylation of histone-H3 at ser28, as evidenced by ICC and western blot (Fig. 6A and B). These data raise the possibility that a ZM-sensitive AURK is an oocyte histone-H3 kinase and that histone-H3 phosphorylation may be influencing normality of oocyte metaphase chromatin condensation and subsequent separation and segregation of homologues.
Figure 5:
Representative micrographs and western blot demonstrating inhibition of oocyte aurora kinases (AURK) results in ser10-histone-H3 hypophosphorylation.
(A) Culture of GV-intact oocytes for 2 h to allow germinal vesicle breakdown(GVBD) in presence of 10 µM ZM447439 resulted in a total lack of ser10-Histone-H3 phosphorylation (d), compared with controls (a). A similar reduction in ser10 phosphorylation was observed following 7 h (MI) of culture in the presence of ZM (j) compared with controls (g). Chromatin was stained with Hoescht and is pictured in blue (b,e,h,k). Overlays are also indicated (c,f,i,l). (B) Western blot analysis confirmed that ZM treatment inhibits ser10 phosphorylation (n = 100 oocytes/lane).
Figure 6:
Representative micrographs and western blot demonstrating inhibition of oocyte aurora kinases (AURK) results in ser28-histone-H3 hypophosphorylation.
Chromatin is stained in blue, while phospho-ser28-histone-H3 is stained in red. (A) Culture of GV-intact oocytes for 2 h to allow germinal vesicle breakdown(GVBD) in presence of 10 µM ZM447439 (ZM) resulted in a total lack of ser28 phosphorylation (b), compared with controls (a). A similar reduction in ser28 phosphorylation was observed following 7 h (MI) of culture in the presence of ZM (d) compared with controls (c). (B) Western blot analysis confirmed that ZM treatment inhibits ser28 phosphorylation (n = 100 oocytes/lane).
Discussion
Maintaining integrity of chromatin remodeling is especially important in the oocyte considering its extreme susceptibility to aneuploidy, primarily during the first meiosis (Hassold and Hunt, 2001). AURKs are a family of serine/threonine kinases that regulate various structural elements and mechanistic events associated with the dynamic process of chromatin remodeling. Therefore, examination of AURKs during oocyte maturation is of interest when attempting to discern causative factors and molecular signaling pathways involved in aberrant oocyte chromosome modifications. We have determined that mouse oocytes contain transcripts for all three Aurk isoforms: AurkA, AurkB and AurkC and that levels of these transcripts increase significantly as oocytes gain meiotic competence, but do not change as oocytes progress through meiosis to MII. The predominant Aurk isoform transcript in meitocally competent and maturing oocytes appears to be AurkA. In agreement with these findings, during preparation of this manuscript, transcripts for AurkA, AurkB and AurkC were also reported in fully-grown immature bovine oocytes, with AurkA as the predominant isoform (Uzbekova et al., 2007).
To determine effects of AURK on oocyte maturation, we utilized the highly selective pharmacological AURK inhibitor, ZM. Inhibition of AURKs via ZM occurs through blockage of the ATP binding site at an adjacent cleft not present in other kinases (Ditchfield et al., 2003). Although ZM is a selective inhibitor of AURKs, it does inhibit other kinases, including CDK1, MAPK and CDC25. However, concentrations of ZM much higher than those utilized in the majority of our studies (20 µM) had no effect on CDK1, CDC25 or MAPK activities in Xenopus egg extracts, indicating ZM did not directly affect these kinases, or affect any upstream regulatory kinases involved in their activation (Gadea and Ruderman, 2005). Furthermore, cellular characteristics and phosphorylation patterns following inhibition of CDK1 (Marchal et al., 2001; Kubelka et al., 2002; Swain et al., 2003; Bui et al., 2004) and MAPK (Tong et al., 2003; Yu et al., 2007) in mammalian oocytes are dramatically different than those observed following ZM treatment in this study; suggesting observed effects are indeed the result of AURK inhibition. It should be mentioned that ZM displays differential inhibitory action toward different AURK isoforms, demonstrating inhibition of AURKB ∼20 times more potently than AURKA (Girdler et al., 2006). Thus, differential phenotypes observed in our study using lower concentrations of ZM may indicate AURKB-specific functions. Future experiments will attempt to determine if lower doses of ZM, as well as utilization of other AURK inhibitors with differential inhibitory actions, can verify this and determine isoform-specific functions within the mammalian oocyte.
AURK do not appear to play a role in oocyte meiotic resumption or regulation of oocyte nuclear envelope (NE) integrity during GVBD. Although ZM appeared to cause a slight delay in NE disassembly, no significant differences were apparent at any dose of ZM examined other than 2.5 μM. This is in agreement with findings that inhibition of bovine oocyte AURKs with VX680 has no effect on GVBD (Uzbekova et al., 2007). However, these findings are in contradiction to a study utilizing pig oocytes, which demonstrated lack of GVBD following exposure to elevated doses of ZM (7–10 µM; Jelinkova and Kubelka, 2006). Difference may be the result of varying experimental conditions or species-specific differences, as Jelinkova and Kulbelka (2006) utilized porcine cumulus–oocyte complexes from abattoir ovaries, whereas the current study utilized denuded oocytes obtained from gonadotropin stimulated mice.
In our study, inhibition of AURKs during 16 h of culture inhibited progression to MII and polar body extrusion, similar to developmental observations in clam oocytes following treatment with another AURK inhibitor, Hesperadin (George et al., 2006), and bovine oocytes treated with VX680 (Uzbekova et al., 2007). Failure to complete cytokinesis has also been observed following AURK inhibition during mitosis (Ditchfield et al., 2003). Evidence exists suggesting AURKB may be the specific isoform responsible for failure of oocytes to extrude the first polar body, as specific inhibition of AURKB in Drosophila cells prevented cytokinesis (Adams et al., 2001; Giet and Glover, 2001). Interestingly, AURKB was localized to the region of the contractile ring in bovine MII oocytes; (Uzbekova et al., 2007). However, AURKA and AURKC were also found in the vicinity of the contractile ring in bovine oocytes, confounding interpretation. Whether failure to complete cytokinesis and extrude the polar body in our study is a primary effect of AURK inhibition on cytokinesis, or a secondary effect due to chromosome remodeling or spindle defects remains to be elucidated.
To begin to determine possible causes for oocyte arrest prior to MII, we examined effects of AURK inhibition on chromatin remodeling and spindle formation during the prophase I–MI transition. Inhibition of AURKs during this time point resulted in improper positioning of chromatin, as evidenced by scattering throughout the meiotic spindle. This is in agreement with recent studies of AURK inhibition in mouse (Wang et al., 2006), pig (Jelinkova and Kubelka, 2006) and bovine oocytes (Uzbekova et al., 2007) reporting abnormal chromosome positioning at MI. Additionally, chromosomal spreading in our study indicates AURK inhibition negatively affects ability of MI oocyte chromosomes to condense properly and resolve bivalents. This may be due to premature decondensation, similar to that observed following AURK inhibition in Xenopus egg extracts (Gadea and Ruderman, 2005). It has been reported that AURKB inhibition via RNAi is responsible for chromosome misalignment in Drosophila cultured cells and results in amorphous chromatin (Adams et al., 2001) and only partial condensation (Giet and Glover, 2001). Thus, AURKB may be the AURK isoform responsible for chromatin defects observed in mammalian oocytes in this study. Indeed, a recent report demonstrates AURKB localized to condensed chromatin in MI and MII bovine oocytes (Uzbekova et al., 2007).
Failure of ZM-treated oocytes to progress to MII does not appear to be the result of inability to polymerize microtubules or form MTOCs, as indicated by β-tubulin and pericentrin staining. This is in contrast to AURK inhibition studies in Xenopus egg extracts, where ZM treatment resulted in failure to form the mitotic spindle (Gadea and Ruderman, 2005). However, fidelity of spindle function in our study remains in question, as spindle morphology and MTOC localization was disrupted following AURK inhibition. AURKs, such as AURKA, regulate several components of the spindle apparatus and spindle poles (see review, Ducat and Zheng, 2004). Indeed, AURKA localized to spindle poles in mouse (Yao et al., 2004) and pig oocytes (Yao and Sun, 2005), but not bovine oocytes (Uzbekova et al., 2007) and specific neutralization of the kinase resulted in disorganization of the meiotic spindle. This disorganization is in agreement with initial reports of ZM influences on intact somatic cells (Ditchfield et al., 2003). Thus ZM inhibition of oocyte AURKA may account for observed defective spindle phenotypes. Alternatively, condensed chromosomes direct formation of the spindle apparatus via nucleation/stabilization of microtubules (Merdes and Cleveland, 1997; Khodjakov et al., 2000). Therefore, defects in meiotic spindle morphology and scattering of chromatin observed in these experiments may be the result of aberrant chromatin condensation, possibly controlled by AURKB (see review, Shannon and Salmon, 2002). Reports in Xenopus mitotic cell-free extracts indicate AURK inhibition does interfere with chromatin driven microtubules assembly (Gadea and Ruderman, 2005).
To begin to determine if the inability of ZM-treated oocytes to reach MII following AURK inhibition was due only to defects incurred during the prophase–MI transition, or if AURKs had roles at other meiotic transition time points, we matured oocytes in vitro to time points consistent with MI and AI oocytes, thus allowing normal spindle formation and chromatin remodeling to occur. Subsequently, we then cultured oocytes in presence of ZM to a time point consistent with MII. Inhibition of AURKs during the MI-MII transition resulted in a portion of oocytes failing to segregate chromosomes and extrude the first polar body, while those that did complete MII displayed severely scattered chromatin. Thus, it appears as if AURKs may not only control spindle formation and chromatin condensation during early meiotic events, but also regulate separation and/or segregation of oocyte chromosomes during later meiosis. Effects may be directly on meiotic spindle components, possibly regulated by AURKA. Alternatively, defective chromosome remodeling could also be due to ZM inhibition of AURKB. Aurora B regulates kinetochores and their interactions with microtubules (Kaitna et al., 2002; Cimini et al., 2006). Another possible explanation for aberrant separation/segregation following AURK inhibition during late oocyte meiosis may be interferences with regulation of cohesion. Aurora B regulates release of chromosome cohesion during meiosis in Caenorhabditis elegans, apparently via phosphorylation of REC-8 (Rogers et al., 2002). Future studies will attempt to determine if defects include premature separation of sister chromatids due to premature release of cohesion, or if aberrant phenotypes are the result of failure to separate homologous chromosomes.
To begin to determine possible targets of oocyte AURKs responsible for observed defects in chromosomal remodeling, we examined the phoshorylation state of histone-H3 at ser10 and ser28. It is thought that phosphorylation of histone-H3 may cause the histone to act as a receptor or recruitment factor for condensation factors (Hirano, 2000), or possibly reduce the affinity of histone-H3 for DNA and make the relatively compact chromatin fiber more readily accessible to remodeling factors (Hirano, 2000), such as the condensin complex. Condensin is a multi-subunit protein complex that play a central role in chromosome compaction and condensation and is reported to co-localize and bind with phosphorylated histone-H3 (Schmiesing et al., 2000; Ball et al., 2002). Interestingly, AURKB controls association of condensin with chromosomes during mitosis (Lipp et al., 2007; Takemoto et al., 2007), and may be functioning in a similar manner during mammalian oocyte meiosis, though this remains unknown. It should be mentioned that reports for the requirements of histone-H3 phosphorylation in chromatin condensation varies greatly (Van Hooser et al., 1998; Wei et al., 1998, 1999; Goto et al., 1999; Kaszas and Cande, 2000; Schmitt et al., 2002). These contradictions also appear to hold true in regard to oocyte meiosis, with differing reports on temporal and spatial localization and correlation with condensation (Jelinkova and Kubelka, 2006; Wang et al., 2006; Swain et al., 2007). However, contradictions may be explained by differences in experimental conditions or perhaps species-specific differences. Regardless, contradictions in the literature make it increasingly evident that differences in the role of histone phosphorylation exist depending on the organism and/or type of cellular division examined (Fuchs et al., 2006), and that the specific histone-H3 AURK within mammalian oocytes remains unknown.
In summary, these studies indicate AURK plays a significant role in mouse oocyte maturation involving progression to MII, acting in both early (prophase–MI transition) and late meiotic events (MI–MII transition). When AURK is inhibited in early meiosis, microtubules polymerize and MTOCs form, but spindle morphology and MTOC localization is disrupted. Furthermore, metaphase chromatin does not condense or position normally. Inhibition of oocyte AURKs during late meiosis (MI–MII transition), following chromatin condensation and spindle formation, negatively affects separation and segregation of chromosomes. This aberrant chromatin remodeling following AURK inhibition in oocytes appears to be due, in part, to hypophosphorylation of histone-H3 at both ser10 and ser28. We report amplification and sequencing of all three Aurk isoforms transcripts: AurkA, AurkB and AurkC. Levels of these transcripts increase as oocytes achieve meiotic competence, with AurkA being the predominant isoform. Future studies will focus on determining protein expression levels of AURK isoforms, as well as specific functional roles and intracellular targets.
Funding
Funding for research by Gary D. Smith provided by NIH RO1 grant #HD046768-01A2. Support for Jason E. Swain provided by an NIH T32 grant. Partial support for Jingwen Wu provided from the Lyle C. Roll Research Fund to Professor James O. Woolliscroft, Dean of Medical School, University of Michigan.
Acknowledgements
Authors would like to thank Dr Carrie Cosola-Smith for critical reading of this manuscript and Irvine Scientific for supply of media.
References
- Abrieu A, Doree M, Fisher D. The interplay between cyclin-B-Cdc2 kinase (MPF) and MAP kinase during maturation of oocytes. J Cell Sci. 2001;114:257–267. doi: 10.1242/jcs.114.2.257. [DOI] [PubMed] [Google Scholar]
- Adams RR, Maiato H, Earnshaw WC, Carmena M. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J Cell Biol. 2001;153:865–880. doi: 10.1083/jcb.153.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre H, Van Cauwenberge A, Tsukitani Y, Mulnard J. Pleiotropic effect of okadaic acid on maturing mouse oocytes. Development. 1991;112:971–980. doi: 10.1242/dev.112.4.971. [DOI] [PubMed] [Google Scholar]
- Assou S, Anahory T, Pantesco V, Le Carrour T, Pellestor F, Klein B, Reyftmann L, Dechaud H, De Vos J, Hamamah S. The human cumulus–oocyte complex gene-expression profile. Hum Reprod. 2006;21:1705–1719. doi: 10.1093/humrep/del065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball AR, Jr, Schmiesing JA, Zhou C, Gregson HC, Okada Y, Doi T, Yokomori K. Identification of a chromosome-targeting domain in the human condensin subunit CNAP1/hCAP-D2/Eg7. Mol Cell Biol. 2002;22:5769–5781. doi: 10.1128/MCB.22.16.5769-5781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui HT, Yamaoka E, Miyano T. Involvement of histone H3 (Ser10) phosphorylation in chromosome condensation without CDC2 kinase and mitogen-activated protein kinase activation in pig oocytes. Biol Reprod. 2004;70:1843–1851. doi: 10.1095/biolreprod.103.026070. [DOI] [PubMed] [Google Scholar]
- Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842–854. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- Cimini D, Wan X, Hirel CB, Salmon ED. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol. 2006;16:1711–1718. doi: 10.1016/j.cub.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161:267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducat D, Zheng Y. Aurora kinases in spindle assembly and chromosome segregation. Exp Cell Res. 2004;301:60–67. doi: 10.1016/j.yexcr.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Fuchs J, Demidov D, Houben A, Schubert I. Chromosomal histone modification patterns—from conservation to diversity. Trends Plant Sci. 2006;11:199–208. doi: 10.1016/j.tplants.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Gadea BB, Ruderman JV. Aurora kinase inhibitor ZM447439 blocks chromosome-induced spindle assembly, the completion of chromosome condensation, and the establishment of the spindle integrity checkpoint in Xenopus egg extracts. Mol Biol Cell. 2005;16:1305–1318. doi: 10.1091/mbc.E04-10-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin A-C, Tsukitani Y, Schorderet-Slatkine S. Induction of M-phase entry of prophase-blocked mouse oocytes through microinjection of okadaic acid, a specific phosphatase inhibitor. Exp Cell Res. 1991;192:75–81. doi: 10.1016/0014-4827(91)90159-r. [DOI] [PubMed] [Google Scholar]
- Gavin A-C, Cavadore JC, Schorderet-Slatkine S. Histone H1 kinase activity, germinal vesicle breakdown and M phase entry in mouse oocytes. J Cell Sci. 1994;107:275–283. doi: 10.1242/jcs.107.1.275. [DOI] [PubMed] [Google Scholar]
- George O, Johnston MA, Shuster CB. Aurora B kinase maintains chromatin organization during the MI to MII transition in surf clam oocytes. Cell Cycle. 2006;5:2648–2656. doi: 10.4161/cc.5.22.3444. [DOI] [PubMed] [Google Scholar]
- Giet R, Glover DM. Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J Cell Biol. 2001;152:669–682. doi: 10.1083/jcb.152.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler F, Gascoigne KE, Eyers PA, Hartmuth S, Crafter C, Foote KM, Keen NJ, Taylor SS. Validating Aurora B as an anti-cancer drug target. J Cell Sci. 2006;119:3664–3675. doi: 10.1242/jcs.03145. [DOI] [PubMed] [Google Scholar]
- Goto H, Tomono Y, Ajiro K, Kosako H, Fujita M, Sakurai M, Okawa K, Iwamatsu A, Okigaki T, Takahashi T, et al. Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J Biol Chem. 1999;274:25543–25549. doi: 10.1074/jbc.274.36.25543. [DOI] [PubMed] [Google Scholar]
- Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- Hirano T. Chromosome cohesion, condensation, and separation. Annu Rev Biochem. 2000;69:115–144. doi: 10.1146/annurev.biochem.69.1.115. [DOI] [PubMed] [Google Scholar]
- Hodges C, Hunt P. Simultaneous analysis of chromosomes and chromosome-associated proteins in mamalian oocytes and embryos. Chromosoma. 2002;111:165–169. doi: 10.1007/s00412-002-0195-3. [DOI] [PubMed] [Google Scholar]
- Hsu J, Sun Z, Li X, Reuben M, Tatchell K, Bishop D, Grushcow J, Brame C, Caldwell J, Hunt D, et al. Mitotic phosporylation of histone H3 Is governed by Ipl1/aurora kinase and GLC7/PP1 phosphatase in budding yeast and nematodes. Cell. 2000;102:279–291. doi: 10.1016/s0092-8674(00)00034-9. [DOI] [PubMed] [Google Scholar]
- Hu HM, Chuang CK, Lee MJ, Tseng TC, Tang TK. Genomic organization, expression, and chromosome localization of a third aurora-related kinase gene, Aie1. DNA Cell Biol. 2000;19:679–688. doi: 10.1089/10445490050199063. [DOI] [PubMed] [Google Scholar]
- Jelínková L, Kubelka M. Neither Aurora B activity nor histone H3 phosphorylation is essential for chromosome condensation during meiotic maturation of porcine oocytes. Biol Reprod. 2006;74:905–912. doi: 10.1095/biolreprod.105.047886. [DOI] [PubMed] [Google Scholar]
- Kaitna S, Pasierbek P, Jantsch M, Loidl J, Glotzer M. The aurora B kinase AIR-2 regulates kinetochores during mitosis and is required for separation of homologous Chromosomes during meiosis. Curr Biol. 2002;12:798–812. doi: 10.1016/s0960-9822(02)00820-5. [DOI] [PubMed] [Google Scholar]
- Kaszas E, Cande WZ. Phosphorylation of histone H3 is correlated with changes in the maintenance of sister chromatid cohesion during meiosis in maize, rather than the condensation of the chromatin. J Cell Sci. 2000;113:3217–3226. doi: 10.1242/jcs.113.18.3217. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Cole RW, Oakley BR, Rieder CL. Centrosome-independent mitotic spindle formation in vertebrates. Curr Biol. 2000;10:59–67. doi: 10.1016/s0960-9822(99)00276-6. [DOI] [PubMed] [Google Scholar]
- Kimura M, Matsuda Y, Yoshioka T, Okano Y. Cell cycle-dependent expression and centrosome localization of a third human aurora/Ipl1-related protein kinase, AIK3. J Biol Chem. 1999;274:7334–7340. doi: 10.1074/jbc.274.11.7334. [DOI] [PubMed] [Google Scholar]
- Kubelka M, Anger M, Kalous J, Schultz R, Motlik J. Chromosome condensation in pig ooctyes: lack of a requirement for either cdc2 kinase or MAP kinase activity. Mol Reprod Dev. 2002;63:110–118. doi: 10.1002/mrd.10176. [DOI] [PubMed] [Google Scholar]
- Li X, Sakashita G, Matsuzaki H, Sugimoto K, Kimura K, Hanaoka F, Taniguchi H, Furukawa K, Urano T. Direct association with inner centromere protein (INCENP) activates the novel chromosomal passenger protein, Aurora-C. J Biol Chem. 2004;279:47201–47211. doi: 10.1074/jbc.M403029200. [DOI] [PubMed] [Google Scholar]
- Lipp JJ, Hirota T, Poser I, Peters JM. Aurora B controls the association of condensin I but not condensin II with mitotic chromosomes. J Cell Sci. 2007;120:1245–1255. doi: 10.1242/jcs.03425. [DOI] [PubMed] [Google Scholar]
- Lu Q, Dunn R, Angeles R, Smith G. Regulation of spindle formation by active mitogen-activated protein kinase and protein phosphatase 2A during mouse oocyte meiosis. Biol Reprod. 2002;66:29–37. doi: 10.1095/biolreprod66.1.29. [DOI] [PubMed] [Google Scholar]
- Mailhes JB, Hilliard C, Fuseler JW, London SN. Okadaic acid, an inhibitor of protein phosphatase 1 and 2A, induces premature separation of sister chromatids during meiosis I and aneuploidy in mouse oocytes in vitro. Chromosome Res. 2003;11:619–631. doi: 10.1023/a:1024909119593. [DOI] [PubMed] [Google Scholar]
- Marchal R, Tomanek M, Terqui M, Mermillod P. Effects of cell cycle dependent kinases inhibitor on nuclear and cytoplasmic maturation of porcine oocytes. Mol Reprod Dev. 2001;60:65–73. doi: 10.1002/mrd.1062. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130:791–799. doi: 10.1530/rep.1.00793. [DOI] [PubMed] [Google Scholar]
- Merdes A, Cleveland DW. Pathways of spindle pole formation: different mechanisms; conserved components. J Cell Biol. 1997;138:953–956. doi: 10.1083/jcb.138.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motlik J, Pavlok A, Kubelka M, Kalous J, Kalab P. Interplay between CDC2 kinase and MAP kinase pathway during maturation of mammalian oocytes. Theriogenology. 1998;49:461–469. doi: 10.1016/s0093-691x(97)00418-4. [DOI] [PubMed] [Google Scholar]
- Murnion M, Adams R, Callister D, Allis C, Earnshaw W, Swedlow J. Chromatin-associated protein phosphatase 1 regulates aurora-B and histone H3 phosphorylation. J Biol Chem. 2001;276:26656–26665. doi: 10.1074/jbc.M102288200. [DOI] [PubMed] [Google Scholar]
- Nigg E. Mitotic kinases as regulators of cell division and it’s checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- Rogers E, Bishop JD, Waddle JA, Schumacher JM, Lin R. The aurora kinase AIR-2 functions in the release of chromosome cohesion in Caenorhabditis elegans meiosis. J Cell Biol. 2002;157:219–229. doi: 10.1083/jcb.200110045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai K, Katayama H, Stenoien DL, Fujii S, Honda R, Kimura M, Okano Y, Tatsuka M, Suzuki F, Nigg EA, et al. Aurora-C kinase is a novel chromosomal passenger protein that can complement Aurora-B kinase function in mitotic cells. Cell Motil Cytoskeleton. 2004;59:249–263. doi: 10.1002/cm.20039. [DOI] [PubMed] [Google Scholar]
- Schmiesing JA, Gregson HC, Zhou S, Yokomori K. A human condensin complex containing hCAP-C-hCAP-E and CNAP1, a homolog of Xenopus XCAP-D2, colocalizes with phosphorylated histone H3 during the early stage of mitotic chromosome condensation. Mol Cell Biol. 2000;20:6996–7006. doi: 10.1128/mcb.20.18.6996-7006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Gutierrez GJ, Lenart P, Ellenberg J, Nebreda AR. Histone H3 phosphorylation during Xenopus oocyte maturation: regulation by the MAP kinase/p90Rsk pathway and uncoupling from DNA condensation. FEBS Lett. 2002;518:23–28. doi: 10.1016/s0014-5793(02)02630-3. [DOI] [PubMed] [Google Scholar]
- Shannon KB, Salmon ED. Chromosome dynamics: new light on Aurora B kinase function. Curr Biol. 2002;12:R458–R460. doi: 10.1016/s0960-9822(02)00945-4. [DOI] [PubMed] [Google Scholar]
- Swain JE, Smith GD. Mechanisms of oocyte maturation. In: Tan SL, Chian RC, Buckkett WM, editors. Abingdon: Informa Healthcare; 2007. pp. 83–102. [Google Scholar]
- Swain JE, Smith GD. Reversible phosphorylation and regulation of mammalian oocyte meiotic chromatin remodeling and segregation. In: Gupta SK, Koyama K, Murray JF, editors. Nottingham: Nottingham Press; 2007. pp. 343–358. Gamete Biology: Emerging Frontiers in Fertility and Contraceptive Development. [PubMed] [Google Scholar]
- Swain JE, Wang X, Saunders T, Dunn R, Smith GD. Specific inhibition of mouse oocyte nuclear protein phosphatase-1 stimulates germinal vesicle breakdown. Mol Reprod Dev. 2003;65:96–103. doi: 10.1002/mrd.10258. [DOI] [PubMed] [Google Scholar]
- Swain JE, Ding J, Brautigan DL, Villa-Moruzzi E, Smith GD. Proper chromatin condensation and maintenance of histone H3 phosphorylation during mouse oocyte meiosis requires protein phosphatase activity. Biol Reprod. 2007;76:628–638. doi: 10.1095/biolreprod.106.055798. [DOI] [PubMed] [Google Scholar]
- Takemoto A, Murayama A, Katano M, Urano T, Furukawa K, Yokoyama S, Yanagisawa J, Hanaoka F, Kimura K. Analysis of the role of Aurora B on the chromosomal targeting of condensin I. Nucleic Acids Res. 2007;35:2403–2412. doi: 10.1093/nar/gkm157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SL, Chian RC, Bucketl WM. 2007. In vitro maturation of human oocytes: Basic science to clinical application: Informa Healthcare Abinanlam. [Google Scholar]
- Tong C, Fan HY, Chen DY, Song XF, Schatten H, Sun QY. Effects of MEK inhibitor U0126 on meiotic progression in mouse oocytes: microtuble organization, asymmetric division and metaphase II arrest. Cell Res. 2003;13:375–383. doi: 10.1038/sj.cr.7290183. [DOI] [PubMed] [Google Scholar]
- Tseng TC, Chen SH, Hsu YP, Tang TK. Protein kinase profile of sperm and eggs: cloning and characterization of two novel testis-specific protein kinases (AIE1, AIE2) related to yeast and fly chromosome segregation regulators. DNA Cell Biol. 1998;17:823–833. doi: 10.1089/dna.1998.17.823. [DOI] [PubMed] [Google Scholar]
- Uzbekova S, Arlot-Bonnemains Y, Dupont J, Dalbiès-Tran R, Papillier P, Pennetier S, Thélie A, Perreau C, Mermillod P, Prigent C, et al. Spatio-temporal expression patterns of aurora kinases a, B, and C and cytoplasmic polyadenylation-element-binding protein in bovine oocytes during meiotic maturation. Biol Reprod. 2007;78:218–233. doi: 10.1095/biolreprod.107.061036. [DOI] [PubMed] [Google Scholar]
- Vallente RU, Cheng EY, Hassold TJ. The synaptonemal complex and meiotic recombination in humans: new approaches to old questions. Chromosoma. 2006;115:241–249. doi: 10.1007/s00412-006-0058-4. [DOI] [PubMed] [Google Scholar]
- Van Hooser A, Goodrich DW, Allis CD, Brinkley BR, Mancini MA. Histone H3 phosphorylation is required for the initiation, but not maintenance, of mammalian chromosome condensation. J Cell Sci. 1998;111:3497–3506. doi: 10.1242/jcs.111.23.3497. [DOI] [PubMed] [Google Scholar]
- Wang WH, Sun QY. Meiotic spindle, spindle checkpoint and embryonic aneuploidy. Front Biosci. 2006;11:620–636. doi: 10.2741/1822. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang CM, Ai JS, Xiong B, Yin S, Hou Y, Chen DY, Schatten H, Sun QY. Histone phosphorylation and pericentromeric histone modifications in oocyte meiosis. Cell Cycle. 2006;5:1974–1982. doi: 10.4161/cc.5.17.3183. [DOI] [PubMed] [Google Scholar]
- Wei Y, Mizzen CA, Cook RG, Gorovsky MA, Allis CD. Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena. Proc Natl Acad Sci USA. 1998;95:7480–7484. doi: 10.1073/pnas.95.13.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Yu L, Bowen J, Gorovsky M, Allis C. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell. 1999;97:99–109. doi: 10.1016/s0092-8674(00)80718-7. [DOI] [PubMed] [Google Scholar]
- Yan X, Cao L, Li Q, Wu Y, Zhang H, Saiyin H, Liu X, Zhang X, Shi Q, Yu L. Aurora C is directly associated with Survivin and required for cytokinesis. Genes Cells. 2005;10:617–626. doi: 10.1111/j.1365-2443.2005.00863.x. [DOI] [PubMed] [Google Scholar]
- Yao LJ, Sun QY. Characterization of aurora-a in porcine oocytes and early embryos implies its functional roles in the regulation of meiotic maturation, fertilization and cleavage. Zygote. 2005;13:23–30. doi: 10.1017/s0967199405003059. [DOI] [PubMed] [Google Scholar]
- Yao LJ, Zhong ZS, Zhang LS, Chen DY, Schatten H, Sun QY. Aurora-A is a critical regulator of microtubule assembly and nuclear activity in mouse oocytes, fertilized eggs, and early embryos. Biol Reprod. 2004;70:1392–1399. doi: 10.1095/biolreprod.103.025155. [DOI] [PubMed] [Google Scholar]
- Yu LZ, Xiong B, Gao WX, Wang CM, Zhong ZS, Huo LJ, Wang Q, Hou Y, Liu K, Liu XJ, et al. MEK1/2 regulates microtubule organization, spindle pole tethering and asymmetric division during mouse oocyte meiotic maturation. Cell Cycle. 2007;6:330–338. doi: 10.4161/cc.6.3.3805. [DOI] [PubMed] [Google Scholar]