Abstract
Percutaneous image-guided radiofrequency ablation (RFA) of tumors has the potential risk for thermal damage to nearby normal collateral tissues. Thus, the goal of creating a sufficient area of tumor necrosis must be weighed against the risk for injury to collateral tissues. In this study, remote thermistors were used to monitor temperatures near collateral structures during tumor RFA. Four unique cases are described. When temperature-sensitive structures are near the target lesion, remote thermometry could further increase the safety of this evolving minimally invasive procedure.
Percutaneous radiofrequency ablation (RFA) is an emerging treatment option for a variety of diseases. It is has been used most extensively to treat unresectable solid tumors, including neoplasms in the liver, kidney, adrenal, spleen, prostate, bone and soft tissue, lung, and breast (1). Most antineoplastic therapies must balance the goal of tumor destruction with the potential damage to normal, non-target tissue. For example, collateral damage is a major limitation of radiation therapy. RFA induces cell death by coagulation necrosis, with temperature being the direct determinant of margins defining a thermal sphere of necrosis. Elevation of tissue temperature to a range of 50° to 60°C induces irreversible cellular damage within minutes. With higher temperature elevation (60°–100°C), the irreversible damage is essentially instantaneous (1,2). These elevations of temperature can also damage adjacent, non-target tissues. Different tissues have variable thermal thresholds (3). For instance, intentional nerve lesioning to treat pain has been described with radiofrequency (RF) heating to approximately 45°C (4-6), while myocardial injury occurs when a temperature of approximately 50°C has been reached (7). A temperature of 45°C has been used as the upper limit of heating for hyperthermia near the bowel (8,9).
Intraprocedural temperature monitoring near adjacent, susceptible organs can provide a direct method to monitor and thereby protect non-target tissue. This method has been described with transrectal ultrasound hyperthermia of prostate cancer (10), as well as transurethral prostatic electrovaporization (11) and transurethral microwave therapy for benign prostatic hyperplasia where thermocouple sensors were positioned along the anterior rectal wall. Although some available RFA systems incorporate thermistors to evaluate tissue temperatures at the tip of the active RFA probe (Radionics, Burlington, MA), or near the margin and in between active tips (RITA Medical, Mountain View, CA), these thermistors cannot be deployed separately from the probe itself. Although these thermistors are directed to the target tissue, they are poorly positioned to provide precise temperature information about specific non-target tissue. This study describes the use of separate, independent thermistors placed near non-target tissue as a means of increasing the safety of RFA in specific clinical settings.
MATERIALS AND METHODS
Institutional review board approval and patient consent were obtained for all patients involved in this report. Intraprocedural temperature monitoring with thermistors was performed during tumor RFA in four patients. In all cases, a 10–15-cm cool-tip probe (Radionics) with a 2- or 3-cm non-insulated tip was used for the ablation. Thermistors were positioned with computed tomography (CT) and ultra-sound (US) guidance according to presumed approximate anatomic location of sensitive structures or near neurovascular bundle if visible on CT. Temperature was sampled by the thermistors on the probe and the independent thermistors continuously, with digital readout. In patients 1–3, a 25-gauge accessory thermistor acting as a stylet inside a 22-gauge SMK pole needle (Radionics) was used for the temperature measurements with a digital TCA-2 monitor (Radionics; Fig 1). In patient 4, an endorectal probe with three linear thermistors was used. The rectal thermistor was connected to an adaptive phased array microwave prostate ablation generator and thermistor system (Microfocus BPH 800; Celsion, Columbia, MD; Fig 2). The procedures were performed with CT and US guidance and standard conscious sedation with midazolam and fentanyl. When thermal spheres were planned near nerves, frequent neurologic examinations were performed. When thermal spheres were planned near bowel, a pro-peristaltic maneuver was performed (polyethylene glycol 128 ounces by mouth during a 3–4 hour period 24 hours before the procedure).
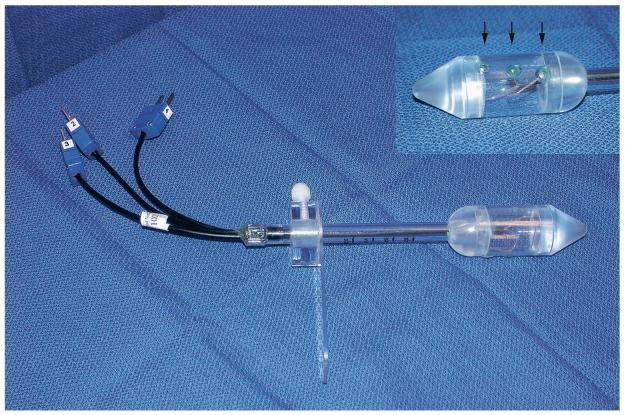
Figure 1.
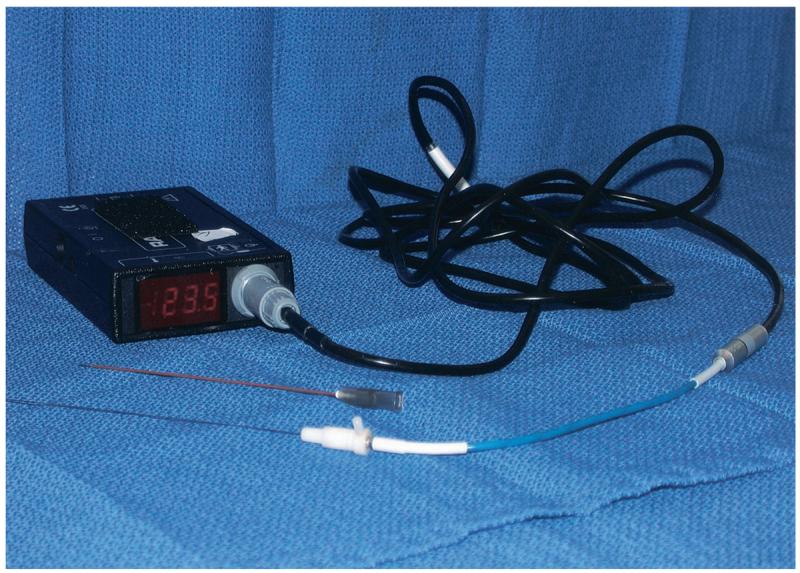
A 25-gauge accessory thermistor and digital TCA-2 monitor (Radionics).
Figure 2.
Endorectal probe (Celsion). The insert shows the three linear thermistors on the probe head (arrows).
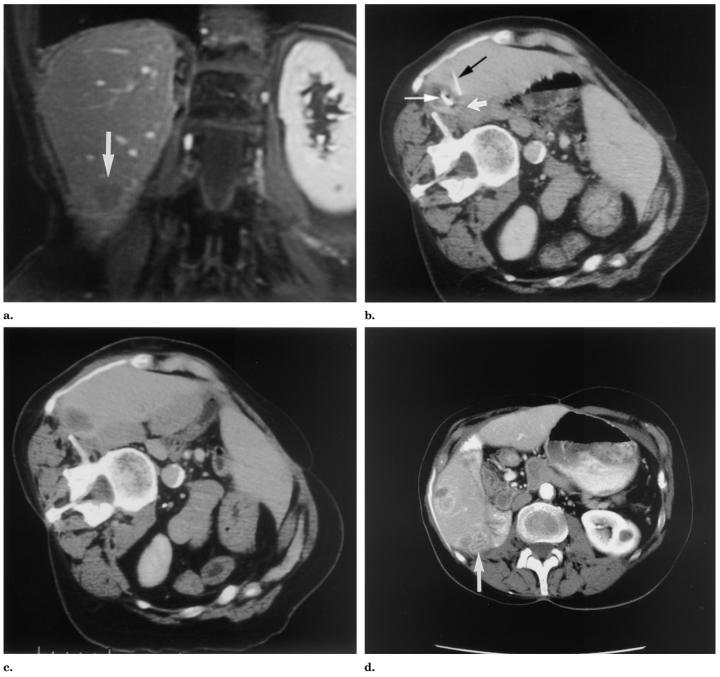
Patient 1 was a 68-year-old woman with a 2-cm liver metastasis from renal cell carcinoma. The liver metastasis was treated with RFA to render the liver disease-free. However, she had two other isolated metastases to her iliac bone and superficial flank, treated with radiation therapy and surgery, respectively. RFA was chosen because of the presence of extrahepatic metastases. The inferior margin of the liver lesion was touching the colon. An independent remote thermistor was placed in the liver near the colon 2 cm from the RF probe at the lesion margin (Fig 3).
Figure 3.
Patient 1: (a) Gadolinium contrast-enhanced T1-weighted coronal MR image (TR = 120, TE = 2) obtained 7 days before RFA for a 2-cm right liver lobe metastasis from renal cell carcinoma. The inferior lesion margin is in close approximation to the colon (arrow). (b,c) Delayed contrast-enhanced CT images obtained during and immediately after RFA. The intraprocedural image (b) shows the RF probe (thin white arrow) and thermistor (black arrow) at the margin of the thermal lesion near colon (thick white arrow), which is medial and inferior to lesion but not well seen on this image. The postprocedural image (c) shows the proximity of the low attenuation thermal lesion to the colon. (d) The 15-month follow-up contrast-enhanced CT shows recurrence along the bowel (arrow); however, without signs of damage to bowel.
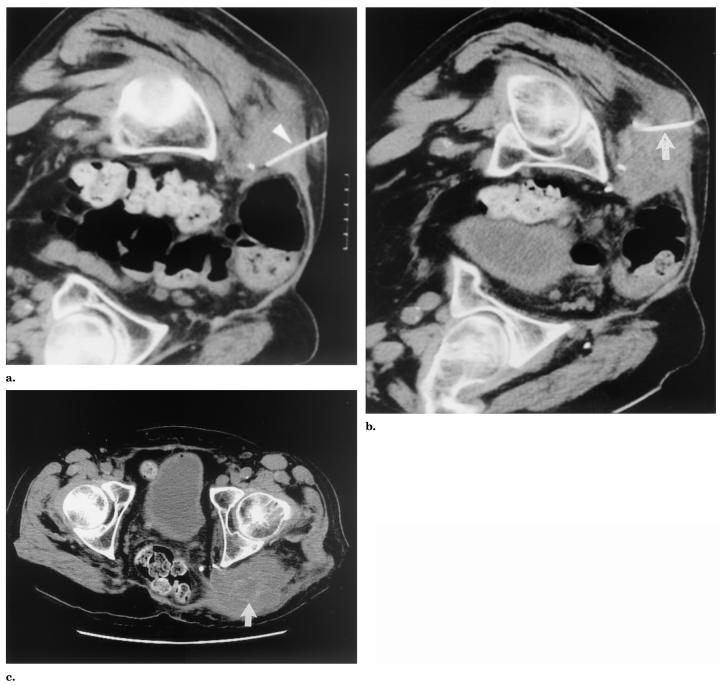
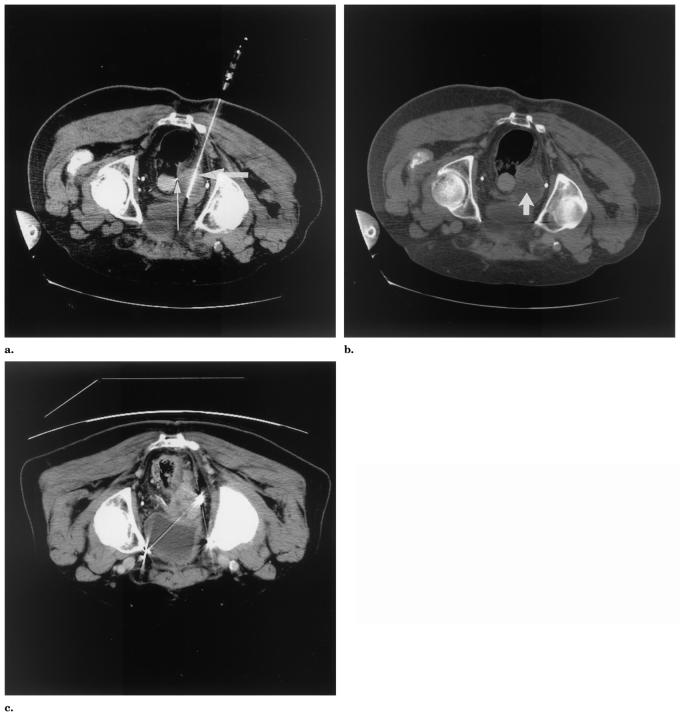
Patient 2 was a 57-year-old man with a history of chordoma with multiple metastases. RFA was applied to a 7-cm painful pararectal-gluteal tumor for pain control and to try to decrease sedating opiate dependence. Thermistors were positioned on either side of the mass, with one being positioned next to the rectum and the other next to the sciatic nerve (Fig 4).
Figure 4.
Patient 2: non–contrast-enhanced CT images (a, b) obtained during RFA for a 7-cm pararectal-gluteal tumor in a patient with metastatic chordoma. Thermistors (white arrowheads in a and b) positioned in target tissue adjacent to rectum (a) and sciatic nerve (b). The 5-month follow-up non–contrast-enhanced CT (c) shows increased size of tumor indicating regrowth (arrow).
Patient 3 was a 49-year-old woman with a history of breast cancer and a 4-cm painful left axillary metastasis, also treated with RFA for pain control. A thermistor was placed approximately between the mass and the lateral thoracic and ulnar nerves 2.5 cm from the RF probe (Fig 5).
Figure 5.
Patient 3: CT images obtained during (a) and immediately after (b) RFA for a 4-cm painful left axillary mass. (a) The pre-ablation non–contrast-enhanced CT image shows the RF probe (arrow) and the thermistor (arrowhead) adjacent to neurovascular structures likely including ulnar and lateral thoracic nerves at the edge of the tumor (seen as punctuate densities near the arrowhead, deep to the tumor). (b) The postablation contrast-enhanced CT image shows the low attenuation in the treated mass (arrow). (c) The 1-month follow-up contrast-enhanced CT shows continued low attenuation (arrow).
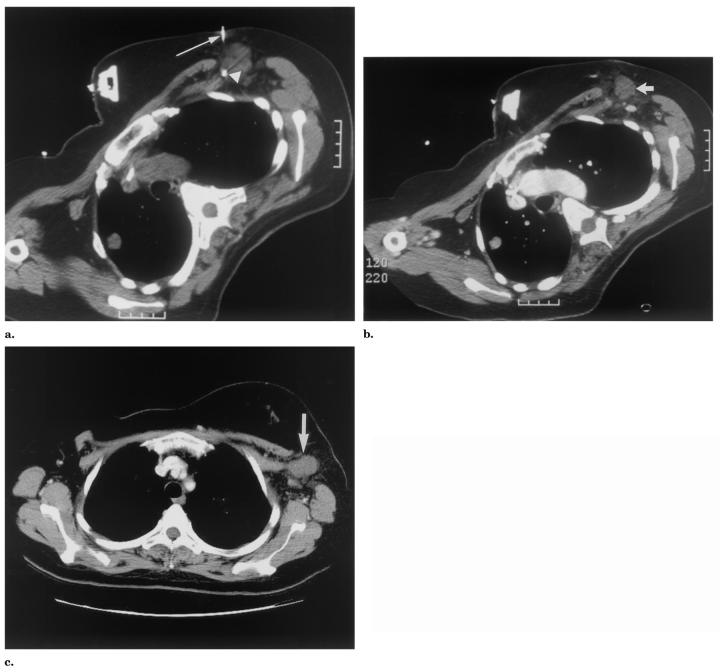
Patient 4 was a 72-year-old man with a history of metastatic prostate cancer status post radical retropubic prostatectomy and chemotherapy who had recurrent disease in the pelvis and was treated with RFA for pain control. Specifically, the patient experienced significant pain as well as hematuria and urinary outlet obstruction from a 5-cm perivesical, perirectal mass. An accessory endorectal probe/thermistor was positioned in the rectum near the tumor margin, 2.1 cm from the RF probe with three side-mounted thermistors directed lateral toward the tumor (Fig 6).
Figure 6.
Patient 4: Non–contrast-enhanced CT images obtained during (a) and immediately after (b) RFA for a 5-cm prostate cancer recurrence near the rectum. (a) The RF probe (thick arrow) was in the epicenter of the mass and the endorectal temperature probe (thin arrow) was near the tumor margin. (b) In the postprocedural image, a large, central, low-attenuation thermal lesion can be seen in the tumor. (c) The 7-month follow-up contrast-enhanced CT shows relatively stable size but recurrence of tumor based on enhancement.
RESULTS
The four patients underwent RFA treatments of various durations. Patients two and three received two sequential RFA treatments during one session. For patient 2, the pararectal thermistor was removed before the second, more remote superficial treatment. The temperature measurements for the four patients are shown in the Table. There was a brief time delay between cessation of the RFA current and the thermal peak. No patient experienced acute or delayed complications from the procedures. Rectal and urinary symptoms were unchanged after RFA in all patients.
Temperature Measurements of Target and Non-Target Tissues in Four Patients Undergoing Tumor RFA
| Target tissue |
Non-Target Tissue |
||||
|---|---|---|---|---|---|
| Patient | Histology/Location | Maximum Temperature During Cooling (°C) |
Non-Target Tissue | Maximum Temperature (°C) |
Time of Maximum Observed Remote Temperature (minutes) |
| 1 | Renal cell carcinoma/liver | 65 | Colon | 47 | 9 |
| 2 | Chordoma/pararectal | 88 | Rectum (1st ablation) | 44.8 | 9 |
| 88 | Sciatic nerve (1st ablation) | 42.7 | 9 | ||
| 94 | Sciatic nerve (2nd ablation) | 40.7 | 1 | ||
| 3 | Breast cancer/axilla | 77 | Ulnar/lateral thoracic nerves (1st ablation) | 41.7 | 12 |
| 79 | Ulnar/lateral thoracic nerves (2nd ablation) | 38.3 | 8 | ||
| 4 | Prostate carcinoma/pararectal | 62 | Rectum | 44.6 | 8 |
Note.—Maximum temperatures during cooling in the target tissue were obtained after the generator and internal saline cooling pump were turned off. For patient 2, the pararectal thermistor was removed before the second more superficial ablation. Patient 3 RFA was stopped intermittently for radiating pain across the chest wall, and transient alteration in neurological examination. For patient 4, the current was stopped intermittently because of high temperature information from the rectal thermistor.
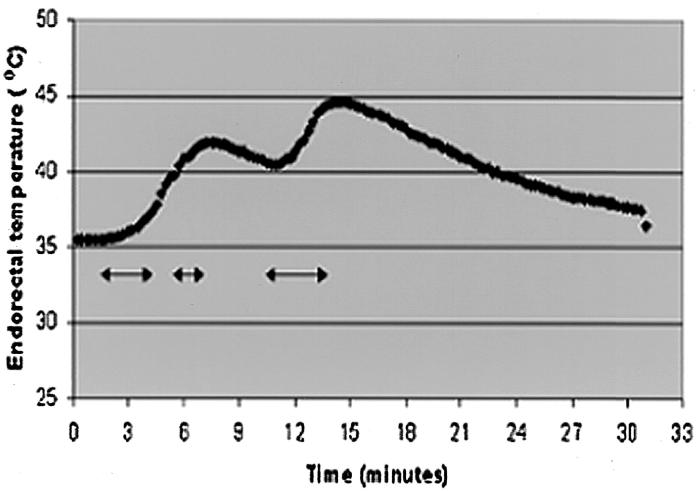
The recommended treatment protocol with the RFA system (Radionics) is 12 minutes to obtain the predetermined desired volume. In this study treatment durations were modified and shortened by remote thermometry in three of four patients. In the fourth patient, treatment was shortened by pain (Table). Maximum temperatures used for terminating the RFA were 47°C, 44.8°C, and 44.6°C. This temperature does not accurately represent bowel or nerve temperature because the thermistors were not actually inside these structures but just nearby. In patient 2, second ablation, the treatment was arrested before the normal 12 minutes because of the increase in echogenicity surrounding the tumor, which corresponds to the formation of tissue and water vapor microbubbles from the heated tissue and provides sonographic evidence of complete ablation of the small accessory tumor. Although US is a poor surrogate marker for completeness of ablation, in this case the tumor was very small and the echoes encompassed an area much larger than the tumor. In patient 3, treatment one, the total ablation time was the usual 12 minutes. However, this ablation was performed during a total time period of 30 minutes because of intermittent stopping for referred pain, which may be a harbinger of potential nerve damage. The goal of positioning remote thermistors was to prevent lethal heating at the non-target sites. In the case of patients 1, 2, and 4, better estimates of the thermal margin of treatment were made by monitoring temperature in or adjacent to colon or rectum, thus ensuring that elevations approaching 50°C did not occur. In patient 4, a real-time temperature graph obtained by the endorectal thermistor (Fig 7) resulted in the intermittent stopping of the treatment.
Figure 7.
Time-temperature curve obtained from the endorectal probe, with use of the thermistor closest to the lesion. The baseline temperature was 35.5°C. Treatment was initiated at 1.5 minutes and the arrows represent the time period of active ablation. The two peaks represent the maximum temperatures of 41.9°C and 44.6°C. Treatment was arrested shortly before these peaks because of the risk of higher temperatures to the rectum.
In patients 2 and 3, peripheral nerves were non-target tissues of interest. The maximum temperatures at these structures were kept below the 45°C that has been reported to be the threshold for RFA thermal damage for nervous tissue. The second treatment sphere in patient two was further away from the nerve, and thus showed only decreasing remote temperatures, consistent with ongoing cooling after the first treatment.
Patients 1 and 2 had tumors regrow along the margin near sensitive structures on 15- and 5-month follow-up, respectively. Patient 3 had no tumor regrowth evident on 1-month radio-graphic follow-up. Patient 4 had stable size at 7-month follow-up CT, but recurrence of tumor based on enhancement. No patient had clinical or radio-graphic evidence for thermal damage to sensitive structures (nerve and bowel).
DISCUSSION
Tumor RFA has a reported complication rate of less than 3% in many series (12,13). However, much higher complication rates have recently been reported in abstract form (14). This includes not only needle-related complications, but also thermal damage to non-target structures. The transition from dead to uninjured tissue is usually thin in most cases of liver RFA (15). While this narrow transition zone facilitates fairly precise treatment, the exact thermal lesion margins remain somewhat unpredictable. Reported instances of thermal damage to normal structures include, injury to the gall-bladder, bile ducts, and bowel (16,17). A number of strategies to protect non-target tissue have been described, including prophylactic measures as well as feedback methods. Prophylactic methods include careful preprocedural planning, ice bags, pro-peristaltic medications, chilled enemas, bladder emptying, and paracentesis. Monitoring or feedback methods include intraprocedural neurological exam and remote thermometry (18). Prophylactic measures are useful, but do not provide direct quantified feedback to decrease risk. Feedback methods such as remote thermometry allow change of treatment during the procedure. Remote thermistors may be placed independently of the RFA probe, and can measure intraprocedural non-target tissue temperatures to protect tissue and improve the safety of RFA. While several previous RF studies have employed temperature sensors to analyze the distribution of RF induced heating in experimental models (4,19), the use of such sensors as a safety mechanism to avoid complications of thermal damage to non-target tissue in humans is a natural next step. Goldberg et al employed remote sensors in ex vivo animal experiments to determine coagulation sphere diameters (17). Dupuy et al used remote thermistors in animal experiments to analyze temperature distribution as RF energy was applied to pig vertebral bodies in vivo (4) In the four patients described in this study, remote thermometry allowed monitoring temperature changes near various vital structures. According to the remote temperature information, the RFA treatment was modified in energy delivery or in treatment duration to limit risk to collateral structures. This temperature information may guide thermal lesioning based on the thermal thresholds of damage to that specific tissue (eg, lower for nerves). Proper positioning of the thermistor between the RFA probe and the collateral, at-risk structure makes overestimation of temperature more likely than underestimation, if the thermistor is closer to the heat than the collateral tissue. This could result in undertreatment of tumors with resulting regrowth as may have happened in three of four patients in this study. However, the alternative to undertreatment could be permanent or catastrophic thermal damage of non-target organs. Development of a tumor specific heating adjuvant would have great benefit in such cases.
The maximum equilibrium temperature after shutting off the current (with cool tip RFA probe) corresponded to ablation volume and low temperatures may suggest persistent perfused and viable tissue at the needle tip (20).
Thermometry may be a more reliable surrogate marker of tissue damage than currently employed imaging parameters, which may be unreliable (especially US without contrast material). Ongoing evaluation with US during RFA may be difficult because of echogenic organic microbubbles released from cooking tissue. An additional 22-gauge needle thermistor or endorectal probe has minimal risk, given the added benefits, and gives continuous information, unlike other imaging modalities.
Intraprocedural temperature monitoring may play an increasingly important role as RFA technology evolves. For tumors near collateral structures, there is a trade-off between aggressive treatment, which risks complications, and undertreatment, which risks residual tumor. Any thermometry method may help the operator walk this tightwire. A clear limitation of the monitoring technique described here is that temperature information is provided for discrete points only. The ideal method would provide three-dimensional, volumetric data, thus more reliably indicating the temperature changes in the entire tissue of interest. Eventually, magnetic resonance (MR) imaging thermometry may provide such real-time volumetric information. Along with the technique described here, MR imaging thermometry may have added importance with the development of larger treatment spheres with multiple array electrodes, saline-enhanced RFA, energy pulsing, and internally-cooled needles. Independent thermometry remote from the treatment probe can provide information on the extent of tissue necrosis in the target lesion and also may help preserve vital structures in the area surrounding the lesion.
Abbreviation
- RFA
radiofrequency ablation
Footnotes
None of the authors have identified a potential conflict of interest.
References
- 1.Wood BJ, Ramkaransingh JR, Fojo T, Walther MM, Libutti SK. Percutaneous tumor ablation with radiofrequency. Cancer. 2002;94:443–451. doi: 10.1002/cncr.10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nahum Goldberg S, Dupuy DE. Image-guided radiofrequency tumor ablation: challenges and opportunities–part I. J Vasc Interv Radiol. 2001;12:1021–1032. doi: 10.1016/s1051-0443(07)61587-5. [DOI] [PubMed] [Google Scholar]
- 3.Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia. 1994;10:457–483. doi: 10.3109/02656739409009351. [DOI] [PubMed] [Google Scholar]
- 4.Dupuy DE, Hong R, Oliver B, Goldberg SN. Radiofrequency ablation of spinal tumors: temperature distribution in the spinal canal. AJR Am J Roentgenol. 2000;175:1263–1266. doi: 10.2214/ajr.175.5.1751263. [DOI] [PubMed] [Google Scholar]
- 5.Yamane T, Tateishi A, Cho S, et al. The effects of hyperthermia on the spinal cord. Spine. 1992;17:1386–1391. doi: 10.1097/00007632-199211000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Froese G, Das RM, Dunscombe PB. The sensitivity of the thoracolumbar spinal cord of the mouse to hyperthermia. Radiat Res. 1991;125:173–180. [PubMed] [Google Scholar]
- 7.Tungjitkusolmun S, Woo EJ, Cao H, Tsai JZ, Vorperian VR, Webster JG. Finite element analyses of uniform current density electrodes for radio-frequency cardiac ablation. IEEE Trans Biomed Eng. 2000;47:32–40. doi: 10.1109/10.817617. [DOI] [PubMed] [Google Scholar]
- 8.Larson BT, Bostwick DG, Corica AG, Larson TR. Histological changes of minimally invasive procedures for the treatment of benign prostatic hyperplasia and prostate cancer: clinical implications. J Urol. 2003;170:12–19. doi: 10.1097/01.ju.0000072200.22089.c3. [DOI] [PubMed] [Google Scholar]
- 9.Sugarbaker PH, Sugarbaker C, Stephens AD, Chang D. Radiofrequency hyperthermia in the palliative treatment of mucinous carcinomatosis of appendiceal origin: optimizing and monitoring heat delivery in western patients. Int J Hyperthermia. 2000;16:429–441. doi: 10.1080/026567300416721. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz MD, Kaplan ID, Svensson GK, Hynynen K, Hansen MS. Feasibility and patient tolerance of a novel transrectal ultrasound hyperthermia system for treatment of prostate cancer. Int J Hyperthermia. 2001;17:31–37. doi: 10.1080/02656730150201570. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan SA, Te AE. Transurethral electrovaporization of the prostate: a novel method for treating men with benign prostatic hyperplasia. Urology. 1995;45:566–572. doi: 10.1016/S0090-4295(99)80044-2. [DOI] [PubMed] [Google Scholar]
- 12.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. doi: 10.1097/00000658-199907000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solbiati L. New applications of ultra-sonography: interventional ultrasound. Eur J Radiol. 1998;27(suppl 2):S200–S206. doi: 10.1016/s0720-048x(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 14.Elias D, Risse O, Kuoch V, Letoublon C, Smayra T. Complications of percutaneous and per-operative radiofrequency ablation of liver tumors. Scientific Paper at Society for Cardiovascular and Interventional Radiology annual meeting. J Vasc Interv Radiol. 2002;13:S63. [Google Scholar]
- 15.Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologicpathologic correlation. Cancer. 2000;88:2452–2463. [PubMed] [Google Scholar]
- 16.Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities–part II. J Vasc Interv Radiol. 2001;12:1135–1148. doi: 10.1016/s1051-0443(07)61670-4. [DOI] [PubMed] [Google Scholar]
- 17.Livraghi T, Solbiati L, Meloni F, Ierce T, Goldberg SN. Complications after cool-tip RF ablation of liver cancer: initial report of the Italian Multicenter Cool-tip RF Study Group (abstract) Radiology. 2000;217(suppl):18. [Google Scholar]
- 18.Wood BJ, Mikityansky I, Pavlovich C, et al. Avoiding complications in radiofrequency tumor ablation. Scientific Paper at Society for Cardiovascular and Interventional Radiology annual meeting. J Vasc Interv Radiol. 2001;12S:42. [Google Scholar]
- 19.Goldberg SN, Stein MC, Gazelle GS, Sheiman RG, Kruskal JB, Clouse ME. Percutaneous radiofrequency tissue ablation: optimization of pulsed-radiofrequency technique to increase coagulation necrosis. J Vasc Interv Radiol. 1999;10:907–916. doi: 10.1016/s1051-0443(99)70136-3. [DOI] [PubMed] [Google Scholar]
- 20.Chang I, Mikityansky I, Wary-Cahen D, Pritchard WF, Karanian J, Wood BJ. Effects of perfusion of radiofrequency ablation of swine kidneys. Radiology. doi: 10.1148/radiol.2312021248. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]