Abstract
Thermal injury to collateral structures is a known complication of thermal ablation of tumors. The authors present the use of CO2 dissection and inserted balloons to protect the bowel during percutaneous radiofrequency (RF) ablation and cryotherapy of primary and locally recurrent renal cell carcinoma. These techniques offer the potential to increase the number of tumors that can be treated with RF ablation or cryotherapy from a percutaneous approach.
Radiofrequency (RF) ablation and cryotherapy are current percutaneous options for the treatment of renal cell carcinoma (RCC) (1-4). Although major complication rates for RF ablation are low (5), thermal injury resulting in perforation of the gastrointestinal tract is one of the most serious complications. Bowel perforations have been reported in RF ablation of liver tumors (5,6). Although perforations of the gastrointestinal tract have not been reported in the relatively small number of cases of RF ablation for RCC, thermal injury resulting in ureteral stricture (7) and stricture in the collecting system (8) have been reported. In cryotherapy of the kidney, complete small bowel obstruction (2) and ureteropelvic junction stricture (9) have been reported in animal studies; pancreatic injury has been reported in a human series (10).
There are several options for protecting vital structures adjacent to tumors from thermal injury. Some vital structures can be actively cooled (11,12) or heated (13) by fluid infusions. In a laparoscopic approach, the vital structure can be mechanically separated from the tumor. In a percutaneous approach, thermocouples have been inserted adjacent to vital structures to monitor and modify the heating (14) or cooling (15) regimens so that the temperature adjacent to the vital structure does not exceed a critical value. Saline solution or CO2 injection has also been used with cryotherapy (16,17) and microwave thermal therapy (18) to separate vital structures from the target tumor. For RF ablation of normal porcine kidneys, Rendon et al (19) developed hydrodis-section and gas dissection techniques to protect perirenal structures from thermal injury that could occur as a result of their lack of perirenal fat. Recently, Farrell et al (20) used hydrodis-section with sterile water to protect the bowel during RF ablation of RCC. Yamakado et al (21) employed balloon catheters to displace duodenum or stomach adjacent to liver tumors that were treated with RF ablation. Herein we report the use of CO2 dissection and balloon distraction to protect the bowel during percutaneous RF ablation and cryotherapy of RCC, respectively.
CASE REPORTS
Treatments were performed under separate protocols approved by institutional review boards for RF ablation and cryotherapy of renal tumors. The patients gave written informed consent before the procedures.
Case 1
A 47-year-old man with von Hippel–Lindau (VHL) disease had been followed at our institution for 3 years. Previous to his referral to our institution, he underwent partial left nephrectomy for RCC. Baseline contrast material–enhanced abdominal computed tomography (CT) showed several cystic lesions in both kidneys; however, there were three index lesions in the cortex of the lower pole of the right kidney: an anterior cystic lesion measuring 1.4 cm × 1.4 cm, a posterior solid lesion measuring 1.6 cm × 1.7 cm, and a lateral lesion with cystic and solid components measuring 0.6 cm × 0.6 cm. Three years later, the anterior lesion became solid and slightly decreased in size to 1.1 cm × 1.4 cm. The posterior solid lesion increased in size minimally to 1.8 cm × 1.8 cm. The lateral lesion remained stable for 2 years and increased in size to 1.9 cm × 2.0 cm in the third year. All three tumors were exophytic by the third year. The patient was asymptomatic from a renal point of view. His baseline serum creatinine level was 1.2–1.4 mg/dL (normal range, 0.9–1.4 mg/dL). Because of the rapid growth in the lateral lesion over a period of 1 year and the history of VHL, the decision was made to treat all three tumors with RF ablation. Because VHL is characterized by the development of bilateral, multifocal, and metachronous RCC tumors, needle biopsy was not performed to confirm the diagnosis of RCC.
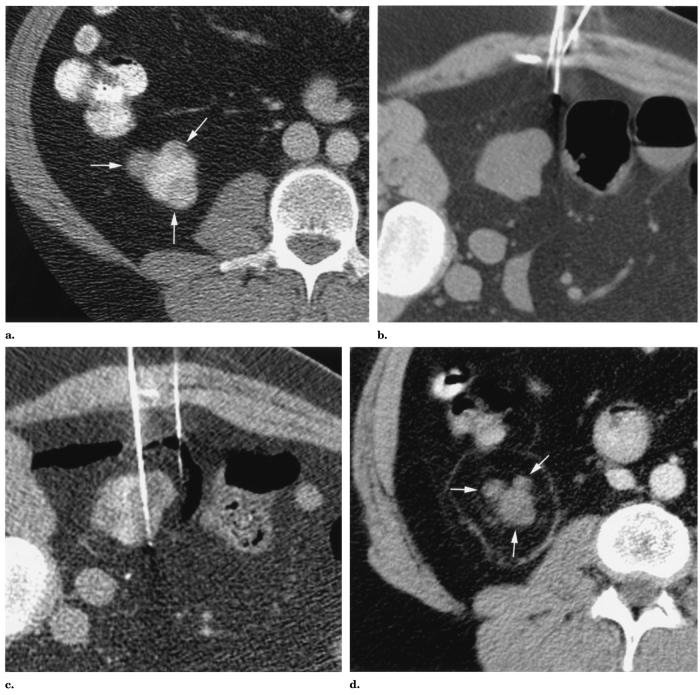
The RF ablation procedure was performed under general anesthesia. The patient was placed in a left lateral decubitus position on the CT table for ideal visualization and access. CT (Lightspeed QX/i; GE Medical Systems, Milwaukee, WI) and ultrasonography (US) (Aspen; Acuson, Mountain View, CA) were used for guidance. The last supine CT before RF ablation showed the proximity of the lateral tumor to the colon (Fig 1a). With the patient in a left lateral decubitus position, the right kidney and colon shifted so that the closest spacing between the lateral tumor and colon was 0.3 cm (Fig 1b). Because of the concern for thermal injury to the colon, a 22-gauge Chiba needle was placed in the gap between the lateral tumor and colon. CO2 from syringes was injected into this gap. Approximately 1,200 mL of CO2 at approximately 1 atm was injected into the gap over the course of the ablation (approximately 75 minutes). The closest spacing between the lateral tumor and colon increased to 1.5 cm with CO2 injection (Fig 1c). Low-current (40 mA) CT was performed at several-minute intervals during the ablation to determine adequacy of insufflation. Slight manual resistance to injection was also used as a surrogate marker of increased intra-abdominal pressure.
Figure 1.
Images from a 47-year-old man with VHL disease. (a) Transverse contrast material–enhanced CT image with the patient supine at the level of the lower pole of the right kidney shows three enhancing solid tumors (arrows) in this lower pole. (b) Transverse unenhanced CT image with the patient in a left lateral decubitus position at the level of the lower pole of the right kidney shows that the lateral exophytic renal tumor is separated from the colon by 0.3 cm. A 22-gauge Chiba needle is being positioned to enter the space between the lateral tumor and colon. (c) Transverse unenhanced CT image with the patient in a left lateral decubitus position at the level of the lower pole of the right kidney. A 22-gauge Chiba needle has been placed in the space between the lateral renal tumor and colon. CO2 has been injected to increase the distance between the lateral tumor and colon to 1.5 cm. A needle electrode has been placed to traverse the lateral and anterior renal tumors. (d) Transverse contrast-enhanced CT image at the level of the lower pole of the right kidney 11.5 months after RF ablation of the three lower-pole tumors shows enhancement of none of the tumors (arrows). There is a ring of burned fat typical of RF ablation of exophytic renal tumor.
An internally cooled 17-gauge needle electrode with a 3-cm active distal tip (Radionics, Burlington, MA) was percutaneously placed through the lateral and anterior tumors and subsequently repositioned to traverse the posterior tumor. RF was applied in pulsed impedance-controlled mode with a 200-W, 480-kHz generator (Radionics) for 12 minutes in the lateral and anterior tumors and 12 minutes in the posterior tumor. Finally, an additional 4-minute treatment was applied in the region of the anterior tumor in an area possibly insufficiently treated based on CT images.
The patient had an uneventful recovery. Serum creatinine level increased to 1.5 mg/dL the day after RF ablation, and he was discharged home that day. At 11.5 months follow-up, the patient remained asymptomatic; serum creatinine level decreased to 1.4 mg/dL. Follow-up CT at this time showed no evidence of colonic injury. The three treated tumors at the inferior pole of the right kidney did not enhance as they had before ablation (Fig 1d). In fact, all ablated tumors decreased in size. The anterior tumor measured 0.8 cm × 0.7 cm, the posterior tumor measured 1.2 cm × 1.2 cm, and the lateral tumor measured 1.1 cm × 1.3 cm.
Case 2
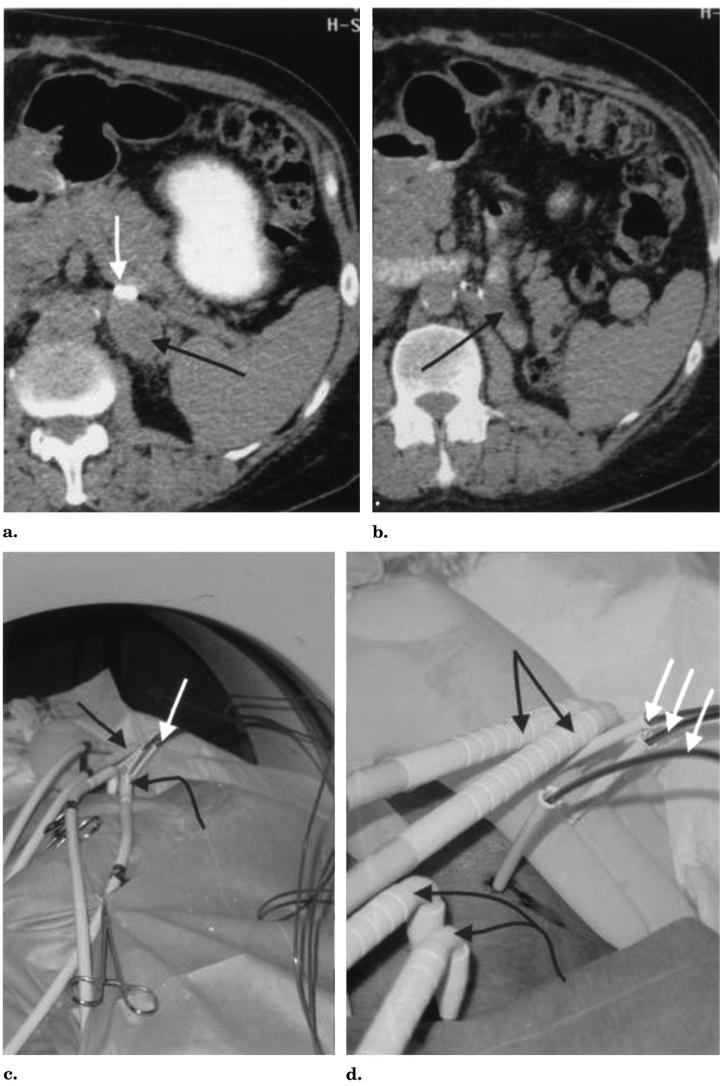
A 65-year-old man with locally recurrent RCC after left nephrectomy presented with a growing soft tissue mass in the left retroperitoneum (Fig 2a,b). CT-guided biopsy confirmed RCC when the mass measured 3.5 cm × 2.5 cm. The patient's impaired renal function (creatinine level of 3.2 mg/dL) precluded intravenous contrast material administration during the planning CT performed in a prone position. However, with the administration of oral contrast material, it was noted that the small bowel lay directly adjacent to the inferior tumor margin. In addition, the tumor abutted the tail of the pancreas and had increased in size to 4.0 cm × 3.0 cm in a 2-month span. To ablate this tumor with cryo-therapy, it was necessary at a minimum to protect the adjacent small bowel. The plan was to percutaneously place multiple balloons along the inferior and superolateral margins of the tumor to separate the bowel from the tumor.
Figure 2.
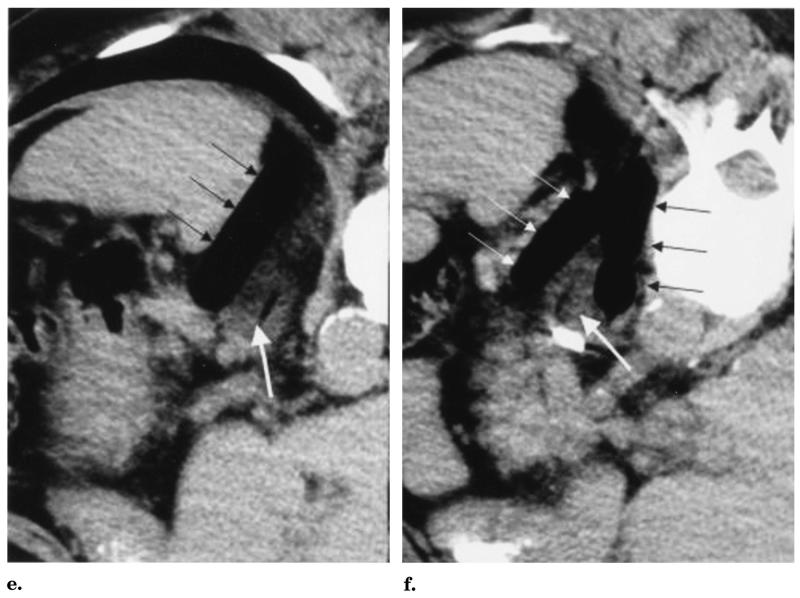
Images from a 65-year-old man with recurrent RCC after left nephrectomy. (a) Transverse unenhanced CT images with oral contrast material show the recurrent RCC mass (black arrow) and (b) bowel adjacent to the inferior margin of the mass (black arrow). The white arrow in a denotes a surgical clip just anterior to the mass. (c,d) Black arrows show L-shaped cryoprobes allowing easy CT gantry clearance. White arrows show three balloon catheters inserted through 3-mm sheaths. The straight black arrows denote the cryoprobes through the upper retroperitoneal mass, and the curved black arrows show the cryoprobes for the isolated paraaortic node. (e,f) Transverse unenhanced CT images with oral contrast material with the patient in a prone right anterior oblique position. (e) The single white arrow shows the residual air tract immediately after cryoprobe removal in the lower-attenuation frozen mass. The thinner arrows show the upper lateral balloon and (f) the two lower balloons distracting and protecting the adjacent bowel. Note the same surgical clip seen in a below the white arrow in f.
Cryotherapy was performed with use of CT guidance under local anesthesia with intravenous sedation. The patient was placed in a prone, slightly right anterior oblique position for comfort. CT fluoroscopy (Somatom Plus 4 with the Care-Vision fluoros-copy package; Siemens, Forchheim, Germany) was used for all needle and cryoprobe placements. The balloons were placed first (Fig 2c-f). A Fast-track dilator–sheath system (Endo-care, Irvine, CA) allowed direct puncture into the retroperitoneum with a 14-gauge needle. An 11-F dilator with a 3-mm Teflon sheath designed to accept the Endocare 2.4-mm cryoprobe was advanced over the needle. The gantry of the CT scanner was easily avoided with use of a simple technique of cranial angulation of the needle course. The 14-gauge component of the Fast-track system was carefully placed along the superior and inferior margins of the retroperitoneal tumor. The cranial angulation also allowed the balloon course to better traverse the cranial and caudal extent of the tumor (Fig 2e,f). After placement of the sheaths, three 2.0-cm-diameter, 4.0-cm-length Controlled Radial Expansion esophageal dilator balloons (Boston Scientific/Microvasive, Boston, MA) were placed directly through the sheaths without guide wires. With the balloon tips in their desired distal extent, the sheaths were retracted to expose the balloons for inflation.
The cryotherapy equipment was an argon–helium gas system with 2.4-mm-diameter L-shaped cryoprobes (Endocare) that readily allowed CT gantry clearance. The ice-ball diameters for these probes may vary according to the tissue and blood supply, but their freeze lengths are consistent at approximately 4.5 cm. Ice-ball diameters refer to the outer 0°C margin, but cytotoxic temperatures (<−20°C) are known to occur 3–5 mm behind visualized ice margins (13,15). For soft tissues (eg, liver and kidney), a conservative ice-ball diameter estimate for the 2.4-mm probe is 2.0 cm. Therefore, two cryoprobes were placed into the tumor with more direct axial approaches than the balloons to avoid puncturing their proximal course (Fig 2c,d). A 20-minute freeze followed by a 10-minute thaw and a 20-minute re-freeze produced visible ice (attenuation 40–60 HU lower) throughout the retroperitoneal tumor (Fig 2e,f). Given the lack of other treatment options in this nonoperative candidate, two additional probes were placed into a developing 2-cm paraaortic node to assist local control.
The patient experienced minimal discomfort during and after CT-guided percutaneous cryotherapy of his retroperitoneal recurrence. He was discharged 6 hours after the procedure without evidence of bowel symptomatology. At 4-week follow-up, the patient continued to have no abdominal symptoms, and pancreatic enzyme levels did not increase. CT at this time showed no evidence of bowel injury or growth of the retroperitoneal recurrence and paraaortic node.
DISCUSSION
The incidence of RCC has been increasing (22,23). For localized RCC, the traditional treatment has been open radical nephrectomy. More recently, for small (<4 cm) localized RCC, because the long-term cancer-free survival after nephron-sparing surgery is similar to that after radical nephrectomy, there has been considerable interest in developing less-invasive surgery such as laparoscopic partial nephrectomy and minimally invasive therapies like RF ablation and cryotherapy (1,24,25). Currently, minimally invasive therapies are reserved for highly selected patients, including those with comorbidities that make surgery highly risky, those with a solitary kidney, and those with VHL disease. Exclusion criteria include presence of metastatic disease from RCC, uncontrolled coagulopathy, lack of a safe percutaneous route, and tumor adjacent to bowel. This article demonstrates two techniques, CO2 dissection and balloon distraction, to circumvent the last exclusion criterion. These techniques will increase the possibilities for percutaneous treatment of localized small RCC.
The perirenal space contains a variable amount of fat, which is useful for two reasons. First, because the thermal conductivity of fat is approximately three times lower than that of the kidney, the perirenal fat acts as an insulator against temperature change (26). Second, the insulating property of the perirenal fat can protect adjacent structures from either extreme of thermal injury. The distance of intervening fat needed to protect an adjacent vital structure during ablation of an exophytic tumor is largely dependent on the sensitivity of the structure to thermal injury. Bowel is perhaps the most sensitive because full-thickness wall necrosis is predisposed to infection from internal enteric bacteria and ensuing perforation.
CO2 dissection provides thermal protection by increasing the distance between the tumor to be ablated and an adjacent vital structure and, more importantly, by creating a thermal blanket consisting of an exchangeable media with poor thermal conductivity. The advantage of CO2 dissection over hydrodissection with water is the lower thermal conductivity of CO2: for example, at room temperature, the thermal conductivity of CO2 is a factor of approximately 37 lower than that of liquid water (27). The main disadvantages of this technique are that, when the CO2 is introduced, it obscures visualization of the kidney on US and may not stay localized. The amount of CO2 administered from syringes, 1.2 L at approximately 1 atm, is a compromise between the requirement to physically separate the colon from the lateral tumor by at least 1 cm and patient safety because the pressure inside the abdominal cavity is not monitored. Therefore, caution is used in administering the CO2. The volume of CO2 used is also a function of the duration of RF ablation. Because CO2 is eventually reabsorbed, CT images are obtained at several-minute intervals to check the separation of bowel from tumor. If this distance decreases from the initial 1.5 cm, additional CO2 is injected. For comparison, during laparoscopic retroperitoneal and pelvic surgeries at our institution, the volume of CO2 infused ranges from 88 L to 950 L at approximately 1 atm (technically <51 mm Hg greater than atmospheric pressure). Future attempts to use this technique may incorporate miniature laparoscopic ports (eg, Auto Suture MiniPort 2 mm introducer; United States Surgical Corporation, Norwalk, CT), which can be used for gas dissection and pressure monitoring, similar to laparoscopic insufflation. The maximum outer diameter of the needle for this port is 0.110 inches or 8.4 F; hence, removal of this port does not require fascial closure.
Similarly, balloon distraction provides thermal protection by displacing the adjacent vital structure from the tumor and by decreasing heat conduction with a material with a low thermal conductivity, air. CT was used for guidance in these cases, and a potential disadvantage of this technique may occur if US is used for guidance: the balloons limit windows for US. A second disadvantage is that multiple balloons may be needed to protect the vital structure. However, there is no risk of overinsufflation, and the air stays well-localized at the protection site. The latter is a distinct benefit over saline solution and CO2, which have a tendency to move out of any area under pressure from adjacent structures.
In summary, we have demonstrated that CO2 dissection and balloon distraction show promise in allowing percutaneous treatment of RCC in which the tumor lies in close proximity to the bowel. From two successful cases, it is not possible to draw definite conclusions about the safety and efficacy of these techniques in percutaneous RF ablation or cryotherapy; however, we believe that these techniques merit trials involving a larger number of selected patients.
Abbreviations
- RCC
renal cell carcinoma
- RF
radiofrequency
- VHL
von Hippel–Lindau
Footnotes
None of the other authors have identified a conflict of interest.
References
- 1.Chin JL, Pautler SE. New technologies for ablation of small renal tumors: current status. Can J Urol. 2002;9:1576–1582. [PubMed] [Google Scholar]
- 2.Desai MM, Gill IS. Current status of cryoablation and radiofrequency ablation in the management of renal tumors. Curr Opin Urol. 2002;12:387–393. doi: 10.1097/00042307-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Lowry PS, Nakada SY. Renal cryotherapy: 2003 clinical status. Curr Opin Urol. 2003;13:193–197. doi: 10.1097/00042307-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Shingleton WB, Sewell PE., Jr Percutaneous renal tumor cryoablation with magnetic resonance imaging guidance. J Urol. 2001;165:773–776. [PubMed] [Google Scholar]
- 5.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 6.de Baere T, Risse O, Kuoch V, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003;181:695–700. doi: 10.2214/ajr.181.3.1810695. [DOI] [PubMed] [Google Scholar]
- 7.Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Renal cell carcinoma: clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology. 2003;226:417–424. doi: 10.1148/radiol.2262012062. [DOI] [PubMed] [Google Scholar]
- 8.Mayo-Smith WW, Dupuy DE, Parikh PM, Pezzullo JA, Cronan JJ. Imaging-guided percutaneous radiofrequency ablation of solid renal masses: techniques and outcomes of 38 treatment sessions in 32 consecutive patients. AJR Am J Roentgenol. 2003;180:1503–1508. doi: 10.2214/ajr.180.6.1801503. [DOI] [PubMed] [Google Scholar]
- 9.Campbell SC, Krishnamurthi V, Chow G, Hale J, Myles J, Novick AC. Renal cryosurgery: experimental evaluation of treatment parameters. Urology. 1998;52:29–33. doi: 10.1016/s0090-4295(98)00169-1. [DOI] [PubMed] [Google Scholar]
- 10.Lee DI, McGinnis DE, Feld R, Strup SE. Retroperitoneal laparoscopic cryoablation of small renal tumors: intermediate results. Urology. 2003;61:83–88. doi: 10.1016/s0090-4295(02)02004-6. [DOI] [PubMed] [Google Scholar]
- 11.Dominique E, El Otmany A, Goharin A, Attalah D, de Baere T. Intraductal cooling of the main bile ducts during intraoperative radiofrequency ablation. J Surg Oncol. 2001;76:297–300. doi: 10.1002/jso.1049. [DOI] [PubMed] [Google Scholar]
- 12.Schultze D, Morris CS, Bhave AD, Worgan BA, Najarian KE. Radiofrequency ablation of renal transitional cell carcinoma with protective cold saline infusion. J Vasc Interv Radiol. 2003;14:489–492. doi: 10.1097/01.rvi.0000064852.87207.0b. [DOI] [PubMed] [Google Scholar]
- 13.Cozzi PJ, Lynch WJ, Robson N, Vonthethoff L, Lumley T, Morris DL. In vitro and in vivo assessment of urethral warming catheters for the transperineal cryoablation of prostatic carcinoma. Br J Urol. 1996;78:589–595. doi: 10.1046/j.1464-410x.1996.16516.x. [DOI] [PubMed] [Google Scholar]
- 14.Diehn FE, Neeman Z, Hvizda JL, Wood BJ. Remote thermometry to avoid complications in radiofrequency ablation. J Vasc Interv Radiol. 2003;14:1569–1576. doi: 10.1097/01.rvi.0000096769.74047.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahn DK, Lee F, Badalament R, Kumar A, Greski J, Chernick M. Targeted cryoablation of the prostate: 7-year outcomes in the primary treatment of prostate cancer. Urology. 2002;60(2 suppl 1):3–11. doi: 10.1016/s0090-4295(02)01678-3. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman CS, Bachman B, Littrup PJ, et al. Office-based ultrasound-guided cryoablation of breast fibroadenomas. Am J Surg. 2002;184:394–400. doi: 10.1016/s0002-9610(02)01010-3. [DOI] [PubMed] [Google Scholar]
- 17.Onik G. Image-guided prostate cryo-surgery: state of the art. Cancer Control. 2001;8:522–531. doi: 10.1177/107327480100800607. [DOI] [PubMed] [Google Scholar]
- 18.Sherar MD, Gertner MR, Yue CK, et al. Interstitial microwave thermal therapy for prostate cancer: method of treatment and results of a phase I/II trial. J Urol. 2001;166:1707–1714. doi: 10.1016/s0022-5347(05)65658-3. [DOI] [PubMed] [Google Scholar]
- 19.Rendon RA, Gertner MR, Sherar MD, et al. Development of a radiofrequency based thermal therapy technique in an in vivo porcine model for the treatment of small renal masses. J Urol. 2001;166:292–298. [PubMed] [Google Scholar]
- 20.Farrell MA, Charboneau JW, Callstrom MR, Reading CC, Engen DE, Blute ML. Paranephric water instillation: a technique to prevent bowel injury during percutaneous renal radiofrequency ablation. AJR Am J Roentgenol. 2003;181:1315–1317. doi: 10.2214/ajr.181.5.1811315. [DOI] [PubMed] [Google Scholar]
- 21.Yamakado K, Nakatsuka A, Akeboshi M, Takeda K. Percutaneous radiofrequency ablation of liver neoplasms adjacent to the gastrointestinal tract after balloon catheter interposition. J Vasc Interv Radiol. 2003;14:1183–1186. doi: 10.1097/01.rvi.0000086530.86489.05. [DOI] [PubMed] [Google Scholar]
- 22.American Cancer Society . Cancer facts and figures. American Cancer Society; Atlanta: 2003. p. 4. [Google Scholar]
- 23.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 24.Reddan DN, Raj GV, Polascik TJ. Management of small renal tumors: an overview. Am J Med. 2001;110:558–562. doi: 10.1016/s0002-9343(01)00650-7. [DOI] [PubMed] [Google Scholar]
- 25.Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001;166:6–18. [PubMed] [Google Scholar]
- 26.Duck FA. Physical properties of tissue. Academic Press; San Diego: 1990. pp. 9–26. [Google Scholar]
- 27.Weast RC, Astle MJ, Beyer WH, editors. CRC handbook of chemistry and physics. 64th ed. CRC Press; Boca Raton, FL: 1983. pp. E2–E10. [Google Scholar]