Abstract
BACKGROUND
Radiofrequency thermal ablation (RFA) is a new minimally invasive treatment for localized cancer. Minimally invasive surgical options require less resources, time, recovery, and cost, and often offer reduced morbidity and mortality, compared with more invasive methods. To be useful, image-guided, minimally invasive, local treatments will have to meet those expectations without sacrificing efficacy.
METHODS
Image-guided, local cancer treatment relies on the assumption that local disease control may improve survival. Recent developments in ablative techniques are being applied to patients with inoperable, small, or solitary liver tumors, recurrent metachronous hereditary renal cell carcinoma, and neoplasms in the bone, lung, breast, and adrenal gland.
RESULTS
Recent refinements in ablation technology enable large tumor volumes to be treated with image-guided needle placement, either percutaneously, laparoscopically, or with open surgery. Local disease control potentially could result in improved survival, or enhanced operability.
CONCLUSIONS
Consensus indications in oncology are ill-defined, despite widespread proliferation of the technology. A brief review is presented of the current status of image-guided tumor ablation therapy. More rigorous scientific review, long-term follow-up, and randomized prospective trials are needed to help define the role of RFA in oncology.
Keywords: radiofrequency thermal ablation, minimally invasive therapy, hyperthermia, tumor ablation
Solid tissue tumors traditionally have been treated with systemic chemotherapy, surgical resection, or local radiation therapy. Many tumors, however, remain poorly responsive to these therapeutic modalities and necessitate the use of alternative treatments. Recent technical advancements in radiofrequency thermal ablation may provide one such option for certain cancer patients.
Heat has been used clinically for thousands of years dating back to ancient Hindu and Greek healers who used it for hemostasis. In fact, Hippocrates is reported as saying that “those diseases that medicine cannot cure, the knife cures; those which the knife cannot cure, fire cures.”1 More recently, heat has been utilized in the surgical setting as electrocautery to control bleeding and to cut tissue. Percutaneous thermal tissue ablation is performed with radiofrequency current by, in effect, transforming the patient into an electrical circuit with adhesive grounding pads on the thighs or back. A needle with a plastic-insulated shaft is placed into the tumor with imaging guidance, in a fashion similar to a routine percutaneous biopsy. Alternating radiofrequency current agitates ions in the tissue surrounding the needle, creating frictional heat, which denatures and destroys tissue at predictable temperatures, in a relatively predictable volume.
Multiple percutaneous image-guided therapies currently are available for thermal ablation of localized solid tumors. Thermal sources for these treatment modalities include high-intensity ultrasound, laser, microwave, and radiofrequency.2,3 Radiofrequency ablation (RFA) is a safe, predictable, and inexpensive option and has emerged as the thermal modality that most easily can create large volumes of tissue necrosis. The predictability of RFA is adequate to limit collateral damage and complications, however, is limited by biologic and anatomic variability of tissue.
RFA has been used over many years for the treatment of cardiac conduction abnormalities, trigeminal neuralgia4 and osteoid osteomas.5 In addition, RFA recently has been used in the treatment of neoplasms in the liver,6–14 kidney,15–17 adrenal,18 spleen,19 prostate,20,21 bone and soft tissue,22 lung,23 and breast.24 The ablation of metastatic pheochromocytomas has been described as well.25 However, the most promising and most common clinical application has been for liver tumors. Three RFA systems are 510-K cleared by the Federal Drug Administration (FDA) for soft tissue tumor ablation (Radionics Inc., Burlington, MA; RadioTherapeutics Corp., Mountain View, CA; RITA Medical Systems, Mountain View, CA). Two of these also are cleared by the FDA (510-K) for unresectable liver tumor ablation specifically. Thus, although the technique is in its infancy, it is not an experimental procedure for the liver.
HEPATIC NEOPLASMS
Hepatocellular carcinoma (HCC) is the most common solid tumor occurring worldwide with an annual incidence of at least 1 million new patients.26 The death rate from HCC approximates its occurrence rate due to its rapidity of progression coupled with a lack of effective therapies.27 Resection or transplantation provides the optimal potentially curative or survival-enhancing treatment. Percutaneous alcohol injection therapy also has proven to be an effective local treatment for local or isolated HCC, and transcatheter hepatic arterial chemoembolization also may play a palliative role for larger, inoperable HCC.
The liver is the most common site of metastatic colorectal adenocarcinoma. The optimal treatment for small or solitary disease is surgical tumor resection. Most patients, however, are not ideal surgical candidates secondary to tumor size, multifocal disease, and inadequate functional hepatic reserve or tumor proximity to nearby biliary and vascular structures. In fact, 80% of colorectal carcinoma liver metastases are unresectable, and most resected tumors recur with only a 30 − 40% 5-year survival rate.28,29 This leaves many patients with suboptimal therapeutic options. Local disease control in solitary lesions improves survival, and extrapolation of these data has been used as a theoretic justification for RFA for colorectal carcinoma liver metastases. Although the long-term data are somewhat sparse, RFA may prove to be a safe and effective treatment option for patients with unresectable hepatic tumors.6,14
PROCEDURE
RFA techniques are in their infancy, and there is rapid evolution of the specific developing and marketed techniques. Therefore, there is a significant variability in how RFA is applied and practiced by different investigators or physicians, which complicates the scientific evaluation of the technique, as well as the patient's and oncologist's management algorithms. Thus, the procedure details reported represent the most common methods of use.
Hepatic tumor ablation with RFA frequently is performed percutaneously but also may be implemented during open or laparoscopic surgery.10 It most often is completed as an outpatient procedure with conscious sedation or, less commonly, under general anesthesia. With monopolar RFA, the patient is, in effect, transformed into an electrical circuit by placing grounding pads on the thighs. Subsequently, the needle entry site is locally anesthetized down to the liver capsule with lidocaine or bupivacaine. A small radiofrequency needle electrode (14 −17.5 gauge) comprised of an insulated shaft with a noninsulated distal tip then is inserted percutaneously and guided with real-time ultrasound, computed tomography (CT) scan or magnetic resonance imaging (MRI) imaging into the tumor.
Once properly positioned within the tumor, the monopolar radiofrequency needle electrode is activated, resulting in a transfer of electrical current from the noninsulated distal tip into the surrounding tissue. Alternating current flows toward the previously placed grounding pads causing ionic agitation of the surrounding cells, ultimately leading to the production of frictional heat. As tissue temperatures increase between 60 −100 °C, there is an instantaneous induction of irreversible cellular damage referred to as coagulation necrosis.30–32 Lower temperatures (50−60 °C) may induce coagulation in minutes. However, temperatures lower than 50−60 °C generally are not used for ablation, because more than 30−40 minutes of heating are required in those cases. During the months after treatment, fibrosis and scar tissue gradually replace the necrotic area.
A typical treatment session is comprised of 10−30 minutes of active ablation and commonly produces a 3.0−5.5-cm spherical zone of coagulation necrosis (Fig. 1). The treatment area is monitored ultrasonographically for increasing echogenicity during the procedure. This increase in echogenicity corresponds to the formation of tissue and water vapor microbubbles from the heated tissue and is used to roughly estimate the boundaries of the treatment sphere. Multiple treatment spheres can be overlapped to ensure complete desiccation of the tumor along with a circumferential rim of normal hepatic tissue, thus decreasing the probability of local tumor recurrence.
FIGURE 1.
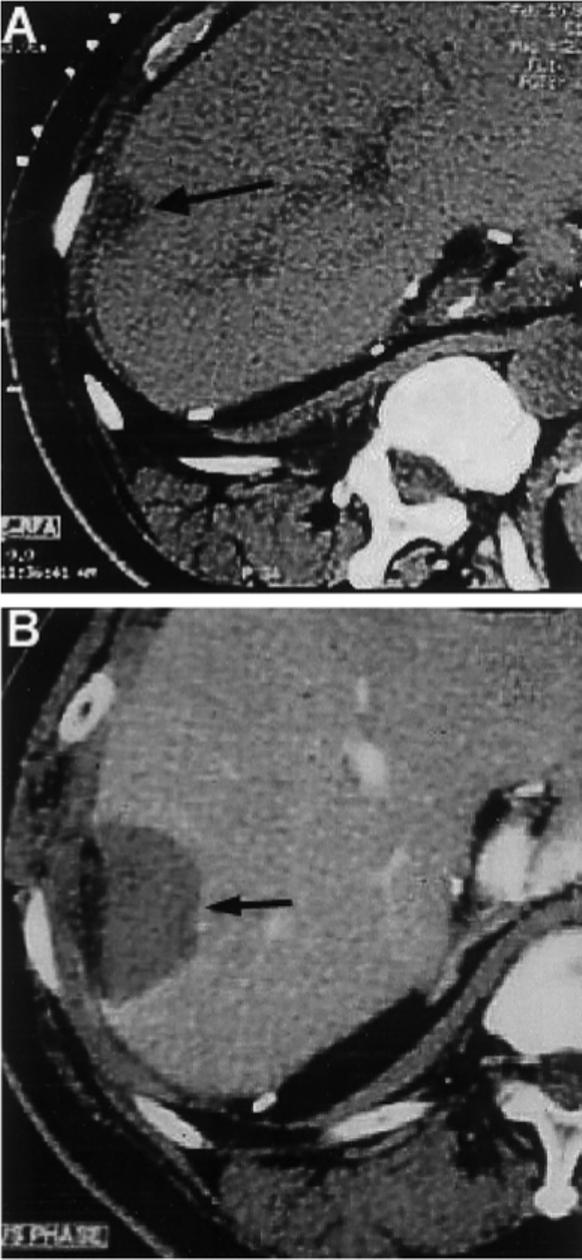
Solitary hepatocellular carcinoma recurrence before and after percutaneous radiofrequency ablation. (A) Computed tomography (CT) scan before percutaneous ablation with radiofrequency demonstrated a solitary isolated hepatocellular carcinoma recurrence in the liver (arrow) in a patient who had undergone multiple previous liver resections. (B) Enhanced CT scan performed 2 months later demonstrated no enhancement (arrow) in the treatment sphere, which included tumor and a 1-cm margin of adjacent normal liver. Lack of enhancement here in the thermal lesion was consistent with coagulation necrosis and complete treatment.
Recent developments in radiofrequency tumor ablation involve three-dimensional enlargement of the treatment sphere to permit the ablation of neoplasms varying in size and shape. In fact, several methods of expanding this sphere currently are available. When using a simple monopolar needle electrode, the current transferred to the surrounding tissue is inversely proportional to the square of the distance from the electrode.14 Consequently, there is a drastic decline in tissue temperature as heat travels farther from the needle electrode, causing a reduction in lesion dimension.33,34 The use of a multiple array electrode is one way to circumvent this obstacle. The multiple array electrode is a coaxial system comprised of several inner hook electrodes within a needle shaft. Once inside the tumor, the inner hooks are deployed and expand similar to an umbrella, thus creating a series of active electrodes, which effectively increase the dimension of ablation.11,14,35
Saline-enhanced RFA is another strategy designed to amplify the ablative sphere and involves the introduction of normal or hypertonic saline into the target tissue via a cannulated radiofrequency (RF) probe.36–39 The infused saline solution either facilitates the conduction of energy or improves thermal transmission away from the RF needle and thus expands the thermal zone. However, saline injection may sacrifice uniformity or predictability as the saline may diffuse in an irregular fashion.
The use of an internally cooled RF probe also has been shown to be effective in increasing lesion dimension.40–42 As tissue is heated to temperatures > 105 °C, it begins to boil and vaporize. Vaporization near the probe serves as an insulating shell that inhibits the propagation of heat through the tumor and therefore decreases the volume of tissue treated. In addition, rapid tissue destruction can produce overcooking or charring of the tissue immediately surrounding the needle electrode. As with tissue vaporization, this charring effect limits the quantity of energy deposited into the target zone and causes incomplete destruction of cells farthest from the needle. Internally cooled RF probes selectively limit the increase in temperature in tissue closest to the needle electrode and permit greater overall energy deposition. The probe is comprised of two internal, closed lumens within a closed needle tip. One lumen transports chilled perfusate to the distal tip whereas the other returns the warmed solution to an outer collection assembly. By preventing charring and vaporization, cooled RF probes are capable of producing lesions of greater dimension.
Energy pulsing is yet another technique applied to augment overall energy transfer while avoiding vaporization and charring. This approach uses a rapid alternation of high and low energy deposition to preferentially cool tissue proximal to the probe while maintaining continual heating of more distant tissue.32 As a result of the increased thermal zone, a greater volume of necrosis is achieved.43 Combining energy pulsing with an internally cooled RF probe synergistically produces greater tissue ablation than either method alone.44
An additional barrier to successful RFA of hepatic neoplasms is the treatment of hypervascular tumors or tumors adjacent to blood vessels. The presence of blood vessels within or near hepatic tumors causes the conduction of thermal energy away from the target tissue and into the relatively cooler blood. Perfusion-mediated cooling limits the heating of perivascular tissue and leads to the production of irregularly shaped treatment spheres.45–47 The primary impediment caused by this “heat-sink” effect is the sparing of tumor cells nearest to blood vessels, resulting in subsequent tumor recurrence. Given this phenomenon of perfusion-mediated cooling, several methods of hepatic blood flow reduction have been designed to increase lesion dimension and to ensure perivascular tumor death. Surgical cross-clamping or wrapping of the portal vein and hepatic artery at the porta hepatis (Pringle maneuver) during RFA has been shown to increase lesion size (Fig. 2).45,47 Angiographic hepatic arterial balloon occlusion also has shown positive results. However, because of the dual blood supply of the liver, balloon occlusion may have clinical limitations.45 In addition, selective transcatheter chemoembolization with polyvinyl alcohol particles, cisplatin, doxorubicin, and mitomycin C in conjunction with RFA has demonstrated promising results.48
FIGURE 2.
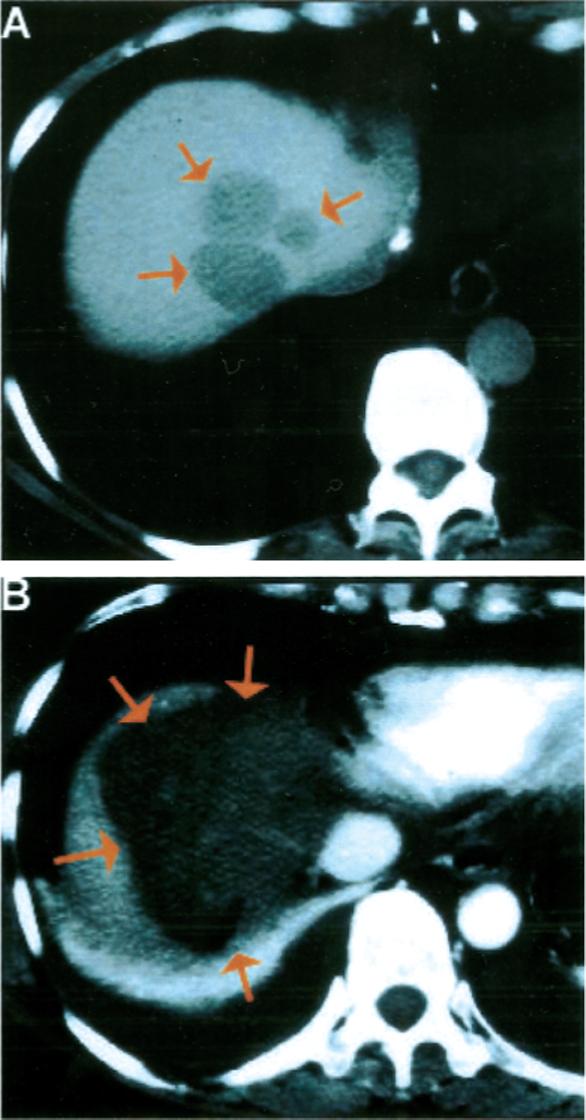
Colorectal carcinoma metastasis to the liver treated with open surgical radiofrequency ablation. (A) Contrast-enhanced computed tomography (CT) scan of the liver demonstrated three adjacent hepatic metastases (arrows) from colorectal carcinoma at the extreme dome of the liver in a difficult-to-treat location adjacent to the inferior vena cava and hepatic veins. (2) Contrast-enhanced CT scan of the liver on the day after treatment demonstrated the thermal lesion encompassing the region of liver containing the metastases, suggesting complete treatment. A pleural effusion also was noted, caused by the proximity of the diaphragm to the thermal lesion.
Hepatic tissue composition is another factor playing a primary role in the determination of treatment size. Primary HCCs are generally soft and encapsulated within a hardened, cirrhotic liver parenchyma. During RFA therapy, this rigid periphery acts as an insulating wall, thus permitting increased temperatures within the tumor. This has been referred to as the “oven effect”.9 Conversely, tumors metastatic to the liver tend to be hard and dense in comparison to the surrounding healthy liver. They also tend to have irregular, infiltrating borders, and thus are more difficult to treat percutaneously with RFA than HCC. Consequently, a larger HCC may be ablated more effectively than a similar-sized colorectal metastasis. Inclusion criteria for size may be appropriately adjusted for cell type.
On termination of the treatment session, the active RF probe is removed slowly from the treatment site. This allows for cauterization of the needle tract, thereby minimizing postprocedure bleeding and preventing seeding of the needle tract by tumor cells inadvertently attached to the needle electrode.
A postprocedure CT scan, which often displays a nonenhancing region ideally enveloping the previous tumor location, also is obtained. Close imaging follow-up with CT and MRI plays a vital role in the management of the post-RFA patient. Recurrences or regrowths can be considered for repeat RFA if the disease is discovered early, before the tumor geometry, location, or distribution become unfavorable. However, neoplastic tissue may be difficult to differentiate from scar tissue in the thermal lesion on CT and MRI. Neoplasm may have a characteristic nodular or irregular footprint along the usual thermal lesion margin, which characteristically shows uniform, regular, and thin contrast enhancement for months after RFA. Positron emission tomography scan may be useful in the pre-RFA evaluation, as well as to differentiate posttreatment morphologic changes from metabolically active tumor.
RESULTS IN THE LIVER
There is evidence to suggest that RFA can provide local, short-term control of small liver malignancies. In one of the largest series to date, only a 1.8% early recurrence rate with median follow up of 15 months was reported in 169 primary and metastatic liver tumors in 123 patients. However, 28% of patients developed metastatic disease elsewhere. The literature concerning RFA for small liver lesions repeats this trend of high rates of local control, however, also with moderate rates of subsequent development of disease elsewhere. A four-series review of RFA for small HCC (<3 cm dimension) revealed a near-complete necrosis rate of 90% at 6 months; however, only approximately 67% of the patients remained tumor free during the 12−23-month mean follow-up.49 Results for metastatic liver tumors are slightly less promising. Dodd et al. summarized several clinical series for RFA of similar small (<3 cm dimension) liver metastases as a 52−67% complete ablation rate at 1 year, with survival rates of 96%, 64%, and 40% at 1, 3, and 5 years, respectively.50 Only time will tell whether these short-term results will translate into improved long-term survival, although preliminary results are quite promising.14
Cryoablation has been used for many more years as an effective method of tissue destruction during open hepatic surgery. However, it may be prone to more complications than RFA, is more expensive, and is less easily performed laparoscopically or percutaneously, because of the larger probe size.51,52 Pearson et al. reported that RFA had a lower recurrence and complication rates compared with intraoperative cryoablation, in a nonrandomized study of 146 patients with inoperable liver tumors.51 Cryoablation also may have longer hospital and intensive care unit stays and more blood loss, thrombocytopenia, pleural effusion, and inflammatory response than RFA.52,53 To our knowledge, no randomized, controlled prospective studies have been reported to date comparing newer small-probe cryoablation with RFA, and the comparison remains controversial and highly subjective.
OTHER ONCOLOGIC APPLICATIONS
RFA also has been studied as a minimally invasive, nephron-sparing option for the treatment of renal tumors (Fig. 4).15–17 It may be well suited for patients who are poor surgical candidates or have multiple comorbid illnesses, a solitary kidney, renal insufficiency, or unresectable tumors. It also is a viable treatment alternative for patients in whom renal preservation is desired, such as those with von Hippel–Lindau disease or other conditions that predispose patients to the development of multiple bilateral renal malignancies. Preliminary reports using RFA for exophytic or small (<3 cm dimension) renal cell carcinoma show a high rate of local control by CT scan criteria on short-term follow-up17,62 (C Pavlovich work in press in J Urology). This potentially could prolong renal function in patients with hereditary, recurrent metachronous renal tumors, such as von Hippel–Lindau disease.
FIGURE 4.
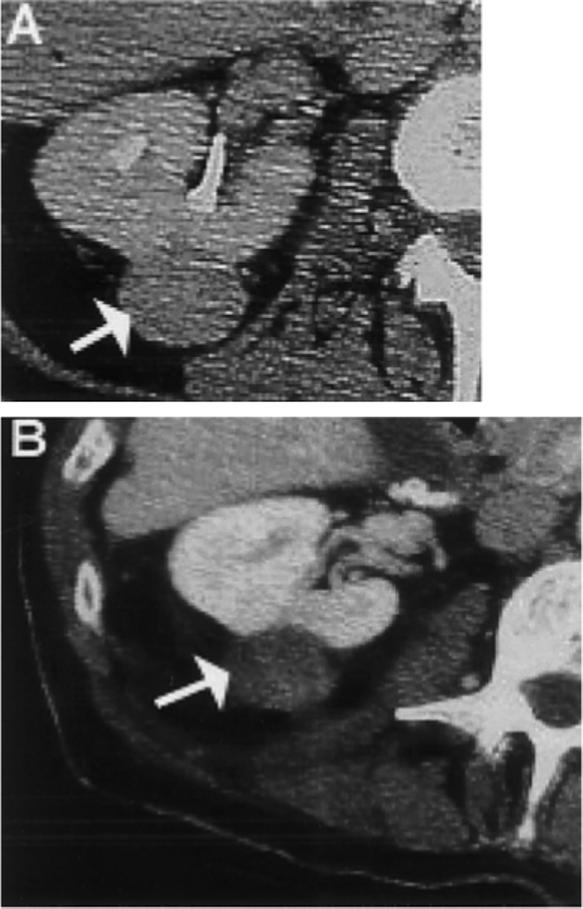
Renal cell carcinoma treated with percutaneous radiofrequency ablation. (A) Contrast-enhanced CT scan of right kidney demonstrated enhancing renal cell carcinoma (arrow). (B) Contrast-enhanced CT scan after percutaneous tumor ablation with a 17-gauge radiofrequency ablation probe demonstrated lack of prior enhancement in tumor (arrow) consistent with complete treatment.
RFA of primary and secondary lung malignancies has been reported recently. Although somewhat speculative, RFA eventually could help in the management of nonsurgical patients or as an adjunctive therapy to conventional chemotherapy or radiotherapy.23 RFA for breast carcinoma, such as lung carcinoma, is at an early stage of clinical development. Five patients with locally advanced invasive breast carcinoma were treated with RFA just before surgical resection, with complete cell death in four of five.24 In the absence of further study, the exact role or clinical indication for breast and lung RFA remains completely speculative.
Treatment options for metastatic adrenocortical carcinoma are limited. The lack of effective chemo-therapy or radiation therapy has led to a reliance on repeat surgical resection in the management of recurrent and metastatic adrenocortical carcinoma. Repeated surgical resection can prolong survival; however, the associated morbidity and cost can be high. RFA can eliminate radiographic evidence for disease in select patients with recurrent adrenocortical carcinoma (Fig. 3).18 Extrapolation from the surgical literature suggests that repeated local disease control with ablation might benefit patients with such diseases in which aggressive surgical management prolongs survival. RFA provides a nonsurgical option for tissue destruction in the inoperable patient.
FIGURE 3.
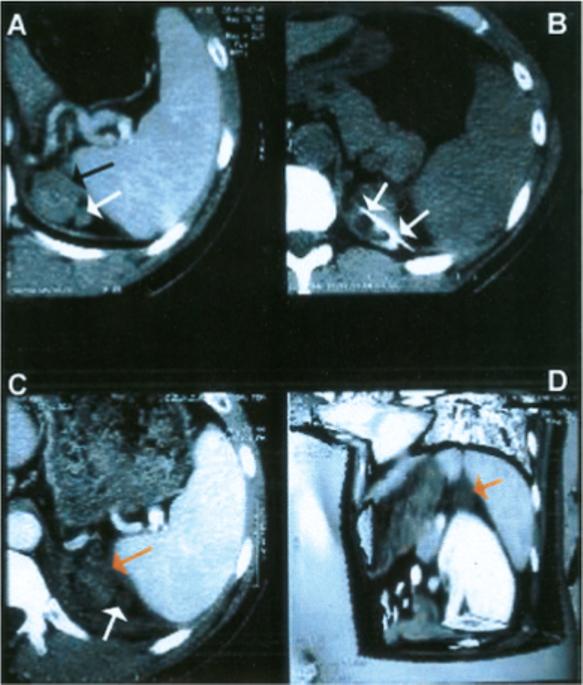
Adrenocortical carcinoma recurrence treated with percutaneous radiofrequency ablation. (A) Contrast-enhanced computed tomography (CT) scan demonstrated enhancing bilobed tumor (arrow) in adrenal bed. (B) CT scan during treatment demonstrated ablation needle (arrows) in tumor. (C) Enhanced CT scan after treatment depicted a lack of enhancement in tumors (arrows) and sliver of adjacent spleen, consistent with coagulative necrosis and cell death. (D) Three-dimensional enhanced CT image with planes cut away demonstrated treated tumor (arrow), with close proximity of adjacent nontarget organs (spleen, pancreas, kidney, stomach).
Similar to hepatic malignancies, local splenic neoplasms traditionally have been treated with surgery, which remains the definitive treatment of choice. RFA is a minimally invasive option that may be less expensive and safer for certain patients. RFA also may be a beneficial alternative for patients who pose a high surgical risk, have comorbid disease, or have concomitant tumors. Image-guided spleen biopsies have been performed with relatively little morbidity.54–56 Much like percutaneous biopsies, splenic probe placement during RFA should target the tumor while penetrating as little spleen as necessary.57 Although it may be an uncommon clinical scenario, focal splenic disease may be safely targeted with percutaneous RFA, thus preserving normal parenchyma and function.19
Conventional surgery for benign prostatic hypertrophy and prostate carcinoma often runs the risk of postsurgical morbidity including erectile dysfunction. RFA may provide a reliable method of predictable gland destruction without significant injury to the nearby bladder base, urethra, or rectum.20,21 Laser, microwave, focused ultrasound, cryotherapy, and brachytherapy are other successful minimally invasive methods of destroying prostatic tissue.
RFA also may provide a means for the palliation of pain unresponsive to other therapies.22,58 RFA has been used for over 10 years to effectively treat benign bone tumors such as osteoid osteomas.5 It also has been described recently in the treatment of metastatic bone disease.25,59 Preliminary data suggest that RFA may be efficacious in the short-term local control of painful bone and peripheral soft tissue tumors.59,61 This rapid, short-term response could lessen the use of narcotics in palliative care.
COMPLICATIONS
The complication rate of RFA in the treatment of hepatic neoplasms has been estimated as less than 3%.14,60 Complications include bleeding, effusion, fever, and infection and usually are managed nonoperatively. Complications may be specific to thermal damage (grounding pad burns, damage to adjacent normal structures) or related to other risks of needle manipulation (seeding, bleeding, infection). The 14−17.5-gauge needles used in RFA are the same sizes as those used in percutaneous biopsies, and some have the added benefit of cauterizing the needle track to decrease bleeding and tumor seeding, resulting in the low rate of postablation bleeding. In addition, the predictable nature of RFA commonly assigns a relatively low risk of collateral damage to nearby structures. In fact, the heat sink effect of adjacent blood vessels serves to protect vascular integrity from thermal damage. However, this protective effect also preserves the viability of neighboring tumor cells and increases the likelihood of local recurrence.
CONCLUSIONS
Percutaneous RFA has become a credible addition to the arsenal of minimally invasive cancer therapies. Image-guided local ablation is based on the premise that local disease control can have a positive effect on survival in selected patient populations. In the Western hemisphere, most patients that might benefit from RFA have colorectal carcinoma metastases to the liver. Recent technical developments allow RFA to be performed in a safe, predictable, and inexpensive manner with low complication rates and minimal patient discomfort. Currently, RFA is not a surgical substitute, and long-term follow-up data are pending; however, short-term results indicate that RFA can be utilized as a practical, safe, and effective treatment alternative for many cancer patients.
Acknowledgments
Bradford Wood receives support in the way of equipment loans from Radionics, Inc., Burlington, MA (a division of Tyco Health); from RITA Medical, Inc., Mountain View, CA; and from Radiotherapeutics Corp., Mountain View, CA. He owns privately purchased stock in RITA Medical, Inc., and in Boston Scientific Inc., who market for Radiotherapeutics in North America.
Footnotes
The views expressed herein do not necessarily reflect the views of the National Institutes of Health.
This article is a U.S. Government work and, as such, is in the public domain in the United States of America.
REFERENCES
- 1.Adams F. Genuine works of Hippocrates. William Wood and Company; New York: 1886. translator. [Google Scholar]
- 2.DeSanctis JT, Goldberg SN, Mueller PR. Percutaneous treatment of hepatic neoplasms: a review of current techniques. Cardiovasc Intervent Radiol. 1998;21:273–96. doi: 10.1007/s002709900263. [DOI] [PubMed] [Google Scholar]
- 3.Scudamore CH, Patterson EJ, Shapiro J, Buczkowski AK. Liver tumor ablation techniques. J Invest Surg. 1997;10:157–64. doi: 10.3109/08941939709032152. [DOI] [PubMed] [Google Scholar]
- 4.Oturai AB, Jensen K, Eriksen J, Madsen F. Neurosurgery for trigeminal neuralgia: comparison of alcohol block, neurectomy and radiofrequency coagulation. Clin J Pain. 1996;12:311–5. doi: 10.1097/00002508-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80:815–21. doi: 10.2106/00004623-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Jiao LR, Hansen PD, Havlik R, Mitry RR, Pignatelli, Habib N. Clinical short-term results of radiofrequency ablation in primary and secondary liver tumors. Am J Surg. 1999;177:303–6. doi: 10.1016/s0002-9610(99)00043-4. [DOI] [PubMed] [Google Scholar]
- 7.Patterson EJ, Scudamore CH, Buczowski AK, Owen DA, Nagy AG. Radiofrequency thermal ablation in surgery. Surg Overview Surg Technol Int. 1997;6:69–75. [PubMed] [Google Scholar]
- 8.Rossi S, Stasi MD, Buscarini E, Cavanna L, Quaretti P, Squassante E, et al. Percutaneous RF interstitial thermal ablation in the treatment of small hepatocellular carcinoma. Cancer J Sci Am. 1995;1:73–81. [PubMed] [Google Scholar]
- 9.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655–61. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 10.Buscarini L, Rossi S, Fornari F, Stasi MD, Buscarini E. Laparoscopic ablation of liver adenoma by radiofrequency electrocautery. Am Soc Gastrointest Endosc. 1995;41:68–70. doi: 10.1016/s0016-5107(95)70279-2. [DOI] [PubMed] [Google Scholar]
- 11.Rossi S, Buscarini E, Garbagnati F, Di Stasi M, Quaretti P, Rago M, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1997;170:1015–22. doi: 10.2214/ajr.170.4.9530052. [DOI] [PubMed] [Google Scholar]
- 12.Elias D, Debaere T, Muttillo I, Cavalcanti A, Coyle C, Roche A. Intraoperative use of radiofrequency treatment allows an increase in the rate of curative liver resection. J Surg Oncol. 1998;67:190–1. doi: 10.1002/(sici)1096-9098(199803)67:3<190::aid-jso9>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Curley SA, Vecchio R. New trends in the surgical treatment of colorectal cancer liver metastases. Tumori. 1998;84:281–8. doi: 10.1177/030089169808400301. [DOI] [PubMed] [Google Scholar]
- 14.Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. doi: 10.1097/00000658-199907000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGovern FJ, Wood BJ, Goldberg SN, Mueller PR. Radiofrequency ablation of renal cell carcinoma via image guided needle electrode. J Urol. 1999;161:599–600. [PubMed] [Google Scholar]
- 16.Zlotta AR, Wildschutz T, Raviv G, Peny M, van Gansbeke D, Noel J, et al. Radiofrequency interstitial tumor ablation (RITA) is a possible new modality for treatment of renal cancer: ex vivo and in vivo experience. J Endourol. 1997;11:251–8. doi: 10.1089/end.1997.11.251. [DOI] [PubMed] [Google Scholar]
- 17.Gervais DA, Mcgovern FJ, Wood BJ, Goldberg SN, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: early clinical experience. Radiology. 2000;217:665–72. doi: 10.1148/radiology.217.3.r00dc39665. [DOI] [PubMed] [Google Scholar]
- 18.Abraham J, Fojo T, Wood BJ. Radiofrequency ablation of metastatic lesions in adrenocortical cancer. Ann Intern Med. 2000;133:312–3. doi: 10.7326/0003-4819-133-4-200008150-00028. [DOI] [PubMed] [Google Scholar]
- 19.Wood BJ, Bates S. Radiofrequency thermal ablation of a splenic metastasis. J Vasc Interv Radiol. 2001;12:261–3. doi: 10.1016/s1051-0443(07)61835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Djavan B, Zlotta AR, Susani M, Heinz G, Shariat S, Silverman DE, et al. Transperineal radiofrequency interstitial tumor ablation of the prostate: correlation of magnetic resonance imaging with histopathologic examination. Urology. 1997;50:986–93. doi: 10.1016/S0090-4295(97)00540-2. [DOI] [PubMed] [Google Scholar]
- 21.Zlotta AR, Djavan B, Matos C, Noel J, Peny M, Silverman DE, et al. Percutaneous transperineal radiofrequency ablation of prostate tumor: safety, feasibility, and pathological effect on human prostate cancer. Br J Urol. 1998;81:265–75. doi: 10.1046/j.1464-410x.1998.00504.x. [DOI] [PubMed] [Google Scholar]
- 22.Tillotson CL, Rosenberg AE, Rosenthal DI. Controlled thermal injury of bone. Report of a percutaneous technique using radiofrequency electrode and generator. Invest Radiol. 1989;24:888–92. doi: 10.1097/00004424-198911000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Dupuy DE, Zagoria RJ, Akerley W, Mayo-Smith WW, Kavanagh PV, Safran H. Percutaneous radiofrequency ablation of malignancies in the lung. Am J Roentgenol. 2000;174:57–9. doi: 10.2214/ajr.174.1.1740057. [DOI] [PubMed] [Google Scholar]
- 24.Jeffrey SS, Birdwell RL, Ikeda DM, Daniel BL, Nowels KW, Dirbas FM, et al. Radiofrequency ablation of breast cancer: first report of an emerging technology. Arch Surg. 1999;134:1064–8. doi: 10.1001/archsurg.134.10.1064. [DOI] [PubMed] [Google Scholar]
- 25.Pacek K, Fojo T, Goldstein DS, Eisenhofer G, Walther M, Linehan WM, et al. Radiofrequency ablation (RFA): a novel approach for the treatment of metastatic pheochromocytoma. J Natl Cancer Inst. 2001;93:648–9. doi: 10.1093/jnci/93.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Di Bisceglie AM, Rustgi VK, Hoofnagle JH, Dusheiko KM, Lotze MT. NIH conference. Hepatocellular carcinoma. Ann Intern Med. 1988;108:390–401. doi: 10.7326/0003-4819-108-3-390. [DOI] [PubMed] [Google Scholar]
- 27.Bosch FX. Global epidemiology of hepatocellular carcinoma. In: Okuda K, Tabor E, editors. Liver cancer. Churchill Livingston; New York: 1997. pp. 487–99. [Google Scholar]
- 28.Geoghegan JG, Scheele J. Treatment of colorectal liver metastases. Br J Surg. 1999;86:158–69. doi: 10.1046/j.1365-2168.1999.01013.x. [DOI] [PubMed] [Google Scholar]
- 29.Bakalakos EA, Kim JA, Young DC, Martin EW., Jr. Determinants of survival following hepatic resection for metastatic colorectal cancer. World J Surg. 1998;22:399–405. doi: 10.1007/s002689900404. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg SN, Solbiati L, Gazelle GS, Tanabe KK, Compton CC, Mueller PR. Treatment of intrahepatic malignancy with radio-frequency ablation: radiologic-pathologic correlation in 16 patients. Am J Roentgenol. 1997;168S:121. [Google Scholar]
- 31.Thomsen S. Pathologic analysis of photothermal and photomechanical effects of laser-tissue interactions. Photochem Photobiol. 1991;53:825–35. doi: 10.1111/j.1751-1097.1991.tb09897.x. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. Am J Roentgenol. 2000;174:323–31. doi: 10.2214/ajr.174.2.1740323. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez H, van Sonnenberg E, D'agostine H, Goodacre B, Esch O. Percutaneous tissue ablation by radiofrequency thermal energy as a preliminary to tumor ablation. Minim Invasive Ther. 1993;2:299–305. [Google Scholar]
- 34.McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol. 1990;25:267–70. doi: 10.1097/00004424-199003000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg SN, Gazelle GS, Dawson SL, Rittman WJ, Mueller PR, Rosenthal DI. Tissue ablation with radiofrequency using multiprobe arrays. Acad Radiol. 1995;2:670–4. [PubMed] [Google Scholar]
- 36.Miao Y, Ni Y, Mulier S, Wang K, Hoey MF, Mulier P, et al. Ex vivo experiment on radio-frequency liver ablation with saline infusion through a screw-tip cannulated electrode. J Surg Res. 1997;71:19–24. doi: 10.1006/jsre.1997.5133. [DOI] [PubMed] [Google Scholar]
- 37.Hoey MF, Mulier PM, Shake JG. Intratumoral ablation using radiofrequency energy via screw-tip catheter and saline electrode. PACE. 1995;18:917. [Google Scholar]
- 38.Leveillee RJ, Hoey MF, Hulbert JH, Muller P, Lee D, Jesserun J. Enhanced radiofrequency ablation of canine prostate utilizing a liquid conductor: the virtual electrode. J Endourol. 1996;10:5–11. doi: 10.1089/end.1996.10.5. [DOI] [PubMed] [Google Scholar]
- 39.Livraghi T, Goldberg SN, Monti F, Bizzini A, Lazzaroni S, Meloni F, et al. Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastases. Radiology. 1997;202:205–10. doi: 10.1148/radiology.202.1.8988212. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg SN, Gazelle GS, Solbiati L, Rittman WJ, Mueller PR. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol. 1996;3:636–44. doi: 10.1016/s1076-6332(96)80188-7. [DOI] [PubMed] [Google Scholar]
- 41.Lorentzen T. A cooled needle electrode for radiofrequency tissue ablation: thermodynamic aspects of improved performance compared with conventional needle design. Acad Radiol. 1996;3:556–63. doi: 10.1016/s1076-6332(96)80219-4. [DOI] [PubMed] [Google Scholar]
- 42.Solbiati L, Goldberg SN, Ierace T, Livraghi T, Meloni F, Dellanoce M, et al. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology. 1997;205:367–73. doi: 10.1148/radiology.205.2.9356616. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg SN, Gazelle GS, Solbiati L, Mullin K, Rittman WJ, Mueller PR. Large volume radiofrequency tissue ablation: increased coagulation with pulsed technique [abstract]. Radiology. 1997;205:258. [Google Scholar]
- 44.Goldberg SN, Stein M, Gazelle GS, Kruskal J, Clouse ME. Percutaneous RF tissue ablation: optimization of pulsed-radio-frequency technique to increase coagulation necrosis. J Vasc Interv Radiol. 1999;10:907–16. doi: 10.1016/s1051-0443(99)70136-3. [DOI] [PubMed] [Google Scholar]
- 45.Goldberg SN, Hahn PF, Tanabe KK, Mueller PR, Schima W, Athanasoulis CA, et al. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998;9:101–11. doi: 10.1016/s1051-0443(98)70491-9. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg SN, Hahn PF, Halpern E, Fogle R, Gazelle GS. Radiofrequency tissue ablation: effect of pharmacologic modulation of blood flow on coagulation diameter. Radiology. 1998;209:761–9. doi: 10.1148/radiology.209.3.9844671. [DOI] [PubMed] [Google Scholar]
- 47.Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998;227:559–65. doi: 10.1097/00000658-199804000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buscarini L, Buscarini E, Di Stasi M, Quaretti P, Zangrandi A. Percutaneous radiofrequency thermal ablation combined with transcatheter arterial embolization in the treatment of large hepatocellular carcinoma. Ultraschall Med. 1999;20:47–53. doi: 10.1055/s-1999-14233. [DOI] [PubMed] [Google Scholar]
- 49.McGahan JP, Dodd GD. Radiofrequency ablation of the liver: current status. Am J Roentgenol. 2001;176:3–16. doi: 10.2214/ajr.176.1.1760003. [DOI] [PubMed] [Google Scholar]
- 50.Dodd GD, Soulen MC, Kane RA, Livraghi T, Lees WR, Yamashita Y, et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. Radiographics. 2000;20:9–27. doi: 10.1148/radiographics.20.1.g00ja019. [DOI] [PubMed] [Google Scholar]
- 51.Pearson AS, Izzo F, Fleming RY, Ellis LM, Delrio P, Roh MS, et al. Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg. 1999;178:592–9. doi: 10.1016/s0002-9610(99)00234-2. [DOI] [PubMed] [Google Scholar]
- 52.Bilchik AJ, Wood TF, Allegra D, Tsioulias GJ, Chung M, Rose DM, et al. Cryosurgical ablation and radiofrequency ablation for unresectable hepatic malignant neoplasms: a proposed algorithm. Arch Surg. 2000;135:657–62. doi: 10.1001/archsurg.135.6.657. [DOI] [PubMed] [Google Scholar]
- 53.Chapman WC, Debelak JP, Wright Pinson C, Washington MK, Atkinson JB, Venkatakrishnan A, et al. Hepatic cryoablation, but not radiofrequency ablation, results in lung inflammation. Ann Surg. 2000;231:752–61. doi: 10.1097/00000658-200005000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Malley ME, Wood BJ, Boland GW, Mueller PR. Percutaneous imaging-guided biopsy of the spleen. Am J Roentgenol. 1999;172:661–5. doi: 10.2214/ajr.172.3.10063856. [DOI] [PubMed] [Google Scholar]
- 55.Soderstrom N. How to use cytodiagnostic spleen puncture. Acta Med Scand. 1976;199:1–5. doi: 10.1111/j.0954-6820.1976.tb06683.x. [DOI] [PubMed] [Google Scholar]
- 56.Keogan MT, Freed KS, Paulson EK, Nelson RC, Dodd LG. Image-guided percutaneous biopsy of focal splenic lesions: update on safety and effectiveness. Am J Roentgenol. 1999;172:933–7. doi: 10.2214/ajr.172.4.10587123. [DOI] [PubMed] [Google Scholar]
- 57.Quinn SF, van Sonnenberg E, Casola G, Wittich GR, Neff CC. Interventional radiology in the spleen. Radiology. 1986;161:289–91. doi: 10.1148/radiology.161.2.3763890. [DOI] [PubMed] [Google Scholar]
- 58.Janjan NA, Payne R, Gillis T, Podoloff D, Libshitz HI, Lenzi R, et al. Presenting symptoms in patients referred to a multi-disciplinary clinic for bone metastases. J Pain Symptom Manage. 1998;16:171–8. doi: 10.1016/s0885-3924(98)00069-4. [DOI] [PubMed] [Google Scholar]
- 59.Dupuy DE, Safran H, Mayo-Smith WW, Goldberg SN. Radiofrequency ablation of painful osseous metastases [abstract]. Radiology. 1998;209:389. [Google Scholar]
- 60.Solbiati L. New applications of ultrasonography: interventional ultrasound. Eur J Radiol. 1998;27(Suppl 2):S200–6. doi: 10.1016/s0720-048x(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 61.Wood BJ, Fojo A, Levy EB, Gomez-Horhez, Chang R, Spies J. Radiofrequency ablation of painful neoplasms as a palliative therapy: early experience. J Vasc Interv Radiol. 2000;11S:207. [Google Scholar]
- 62.Pavlovich CP, Walther MM, Choyke PL, et al. Percutaneous radiofrequency ablation of small renal tumors: initial results. J Urology. 2002;167:10–15. [PMC free article] [PubMed] [Google Scholar]


