Abstract
Purpose
With evolving radio frequency technology, the clinical application of radio frequency ablation (RFA) has been actively investigated in the treatment for small renal tumors. We present our intermediate patient outcomes after RFA.
Materials and Methods
Since January 2001, 17 patients with a total of 24 hereditary renal tumors ranging from 1.2 to 2.85 cm were treated with RFA using the 200 W Cool-tip RF System (Radionics, Burlington, Massachusetts) under laparoscopic (9) or percutaneous (8) guidance and had a minimum 1-year followup. A percutaneous approach was considered unsuitable if kidney tumors were contiguous to bowel, ureter or large vessels. Treatment eligibility criteria included an average tumor diameter of less than 3.0 cm, tumor growth during 1 year and solid appearance with contrast enhancement (HU change greater than 20) on computerized tomography (CT). Postoperative followup consisted of CT with and without intravenous contrast, and renal function assessment at regular intervals.
Results
Median patient age was 38 years (range 20 to 51). At a median followup of 385 days (range 342 to 691), median tumor or thermal lesion diameter decreased from 2.26 to 1.62 cm (p = 0.0013), and only 1 lesion (4%), which was located centrally near the hilum, exhibited contrast enhancement (HU change greater than 10) on CT at 12 months. Of the 15 renal tumors ablated laparoscopically, 13 were in direct contact with the bowel and 2 were abutting the ureter, necessitating mobilization before RFA. Laparoscopic ultrasound was used to guide radio frequency electrode placement and monitor the ablation process in these cases. Operative time and intraoperative blood loss (mean ± standard mean of error) were 243 ± 29 minutes and 67 ± 9 cc, respectively. In 1 patient whose ureter was adherent to the tumor a ureteropelvic junction obstruction developed after laparoscopic RFA, requiring open repair.
Conclusions
At the minimum 1-year followup 23 of 24 ablated tumors lacked contrast uptake on CT, meeting our radiographic criteria of successful RFA treatment. RFA treatment of small renal tumors using the Radionics system appears to result in superior treatment outcomes compared to those of earlier series with lower radio frequency power generators. A high wattage generator might attain more consistent energy deposition with subsequent cell death in the targeted tissue due to less convective heat loss.
Keywords: laparoscopy, kidney neoplasms
Recent advances in ablative and imaging technology have led to the application of various minimally invasive modalities in the treatment of small renal tumors. Although still considered experimental in cancer treatment, minimally invasive therapy potentially offers several advantages compared to conventional open renal surgery including shorter convalescence, decreased cost, improved cosmesis and decreased postoperative pain.
Radio frequency interstitial tissue ablation (RFA) is a Food and Drug Administration approved device for treating soft tissue. Energy is delivered to tissues via specially designed needles, resulting in heating the tissues up to 105C, leading to cell death and coagulation necrosis. In recent years an increasing number of tertiary centers have used the RFA based strategy for small renal tumors and the early results have been controversial with several centers reporting incomplete tumor ablation in many treated patients.1–7 High failure rates in these studies can be attributed to several factors such as a learning curve, variable or suboptimal technique, or failure to achieve adequate “kill” temperature within the targeted tumor. It is now recognized that a 50 W radio frequency generator, the system commonly used in early studies, is underpowered and susceptible to energy loss in highly perfused tissues such as the kidney due to a heat sink phenomenon from nearby blood vessels and adjacent normal tissue parenchyma.
Previously we published our initial experience with percutaneous RFA of small, hereditary based renal tumors using a 50 W RFA system and reported modest treatment success at 2 months.2 Since January 2001 we have used a 200 W radio frequency (RF) generator for RFA of small renal tumors and we now report our intermediate results (minimum 1-year followup) with this device.
METHODS
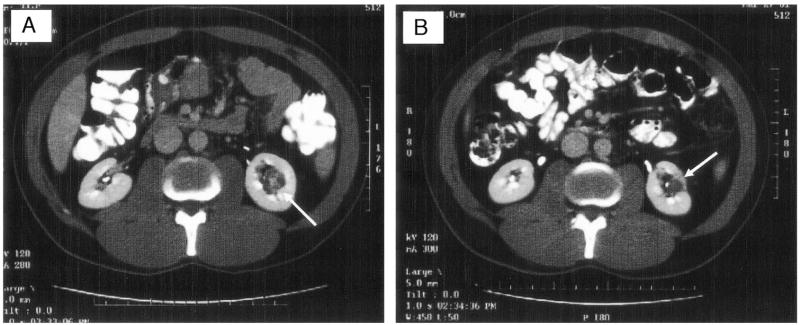
Since January 2001, 17 patients with a total of 24 hereditary based renal tumors underwent RFA treatment and had a minimum 1-year followup. Patients consisted of 9 men and 8 women, and all but 2 (1 each with the Birt-Hogg-Dubé syndrome and hereditary renal cell carcinoma [RCC]) had von Hippel-Lindau (VHL) disease. Treatment eligibility criteria included an average tumor diameter less than 3.0 cm, tumor growth for 1 year, solid appearance with contrast enhancement (HU change greater than 20) on computerized tomography (CT), and 24-hour creatinine clearance greater than 60 ml per minute. There was no selection process in this patient population, with all participants who met the aforementioned inclusion criteria receiving treatment. Medullary and central tumors were not excluded from analysis (see figure). This study was undertaken as part of an Investigational Review Board approved phase II clinical trial to evaluate radio frequency ablation of renal cancer.
Figure.
A, medullary tumor (arrow) is shown in lower pole of left kidney surrounded by abundant perinephric adipose tissue. Tumor was ablated percutaneously. B, year after RFA CT reveals nonenhancing lesion that has decreased in size.
Depending on tumor accessibility as determined by an interventional radiologist (BJW), RFA was performed under percutaneous (8 patients) or laparoscopic (9 patients) guidance. RFA of renal tumor was performed as previously described2 except that the Radionics 200 W system was used instead of the RITA 50 W first generation system. The change was made because the former was the first system available with increased wattage. An additional concern was the possibility of lacerating blood vessels adjacent to a central tumor using a deployable array. With this system the single needle minimizes the risk of this potential problem.
All percutaneous treatment was performed and monitored with CT (with and without contrast as indicated) and real-time ultrasound in the CT scanning suite with patients under conscious sedation (7) or general anesthesia (1). With a laparoscopic approach, upon isolating the targeted tumor(s) away from adjacent normal structures, laparoscopic ultrasound was used to guide the RF electrode placement and monitor the ablation process in all cases. Renal tumors were ablated using the Cool-tip RF System, a 200 W, 480 kHz RFA generator with a 17.5 gauge straight electrode probe that has a 3 cm exposed tip and thermocouple at the electrode tip. Continuous internal cycling of chilled saline through the electrode was used to minimize tissue charring and increased tissue impedance. A single electrode probe powered up to 200 W was used for each RFA procedure. The probe was repositioned for repeat or overlapping ablation if indicated, and up to 4 ablation cycles of 12 minutes each were applied as necessary. Tissue temperatures were recorded at 1 and 3 minutes after RFA as a measure of the cooling curve after treatment, which corresponds to the completeness of ablation (unpublished data). Upon completion of tumor RFA, the probe track was cauterized to 70C without saline perfusion since the probe was being removed to decrease the potential risk of track seeding.
Postoperative followup consisted of CT with and without intravenous contrast, and renal function assessment at 2, 6 and 12 months, semiannually thereafter. A total of 24 urine creatinine clearance collections were performed before RFA and at 6 and 12 months after RFA. RFA treatment was deemed unsuccessful if followup CT demonstrated contrast enhancement (HU change greater than 10). Intraoperative and postoperative complications were noted according to the National Cancer Institute Common Toxicity Criteria.2 The Mann-Whitney U Test was applied to determine the significance between any 2 groups in comparison. All data listed represent mean plus or minus standard mean of error.
RESULTS
Median patient age was 38 years (range 20 to 51) at RFA treatment. Patient and tumor characteristics are listed in table 1. At a median followup of 385 days (range 342 to 691) mean tumor or thermal lesion diameter decreased from 2.26 ± 0.10 to 1.62 ± 0.11 cm (p = 0.0013). Of the 24 tumors treated only 1 (4%) exhibited contrast enhancement (HU change greater than 10) and met radiographic criteria for tumor recurrence. This case was notable because the tumor was located centrally adjacent to the renal hilum, and required laparoscopic ultrasonography for tumor visualization and ablation. The recurrence occurred as a rim of brightly enhancing tissue (HU change of 211) along the tumor/hilar interface. In the remaining 23 tumors mean HU change changed from 69 ± 36 to 1 ± 6 after RFA treatment (p < 0.0001). Treatment parameters and results are listed in table 2.
Table 1.
Patient data
| Pt No. — Age— Sex | Tumor No. | Diagnosis | Approach | Side | Pole | Location | Exophytic | Depth |
|---|---|---|---|---|---|---|---|---|
| 1 —51— M | 1 | Birt-Hogg Dubé syndrome | Percutaneous | Rt | Lower | Lateral | No | Corticomedullary |
| 2—30— M | 2 | VHL | Percutaneous | Rt | Lower | Lateral | No | Corticomedullary |
| 3 —30— M | 3 | VHL | Percutaneous | Lt | Middle | Posterior | Yes | Cortical |
| 4 —44— M | 4 | VHL | Percutaneous | Lt | Lower | Lateral | No | Medullary |
| 5 —44— F | 5 | VHL | Percutaneous | Rt | Lower | Lateral | No | Corticomedullary |
| 6 —63— M | 6 | Hereditary RCC | Percutaneous | Lt | Lower | Posterior | Yes | Cortical |
| 7 —43— F | 7 | VHL | Percutaneous | Lt | Middle | Lateral | Yes | Cortical |
| 8 —36— M | 8 | VHL | Percutaneous | Rt | Lower | Posterior | No | Corticomedullary |
| 9 | VHL | Percutaneous | Lt | Middle | Posterior | No | Medullary | |
| 9 —25— M | 10 | VHL | Laparoscopic | Rt | Middle | Anterior | No | Cortical |
| 11 | VHL | Laparoscopic | Rt | Lower | Lateral | No | Cortical | |
| 10 —41— F | 12 | VHL | Laparoscopic | Rt | Middle | Medial | Yes | Cortical |
| 11 —38— M | 13 | VHL | Laparoscopic | Lt | Lower | Lateral | Yes | Cortical |
| 14 | VHL | Laparoscopic | Lt | Middle | Lateral | No | Corticomedullary | |
| 12 —49— F | 15 | VHL | Laparoscopic | Rt | Middle | Medial | No | Corticomedullary |
| 13 —38— F | 16 | VHL | Laparoscopic | Lt | Lower | Lateral | Yes | Cortical |
| 14 —20— M | 17 | VHL | Laparoscopic | Rt | Middle | Anterior | No | Corticomedullary |
| 18 | VHL | Laparoscopic | Rt | Middle | Posterior | No | Medullary | |
| 19 | VHL | Laparoscopic | Rt | Lower | Lateral | No | Cortical | |
| 15 —29— F | 20 | VHL | Laparoscopic | Lt | Middle | Anterior | Yes | Corticomedullary |
| 10 —44— F | 21 | VHL | Laparoscopic | Lt | Middle | Anterior | Yes | Corticomedullary |
| 22 | VHL | Laparoscopic | Lt | Lower | Lateral | No | Cortical | |
| 11 —42— F | 23 | VHL | Laparoscopic | Lt | Lower | Posterior | Yes | Corticomedullary |
| 24 | VHL | Laparoscopic | Lt | Lower | Lateral | Yes | Cortical |
Table 2.
Treatment parameters
| Tumor No. | RFA Temperature Greater Than 70C* | No. Cycles | Days Followup | Pre-RFA Size(cm) | Post-RFA Size(cm) | HU Change Before RFA | HU Change After RFA |
|---|---|---|---|---|---|---|---|
| 1 | Yes | 2 | 404 | 1.75 | 1.65 | 109 | 8 |
| 2 | Yes | 4 | 342 | 2.68 | 2.25 | 78 | 1 |
| 3 | Yes | 2 | 427 | 1.43 | 1.84 | 66 | 2 |
| 4 | Yes | 4 | 372 | 2.39 | 2.26 | 42 | −7 |
| 5 | Yes | 2 | 385 | 2.05 | 1.45 | 57 | 1 |
| 6 | Yes | 2 | 572 | 2.04 | 1.63 | 78 | 4 |
| 7 | Yes | 1 | 370 | 2.29 | 2.39 | 86 | 6 |
| 8 | Yes | 2 | 578 | 2.65 | 2.30 | 48 | −1 |
| 9 | Yes | 3 | 454 | 2.51 | 2.35 | 40 | −7 |
| 10 | No | 1 | 362 | 2.64 | 1.90 | 76 | 0 |
| 11 | No | 2 | 362 | 2.24 | 1.60 | 172 | 0 |
| 12* | Yes | 2 | 294† | 2.85 | Not applicable | Not applicable | Not applicable |
| 13 | Yes | Not applicable | 355 | 1.80 | 1.39 | 36 | 3 |
| 14 | Yes | Not applicable | 355 | 2.59 | 1.39 | 53 | 3 |
| 15 | Yes | 4 | 363 | 2.79 | 0.99 | 156 | 211 |
| 16 | Yes | 3 | 574 | 2.71 | 2.41 | 78 | 2 |
| 17 | No | 2 | 356 | 2.09 | 1.41 | 53 | 9 |
| 18 | No | 1 | 356 | 2.29 | 1.40 | 64 | −2 |
| 19 | Yes | 3 | 356 | 1.45 | 0.75 | 40 | −15 |
| 20 | No | 4 | 537 | 2.24 | 1.15 | 47 | 5 |
| 21 | Yes | 2 | 537 | 1.69 | 1.50 | 76 | 5 |
| 22 | Yes | 2 | 537 | 1.90 | No longer visible | 72 | |
| 23 | Yes | 2 | 691 | 2.55 | 1.88 | 27 | −6 |
| 24 | Yes | 2 | 691 | 1.20 | No longer visible | 27 |
Size expressed in geometric mean.
RFA temperature measured tumor temperature 1 minute after RFA treatment.
Status after UPJ repair.
Of the 15 renal tumors ablated laparoscopically 13 were in direct contact with bowel and 2 were abutting the ureter, necessitating mobilization before RFA. Laparoscopic ultrasound was used to assess RF electrode placement and monitor the ablation process in all cases. Mean operative time and intraoperative blood loss were 243 ± 29 minutes and 67 ± 9 cc, respectively. Average postoperative stay was 2.9 days (range 2 to 5).
There were no immediate postoperative complications excluding 2 cases of transient gross hematuria after laparoscopic guided RFA of centrally located lesions. One patient who wished a noninvasive treatment had a ureter densely adherent to the adjacent tumor which prevented laparoscopic mobilization. An asymptomatic ureteropelvic junction (UPJ) obstruction developed in this patient, who underwent an open surgical repair after 9 months of conservative management. The previously ablated tumor in this patient was excised at UPJ repair and there was no evidence of viable tumor tissue on pathological examination. There was no statistically significant change in renal function after RFA treatment in this patient cohort. Mean 24-hour creatinine clearance before and after RFA treatment was 115 ± 9 and 102 ± 7 ml per minute respectively.
DISCUSSION
Renal tumors are being detected at increasing rates due to the widespread use of modern imaging techniques. Typically these tumors are found incidentally and tend to be small with lower stage, yielding better survival outcomes than tumors diagnosed in symptomatic patients.8 Recent advances in ablative technology have led to the application of radio frequency interstitial tissue ablation as a minimally invasive strategy for select renal tumors. There are 4 systems with United States Food and Drug Administration clearance for soft tissue ablation with radio frequency energy. The cytotoxic mechanism involves desiccation due to high intracellular temperatures. The ablation process is continuously monitored using temperature and/or impedance feedback aided by various imaging modalities. Imaging and immediate temperature, and impedance monitoring provide predictable real-time control of tissue ablation.
The majority of human experience with RFA has been in the management of liver neoplasms.9, 10 Since the introduction of renal RFA by Zlotta et al in 199711 early results of RFA for renal tumors have been controversial. Rendon et al from Canada reported their experience with percutaneous RFA of small renal tumors (mean 2.4 cm) using the Model 2000 System (Radio Therapeutics Corporation, Sunnyvale, California) followed by immediate or delayed nephrectomy in 10 patients.3 The treatment protocol developed in the porcine model12 allowed for the maximum power input of 75 W for the 3 cm electrode. In the immediate group 5 tumors in 4 patients were treated with open RFA followed immediately by partial or radical nephrectomy. In the delayed group 6 tumors were initially treated with percutaneous RFA 7 days before open resection. On pathological examination 4 of the 5 tumors in the immediate group and 3 of the 6 tumors in the delayed group demonstrated persistent viable cancer cells. It is not clear whether the treatment resulted in satisfactory intratumoral temperatures since tissue temperature during RFA was not reported.
Early pathological and histological examination may be misleading, and tissue may look viable when cells are not actually alive. For example, hematoxylin and eosin stains may give the false impression of viability in nonsurvival studies or when excision occurs immediately after RFA. Vital stains like nicotinamide adenine dinucleotide diaphorase or tetrazolium may provide a more accurate assessment of tissue viability immediately after thermal insult.5, 13 The delayed group treatment failures may represent under treated tumors due to a combination of factors. The kidney is a highly perfused organ, and the convection of heat due to overall perfusion and into blood vessels may result in the under treatment of tumors in the kidney. This finding is especially true when ablation algorithms are applied that were developed specifically for liver tissue or for less perfused tissue. Kidney perfusion is approximately 4 times that of the liver.14 This difference drastically alters the bioheat equation (which estimates the amount of energy which can be deposited in tissue) by increasing the energy lost term.14 In addition, the first generation generator (less than 200 W) may not provide enough power to overcome the heat sink effect from the high tissue perfusion.
Similar unfavorable RFA treatment findings were also noted by the Lahey Clinic group.5 Michaels et al performed RFA in 15 patients with a total of 20 tumors (mean 2.4 cm) followed by partial nephrectomy.5 Ablated with a RITA Model 500 (the first 15 cases) or Model 1500 (the last 5 cases) system, tumors demonstrated viable tumor cells on pathological inspection (hematoxylin and eosin and/or nicotinamide adenine dinucleotide diaphorase staining). The generator delivered up to 110 W of power and tumors were heated to 90 to 110C. It remains to be elucidated whether incomplete tumor destruction was a result of lack of time between RFA and histological analysis. In addition, if the generator only delivered 110 W of power, the technique or algorithm may need to be further optimized for the kidney to allow delivery of more of the available power.
Differences in kidney perfusion or tissue dielectrics (thermal and electrical conductivities) may account for treatment effect shortcomings as well. We have tried to compensate for this effect by treating tumors more than once in similar locations. Normally in the liver 1, 12-minute treatment might suffice for a 3 cm thermal lesion. In the kidney we have found that it may take 2 or 3, 12-minute overlapping treatment sessions to treat the same volume of tissue. These modifications are somewhat subjective and difficult to translate to general practice. Thus there is a need for development of more standardized tissue or organ specific algorithms to facilitate translation of success with this technology in the kidney to the community.
In contrast to the previously mentioned findings 2 recent publications report excellent results from RFA treatment of renal tumors using high wattage generators. Jacomides et al used a RITA Model 1500X system to treat 17 renal tumors (mean 1.96 cm) laparoscopically.7 Five ablated lesions were immediately excised for pathological analysis. At a mean followup of 9.8 months (range 1.5 to 22) all treated tumors left in situ remained recurrence-free on followup imaging. Of the resected tumors only 1 lesion had a focally positive margin. Matlaga et al also reported similar results from an open RFA treatment program (using the Radionics Cool-tip System) of 10 tumors followed by immediate partial or radical nephrectomy.6 Mean tumor size was 3.2 cm (range 1.4 to 8.0). Of the 10 tumors treated 8 were completely ablated on pathological review. Two incompletely treated tumors (a 1 cm and an 8 cm tumor) were notable for insufficient intratumoral temperature achievement after RFA.
Previously we reported our initial results on RFA of small hereditary based renal tumors using the RITA Model 500 electrosurgical generator.2 We reported on 24 ablations performed in 21 patients with renal tumors and at 2-month followup a majority of tumors (19 of 24, 79%) ceased to enhance on contrast CT.2 Further followup is now available on these patients treated with the old generation RITA device (unpublished data). At a median followup of 24 months 10 of the ablated tumors (40%) demonstrated contrast enhancement (HU change greater than 10) on followup CT (unpublished data), 9 of the tumors have been surgically removed and pathologically confirmed as viable clear cell carcinoma and the remaining case has been re-treated with RFA using the higher wattage Radionics system with success based on imaging criteria.
With the 50 W RITA RFA system we have noted that tumor recurrence or persistence was often observed along the medullary portion of the ablated tumor, usually at the interface between the tumor and renal hilum (unpublished data). RFA may be less effective in medullary lesions than in cortical, peripheral or exophytic lesions that have less convective heat loss and more insulating effect from perirenal fat. Low wattage electrosurgical generators are more susceptible to rapid energy loss since heat propagates toward the periphery of the thermal lesion. Energy distribution is further impeded by the development of tissue charring around the probe tip with a subsequent increase in tissue impedance. Potential dielectric disparities in electrical and thermal conductivity between cortex and medulla may also affect the ablation response of central tumors. Compared to centrally located tumors, exophytic, cortical tumors appear to respond well to RFA, likely due to the heat insulating effect by surrounding fat. Therefore, tumor location may have an important role when selecting a suitable candidate, and this factor may be especially important with low wattage electrosurgical generators. Generally speaking, in our experience low watt generators should be avoided in the treatment of RCC.
There were 5 tumors which did not achieve a temperature greater than 70C after treatment (table 2). Tumor location was in the cortex and the medulla. It is assumed that blood vessels adjacent to the tumors acted as a heat sink and resulted in an altered temperature profile. Tumors not heated to more than 70C were re-treated. The needle was sometimes repositioned if a vessel was next to the temperature sensor on its tip. Patients with larger tumors or with contiguous tumors were treated with overlapping RFA fields. All 5 tumors appeared well treated without recurrence on followup.
Our experience with this 200 W system which includes treating 13 centrally located tumors with a deep medullary component has been excellent, with only 1 of 24 ablated tumors showing radiographic evidence of recurrence at 12 months of followup. Differences in patient selection, RFA system used and procedure technique preclude any direct comparison of treatment outcomes from various RFA series reported in the literature, but our data suggest that high wattage RF generators may be less susceptible to the heat sink phenomenon and better suited to overcome anatomical factors such as tumor location and characteristics. With further improvements in RF technology and equipment, more consistent, favorable outcomes may be the result. The exact role of RFA will soon become more clearly defined as part of the minimally invasive therapy arsenal for renal tumors.
CONCLUSIONS
With evolving technology of radio frequency generators and improved probe design, RFA is a promising treatment alternative for small renal tumors. The 200 W power results in superior treatment outcomes compared to low wattage systems, likely due to less convective heat loss and more consistent energy distribution throughout the tumor. Although promising in the treatment of cancer, RFA remains somewhat experimental in the kidney until long-term efficacy is validated. Further development of kidney specific techniques and algorithms should improve outcomes. At this time this technique should be reserved for experienced centers in specific clinical scenarios.
Footnotes
Nothing to disclose.
References
- 1.Gervais DA, McGovern FJ, Wood BJ, Goldberg SN, McDougal WS, Mueller PR. Radio-frequency ablation of renal cell carcinoma: early clinical experience. Radiology. 2000;217:665. doi: 10.1148/radiology.217.3.r00dc39665. [DOI] [PubMed] [Google Scholar]
- 2.Pavlovich CP, Walther MM, Choyke PL, Pautler SE, Chang R, Linehan WM, et al. Percutaneous radio frequency ablation of small renal tumors: initial results. J Urol. 2002;167:10. [PMC free article] [PubMed] [Google Scholar]
- 3.Rendon RA, Kachura JR, Sweet JM, Gertner MR, Sherar MD, Robinette M, et al. The uncertainty of radio frequency treatment of renal cell carcinoma: findings at immediate and delayed nephrectomy. J Urol. 2002;167:1587. [PubMed] [Google Scholar]
- 4.de Baere T, Kuoch V, Smayra T, Dromain C, Cabrera T, Court B, et al. Radio frequency ablation of renal cell carcinoma: preliminary clinical experience. J Urol. 2002;167:1961. doi: 10.1097/00005392-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Michaels MJ, Rhee HK, Mourtzinos AP, Summerhayes IC, Silverman ML, Libertino JA. Incomplete renal tumor destruction using radio frequency interstitial ablation. J Urol. 2002;168:2406. doi: 10.1016/S0022-5347(05)64155-9. [DOI] [PubMed] [Google Scholar]
- 6.Matlaga BR, Zagoria RJ, Woodruff RD, Torti FM, Hall MC. Phase II trial of radio frequency ablation of renal cancer: evaluation of the kill zone. J Urol. 2002;168:2401. doi: 10.1016/S0022-5347(05)64154-7. [DOI] [PubMed] [Google Scholar]
- 7.Jacomides L, Ogan K, Watumull L, Cadeddu JA. Laparoscopic application of radio frequency energy enables in situ renal tumor ablation and partial nephrectomy. J Urol. 2003;169:49. doi: 10.1016/S0022-5347(05)64032-3. [DOI] [PubMed] [Google Scholar]
- 8.Luciani LG, Cestari R, Tallarigo C. Incidental renal cell carcinoma-age and stage characterization and clinical implications: study of 1092 patients (1982–1997) Urology. 2000;56:58. doi: 10.1016/s0090-4295(00)00534-3. [DOI] [PubMed] [Google Scholar]
- 9.McGahan JP, Brock JM, Tesluk H, Gu WZ, Schneider P, Browning PD. Hepatic ablation with use of radiofrequency electrocautery in the animal model. J Vasc Interv Radiol. 1992;3:291. doi: 10.1016/s1051-0443(92)72028-4. [DOI] [PubMed] [Google Scholar]
- 10.Rossi S, Di Stasi M, Buscarini E, Quaretti P, Garbagnati F, Squassante L, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996;167:759. doi: 10.2214/ajr.167.3.8751696. [DOI] [PubMed] [Google Scholar]
- 11.Zlotta AR, Wildschutz T, Raviv G, Peny MO, van Gansbeke D, Noel JC, et al. Radiofrequency interstitial tumor ablation (RITA) is a possible new modality for treatment of renal cancer: ex vivo and in vivo experience. J Endourol. 1997;11:251. doi: 10.1089/end.1997.11.251. [DOI] [PubMed] [Google Scholar]
- 12.Rendon RA, Gertner MR, Sherar MD, Asch MR, Kachura JR, Sweet J, et al. Development of a radiofrequency based thermal therapy technique in an in vivo porcine model for the treatment of small renal masses. J Urol. 2001;166:292. [PubMed] [Google Scholar]
- 13.Crowley JD, Shelton J, Iverson AJ, Burton MP, Dalrymple NC, Bishoff JT. Laparoscopic and computed tomography-guided percutaneous radiofrequency ablation of renal tissue: acute and chronic effects in an animal model. Urology. 2001;57:976. doi: 10.1016/s0090-4295(00)01129-8. [DOI] [PubMed] [Google Scholar]
- 14.Duck FA. Physical Properties of Tissue. London: Academic Press Limited; 1990. Appendix A: Tissue perfusion rates. [Google Scholar]