Chordomas originate in the notochord remnants and are difficult to manage with surgery and radiation therapy because of morbidity to collateral structures, such as nerves, and the high radiation doses that are required. We used percutaneous imaging-guided radiofrequency ablation to treat a patient whose chordoma had metastasized to the pararectal region and was causing local and sciatic pain. To our knowledge, radiofrequency ablation of a chordoma has not been previously reported.
Chordomas are the most commonly occurring primary malignant spinal tumor. This infrequent tumor represents 1−4% of primary bone tumors. Chordomas arise within the axial skeleton, most commonly in the sacrococcygeal region (50−66%) and the base of the skull (approximately 35%); they have a metastatic rate of 5−43% [1]. Patient age at detection is between 30 and 70 years; chordomas are found more often in men than in women (ratio, 2−3:1).
A chordoma usually presents as an expansile, midline bone lesion with marked soft-tissue involvement. Intratumoral calcification is seen on CT in most patients. MR imaging shows a low or intermediate signal intensity on T1-weighted images and very high signal intensity on T2-weighted images, indicating high water content in the tumor. Contrast enhancement is typically seen on CT and MR imaging.
The mainstay of treatment for chordomas is maximal debulking by surgery followed by adjuvant radiation or proton radiation treatment. Unfortunately, chordomas have a relatively high recurrence rate, low disease-free survival rates, and high rate of morbidity from conventional treatments. Surgery to debulk the tumor and reduce local pain can also be considered for palliation [2].
Radiofrequency ablation is a well-established, minimally invasive technique for unresectable liver tumors. Percutaneous radiofrequency ablation can be performed with the patient under local anesthesia and conscious sedation in the outpatient setting. Radiofrequency ablation has promising early results for the treatment of primary and secondary hepatic tumors and carries minimal risk [3]. Radiofrequency ablation has been described for the treatment of metastatic bone tumors and has been used for more than a decade for the treatment of osteoid osteoma. Although this technique has been given clearance for soft-tissue ablation under section 510(k) regulations of the United States Food and Drug Administration, it has not been validated for the treatment of chordomas. We describe the successful application of radiofrequency ablation in a patient with a chordoma.
Technique
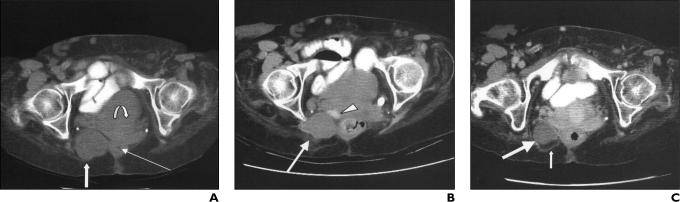
A 62-year-old woman presented with a slowly enlarging pararectal tumor. She had a 16-year history of recurrent sacral chordomas, despite having undergone multiple surgical re-sections, partial sacrectomy, and intraoperative radiotherapy. Previous treatments resulted in progressive residual spinal cord and vascular compromise, with resulting paresis and complete foot drop. This pararectal mass (Fig. 1A) had been followed up for 7 years. However, its growth markedly increased, from 2.0 × 1.5 cm to 4.8 × 4.6 cm, in the 18 months before we performed radiofrequency ablation. Other sacral disease remained stable. It was difficult to differentiate tumor from irradiation damage.
Fig. 1.
62 year-old woman with pararectal chordoma.
A, Contrast-enhanced CT scan obtained before treatment shows nonenhancing pararectal chordoma (thick straight arrow) measuring 4.8 × 4.6 cm, rectum (thin straight arrow), and urinary bladder (curved arrow).
B, Contrast-enhanced CT scan obtained immediately after ablation shows nonenhancing thermal lesion (arrow) has replaced entire tumor. Contrast material is visible in base of bladder (arrowhead). Thermal lesion measured 29 H after uptake of contrast material, compared with 28 H before.
C, Contrast-enhanced CT scan obtained 6 months after ablation shows reduction in tumor size to 3.0 × 3.3 cm (large arrow), with no definite pathologic enhancement to suggest local recurrence. Note linear tissue (small arrow) in fat posterior relative to lesion, likely representing burned fat and scar tissue. The treated mass after contrast enhancement measured 44 H (before contrast administration [not shown], 45 H).
We obtained the patient's written informed consent for radiofrequency ablation treatment, but we did not seek investigational review board approval because the Food and Drug Administration has approved the technique for soft-tissue ablation. We performed the procedure under real-time sonographic and CT guidance using two 17-gauge radiofrequency probes (3- and 2-cm uninsulated tips) connected to a 480-kHz radiofrequency generator (CC-1; Radionics, Burlington, MA) delivering a maximum power of 200 W. The 3-cm probe was perfused with chilled saline in a closed system for 12 min, and the 2-cm probe, for 6 min. The generator was used in impedance-controlled pulsing mode, but no pulsing was required. Baseline impedance was 55 Ω for both sessions.
Radiofrequency ablation was performed after the administration of standard local anesthesia and conscious sedation, including intradermal, subcutaneous 1% lidocaine and IV midazolam and fentanyl. A 180-cm2 grounding pad was placed on each thigh. Maximal current used during the first treatment was 1.6 A, and during the second treatment, 1.4 A. The second treatment was stopped at 6 min because the sonographic echoes from microbubbles approached the edge of the rectum. Maximal temperature after radiofrequency ablation was 92°C during cooling.
Intermittent neurologic examinations were performed during the procedure to minimize risk of nerve damage. The needle track was cauterized using the gradual pull-back technique with current titrated to deliver enough energy to maintain a needle-tip temperature greater than 70°C.
No complications were observed during the procedure or during the 13-month follow-up period. Specifically, no clinical or radiographic evidence of rectal or progressive nerve injury was observed. CT scans obtained immediately after ablation and after injection of IV contrast material showed no contrast enhancement in the target tumor in regions where contrast enhancement had been seen before treatment (Fig. 1B). This loss of enhancement suggests the treated tissue may represent coagulation necrosis. Lack of enhancement in hepatic lesions treated by radiofrequency ablation has been shown to represent coagulation necrosis [4].
The patient was kept overnight for observation and pain control. Before undergoing radiofrequency ablation, the patient had been given prophylactic doses of ampicillin, gentamicin, and metronidazole; after the procedure, she was given ciprofloxacin and metronidazole for 10 days because the needle insertion site was in proximity to her colostomy.
Follow-up CT at 2 and 6 months after treatment revealed progressive reduction in the size of the treated tumor and no evidence of local recurrence (Fig. 1C). Progressive shrinkage and lack of contrast enhancement are the radiographic criteria used to assess for incomplete treatment or recurrence. At 2 months after ablation, the thermal lesion measured 4.6 × 4.4 cm, and at 6 months, 3.0 × 3.3 cm.
Discussion
Percutaneous imaging-guided radiofrequency ablation has been used to treat tumors in the liver, kidney, adrenal gland, lung, breast, bone, head, and neck with promising early results [3]. Unlike other percutaneous techniques, radiofrequency ablation can produce controlled areas of coagulation necrosis as large as 3−7 cm in diameter with a single application. Radiofrequency ablation has been reported as an effective method of local pain control for patients with recurrent tumors [5, 6].
More than half of primary sacral tumors are chordomas. They are extremely difficult to resect completely without sacrificing surrounding neurologic structures, although radical excision is the conventional treatment. However, similar results may be achieved by administering radiation to patients with positive surgical margins. Chordomas are considered radioresistant because they require high radiation-therapy doses that have associated high risks [1].
A wide variety of progression, recurrence, and survival rates have been reported, likely reflecting small studies and diverse patient populations [7]. Chordomas rarely metastasize, but are commonly locally progressive, symptomatic, and slow-growing. These growth characteristics make them attractive candidates for a local ablative therapy such as radiofrequency ablation. Many patients who survive are symptomatic and are not disease-free. Researchers who studied 23 patients over the course of 31 years found a 2.5% survival rate with no symptom progression, a 17% survival rate with no symptoms, and an 8% disease-free survival rate [7]. Surgical excision may provide palliation for large, painful chordomas involving the soft tissues. Because chordomas tend to recur locally, the needle track was cauterized after radiofrequency ablation to decrease this risk.
The high water content of the tumor and its lack of vascularity may contribute to the favorable dielectric properties of the tumor, manifest in a treatment volume that is larger than expected. This outcome may be caused by thermal and electric conductivity that is much higher than that of normal well-vascularized liver or kidney tissue. This finding was confirmed by the unusually high temperatures after ablation, and the lack of pulsing, or significant impedance rise, during ablation. A typical liver lesion treated with an identical system and settings results in a thermal lesion 3 cm in diameter, rather than nearly 5 cm, as was produced in our patient. Although systems are available to treat larger lesions (up to 7 cm), their use might have resulted in damage to adjacent structures. This case shows the wide variability of thermal damage, depending on tissue dielectrics, perfusion, thermal conductivity, and native histology.
Perhaps in the future radiofrequency ablation will find a role as an adjunctive therapy to surgery and radiation therapy. Short-term radiographic success in this patient was shown by a reduction in tumor pain and decrease in tumor burden with an arrest in tumor growth. Radiofrequency ablation may be an effective, minimally invasive treatment for painful or rapidly growing chordomas that are recalcitrant to conventional radiation therapy.
References
- 1.Hug EB, Slater JD. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. Neurosurg Clin N Am. 2000;11:627–638. [PubMed] [Google Scholar]
- 2.Tai PT, Craighead P, Liem SK, Jo BH, Stitt L, Tonita J. Management issues in chordoma: a case series. Clin Oncol. 2000;12:80–86. doi: 10.1007/s001740050116. [DOI] [PubMed] [Google Scholar]
- 3.Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000;217:633–646. doi: 10.1148/radiology.217.3.r00dc26633. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic–pathologic correlation. Cancer. 2000;88:2452–2463. [PubMed] [Google Scholar]
- 5.Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities. II. J Vasc Interv Radiol. 2001;12:1135–1148. doi: 10.1016/s1051-0443(07)61670-4. [DOI] [PubMed] [Google Scholar]
- 6.Wood BJ, Fojo A, Levy EB, Gomez-Horhez J, Chang R, Spies J. Radiofrequency ablation of painful neoplasms as a palliative therapy: early experience. J Vasc Interv Radiol. 2000;11(suppl):207. [Google Scholar]
- 7.Magrini SM, Papi MG, Marletta F, et al. Chordoma: natural history, treatment and prognosis—the Florence Radiotherapy Department experience (1956−1990) and a critical review of the literature. Acta Oncol. 1992;31:847–851. doi: 10.3109/02841869209089717. [DOI] [PubMed] [Google Scholar]