Abstract
Radiofrequency ablation (RFA) has begun to show promise for extrahepatic indications. Although much of the reported work on image-guided RFA of liver neoplasms is quite promising, it is even earlier in the evaluation and validation process for extrahepatic RFA, with few short-term and no long-term studies reported. Although there are much more data for liver RFA with almost 3,000 cases reported in the literature, there are a number of ongoing investigations of RFA for tumors in the kidney, lung, bone, breast, bone, and adrenal gland. Debulking and pain control with RFA present palliative options becoming increasingly popular weapons in the interventionalist's oncology arsenal. Metastatic disease with a wide variety of primary histologies in a myriad of locations may be treated with RFA after a careful consideration of the risk-to-benefit ratio balance. The RFA technique can be slightly different outside the liver. Specifically, differing dielectric tissue characteristics may markedly alter the RFA treatment. Each different RFA system has a unique risk and advantage profile. Extrahepatic indications and contraindications will be suggested. Treatment tips and the unique complications and considerations will be introduced for some of the more common extrahepatic locations.
Why Outside the Liver?
Preliminary data recently support the potential clinical utility of RFA for indications besides unresectable liver tumors. Although safety and outcome issues have not yet been completely addressed, interventionalists have been exploring using RFA to destroy tissue for quality of life and debulking indications, without hard evidence of impact on long-term prognosis. However, when such a procedure may be performed as an outpatient with minimal risk (in the liver: <3 % complications), one can make the argument that RFA presents a reasonable phase I–II treatment alternative for certain patients without other effective options.
Although safety and outcomes issues are only beginning to be addressed, extrapolation from the limited data available as well as from the literature on the liver suggests that RFA may present a safe, less invasive alternative to surgical debulking in some situations. The interventionalist must be sensitive to calling RFA an alternative to surgery because there are no direct hard data to support this for most indications, with no randomized controlled trials of surgery versus RFA. However, the ease, rapid recovery, and relative safety make RFA a realistic option for patients who are not candidates for limited organ resection, with incurable disease or with widespread metastatic disease. In this difficult population, there is a fine balance between prolonging survival and maintaining quality of life for any possibly helpful but unproven palliative procedure.
Radiofrequency Systems
Recent advances in technique have resulted in larger volumes of tissue ablation possible. This has been accomplished with relatively low complication rates, and the improving predictability means less likely collateral damage. Multiple methods for increasing energy and heat deposition with RFA have been attempted. The most successful of these are the coaxially deployed hooks (Christmas tree or umbrella-shaped), the internally cooled probes, and multiple parallel probes.
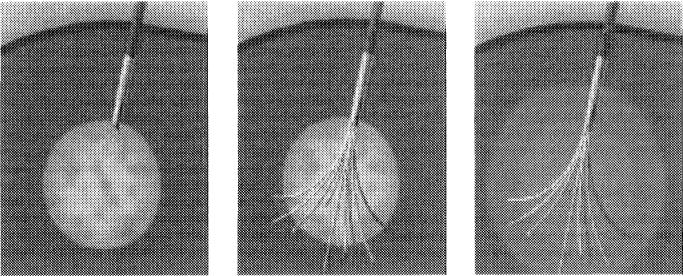
The 3 RFA systems currently available in the United States are (1) RITA Medical Systems, Inc (Fig 1); (2) Radionics Inc., Tyco Healthcare (Fig 2); (3) RadioTherapeutics, Inc., Boston Scientific (Fig 3). They differ in power of the generator, the technique used to maximize treatment volumes, the gauge of the needles, and in the tissue and electrical parameters monitored to optimize energy deposition. Although temperature and impedance are measured in several of the systems, each uses 1 parameter to maximize treatment diameter, and each system has a specific algorithm for treatment, which requires varying degrees of operator input. Only 1 cm diameter of tissue ablation was possible with a single RFA needle until the last few years.
Fig 1.
The RITA needle system has Christmas tree–like hook tines.
Fig 2.
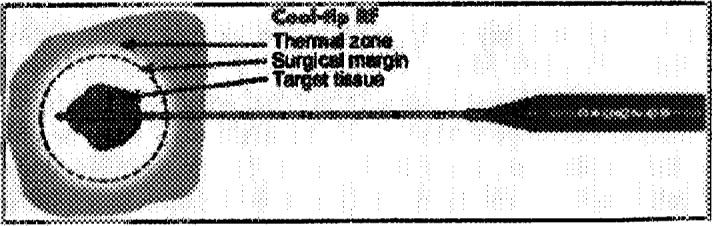
The Radionics system is water cooled.
Fig 3.
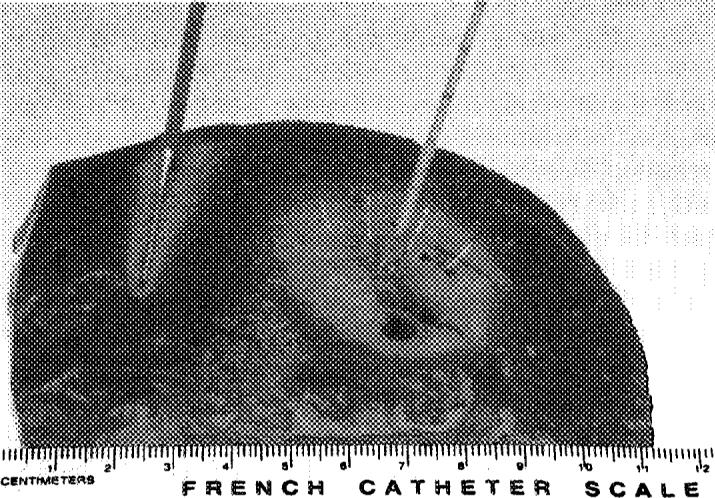
The RadioTherapeutics system has 10 equidistant flower-like tines.
Often, location and size of target may influence our choice of system, since we have all three available. However, in the majority of cases, any one of the 3 systems will get the job done. The most important factor is operator familiarity and comfort, as each system has a learning curve. Each device has specific strengths, weaknesses, and pitfalls, which become more important with RFA outside the liver. The liver is a forgiving organ in which to learn.
Three systems are available to the American market and are Food and Drug Administration 510 K-cleared for “soft tissue ablation.” The use of RFA outside the liver may constitute an “off-label” indication since RFA is not specifically approved for extrahepatic tumors. However, some might interpret the soft tissue indication broadly to include palliation and many other organs and locations. Check with local Institutional Review Boards for interpretation or clarification.
Two of the 3 systems (RITA Medical Systems, Inc., Mountain View, CA, and RadioTherapeutics Inc., Mountain View, CA) use coaxially deployed hooks or inner tines that expand into the tumor after the outer needle is placed into the tumor. The RITA needle has 4, 7, or 9 Christmas tree–like hook tines, and the RadioTherapeutics has 10 equidistant flower-like tines. The coaxial systems have the advantage of keeping the treatment needle stationary if the target is particularly mobile (as might occur with deep breathing or with deep sedation in a lateral dome lesion approached from a caudal subcostal access). They also may deliver a more uniformly spherical thermal lesion. The tips of the RITA hooks have thermocouples that report real-time temperature at the treatment volume margin, as the tissue heats up, which semi-automatically maximizes treatment volume. The RadioTherapeutics and Radionics (Radionics, Inc., Burlington, MA) systems rely predominantly on monitoring impedance to avoid charring at the needle tip. The RadioTherapeutics system algorithm treats the sphere until impedance maximizes or until a time limit is reached. The RITA or RadioTherapeutics needles may be partly deployed to treat smaller lesions, whereas the Radionics needles may have different-size active tips to ablate smaller lesions.
The Radionics system requires a pump that perfuses chilled saline through the hollow ports inside the needles (in a closed system). This decreases charring and vaporization, and thus increases ablation volume. Avoiding the insulating overcooking is similar to avoiding a charbroiled burger that is raw in the middle (away from the needle) and yet charred on the outside (touching the needle). The impedance-controlled pulsing technique allows the tissue around the needle to cool between energy bursts. This will automatically turn the current down to near-zero when the impedance increases to more than 20 ohms above baseline and let the tissue cool before turning it back up to the appropriate level. Radionics also has a triad or triple parallel needles on 1 probe, which creates a 4- to 5-cm diameter treatment sphere. The Radionics system requires the most user input and is a more difficult setup but also provides versatility, delivering a large burn with a small-gauge needle, with different-size active tips and different output adjustments able to treat small nerve ganglia or quite large tumors. The new RITA system has a 7-cm array probe that uses injected hypertonic saline to maximize thermal lesions. The current RadioTherapeutics system gets about a 4-cm lesion.
The Radionics generator has 200 W maximum, whereas the RadioTherapeutics has 90 W (200 W in the newer model), and the RITA has 50-, 150-, or 250-W models. Needle gauges are 17.5G for Radionics, 14G or 15G for RITA, and 15G for RadioTherapeutics. RadioTherapeutics has a truly coaxial system that allows computed tomography scanning with needles in place (without bulky hubs), and also multiple needle placement for treatment planning. However, placing needles in close proximity to a thermal lesion during treatment can alter the shape and size of the thermal sphere. There is also a smaller G needle available for Radionics that may be used for nerve ganglia ablation, smaller applications, or thermometry. The companies tend to leap frog each other with new releases.
Strengths and Weaknesses of Different Systems
One can basically divide the systems available into 4 types.
Single-Tip Probe
Advantages: small-needle gauge with fewer punctures, tapered, noncoring needle, accurate tip placement and monitoring, minimal collateral damage and easier maneuverability and repositioning, easiest to cauterize on the way out to limit bleeding and seeding. Disadvantages: limited treated radius, wider variability of size and shape (usually slightly oval), more dependence on thermal conductivity, thermometry only useful while cooling after treatment, more operator input required, learning curve.
Cluster Triple-Parallel Probe
Similar to single. Advantages: large treatment area. Disadvantages: more variable treatment volumes and shapes, less accurate placement with diverging or converging needle tips affecting volumes, especially in deeper targets.
Umbrella Deployed Probe
Advantages: coaxial option for fitting probe inside CT gantry, uniformity of sphere shape, predictable deployment geometry, more predictable treatment volume and shape, easier to learn. Disadvantages: Coring needle, difficult visualization of multiple hooks during real-time deployment with risk of collateral damage if adjacent to bowel, more complex repositioning and maneuverability, harder to deploy in hard bone, slightly smaller volumes.
Christmas Tree Probe
(With or without interstitial saline injection): Advantages: peripheral thermometry during treatments for on-line feedback (and detection of heat-sink), uniformity of sphere shape, predictable deployment geometry, more predictable treatment volume and shape, easier to learn, larger volumes. Disadvantages: Coring needle, difficult visualization of multiple hooks during real-time deployment with risk of collateral damage if adjacent to bowel, more complex repositioning and maneuverability, harder to deploy in hard bone.
What System Should I Use?
On deciding what system to use, it is truly physician preference—different strokes for different folks (location, importance of minimizing collateral damage, proximity of large vessels, desired treatment volume, importance of uniform lesion formation, bleeding risk, respiratory motion, physician experience, probe pathway). In general, any system or probe is adequate for the majority of liver lesions, but the selection of the appropriate system or probe may be more important for RFA outside the liver. The liver with its dual blood supply is a more forgiving organ than other organs, and collateral structures are often closer to the treatment area with extrahepatic RFA. Different rules operate outside the liver for treatment margin and collateral damage, and there are fewer rules and trends to guide the inexperienced ablationist. The liver is a better location to learn RFA techniques, and extrahepatic indications should not be undertaken without vast experience in the liver under one's belt.
Image Guidance/Technique/Tissue Dielectrics
In liver RFA, ultrasound guidance may be the most common imaging guidance, and extensive percutaneous biopsy skills are required. However, needle placement location is more important with RFA than with biopsy and must be precise to the millimeter. Further, needle placement for extrahepatic RFA may be even more position dependent, with more potential for damage of normal structures. Tissue dielectrics, thermal conductivity, perfusion, and nearby vital structures are more varied outside the liver, and these factors may influence ablation volume and thus have important treatment implications. A wider variety of thermal lesion shapes and volumes are attained outside the more predictable liver. For example, the typically gelatinous chordoma may result in over 4 times the volume as a typical liver lesion even with identical generator settings and treatment times.
Outside the liver, guidance with real-time ultrasound alternating with CT or CT fluoroscopy has definite advantages. The exact tip location can be confirmed, and the proximity to adjacent organs may be directly measured. Contrast may be administered in 50-mL increments to optimize treatment overlap and thermal lesion geometry and to ensure absence of skip areas. Magnetic resonance thermometry is feasible and should prove to be advantageous in the future, but several equipment and production hurdles need to be addressed by the corporate world first. Twenty-two-gauge needle thermometers may now be placed adjacent to vital structures, nerves, or collateral tissue to protect from thermal damage. Nerves have lower thermal thresholds. This can help if nerves are targeted, but this adds a risk if nerves are adjacent collateral structures.
Patient Selection Criteria
Patient selection directly determines outcomes, and the variability in disease-free survival rates and outcomes may reflect this. There is a steep learning curve, therefore, one interventionalist's results or survival rates are not universal. The recent proliferation of RFA systems makes wise patient selection a necessity. Start with small, isolated lesions centimeters away from major vessels, bowel, skin, nerves, or other vital structures. Practice first in the liver and in phantoms. Make sure that the patients, their family, and their oncologist fully understand the nature of the RFA and the exact goals of the treatment, including specific risks (ie, pneumothorax for lung tumors, skin breakdown for superficial tumors). Make sure that the goals are realistic. The procedure should be undertaken only after everyone understands the possible outcomes.
Pre-Procedure Evaluation
Like RFA in the liver, this is truly a team effort, but the partners are potentially more numerous. If the RFA is for pain control or debulking, then surgical oncology, palliative care, and pain service consults may be appropriate. Surgical or medical consultation may be wise depending on the location (ie, kidney—urology; lung—thoracic surgery; adrenal—endocrine; bone—radiation oncology.) The most important step may be the treatment planning ultrasound and CT. The tissue down to the organ capsule is anesthetized, conscious sedation is administered, and a small skin knick is made, through which is placed a guiding needle or the treatment probe.
Monitor treatment spheres with ultrasound and CT such that overlapping spheres create a thermal lesion of coagulation necrosis encompassing the tumor and a margin of normal tissue, if indicated. The margins outside the liver may be adjusted according to indication. For example, in patients with hereditary renal cell carcinoma, we often aim for a several-millimeter margin, such that it is truly nephron sparing because they may need multiple treatments over a lifetime. Planning a margin is the most difficult part of the procedure, and most inexperienced operators undertreat in the liver. Remember, it takes over 6 spheres to create a 3.75-cm thermal lesion to envelope a lesion more than 2 cm plus margins. For very superficial tumors, skin may be protected with ice bags after the skin heats up. Frequent neurologic examinations may protect from nerve injury.1 Pro-peristaltic agents may protect from bowel injury (a moving target is less likely to reach lethal temperature).
RFA Technique
As with large liver lesions, some prefer general anesthesia and overnight observation for patients with painful soft-tissue tumors treated for debulking or palliation. Patients often require short-term patient-controlled analgesia pumps for a few hours after RFA. Bupivicaine (Marcaine) and 30 mg ketorolac (Toradol) IV should be used after the RFA of painful lesions, although ketorolac should only be used for several doses given the renal toxicity issues (and other non-steroidal anti-inflammatory drug precautions). At the end of a single session, the access tract may be cauterized on the way out, which theoretically decreases the risk of needle tract seeding or bleeding. This is possible on 2 of the 3 systems by turning down the output while dragging the needle slowly out, monitoring temperature. To decrease back bleeding, injecting Gelfoam pledgets using a coaxial sheath has been described for the third system.
Future Optimization of RFA
Combining RFA with chemoembolization, bland embolization, vein or artery occlusion, or intravenous chemotherapy may have an additive effect when needed for large lesions or high tumor burden, although the experience is limited. Decreasing the heat sink with embolization will increase the thermal lesion, and RFA will increase the amount of circulating drug deposited from heat-mediated leaky capillaries. There will be optimistic reports in the literature shortly on such combination of modalities, or on combining RFA with chemotherapy, but the exact timing sequence and chemotherapy agents are yet to be optimized.
Manufacturer's Flight Manuals
Radionics
Hook up all lines. Place needle to far end of desired thermal lesion. Turn on generator first, then water pump, after verifying temperature increase. Start with low current (100 to 800 mA) for 1 or 2 minutes before ramping to higher current. In pulsing mode, the maximum current will take care of itself, based on tissue impedance. Treat for 12 minutes. Turn off generator and pump simultaneously and wait 30 seconds for maximum temperature. This is usually 60° to 90°C. If less than 60°, then there is likely a vessel near the probe tip, and a repeat treatment in a slightly different area is required. 1-, 2-, 3-cm single, 2.5-cm triple probe (4- to 5-cm sphere), 200-W generator.
RadioTherapeutics
Deploy hooks slightly proximal to the center of thermal sphere (can be done through coaxial outer needle if desired). Radiofrequency current is applied according to protocol, until “roll-off” occurs at each sequential level of increasing power or until the impedance increase in tissue indicates desiccation. 2-, 3-, 3.5-, 4-cm probes, 100 and 200 W generators.
RITA
XL: Deploy hooks in proximal part of thermal sphere to 2-, 3-, 4-, 5-, 7- ... cm marks, with power set to 50, 70, 90, 110 ... W, and target temperature at 80, 105, 110, 110... and treat for time intervals or until target temperature is reached, then cool down and cauterize the track moving at 5-mm intervals when 75°C is reached. Use forward pressure to avoid outer needle “pull-back” during deployment. If target temperature is not reached at any stage in 3 minutes, increase power by 20 W. Xli is 7-cm sphere with hypertonic saline pump into peripheral interstitium of thermal lesion to increase thermal or electrical conductivity. 3-, 5-, 7-cm probes, 50, 150, 250 W generators.
Specific Extrahepatic Applications
Kidney
Complete or partial nephrectomy has been the treatment of choice for solid renal tumors for many years. Nephron-sparing surgery has also been implemented successfully in recent years. Renal cancers are usually slow-growing tumors that may appear incidentally with relatively low incidence of metastatic disease until the primary tumor has grown to more than 3 cm diameter. Renal RFA is usually safe and well tolerated.
Large vessels near the renal hilum may cause heat-sink effect by convection of heat into flowing blood, leading to under-treated areas. Careful probe placement may limit this effect, even in medullary lesions. Nearby ureter may become strictured if overheated, although the peripheral collecting system is fairly resistant to heat injury. We have had no cases of urine extravasation or urinoma in over 70 kidney tumors treated. Peripheral and exophytic lesions are likely easier, safer, and more accurately targeted, with better outcomes. CT and ultrasound together in combination (or magnetic resonance) optimize probe positioning, especially important for iso-echoic small lesions. RFA lesion size varies according to the size of the electrode, the current, duration of the treatment, and the local blood flow. For small 2- to 3-cm exophytic renal cell carcinomas, full tumor treatment often is accomplished with 2 single 3-cm spheres, although this depends partly on local perfusion issues. The cooling curves are particularly useful when deciding whether to repeat treatments in a slightly different location. For the Radionics system, a maximum temperature above 70°C is ideal. The rapidity of cooling after RFA correlates with volumes treated (unpublished data). The perinephric fat acts as an insulator for heating, and there may be slight dielectric differences between cortex and medulla. The first placement of the RF electrode should be deep, adjacent to the margin of tumor and normal cortex. This theoretically reduces the effective blood flow to the remainder of the mass, thus reducing heat sink effect on subsequent ablations, as well as treating the deep areas that will soon be “gassed out” sonographically by the treatment.
Potential indications for renal RFA include contraindications to nephrectomy, comorbidities, advanced age, solitary kidney, renal insufficiency, hereditary predisposition to kidney tumors (VHL), metachronous or bilateral tumors, and intractable hematuria. Complete lack of enhancement on CT probably correlates to pathologic coagulation necrosis within a few millimeters, and ultrasound is a less accurate predictor of the treatment zone.
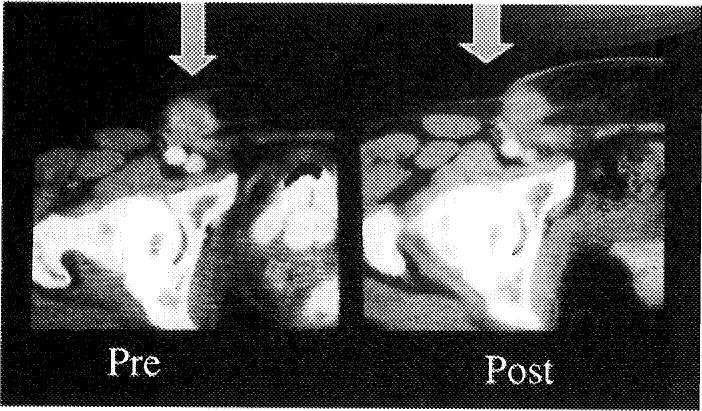
We performed the first renal RFA as a stand-alone treatment in early 1998, and this patient remains disease free by radiographic criteria (Fig 4).2 Since then, more than 25 published papers and at least 3 reviews have appeared on renal RFA, including clinical and animal model experience, demonstrating the overall feasibility, safety, and short-term efficacy of this therapy. We have treated nearly 100 kidney tumors with RFA. We reported our first laparoscopic renal RFA in 2001, and have since successfully treated 18 tumors with this approach, with only 1 complication of a ureteral stricture treated successfully with stenting.3 The solitary blood supply makes perfusion phenomena common, and there may be small infarcts adjacent to the thermal lesion. These can be easily misinterpreted as tumor regrowth on follow-up imaging. Although the data is thin, small (<3 cm) renal tumors can be successfully treated percutaneously in 1 session without complications, with about an 80% to 100% (depending on the system) technical success rate in the short term.4 Conversely large and central renal tumors are more risky and difficult to completely treat.5 For large, central, or particularly vascular renal tumors, the newer devices with higher power give the best chance for complete treatment. Interstitial saline-augmented RFA may show promise when dealing with larger tumors; however, there is a trade-off between size and predictability when using any injectable augmentation, as the fluid may track in an irregular fashion, creating irregular shapes or unpredictable-size lesions. Early generation systems or inexperience may lead to higher rates of incomplete treatment.
Fig 4.
RFA kidney tumor. Enhanced CT scans before and 2 years after radiofrequency ablation (RFA). Peripheral exophytic kidney tumors are easier to treat with RFA.
Bone
Percutaneous RFA has become the standard treatment for osteoid osteoma over the last decade. More recent reports use RFA to treat both benign and malignant bone tumors, including chondroblastoma, chordoma, and metastatic disease. Bone RFA is usually performed under real-time CT guidance. If normal, thick cortex must be traversed, a 14-G coaxial bone biopsy needle is placed through the dense bone, followed by insertion of the RF needle through the outer guiding needle. For benign osteoid osteoma, a 6-minute RFA with a constant temperature of 90°C is the traditional recipe, with the output and impedance less than 10 W and 150 ohms, to ablate the nidus. However, this was described using a different generator than is now in common use. The normal settings required to ablate a 1- to 2-cm region will suffice, regardless of technique. If using the Radionics system, for example, osteoid osteomas can be treated without the internal cooling. The success rate for these small benign tumors is similar to that of surgery, and approximately 90% of patients are cured permanently after a single treatment. RFA has been reported in the vertebral bodies with and without concomitant vertebroplasty, with early reports showing high success rates for pain control. Tumor proximity to the spinal cord or peripheral nerves with disrupted intervening cortical bone may be a contraindication, risking nerve damage. Nerve tissue is more sensitive to thermal damage than most other tissue, although the sensory bundles are usually affected first. Temperature changes within the spinal canal during RFA of the vertebral body have been demonstrated, with toxic temperatures being reached at a distance of 5 mm from the radiofrequency probe tested.6 A second thermometry needle might limit risk in this scenario. Metastatic disease to the bone presents a common clinical dilemma, with suboptimal treatments that may heavily rely on sedating opiates. The traditional conservative treatment of symptomatic skeletal metastasis involves radiation with or without chemotherapy, hormone therapy, or excision, often with poor pain relief and frequent relapse. Radiation effects can take weeks to a month to provide adequate pain relief. There is currently an ongoing American College of Radiology Imaging Network multi-institutional trial studying safety and efficacy of RFA for skeletal metastases using quality of life and pain inventory outcomes.
Pain/Palliation
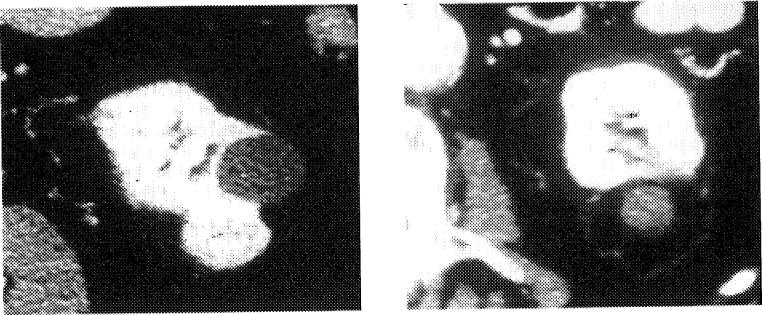
Early reports show promise for RFA of painful bone and soft-tissue tumors that are recalcitrant to conventional radiation and pharmacologic therapies.7 Radiation therapy and opiates may be ineffective or suboptimal options for this difficult clinical problem, which may leave patients overly sedated or in pain during their last months of life. Our pilot study of soft-tissue tumors for pain showed a high response rate between 1 day and 1 week after treatment (Figs 5 and 6). We treated painful tumors from the neck to the leg, including 26 tumors in 14 patients, with promising early results; however, there were limited follow-up data. All 14 patients reported subjective pain relief verbally by 1 week after RFA with 6 of 14 reporting pain improvement 1 day after the procedure. One patient had a delayed wound infection adjacent to a colostomy. This group was comprised of a myriad of histologies and locations, and tumor abutment to vital structures did not preclude RFA. However, in these cases, extreme care was taken to avoid collateral damage to adjacent structures. Pain relief and debulking can be achieved without obtaining clean margins. We have begun a formal National Institutes of Health study of RFA for recalcitrant soft-tissue pain with quality-of-life and pain inventory questionnaires (see www.cc.nih.gov/drd/rfa).
Fig 5.
RFA for palliation. Painful subcutaneous nodule before and after RFA.
Fig 6.
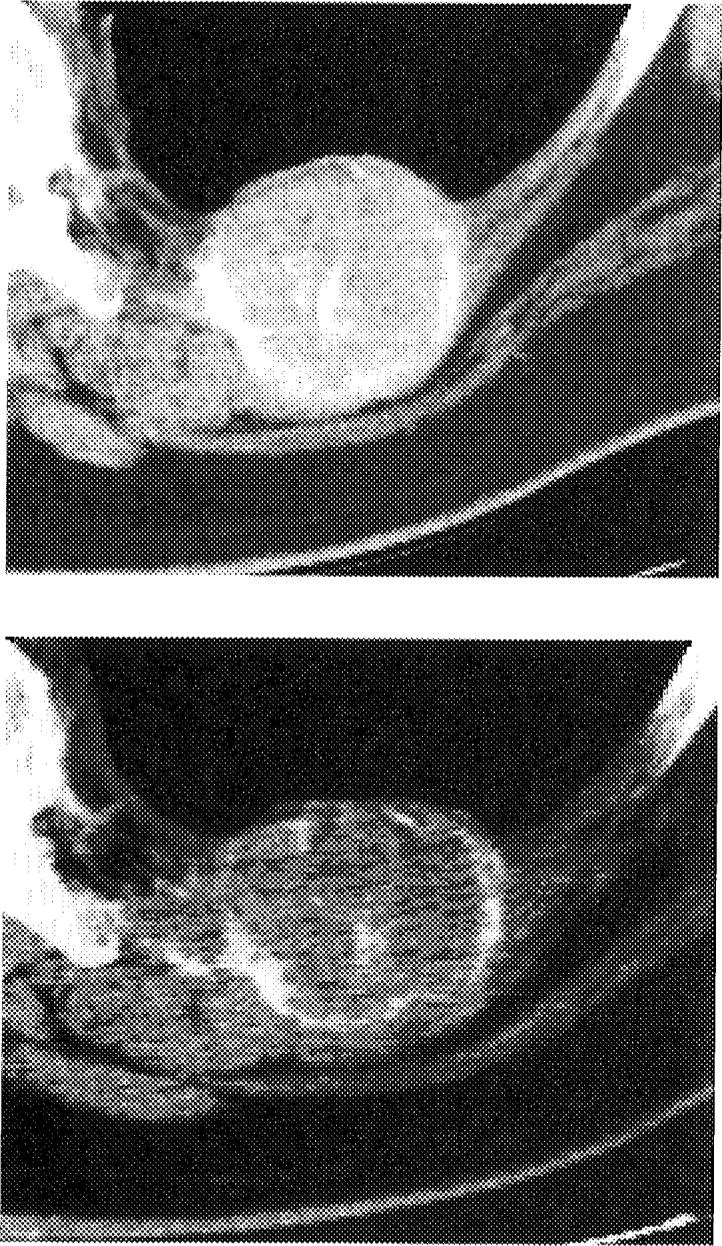
RFA Rib/Bone/Lung: Pre- and post-RFA CT scan of a rib metastasis from adrenocortical carcinoma with loss of enhancement after RFA but persistent high-attenuation calcification. This tumor failed RFA with regrowth several months later along the medial margin.
RFA of nerve ganglia has been effective in the treatment of multiple pain syndromes, including trigeminal neuralgia, celiac ganglion pain, cluster headaches, chronic segmental thoracic pain, cervicobrachialgia, and plantar fascitis. RFA has also been used for inflammatory, idiopathic, and tumor-related pain. Multiple minimally invasive neurodestructive techniques have been safely applied for pain control, including radiofrequency lesioning, cryoanalgesia, and chemical neurolysis with agents such as phenol, alcohol, and hypertonic saline. Neurodestruction, decreased interstitial or intra-tumoral pressure, or decreased pressure on adjacent structures may be the mechanism of pain relief in patients with focal tumor pain.
Lung
There is limited experience for RFA in the lung. First reported in 2000, RFA for primary and metastatic lung tumors is in its infancy. At the Radiological Society of North America 2001, there were 44 patients with lung cancer reported who were treated with RFA in 2 centers; however, results are widely mixed with local recurrence rates from 33% to 74% in short-term follow-up. Pneumothorax rates appear to be in the range of 10% to 20%, with other complications less common, such as bleeding, fistula, hemoptysis, subcutaneous emphysema, effusions, fever, infection, and pain. One peri-procedural death has been reported from bleeding, and 1 patient had a brain infarction, possibly related to cerebral embolism. The safety issues central to lung RFA have not been completely addressed or documented. Specifically, the theoretical risk of cerebral embolism must be addressed in an animal model or large-number human study before claiming safety. The lack of the usual lung-filtering mechanism on the pulmonary vein side and the direct path to the arterial tree represents a major unanswered question for this application. Central tumors close to the hilum may present added risk for bleeding. Chest tube trays should be immediately available at the bedside. The addition of general anesthesia may make pneumothorax more likely. Unilateral intubation may help control bleeding in cases of excessive bleeding.
The surrounding air in adjacent normal lung parenchyma may provide insulation for the thermal lesion, making cooking easier or faster than in the well-vascularized liver. Lack of activity on positron-emission tomography correlates with success and tumor retraction and an unenhancing fibrosis-like process on CT. This technique may be used as an experimental adjunctive therapy to conventional chemotherapy and radiation in inoperable patients. However, before this becomes as common-place as liver, bone, or renal RFA, safety and efficacy need to be addressed more rigorously.
Breast
RFA for breast cancer is also in its infancy, with preliminary results just now reaching press. Large prospective trials have shown that there is no significant change in survival when comparing mastectomy and breast lumpectomy followed by radiation for most breast cancer patients. There has been a trend toward less radical interventions for biopsy and excision in the past decade. Whether this trend will extrapolate to treatment with RFA is another question. Magnetic resonance thermometry and improved detection of breast cancer with magnetic resonance may make this a more accurate guidance method for RFA in the future.
Jeffrey et al8 first described breast RFA in 5 women with locally advanced, invasive breast cancer treated by intra-operative RFA with subsequent pathologic confirmation of cell death within the ablation zone. One of 5 patients postoperatively had a few viable tumor cells lining a cyst, although this study was performed with first-generation techniques. The technique will have to render a near-perfect success rate to compete with surgical options. However, RFA may play a debulking role that may not be in direct competition with excision; for example, RFA in combination with radiation.
An Italian pilot study recently reported in Cancer 26 patients with T1 or T2 breast cancers treated with RFA.11 Twenty-five of 26 had complete coagulation necrosis with a mean treatment diameter of 1.8 cm, although 1 patient had a skin burn, and 1 had viable tumor cells along the shaft of the needle. However, the system used was the one system that cannot cauterize on the way out, which may predispose to this problem. The exact role of RFA in the breast cannot be established before sufficient surgical excision data with pathologic correlation of margins are available. However, if and when future prospective trials show unequivocal clear margins and complete cell death, RFA might then be a minimally invasive option after a positive core biopsy for certain patients with breast cancer. RFA may be tested first in small tumors or in localized, well-circumscribed T1 breast cancers, which are seen well with imaging.
Adrenal
Treatment options for primary and metastatic adrenal tumors are limited. For adrenocortical carcinoma, chemotherapy and radiation therapy play a limited role. However, repeat surgical resection may prolong survival. Extrapolation from this data suggests that local adrenal tumor destruction with RFA may improve survival in select patients. We have treated 15 tumors in 8 patients with primary adrenocortical carcinoma with a high short-term technical success rate for tumors less than 5 cm.9 Pheochromocytoma, aldosteronoma, and metastases to the adrenal may also be treated with RFA, with the appropriate endocrine evaluation (and blockade for pheochromocytoma).10
Other Organs
RFA has also been attempted to a very limited degree in tumors of the prostate, pancreas, brain, thyroid, parathyroid, lymph nodes, bronchus, bowel, retroperitoneum, renal collecting system, pelvis, spleen, head and neck, and bladder. Each location has unique limitations, and risks and should not be undertaken without a thoughtful review of existing options and potential complications. Nerves, vessels, or ducts near the field represent a common scenario. RFA of the capsule-free pancreas may predispose to pancreatitis, for example, and prostate RFA may cause outlet obstruction.
As with many of the extrahepatic applications, upfront consultation with other medical, surgical, oncology, radiation therapy, pain, and palliative care specialists is paramount to successful achievement of realistic goals. One of the most important considerations is knowing when to say, “No.” In the rapidly evolving atmosphere in image-guided oncology, the time for scientific questions and answers is now. The opportunity to conduct quality RFA studies will disappear soon, if clinical practice outpaces basic science background and scientific validation. The window is open; interested interventional radiologists should rise to the occasion before extrahepatic RFA becomes more widespread clinical practice simply by default.
More information on RFA may be found at www.cc.nih.gov/drd/rfa.
References
- 1.Wood BJ, Mikityansky I, Pavlovich C, et al. Avoiding complications in radiofrequency tumor ablation. J Vasc Interv Radiol. 2001;12S:42. [Google Scholar]
- 2.Mcgovern FJ, Wood BJ, Goldberg SN, et al. Radiofrequency ablation of renal cell carcinoma via image guided needle electrodes. J Urol. 1999;161:599–600. [PubMed] [Google Scholar]
- 3.Pautler SE, Pavlovich CP, Mikitansky I, et al. Retroperitoneoscopic-guided radiofrequency ablation of renal tumors. Can J Urol. 2001;8:1330–1333. [PubMed] [Google Scholar]
- 4.Pavlovich CP, Walther MM, Choyke PL, et al. Percutaneous radio-frequency ablation of small renal tumors: initial results. J Urol. 2002;167:10–15. [PMC free article] [PubMed] [Google Scholar]
- 5.Gervais DA, McGovern FJ, Wood BJ, et al. Radio-frequency ablation of renal cell carcinoma: early clinical experience. Radiology. 2000;217:665–672. doi: 10.1148/radiology.217.3.r00dc39665. [DOI] [PubMed] [Google Scholar]
- 6.Dupuy D, Hong R, Oliver B, et al. Radiofrequency ablation of spinal tumors. Temperature distribution in spinal canal. Am J Roentgenol. 2000;175:1263–1266. doi: 10.2214/ajr.175.5.1751263. [DOI] [PubMed] [Google Scholar]
- 7.Dupuy DE, Safran H, Mayo-Smith WW, et al. Radiofrequency ablation of painful osseous metastatic disease. Scientific Paper presented at the Annual Meeting of the Radiological Society of North America. Radiology. 1998;209S:171–172S. [Google Scholar]
- 8.Jeffrey SS, Birdwell RL, Ikeda DM, et al. Radiofrequency ablation of breast cancer: first report of an emerging technology. Arch Surg. 1999;134:1064–1068. doi: 10.1001/archsurg.134.10.1064. [DOI] [PubMed] [Google Scholar]
- 9.Wood BJ, Abraham J, Hvizda JL, et al. Thermal ablation of adrenal tumors with radiofrequency. Cancer. doi: 10.1002/cncr.11084. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pacak K, Fojo T, Goldstein DS, et al. Radiofrequency ablation: A novel approach for treatment of metastatic pheochromocytoma. J Natl Cancer Inst. 2001;93:648–649. doi: 10.1093/jnci/93.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izza F, Thomas R, Debrio P, et al. Radiofrequency ablation in patients with primary breast carcinoma: a pilot study in 26 patients. Cancer. 2001;92:2036–2044. doi: 10.1002/1097-0142(20011015)92:8<2036::aid-cncr1542>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]