Abstract
A patient with renal cell carcinoma underwent external-beam radiation therapy (XRT) to treat a painful chest-wall metastasis. One month later, she underwent radiofrequency (RF) ablation of two metastatic deposits within the liver; one of the target lesions was in the recent irradiation zone and the other was outside of the radiation field. RF ablation within the irradiated liver produced a slightly larger ablation zone with prominent needle tract scarring, and required less energy input than treatment in the unirradiated liver. RF ablation and XRT may interact, possibly producing a synergistic effect. Further study of the potentially adjunctive relationship between these two modalities is warranted.
Current treatment strategies for liver cancer frequently rely on a combination of modalities, including chemotherapy, radiation therapy, and surgery. This multifaceted approach may be more effective than any single therapy. Another tool in the arsenal of cancer therapy is radiofrequency (RF) ablation. RF ablation is a safe and effective technique to destroy focal cancer lesions in a variety of locations, including the liver, bone, and kidney (1-3). Clinical research is currently under way in cancers in the lung, breast, and adrenal gland as well (4-6). RF ablation also has the potential for synergism with traditional cancer treatments, as RF ablation and liposomal chemotherapy have been shown to considerably enhance ablation volumes (7). Although the additive effects of hyperthermia and radiation have been studied at low temperatures (40°C–44°C), there are few data about radiation and the high temperatures of RF ablation (60°C–100°C) (8). Herein we present a case in which the effects of external-beam radiation treatment (XRT) in combination with RF ablation may account for treatment differences seen with RF ablation of liver metastases. The potential synergism between RF ablation and radiation therapy for local control warrants further investigation.
CASE REPORT
In 1969, a 42-year-old woman presented with hematuria secondary to renal-cell carcinoma. She underwent right nephrectomy and XRT to the renal bed; there was no evidence of metastatic disease at that time. Twenty-seven years later, the woman, now 69 years of age, developed biopsy-proven metastases to the pelvis and liver. She was treated with abdominal resection of the pelvic mass followed by XRT, RF ablation of the liver lesion, and administration of interleukin-2, with good response. The following month, the patient developed a painful metastasis to the right chest wall, which was successfully treated with 2,100 cGy of radiation. On this hospital admission, the patient presented with two liver metastases and was referred for RF treatment. There was a 0.9-cm (transverse) by 0.8-cm (anteroposterior) lesion in the anterior section of the left lobe, and a 0.9-cm (transverse) by 0.8-cm (anteroposterior) in the posterior aspect of the right lobe. The region of the posterior liver lesion had received the full dose of radiation treatment on the previous admission, whereas the anterior lesion was exposed to less than 10% of the dose (Fig 1). On positron emission tomography, there was increased fluorinated deoxyglucose metabolism in the anterior lesion and decreased fluorinated deoxyglucose metabolism in the posterior lesion.
Figure 1.
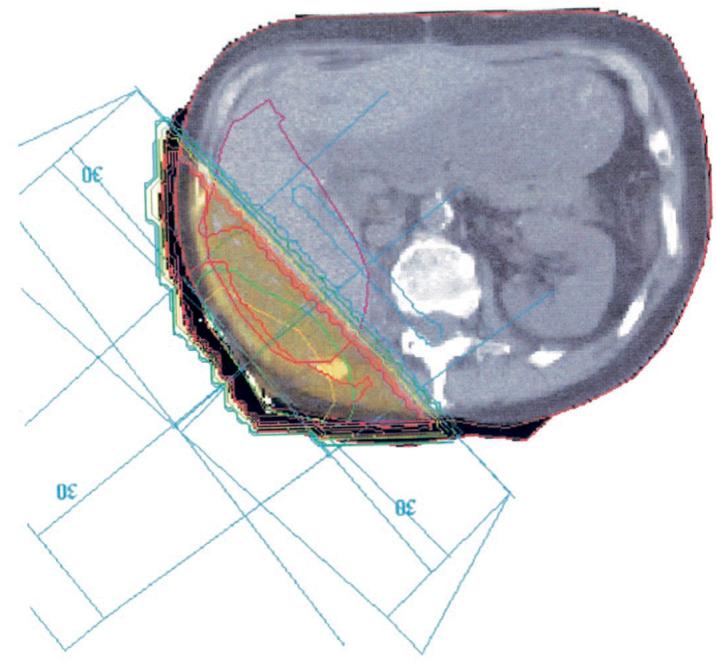
Dosimetry-planning diagram for radiation treatment. The region in yellow received 100% of the radiation dose (2,100 cGy).
Written informed consent was obtained and the patient was treated on an investigational review board–approved study. The patient was placed supine in the interventional computed tomography (CT) suite. Conscious sedation was administered with intravenous fentanyl and midazolam, and 1% lidocaine was administered down to the liver capsule.
The procedure was performed with alternating CT and real-time ultrasound (US) guidance in both grayscale and color modes. A 23-gauge needle was placed under US guidance through an acrylic button to serve as a tandem guide for the 17.5-gauge internally-cooled triple needles with 2.5 cm of exposed active tip on each needle (Cool-tip; Radionics, Burlington, MA). RF current was provided by the Radionics Cool-tip 200-W, 480 kHz generator ablation system in automatic impedance-control mode. The anterior lesion was ablated with a 12-minute treatment, with a maximum current of 1.46 amps, maximum power of 141 W, and time to pulsing of 5 minutes. This was followed by three shorter treatments of 2 minutes each without repositioning to improve postablation temperatures. The posterior lesion was ablated with a similar impedance-controlled 12-minute session and a single 2-minute short treatment. Maximum current and power were 1.41 amps and 137 W respectively, and time to pulsing was 10 minutes. The lesions were monitored during ablation by US with CT verification of subsequent thermal lesions. The needle tract was cauterized upon needle removal during both ablations to prevent back-bleeding and theoretic needle tract seeding. Needle tract cauterization was performed with identical algorithms, with identical goals of maintaining a temperature 70°C while slowly retracting the needle. CT was performed following the procedure after injection of 50 mL of nonionic contrast material. The needle tract to the posterior lesion was more than 1.0 cm wide (Fig 2). There was also a thick rim of enhancement on the high-radiation-dose side of the posterior ablation zone and needle tract (Fig 3). Three months after ablation, magnetic resonance imaging showed a 2.0-cm by 2.0-cm ablation zone around the anterior lesion and a 3.0-cm by 2.0-cm ablation zone around the posterior, irradiated lesion (Fig 4). Seven months after ablation, there is slight shrinkage of the ablation zones in both treated areas (Fig 5). The ablated lesions have remained free of recurrence while the patient's disease progressed elsewhere in the liver.
Figure 2.

Contrast material–enhanced CT image 10 minutes after RF ablation demonstrated the needle tract in the radiation field is more than 1.0 cm wide (arrow). This is much larger than normally seen with use of the same parameters and technique.
Figure 3.

CT of the abdomen shows a normal thin enhancing rim around the ablation zone on the low-dose side (short arrow) and thick parenchymal enhancement adjacent to the high-radiation-dose border (long arrow).
Figure 4.

T1-weighted contrast-enhanced coronal MR image after treatment shows larger ablation zone in the irradiated lesion (bottom image) than in the nonirradiated lesion (top image) with use of similar technique.
Figure 5.
CT of the abdomen 7 months after ablation demonstrates interval shrinkage of thermal lesions without evidence of recurrence. The thermal lesion in the irradiated field remains larger than in the other lesion.
DISCUSSION
XRT and RF ablation, although both well-established and effective local cancer treatments, are not generally used in tandem. The development of metastatic lesions in this patient within and outside the previously irradiated liver provides an opportunity to compare the potential additive effects of RF ablation and radiation therapy. The ablation of the irradiated lesion was subjectively less painful, and the ablation zone and cauterization tract were substantially larger in the irradiated area on postablation CT.
Hyperthermia combined with radiation therapy has been shown to increase local control of advanced malignancies. Adjuvant effect was found to be most significant with a temperature in the tumor's center higher than 42.5°C (8). The application of heat before radiation may improve the complex relationship between local perfusion, interstitial pressure, and oxygen tension in the center of a tumor. In addition, hyperthermia interferes with cellular repair mechanisms of irradiated DNA (9). The timing and treatment parameters of low-temperature hyperthermia induction before XRT are the focus of study in large clinical trials (10,11).
Radiation therapy before RF ablation may hold similar promise for treatment potentiation. Radiation effects on local tissue perfusion could reduce the convection of heat away during RF ablation, thereby increasing energy deposition and thermal lesion volume. Decreased perfusion in irradiated liver could also greatly reduce this “heat-sink” effect that impedes tissue ablation adjacent to larger blood vessels. Mechanical modification of blood flow may improve RF ablation treatment volumes in a similar manner. This may be accomplished with porta hepatis occlusion in the operating suite or with endovascular balloons in the hepatic artery, hepatic vein, or portal vein in the image-guided therapy suite (12,13). In lieu of balloon inflation, targeted ablation of perivascular tumor segments before targeting the more remote regions of tumor may increase ablation size by reducing regional blood flow (14).
In addition to vascular effects, radiation-induced changes of tissue characteristics may also account for the observed RF ablation synergy. Histologic studies of high-dose radiation therapy in the canine liver show parenchymal fibrosis and hepatocyte atrophy in the short term (within 3 months) and arteriolar hyalinosis in the long term (3–5 years) (15). The increased enhancement of tissue outside the ablation zone of irradiated lesion and needle tract was seen on the high-dose side of the radiation field (Fig 3). Early radiation-induced tissue changes could alter tissue dielectrics, thereby allowing greater deposition of RF energy and causing the observed periablative penumbra. The longer time to pulsing of the irradiated lesion (10 minutes versus 5 minutes for the untreated lesion) suggests that more energy may have been deposited during ablation before impedance increased. However, identical algorithms and control parameters were followed. The very similar ablation techniques with differing volumes of ablation implies either that less energy was reflected or removed from the previously radiated lesion or that the tissue was sensitized to the energy deposition.
This is a limited anecdotal report of potential interactions between two complex technologies, so care must be taken to avoid broad assumptions. Confounding variables may have accounted for the observed phenomenon; these include the peripheral location of the anterior lesion (which limits ablation size by abutting the liver capsule) and natural local perfusion differences. In fact, no general conclusions may be derived from this case, except that further study is warranted. In this one patient, similar RF ablation algorithms resulted in a substantially larger volume of necrosis, raising the possibility of adjuvant effects. Further basic science and clinical research is needed to clearly elucidate the relationship between RF ablation and XRT and their potential combined application for focal malignancy.
Abbreviations
- RF
radiofrequency
- XRT
external-beam radiation therapy
Footnotes
None of the authors have identified a potential conflict of interest.
References
- 1.Bleicher RJ, Allegra DP, Nora DT, et al. Radiofrequency ablation in 447 complex unresectable liver tumors: lessons learned. Ann Surg Oncol. 2003;10:52–58. doi: 10.1245/aso.2003.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Callstrom MR, Charboneau JW, Goetz MP, et al. Painful metastases involving bone: feasibility of percutaneous CT- and US-guided radio-frequency ablation. Radiology. 2002;224:87–97. doi: 10.1148/radiol.2241011613. [DOI] [PubMed] [Google Scholar]
- 3.Gervais DA, McGovern FJ, Arellano RS, et al. Renal cell carcinoma: clinical experience and technical success with radio-frequency ablation of 42 tumors. Radiology. 2003;226:417–424. doi: 10.1148/radiol.2262012062. [DOI] [PubMed] [Google Scholar]
- 4.Dupuy DE, Zagoria RJ, Akerley W, et al. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174:57–59. doi: 10.2214/ajr.174.1.1740057. [DOI] [PubMed] [Google Scholar]
- 5.Izzo F, Thomas R, Delrio P, et al. Radiofrequency ablation in patients with primary breast carcinoma: a pilot study in 26 patients. Cancer. 2001;92:2036–2044. doi: 10.1002/1097-0142(20011015)92:8<2036::aid-cncr1542>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 6.Wood BJ, Abraham J, Hvizda JL, et al. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer. 2003;97:554–560. doi: 10.1002/cncr.11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg SN, Kamel IR, Kruskal JB, et al. Radiofrequency ablation of hepatic tumors: increased tumor destruction with adjuvant liposomal doxorubicin therapy. AJR Am J Roentgenol. 2002;179:93–101. doi: 10.2214/ajr.179.1.1790093. [DOI] [PubMed] [Google Scholar]
- 8.Xia T, Sun Q, Shi X, Fan N, Hiraoka M. Relationship between thermal parameters and tumor response in hyperthermia combined with radiation therapy. Int J Clin Oncol. 2001;6:138–142. doi: 10.1007/pl00012096. [DOI] [PubMed] [Google Scholar]
- 9.Kampinga HH, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevance. Int J Radiat Biol. 2001;77:399–408. doi: 10.1080/09553000010024687. [DOI] [PubMed] [Google Scholar]
- 10.van der Zee J, Gonzalez DG, van Rhoon GC, van Dijk JDP, van Putten WLJ, Hart AAM. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumors: a prospective, randomized, multicentre trial. Lancet. 2000;355:1119–1125. doi: 10.1016/s0140-6736(00)02059-6. [DOI] [PubMed] [Google Scholar]
- 11.Sakurai H, Hayakawa K, Mitsuhashi N, et al. Effect of hyperthermia combined with external radiation therapy in primary non-small cell lung cancer with direct bony invasion. Int J Hyperthermia. 2002;18:472–483. doi: 10.1080/02656730210146917. [DOI] [PubMed] [Google Scholar]
- 12.de Baere T, Bessoud B, Dromain C, et al. Percutaneous radiofrequency ablation of hepatic tumors during temporary venous occlusion. AJR Am J Roentgenol. 2002;178:53–59. doi: 10.2214/ajr.178.1.1780053. [DOI] [PubMed] [Google Scholar]
- 13.Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Okita K. Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow. Cancer. 2002;95:2353–2360. doi: 10.1002/cncr.10966. [DOI] [PubMed] [Google Scholar]
- 14.Lu DSK, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol. 2002;178:47–51. doi: 10.2214/ajr.178.1.1780047. [DOI] [PubMed] [Google Scholar]
- 15.Cromheecke M, Grond AJK, Szabo BG, Hoekstra HJ. Short- and long-term histopathological changes in the canine liver following single high-dose intraoperative radiation therapy (IORT) Int J Radiat Biol. 1999;75:1437–1448. doi: 10.1080/095530099139304. [DOI] [PubMed] [Google Scholar]



